Abstract
Large interindividual variance is observed in both response and toxicity associated with chemotherapy. Our goal is to identify factors that contribute to chemotherapy-induced toxicity. To this end, we used EBV-transformed B-lymphoblastoid HapMap cell lines derived from 30 Yoruban trios (African descent) and 30 Centre d' Etude du Polymorphisme Humain (CEPH) trios (European descent) to evaluate population- and gender-specific differences in cytotoxicity of carboplatin, cisplatin, daunorubicin, and etoposide using a high-throughput, short-term cytotoxicity assay. The IC50 was compared for population- and gender-specific differences for the four drugs. We observed large interindividual variance in IC50 values for carboplatin, cisplatin, daunorubicin, and etoposide for both Yoruban and CEPH populations (range from 8- to 433-fold). Statistically significant differences in carboplatin and daunorubicin IC50 were shown when comparing Yoruban cell lines (n = 89) to CEPH cell lines (n = 87; P = 0.002 and P = 0.029, respectively). This population difference in treatment induced cytotoxicity was not seen for either cisplatin or etoposide. In the Yoruban population, cell lines derived from females were less sensitive to platinating agents than males [median carboplatin IC50, 29.1 versus 24.6 μmol/L (P = 0.012); median cisplatin IC50, 7.0 versus 6.0 μmol/L (P = 0.020) in female and male, respectively]. This difference was not observed in the CEPH population. These results show that population and gender may affect risk for toxicities associated with certain chemotherapeutic agents.
Introduction
Treatment-induced toxicity has hindered the use of chemotherapeutic agents to their full potential. Different individuals respond differently to the same chemotherapeutic agent. Genetic, physiologic, and environmental factors are likely to contribute to individual variation (1). To improve the success of chemotherapy, a better understanding of the interindividual variation in both response and toxicity of chemotherapy is necessary. Limited clinical trials have suggested that population is a significant predictor of chemotherapy response (2) as well as toxicity (3). Furthermore, female gender has been indicated as a predictor of treatment response in non–small-cell lung cancer (4, 5), retinoblastoma (6, 7), and glioblastoma (8). Specifically in patients receiving carboplatin therapy, females are more likely than males to experience pulmonary toxicity or to develop oral mucositis (9, 10).
Although these clinical studies have suggested population and gender roles in the frequency of toxicities associated with chemotherapy, the underlying cause of these differences is not known. Clinical drug responses reflect not only properties intrinsic to the target cell but also host metabolic properties, drug-drug interactions, and pharmacokinetics. Therefore, to evaluate population and gender effects and to begin to understand how genetic variations contribute to these effects, we used cell lines from the International HapMap Consortium4 derived from healthy individuals from Yoruba (Africa) and Utah (United States) to study differences in the cytotoxic effect of chemotherapy. The HapMap cell lines have publicly available genetic information that will allow us to further study the genetic variants important in observed differences. In this article, we evaluated the influence of population and gender on four chemotherapeutic agent–induced cytotoxicities using HapMap cell lines.
Materials and Methods
Materials
EBV-transformed B-lymphoblastoid cell lines from 30 trios (mother, father, and child) of healthy Yorubans (Ibadan, Africa) and 30 trios of Centre d' Etude du Polymorphisme Humain (CEPH; Caucasians of Northern and Western European descent) were used. These cell lines were obtained from Coriell Cell Repositories (Camden, NJ).5 RPMI 1640, containing 4.5 g/L glucose and l-glutamine, was purchased from Mediatech, Inc. (Herndon, VA). Fetal bovine serum was purchased from HyClone (Logan, UT). PBS was purchased from Life Technologies, Inc. Invitrogen (Carlsbad, CA). Carboplatin, cisplatin, and DMSO were purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Daunorubicin and etoposide were obtained from National Cancer Institute Drug Synthesis and Chemistry Branch (Bethesda, MD).
