Abstract
Preclinical data indicate that α6β4 integrin signaling through Ras homolog gene family, member A, plays an important role in tumor cell motility. The objective of this study was to determine whether the combined expression of α6β4 integrin and neuroepithelioma transforming gene 1 (Net1), a guanine nucleotide exchange factor specific for Ras homolog gene family member A, is associated with adverse clinical outcome in breast cancer patients. Immunohistochemical expression of each protein was evaluated in a tumor tissue microarray prepared from the primary tumors of 94 node-positive patients with invasive breast carcinoma treated with total mastectomy and doxorubicin-based chemotherapy without radiation with a median follow-up of 12.5 years. Associations between staining results and multiple clinicopathologic variables were investigated. Although there was no significant association between α6β4 integrin or Net1 expression and clinical outcome when each marker was considered individually, coexpression of α6β4 and Net1 was associated with decreased distant metastasis–free survival (P = 0.030). In the subset of patients with hormone receptor–positive tumors, coexpression of α6β4 and Net1 was associated with a decrease in distant metastasis–free and overall survival (P < 0.001 and P = 0.006, respectively). Although an association between human epidermal growth factor receptor 2 expression and coexpression of α6β4 and Net1 (P = 0.008) was observed, coexpression of α6β4 and Net1 (hazard ratio, 1.63; P = 0.02) and lymphovascular invasion (hazard ratio, 2.35; P = 0.02) were the only factors independently associated with the development of distant metastasis in multivariate analysis. These findings suggest that coexpression of α6β4 integrin and Net1 could be a useful biomarker for aggressive disease in node-positive breast cancer patients.
Introduction
The α6β4 integrin is a protein heterodimer that functions as a receptor for laminin isoforms, including laminin-5, a component of epithelial basement membranes (1-10). Preclinical data suggest that α6β4 signaling plays an important role in tumor cell motility and invasion (2-5, 7, 11-13). Rho family small G proteins function as GTPases downstream of integrins (14-16). The α6β4 integrin has been shown to signal through Ras homolog gene family member A (RhoA), and α6β4-mediated activation of RhoA is essential for the ability of this integrin to promote carcinoma migration and invasion (5, 7). Neuroepithelioma transforming gene 1 (Net1) is a RhoA-specific guanine nucleotide exchange factor, which controls the activation state of RhoA (17-23).
Many integrins are difficult to evaluate by immunohistochemistry in archival tissues (24, 25), but we recently found that a modified heat-induced antigen retrieval method greatly improves immunohistochemical staining for the β4 integrin subunit in formalin-fixed, paraffin-embedded tissue sections. The objective of this study was to examine the expression of α6β4 integrin and Net1 in the primary tumors of a group of patients with invasive breast carcinoma treated with doxorubicin-based chemotherapy and long clinical follow-up to determine whether coexpression of these proteins has a greater association with clinical outcome than expression of either protein alone.
Materials and Methods
Patients
This study was approved by The University of Texas M.D. Anderson Cancer Center institutional review board. Patients included in this retrospective study were treated on Protocol DM86-12, a randomized study comparing 6 cycles of 5-fluorouracil, doxorubicin, and cyclophosphamide in the adjuvant setting to 6 cycles of fluorouracil, doxorubicin, and cyclophosphamide followed by 4 cycles of methotrexate and vinblastine. Although patients ≥50 y of age with estrogen receptor–positive disease were randomized to receive tamoxifen or 6 cycles of fluorouracil, doxorubicin, and cyclophosphamide plus 4 cycles of methotrexate and vinblastine, those who received tamoxifen were excluded from our retrospective study, so all patients in our study received doxorubicin-based chemotherapy without tamoxifen. The previous clinical protocol failed to show any benefit from the addition of four cycles of methotrexate and vinblastine to six cycles of fluorouracil, doxorubicin, and cyclophosphamide, so both groups were regarded as having similar doxorubicin-based chemotherapy (26).
Inclusion criteria for this retrospective study were resectable stages II and IIIA breast cancer with axillary lymph node metastases, surgical treatment with mastectomy and axillary dissection without irradiation, age younger than 75 y at diagnosis, no evidence of distant disease at diagnosis, and no history or concurrent malignancy. Additional entry criteria included availability of sufficient archival paraffin-embedded tumor tissue from the primary breast tumor to obtain cores for tissue microarrays. Ninety-four patients met the study criteria. All patients had surgery done at M.D. Anderson Cancer Center between 1986 and 1994.