Cell Growth Inhibition Experiments
Cell lines were maintained in RPMI 1640 supplemented with 15% fetal bovine serum and 1% l-glutamine. Cell lines were passaged thrice a week and seeded at a concentration of 350,000 cells/mL at 37°C in a 95% humidified 5% CO2 atmosphere. Drug treatment was quantified at passages 3 to 8. Cell growth inhibition experiments were assessed using alamarBlue® assay (BioSource International, Inc., Camarillo, CA). Briefly, at the time of passage, cell suspensions (100 μL, 1 × 105 cells/mL) were added into 96-well round-bottomed plates (Corning, Inc., Corning, NY). Experiments were conducted only on cell lines showing >85% viability on the day of plating. After 24-h incubation, treatment was started by adding 100 μL of increasing concentrations of treatment solutions. The treatment concentrations and exposure times were optimized in our laboratory so that we observed a full spectrum of cytotoxicity. Carboplatin was dissolved in water. Cisplatin and etoposide were dissolved in DMSO. Daunorubicin was dissolved in PBS. Carboplatin concentrations used were 0 (medium only), 2.5, 5, 10, 20, 40, 80, 120, and 160 μmol/L. Cisplatin concentrations used were 0, 0.1% DMSO (vehicle control), 0.5, 1, 2.5, 5, 10, 20, 40, and 80 μmol/L. Daunorubicin treatment concentrations used were 0 (medium only), 0.0125, 0.025, 0.05, 0.1, 0.2, and 1 μmol/L. Etoposide treatment concentrations used were 0.025% DMSO (vehicle control), 0.02, 0.1, 0.5, and 2.5 μmol/L. Cells were treated with cisplatin for 48 h and with carboplatin, daunorubicin, and etoposide for 72 h. All experiments were conducted at least twice in triplicate for each concentration. Twenty microliters of alamarBlue® were added to each well 24 h before colorimetric detection to determine the percent cell growth inhibition relative to control. Absorbances at 570 and 600 nm were recorded using a Synergy HT plate reader (BioTek Instruments, Inc., Winooski, VT). Percent survival was quantified according to the manufacturer's protocol.6
Data Analysis
IC50 was determined by curve fitting of percent cell survival against concentrations of drug. To examine the population and gender effects on drug treatment, a general linear model was constructed with IC50 values transformed using the natural logarithm (ln) as the dependent variable and dichotomous indicators for population and gender as the independent variables. The dependent variable was transformed to satisfy the assumption of normality. Trios were treated as units of analysis, and members of different families were considered independent. The covariance structure within a trio was modeled using a Toeplitz structure with two diagonal bands, such that the trios are ordered father, offspring, and then mother. With this covariance structure, mother and father IC50 values are independent but the offspring's value is allowed to covary with both father and mother's values. The same general linear model was also constructed with the ln transformed percent cell survival as the dependent variable at each drug treatment concentration. In addition, to explore within population and within gender effects, separate models were constructed within each gender with only an indicator of population as a predictor. Similarly, separate models were also constructed within each population with only an indicator of gender as a predictor. P < 0.05 was considered statistically significant. All models were programmed using the PROC MIXED procedure in SAS/STAT software (version 9.1 of the SAS System for Windows, SAS Institute, Inc., Cary, NC). The REPEATED statement was used to allow modeling the Toeplitz covariance structure.
Results
Cell Viability after Drug Treatment
EBV-transformed B-lymphoblastoid cell lines derived from Yoruban trios (n = 89) and CEPH trios (n = 87) were examined using the alamarBlue® assay. Four cell lines (one Yoruban and three CEPH) failed to reach 85% viability on the experiment day on more than three attempts and therefore were not further evaluated. Carboplatin, cisplatin, daunorubicin, and etoposide all showed dose-dependent cytotoxicity (Fig. 1; Table 1). The same cell line showed different sensitivity to each drug (Fig. 1). We observed considerably large interindividual variation in IC50 in both Yoruban and CEPH cell lines. Within the Yoruban population, there were 12-, 17-, 54-, and 222-fold variations for carboplatin, cisplatin, daunorubicin, and etoposide IC50s, respectively. We also observed 8-, 49-, 40-, and 433-fold variations in IC50s for CEPH.
Figure 1.

Sensitivity of lymphoblastoid cell lines derived from CEPH and Yorubans to four chemotherapeutic drugs. Percent cell survival after carboplatin (■), cisplatin (▲), daunorubicin (▼), and etoposide (◆) 72-h treatment (except cisplatin, exposure time = 48 h) in cell lines derived from Yoruban (A; solid symbols) and CEPH (B; open symbols) populations. Points, mean (n = 89 for Yoruban and n = 87 for CEPH); bars, SD.
Table 1. Median and range of carboplatin, cisplatin, daunorubicin and etoposide IC50s in Yoruban and CEPH.
| Drug | Yoruban |
CEPH |
||
|---|---|---|---|---|
| Female (μmol/L) | Male (μmol/L) | Female (μmol/L) | Male (μmol/L) | |
| Carboplatin*,† | 29.1 (15.3 – 141.1) | 24.6 (11.8 – 70.3) | 20.4 (10.4 – 49.2) | 20.1 (8.6 – 70.4) |
| Cisplatin† | 7.0 (2.2 – 37.4) | 6.0 (2.2 – 27.1) | 5.2 (1.8 – 25.8) | 5.1 (1.5 – 73.9) |
| Daunorubicin* | 0.05 (0.02 – 0.87) | 0.05 (0.02 – 0.27) | 0.04 (0.01 – 0.23) | 0.04 (0.01– 0.30) |
| Etoposide‡ | 0.47 (0.10 – 17.89) | 0.37 (0.09 – 5.38) | 0.43 (0.04 – 7.00) | 0.46 (0.11 – 17.31) |
Statistically significant difference between Yoruban and CEPH.
Statistically significant difference between female and male in Yoruban.
Statistically significant difference between female and male in CEPH.
Population Effect on Drug-Induced Cytotoxicity
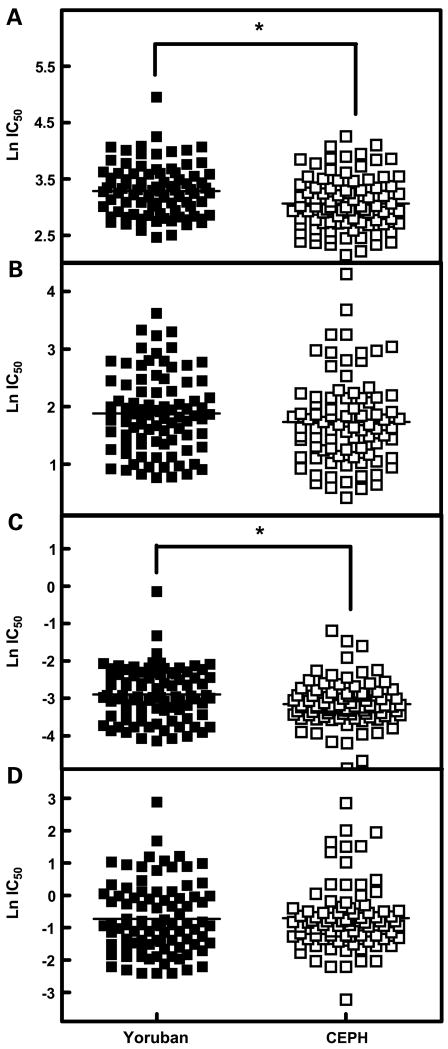
Carboplatin IC50 was higher in the Yoruban versus the CEPH population with a median IC50 of 26.8 μmol/L (Yoruban) versus 20.2 μmol/L (CEPH). Similarly, median daunorubicin IC50 was 0.05 μmol/L for Yoruban versus 0.04 μmol/L for CEPH-derived cells. This was statistically significant with P = 0.002 and P = 0.029 for carboplatin and daunorubicin, respectively (Table 1; Fig. 2A and C). In models of females and males separately, population was statistically significant within females (P = 0.0006) and not significant within males (P = 0.298) for carboplatin (Fig. 3A). One Yoruban cell line had high IC50 (indicating high resistance) to all four drugs. After removing this potential outlier, population difference remained statistically significant for carboplatin (P = 0.003) and was trending towards significance for daunorubicin (P = 0.051). The general linear models described above were also constructed with the ln transformed percent cell survival as the dependent variable for each treatment dose. Population was a statistically significant predictor of cell survival at all doses for carboplatin (10, 20, 40, and 80 μmol/L) and at 0.1 and 0.2 μmol/L doses for daunorubicin.
Figure 2.
Effect of population on sensitivity of cell lines to four chemotherapeutic drugs. The ln IC50s of carboplatin (A), cisplatin (B), daunorubicin (C), and etoposide (D) were calculated and plotted in cell lines derived from Yoruban and CEPH populations. Data presented as each individual cell line ln IC50 in Yoruban (■) and CEPH (□). Horizontal line, median value. *, P < 0.05.
Figure 3.