Antibodies
A monoclonal rat antihuman antibody directed against the β4 integrin subunit was purchased (clone 439-9B, BD Biosciences), and a rabbit polyclonal antibody to the C-terminal eighteen amino acids of Net1 was produced. The anti-Net1 antibody was purified using protein A-Sepharose beads and concentrated using an Amicon stirred cell containing a 30-kDa Ultracel YM-30 filter (Millipore). Bovine serum albumin was added to a concentration of 0.1 mg/mL, and the mixture was dialyzed overnight in PBS plus 10% glycerol. After dialysis, extra glycerol was added to a final concentration of 30%.
Immunohistochemical Staining
Tissue microarrays were prepared from the paraffin blocks of the primary breast tumors using a manual tissue puncher or array (Beecher Instruments). Up to 6 cores, 0.6 mm in diameter, were cut from each primary tumor. Before α6β4 integrin staining, slides were placed in a plastic pressure cooker in citrate buffer and heated at 20% power in a 1,300-W microwave oven for 5 min × 7 with 30 s intervals between each heating period. No antigen retrieval method was used for Net1. Peroxidase activity was inhibited using 3% H2O2 in methanol for 5 min, and slides were blocked with 15 μL/mL goat serum in PBS at room temperature for 30 min. The primary anti-β4 subunit antibody (1:100 dilution) was applied at 4°C overnight, followed by secondary biotinylated antirat IgG (1:200, Vector Labs) at room temperature for 1 h. The purified anti-Net1 antibody (5 μg/mL in PBS plus 3% bovine serum albumin) was applied at room temperature for 1 h, followed by secondary biotinylated rabbit antigoat IgG (1:200, Vector Labs). Nonimmune goat serum was used as a negative control. Staining was done using the ABC kit (Vector Labs) and a standard avidin-biotin peroxidase method.
The immunohistochemical stains were scored without knowledge of the clinical outcome. Staining for α6β4 integrin was scored as positive if at least 5% of invasive tumor cells had membranous and/or cytoplasmic staining because we previously observed membranous and/or cytoplasmic staining in tumors with β4 mRNA expression (27). Weak cytoplasmic staining for Net1 was observed with the nonimmune serum negative control, so only cytoplasmic staining results clearly above the background staining were considered positive. No nuclear staining was observed with the nonimmune serum negative control, and nuclear staining in at least 10% of invasive tumor cells was considered positive for Net1.
Statistical Analyses
Fisher's exact test was used to evaluate associations between immunohistochemical staining results and multiple clinicopathologic variables. All Ps were two sided. Survival estimates were calculated using the Kaplan-Meier product limit method, and the two-sided log-rank test was used to test the association between particular factors and survival. Ten-year survival estimates were expressed ± SE. Multivariate analysis was done using the Cox proportional hazards regression model. All statistical analyses were carried out using SSPS 12.0 for Windows (SPSS, Inc.).
Results
The mean age of the patients was 49 years (range, 28-74 years). Clinical tumor stages included 25 T1, 57 T2, 7 T3, and 5 Tx tumors. The mean tumor size was 3.0 cm (range, 0.5-10 cm). Eighty-one patients had invasive ductal carcinoma, 10 had invasive lobular carcinoma, 2 had mixed invasive ductal and lobular carcinoma, and 1 had invasive papillary carcinoma. Sixtumors were grade 1, 40 were grade 2, and 48 were grade 3. Lymphovascular invasion was present in 39 cases and absent in 55. Ninety-two patients were staged as N1, and two were staged as N2. An average of 18 lymph nodes were removed at axillary dissection (range, 5-48), and the average number of positive nodes was 4 (range, 1-30). The mean clinical follow-up was 130 months (range, 3-226 months). Additional clinicopathologic features of this patient population are summarized in the first column of Table 1.
Table 1. α6β4 and Net1 expression according to clinicopathologic variables.
| Variable | Total patients | α6β4 positive* | α6β4 negative* | Net1 positive* | Net1 negative* | α6β4 and Net1 positive* | α6β4 and/or Net1 negative* |
|---|---|---|---|---|---|---|---|
| Age (y) | P = 0.47 | P = 0.12 | P = 0.60 | ||||
| 20-35 | 7 | 5 | 2 | 2 | 5 | 1 | 6 |
| 36-50 | 52 | 24 | 26 | 21 | 29 | 9 | 40 |
| 51-70 | 34 | 15 | 19 | 7 | 25 | 3 | 29 |
| >70 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| Race | P = 0.10 | P = 0.76 | P = 0.63 | ||||
| Black | 8 | 4 | 4 | 4 | 4 | 2 | 6 |
| White | 66 | 30 | 35 | 21 | 42 | 8 | 54 |
| Hispanic | 14 | 5 | 9 | 5 | 9 | 2 | 12 |
| Other | 6 | 5 | 0 | 1 | 4 | 1 | 4 |
| Menopausal status | P = 0.29 | P = 0.69 | P = 0.76 | ||||
| Pre | 51 | 27 | 22 | 19 | 30 | 7 | 41 |
| Post | 39 | 16 | 23 | 11 | 26 | 5 | 32 |
| Unknown | 4 | 1 | 3 | 1 | 3 | 1 | 3 |
| Tumor size (cm) | P = 0.23 | P = 0.38 | P = 0.83 | ||||
| ≤1 | 6 | 2 | 4 | 0 | 6 | 0 | 6 |
| 1.1-2.0 | 19 | 9 | 9 | 8 | 9 | 3 | 14 |
| 2.1-3.0 | 30 | 15 | 14 | 8 | 21 | 3 | 25 |
| 3.1-4.0 | 19 | 12 | 7 | 7 | 12 | 4 | 15 |
| 4.1-5.0 | 8 | 4 | 4 | 4 | 4 | 2 | 6 |
| ≥5.0 | 7 | 2 | 5 | 3 | 4 | 1 | 6 |
| Unknown | 5 | 0 | 5 | 1 | 3 | 0 | 4 |
| Tumor grade | P = 0.036 | P = 0.15 | P = 0.80 | ||||
| 1 | 6 | 0 | 6 | 3 | 0 | 0 | 5 |
| 2 | 40 | 18 | 21 | 16 | 23 | 5 | 33 |
| 3 | 48 | 26 | 21 | 12 | 34 | 8 | 38 |
| Histologic type | P = 0.007 | P = 0.77 | P = 0.59 | ||||
| Ductal | 81 | 42 | 37 | 26 | 52 | 13 | 64 |
| Lobular | 10 | 1 | 9 | 4 | 5 | 0 | 9 |
| Other | 3 | 1 | 2 | 1 | 2 | 0 | 3 |
| No. of lymph node metastases | P = 0.060 | P = 0.24 | P = 0.22 | ||||
| 1-3 | 65 | 34 | 30 | 25 | 37 | 12 | 50 |
| 4-9 | 20 | 9 | 10 | 4 | 16 | 1 | 18 |
| >9 | 9 | 1 | 8 | 2 | 6 | 0 | 8 |
| Lymphovascular invasion | P = 0.091 | P = 0.38 | P = 1.00 | ||||
| Present | 39 | 23 | 16 | 11 | 27 | 5 | 33 |
| Absent | 55 | 21 | 32 | 20 | 32 | 8 | 43 |
| ER* | P = 1.00 | P = 0.82 | P = 0.76 | ||||
| Positive | 56 | 26 | 30 | 19 | 35 | 7 | 47 |
| Negative | 35 | 17 | 18 | 11 | 24 | 6 | 29 |
| PR* | P = 0.14 | P = 0.82 | P = 0.53 | ||||
| Positive | 42 | 16 | 26 | 14 | 26 | 4 | 36 |
| Negative | 47 | 26 | 21 | 15 | 32 | 8 | 39 |
| HER2* | P = 0.025† | P = 0.59† | P = 0.008† | ||||
| 0 | 53 | 20 | 33 | 17 | 34 | 3 | 48 |
| 1+ | 13 | 6 | 7 | 3 | 10 | 2 | 11 |
| 2+ | 5 | 3 | 2 | 2 | 3 | 1 | 4 |
| 3+ | 20 | 14 | 6 | 8 | 12 | 7 | 13 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Positive and negative values combined for all categories of each variable do not equal 94 because some cores in the tissue microarrays had insufficient tumor and/or were technically unsuitable for evaluation. Total number of patients with scores for α6β4, NET1, both α6β4 and NET1, estrogen receptor, progesterone receptor, and HER2 were 92, 90, 89, 91, 89, and 91, respectively.
P values are for associations with HER2 dichotomized to HER2 = 0 to 2+ versus HER2 = 3+.