Effect of gender on sensitivity of cell lines to four chemotherapeutic drugs. The ln IC50s of carboplatin (A), cisplatin (B), daunorubicin (C), and etoposide (D) were calculated and plotted in cell lines derived from Yoruban and CEPH populations. Data presented as each individual cell line ln IC50 in Yoruban female (●), Yoruban males (▲), CEPH females (○), and CEPH males (Δ). *, P < 0.05.
Cisplatin showed similar population differences in cytotoxicity, with a higher median IC50 in Yoruban (6.3 μmol/L) compared with CEPH (5.1 μmol/L), although this effect was not found to be statistically significant (Table 1; Fig. 2B).
Etoposide IC50 values for Yoruban (0.40 μmol/L) and CEPH (0.43 μmol/L) were not significantly different (P = 0.838; Table 1; Fig. 2D).
Gender Effect on Drug-Induced Cytotoxicity
There were 42 males and 44 females in the CEPH population and 51 males and 37 females in the Yoruban population. In the Yoruban population, cell lines derived from females were less sensitive to platinating agents than cell lines derived from males. The median carboplatin IC50 was 29.1 for females versus 24.6 μmol/L for males, and median cisplatin IC50 was 7.0 for females versus 6.0 μmol/L for males (Table 1; Fig. 3A and B). The F test showed that gender was a significant predictor of ln (IC50) values for carboplatin (P = 0.012) and cisplatin (P = 0.020) in this population. After removing the potential female Yoruban outlier, these gender differences remained statistically significant. There was a trend of higher percent cell survival in Yoruban females than in Yoruban males at each treatment concentration for both carboplatin and cisplatin (data not shown).
The gender difference in response to drug was not seen for daunorubicin and etoposide in the Yoruban population (Fig. 3C and D). Additionally, we did not observe a gender difference for carboplatin, cisplatin, and daunorubicin cytotoxicity in the CEPH population (Fig. 3A–C), although gender did play a role in the response to etoposide in CEPH [median IC50, 0.43 μmol/L (female) versus 0.46 μmol/L (male); P = 0.048; Table 1; Fig. 3D].
Discussion
Our study showed a wide range of interindividual variation in cytotoxic response to carboplatin, cisplatin, daunorubicin, and etoposide using lymphoblastoid cell lines from normal individuals. Significant differences in sensitivity to carboplatin- and daunorubicin-induced cytotoxicity in cell lines derived from individuals of African and European descent imply that populations may vary in their susceptibility to chemotherapeutic toxicity. Cell lines from Yoruban females are less sensitive to carboplatin than Caucasian females. Yoruban female cell lines are also less sensitive to carboplatin and cisplatin than cell lines from their male counterparts. The population and gender effects associated with chemotherapy seem to be dependent on the chemotherapeutic drug.
The effect of population on antitumor activity and toxicity associated with chemotherapy has not been well studied. However, our model focuses on differences in normal cells, not tumor cells. A few clinical trials do support the role of population in response and toxicity of chemotherapy. For example, Millward et al. (2) have shown that ethnicity was a significant predictor of response to docetaxel and carboplatin treatment in advanced non–small-cell lung cancer. Furthermore, African American head and neck cancer patients were more likely to develop cisplatin-induced nephrotoxicity (11) and less likely to develop hypothyroidism when compared with Caucasians (12). Hansen et al. (3) observed cardiotoxicity more frequently in African American patients who received doxorubicin. HapMap cell lines from different populations will allow researchers to begin to unravel the underlying genetic variants that result in these population differences in drug-induced toxicities observed clinically by evaluating publicly available genotypic data4 and expression data.7
One limitation that we acknowledge is that the collection time for the CEPH cell lines was ∼15 years earlier than that for the Yoruban cell lines. This may contribute to the phenotypic difference observed between these two populations; however, we only observed population differences for two drugs among the four drugs tested. More likely, different sensitivities to drugs are due to differences in frequencies of specific genetic variants that may lead to differences in gene expression or function of the protein between the populations. We are currently investigating genetic variants and expression differences that contribute to these differences in sensitivity to drug. Dabholkar et al. (13) have indicated that the increased expression of xeroderma pigmentosum group A, a nucleotide excision repair protein, in ovarian cancer is associated with resistance to platinum treatment. Our preliminary gene expression analysis showed significant higher xeroderma pigmentosum group A expression in Yoruban when compared with CEPH (data not shown). This was in agreement with our hypothesis that higher DNA repair protein expression in Yoruban leads to less sensitivity (higher IC50) to carboplatin in the same population.