Although primary tumor tissue from 94 patients was included in the tissue microarrays, a few cores had insufficient tumor and/or were technically unsuitable for evaluation. Satisfactory immunohistochemical stains for α6β4 integrin and Net1 were obtained in 92 and 90 patients, respectively. Staining for α6β4 in normal breast tissue adjacent to tumor was observed in the myoepithelial cell layer of normal breast ducts and was absent from the luminal cell layer (Fig. 1A). Staining for α6β4 in normal myoepithelial cells was cytoplasmic and membranous and polarized predominantly on the basal aspect of the cells. Staining for Net1 in normal breast tissue was observed in myoepithelial and luminal ductal epithelial cells and was predominantly nuclear (Fig. 1C).
Figure 1.
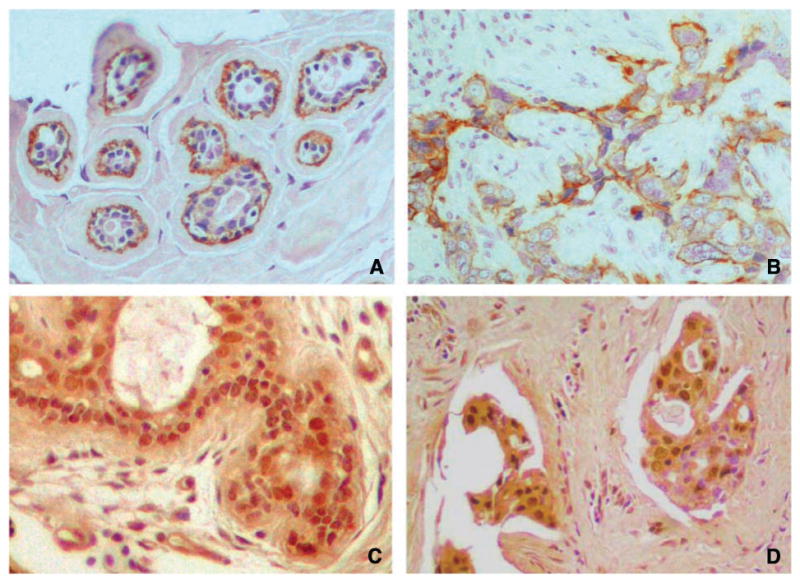
Photomicrographs of paraffin-embedded tissue microarray sections with immunohistochemical staining for α6β4 integrin in normal breast tissue, in which staining is limited to the myoepithelial cell layer of breast epithelium (A); α6β4 integrin in invasive breast carcinoma, with predominantly membranous and focal cytoplasmic staining (B); Net1 in normal breast tissue, in which there is strong nuclear staining (C); and Net1 within nuclei of invasive breast carcinoma cells (D; immunoperoxidase). Original magnification, ×200.
Forty-four patients (48%) had membranous and/or cytoplasmic expression of α6β4 in tumor cells (Fig. 1B). Thirty-one patients (33%) had nuclear expression of Net1 in tumor cells (Fig. 1D). Only three patients had cytoplasmic staining of tumor cells for Net1. Because there were too few patients with cytoplasmic Net1 staining in tumors for meaningful analysis, only nuclear staining for Net1 was evaluated in this study, and Net1 expression in this report refers to nuclear Net1 staining. Thirteen (14%) of the 89 patients with available results for α6β4 integrin and Net1 had coexpression of the 2 markers.
By univariate analysis, there was no significant association between α6β4 integrin or Net1 expression and locoregional recurrence-free (LRF), distant metastasis–free, or overall survival when each marker was considered individually. In contrast, coexpression of α6β4 and Net1 was significantly associated with decreased distant metastasis–free survival (n = 89; two-sided log-rank test, P = 0.030), and there was a trend toward decreased overall survival (P = 0.055; Fig. 2). The 10-year actuarial distant metastasis–free survival was 68% ± 6% versus 34% ± 15% for lack of coexpression versus coexpression, respectively, and the 10-year actuarial overall survival was 72% ± 5% versus 50% ± 15%. When analysis was restricted to hormone receptor–positive (estrogen receptor–positive and/or progesterone receptor–positive) patients, the association between coexpression of α6β4 and Net1 and patient outcome was more significant. In hormone receptor–positive patients (n = 59), coexpression of α6β4 and Net1 was associated with a decrease in distant metastasis–free and overall survival (P < 0.001 and P = 0.006, respectively; Fig. 3). The 10-year actuarial distant metastasis–free survival was 64% ± 7% versus 0% ± 0% for lack of coexpression versus coexpression, respectively, and the 10-year actuarial overall survival was 70% ± 6% versus 34% ± 20%.
Figure 2.
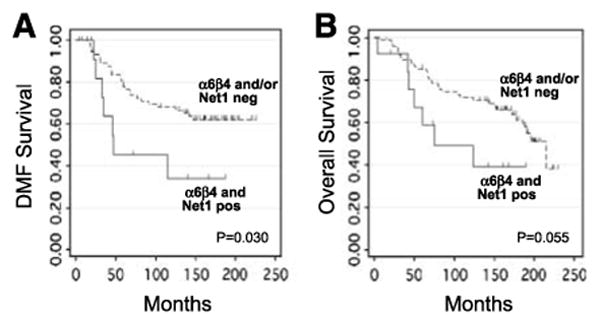
Kaplan-Meier curves of distant metastasis–free (A) and overall (B) survival according to α6β4 integrin and Net1 coexpression (n = 89).
Figure 3.
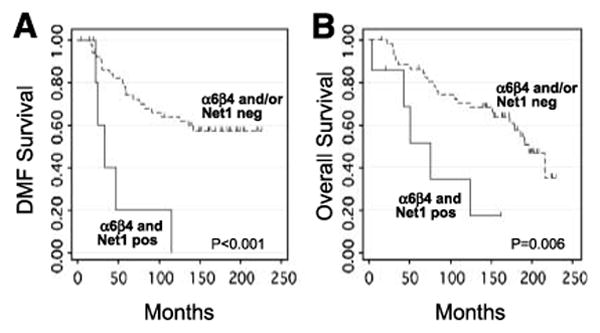
Kaplan-Meier curves of distant metastasis–free (A) and overall (B) survival in hormone receptor–positive patients according to α6β4 integrin and Net1 coexpression (n = 59).
To determine whether α6β4 integrin and Net1 were significantly associated with other factors known to be associated with increased risk, expression of each protein was evaluated in relation to multiple clinicopathologic characteristics (Table 1). A significant association was observed between α6β4 and human epidermal growth factor receptor 2 (HER2) expression when HER2 was dichotomized to HER2 = 0 to 2+ and HER2 = 3+. Fourteen (70%) of 20 patients with HER2 = 3+ had α6β4 expression compared with 29 (41%) of 71 patients with HER2 = 0 to 2+ (Fisher's exact test, P = 0.025). Associations were also observed between α6β4 expression and histologic type and tumor grade. Only 1 (10%) of 10 invasive lobular carcinomas was α6β4 positive compared with 42 (53%) of 79 invasive ductal carcinomas (P = 0.007). None of the 6 grade 1 tumors was α6β4 positive, but 18 (46%) of 39 grade 2 tumors and 26 (55%) of 47 grade 3 tumors with available α6β4 staining results were α6β4 positive (P = 0.036). When α6β4 was dichotomized to negative or low (score, 0 or 1) versus moderate or high (score, 2 or 3), there was an inverse association between α6β4 and estrogen receptor expression. Only 3 (5%) of 56 estrogen receptor–positive patients had moderate or high α6β4 expression compared with 7 (20%) of 35 estrogen receptor–negative patients (P = 0.041).
No significant association was observed between Net1 and any of the clinicopathologic features evaluated. However, an association between HER2 and coexpression of α6β4 and Net1 was observed that was stronger than the association between HER2 and α6β4 alone. Only 6 (8%) of 71 patients with HER2 = 0 to 2+ had coexpression of α6β4 and Net1 compared with 7 (35%) of 20 patients with HER2 = 3+ (P = 0.008).
Standard clinicopathologic features associated with patient outcome in this cohort were evaluated by univariate analysis to select those factors to include in multivariate analysis. Greater than 20% positive lymph nodes and HER2 expression were associated with decreased LRF survival (P = 0.013 and P = 0.014, respectively), and patient age of <40 years, tumor size of >2 cm, lymphovascular invasion, and HER2 expression were associated with decreased distant metastasis–free survival (P = 0.041, P = 0.040, P = 0.040, and P = 0.049, respectively). Tumor size of >2 cm, tumor grade 2 or 3, >20% positive lymph nodes, and HER2 expression were each associated with decreased overall survival (P = 0.016, P = 0.047, P = 0.021, and P = 0.001, respectively), and Hispanic race was associated with improved overall survival (P = 0.037).