Multiple clinical trials have also suggested that female gender is a predictor of chemotherapy response as well as toxicity. Female gender has been found to be significantly associated with an increased likelihood of receiving second-line therapy to improve survival and quality of life in patients with non–small-cell lung cancer after initial carboplatin and paclitaxel therapy (14). In terms of response, a retrospective analysis on stage III non–small-cell lung cancer indicated that females survived longer than males overall and had longer local recurrence-free survival when treated with high-dose hyperfractionated radiation therapy and concurrent carboplatin and etoposide therapy (4). In terms of toxicity associated with gemcitabine and carboplatin, female gender was associated with more profound changes in posttreatment diffusion capacity for carbon monoxide, an indicator of pulmonary toxicity (9). Female gender is also associated with developing oral mucositis, a standard-dose chemotherapy toxicity for patients treated with multiple chemoregimens (10).
Our study showed significantly higher IC50 for platinating agents in cell lines derived from Yoruban females than in those derived from Yoruban males. This gender difference could translate into lower toxicity of these agents in Yoruban females. Evaluation of gender effects on chemotherapeutic-induced toxicity in a target population (i.e., Africans) may be warranted. When the gene expression of lymphoblastoid cell lines derived from 115 female and 118 males CEPH individuals was compared using the Affymetrix Human Genome Focus Array,7 we identified four genes (FOXO1A, JUP, KLF2, and ZNF706) with higher expression in females and six genes (CTNND1, FEZ1, FFAR2, LGALS1, LMNA, and TNFSF9) with higher expression in males (15). Therefore, it is plausible that the gender difference in phenotype may be the result of epigenetic, posttranscriptional, or posttranslational regulation and/or hormone-related or baseline expression differences. We are currently working on identifying genetic variants that contribute to gender differences using expression array data.
Whereas tumor cell lines may be more appropriate for the study of the antitumor response to chemotherapeutic agent effects, the goal of our model is to eventually identify genetic variants in patients before treatment with chemotherapy so that patients at risk for toxicities can be given a dose-reduced regimen. One limitation of the model is that it only represents one tissue and may not represent protein expression in a tissue of known toxicity. Therefore, we may not capture the genetic contribution of all genes contributing to chemotherapy-induced cytotoxicity in various tissues. However, given the extensive genetic information, these HapMap cell lines remain to be one of the best tools to identify genetic variants that may predict toxicities. The cell lines will be used to identify genes important in the pharmacodynamics of the drug without confounding variables from pharmacokinetic parameters. There is some concern that phenotypes of lymphoblastoid cell lines may be altered by EBV-mediated transformation. However, Huang et al. (16) have shown that the morphology of the transformed cells was similar to that of phytohemagglutinin-stimulated peripheral blood lymphocytes, and the observation on the variation in gene expression profiles in lymphoblastoid cell line clusters by families (17) indicates that genetic factors, such as single-nucleotide polymorphisms, drive gene expression; therefore, it is more likely that phenotypic variation is related to genetic variables that control expression.
Our ultimate goal is to improve therapy through the identification of genetic variants dictating chemotherapeutic agent–induced toxicity. To this end, we chose cell lines that are part of the International HapMap consortium and have publicly available genotypic data, which will allow us to compare the frequency and spectrum of genetic variants in target genes from individuals of African and European descent associated with susceptibility to drugs. To our knowledge, we are the first to illustrate a significant population and gender difference in susceptibility to drug-induced cytotoxicity in these HapMap cell lines. Further efforts to identify genetic variants or expression differences that provide mechanistic explanations for population and gender differences in chemotherapeutic agent–induced cytotoxicity will follow.
Acknowledgments
We thank Dr. Jeong-Ah Kang for excellent technical support in maintaining the cell lines and Cheryl Roe for critical review of the manuscript.
Grant support: This Pharmacogenetics of Anticancer Agents Research Group (http://pharmacogenetics.org) study was supported by NIH/National Institute of General Medical Sciences grant GM61393 and by NIH/National Institute of General Medical Sciences Pharmacogenetics Research Network and Database (U01GM61374, http://pharmgkb.org/; Russ Altman, Principal Investigator).
Footnotes
References
- 1.Spitz MR, Wu X, Mills G. Integrative epidemiology: from risk assessment to outcome prediction. J Clin Oncol. 2005;23:267–75. doi: 10.1200/JCO.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 2.Millward MJ, Boyer MJ, Lehnert M, et al. Docetaxel and carboplatin is an active regimen in advanced non-small-cell lung cancer: a phase II study in Caucasian and Asian patients. Ann Oncol. 2003;14:449–54. doi: 10.1093/annonc/mdg118. [DOI] [PubMed] [Google Scholar]
- 3.Hasan S, Dinh K, Lombardo F, Kark J. Doxorubicin cardiotoxicity in African Americans. J Natl Med Assoc. 2004;96:196–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Jeremic B, Milicic B, Dagovic A, Aleksandrovic J, Milisavljevic S. Stage III non-small-cell lung cancer treated with high-dose hyperfractionated radiation therapy and concurrent low-dose daily chemotherapy with or without weekend chemotherapy. Am J Clin Oncol. 2004;27:350–60. doi: 10.1097/01.coc.0000071463.72269.2a. [DOI] [PubMed] [Google Scholar]
- 5.Jeremic B, Milicic B, Dagovic A, Aleksandrovic J, Nikolic N. Pretreatment clinical prognostic factors in patients with stage IV non-small cell lung cancer (NSCLC) treated with chemotherapy. J Cancer Res Clin Oncol. 2003;129:114–22. doi: 10.1007/s00432-002-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunduz K, Gunalp I, Yalcindag N, et al. Causes of chemoreduction failure in retinoblastoma and analysis of associated factors leading to eventual treatment with external beam radiotherapy and enucleation. Ophthalmology. 2004;111:1917–24. doi: 10.1016/j.ophtha.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Mashayekhi A, Carter J, Shelil A, Meadows AT, Shields JA. Chemoreduction for retinoblastoma. Analysis of tumor control and risks for recurrence in 457 tumors. Am J Ophthalmol. 2004;138:329–37. doi: 10.1016/j.ajo.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Puchner MJA, Herrmann HD, Berger J, Cristante L. Surgery, tamoxifen, carboplatin, and radiotherapy in the treatment of newly diagnosed glioblastoma patients. J Neurooncol. 2000;49:147–55. doi: 10.1023/a:1026533016912. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulou I, Efstathiou E, Samakovli A, et al. A prospective study on lung toxicity in patients treated with gemcitabine and carboplatin: clinical, radiological and functional assessment. Ann Oncol. 2004;15:1250–5. doi: 10.1093/annonc/mdh311. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg SL, Chiang L, Selina N, Hamarman S. Patient perceptions about chemotherapy-induced oral mucositis: implications for primary/secondary prophylaxis strategies. Support Care Cancer. 2004;12:526–30. doi: 10.1007/s00520-004-0640-3. [DOI] [PubMed] [Google Scholar]
- 11.Shord SS, Thompson DM, Krempl GA, Hanigan MH. Effect of concurrent medications on cisplatin-induced nephrotoxicity in patients with head and neck cancer. Anticancer Drugs. 2006;17:207–15. doi: 10.1097/00001813-200602000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Mercado G, Adelstein DJ, Saxton JP, Secic M, Larto MA, Lavertu P. Hypothyroidism. Cancer. 2001;92:2892–7. doi: 10.1002/1097-0142(20011201)92:11<2892::aid-cncr10134>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest. 1994;94:703–8. doi: 10.1172/JCI117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensing TA, Schell MJ, Lee JH, Socinski MA. Factors associated with the likelihood of receiving second line therapy for advanced non-small cell lung cancer. Lung Cancer. 2005;47:253–9. doi: 10.1016/j.lungcan.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Bleibel WK, Roe CA, Cox NJ, Dolan ME. Gender-specific differences in expression in lymphoblastoid cell lines. Pharmacogenetics and genomics. 2007 doi: 10.1097/FPC.0b013e3280121ffe. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CC, Moore GE. Chromosomes of 14 hematopoietic cell lines derived from peripheral blood of persons with and without chromosome anomalies. J Natl Cancer Inst. 1969;43:1119–28. [PubMed] [Google Scholar]
- 17.Cheung V, Conlin L, Weber T, et al. Natural variation in human gene expression assessed in lymphoblastoid cells. Nat Genet. 2003;33:422–5. doi: 10.1038/ng1094. [DOI] [PubMed] [Google Scholar]