Using the factors found to have significance in univariate analysis as stated above, multivariate analysis using the Cox proportional hazards regression model was done to determine whether α6β4 and Net1 coexpression had independent prognostic significance. The only factors independently associated with decreased distant metastasis–free survival were coexpression of α6β4 and Net1 (hazard ratio, 1.63; P = 0.024) and lymphovascular invasion (hazard ratio, 2.35; P = 0.020). Only HER2 expression (hazard ratio, 1.55; P = 0.001) and Hispanic race (hazard ratio, 0.10; P = 0.023) were independently associated with overall survival (Table 2).
Table 2. Cox multivariate analysis of distant metastasis–free and overall survival.
| Outcome measure | Variable | HR (95% CI) | P |
|---|---|---|---|
| Distant metastasis–free survival | Lymphovascular invasion | 2.35 (1.15-4.80) | 0.020 |
| α6β4 and Net1 coexpression | 1.63 (1.07-2.48) | 0.024 | |
| Overall survival | HER2 expression | 1.55 (1.20-2.00) | 0.001 |
| Hispanic race | 0.10 (0.01-0.73) | 0.023 |
Abbreviation: HR, hazard ratio.
Discussion
The results of this study show that α6β4 integrin and Net1 coexpression is independently associated with the development of distant metastasis. This finding supports the hypothesis that α6β4 signaling in the presence of Net1 may play an important mechanistic role in the development of distant metastasis. As an early step in tumor cell migration, α6β4 integrin activates RhoA to stimulate the actin-myosin contraction necessary for the generation of traction forces at the leading edge of invasive tumor cells (4, 5, 7). Although the precise mechanism for α6β4-mediated metastasis is unclear, a pathway that regulates cytoskeletal changes and is known to be involved in tumor cell migration may play an important role in those aspects of tumor cell adhesion and motility related to metastasis.
Net1 is a RhoA-specific guanine nucleotide exchange factor that controls RhoA activation (20-23). Multiple nuclear localization signal sequences present in its amino terminus allow Net1 to be localized to the cell nucleus, although export of Net1 from the nucleus to the cytoplasm is required for RhoA-mediated cytoskeletal rearrangements (20, 21, 23). We had difficulty detecting cytoplasmic expression of Net1 partly because faint cytoplasmic staining was apparent even with the preimmune serum negative control, and it was difficult to discern low-level expression above the background cytoplasmic staining level. This was not a problem when evaluating nuclear expression because there was no background nuclear staining using the preimmune serum control. Preliminary data from the laboratory of one of the authors of this report (J.A. Frost) suggest that the half-life of cytoplasmic Net1 is very short6, which may also account for the difficulty in detecting cytoplasmic Net1. For these reasons, we considered nuclear expression of Net1 as a surrogate marker of a tumor capable of exporting Net1 from the nucleus. Because Net1 controls RhoA activation, we hypothesized that Net1 would be a better indicator of RhoA activity than RhoA expression.
In a recent gene expression profiling data analysis, Lu et al. (9) found that a 65-gene “β4 signature” derived from the top 0.1% of genes that correlated with β4 integrin subunit expression predicted increased tumor recurrence and decreased patient survival when applied to four independent data sets. Their analysis was based on the hypothesis that a group of genes involved in α6β4 signaling is more likely to be associated with clinical outcome than β4 subunit gene expression alone. Although we found no significant association between α6β4 protein expression alone and clinical outcome, coexpression of α6β4 and Net1 was significantly associated with decreased distant metastasis–free and overall survival in hormone receptor–positive patients by univariate analysis, and in the entire patient cohort, α6β4 and Net1 coexpression was independently associated with decreased distant metastasis–free survival by multivariate analysis. Our findings support the concept that evidence of α6β4 integrin–RhoA signaling is more predictive of outcome than α6β4 expression alone.
Unlike Lu et al. (9), who reported no association between α6β4 expression and HER2 expression either by immunohistochemistry in a group of archival invasive breast carcinomas or by regression analysis of expression profiling data retrieved from a combined data set of 315 invasive breast carcinomas, we found an association between α6β4 and HER2 and an even stronger association between α6β4 and Net1 coexpression and HER2. The association between α6β4 and HER2 that we observed is consistent with in vitro studies that have found colocalization of and cooperative signaling between α6β4 integrin and HER2 (11, 28). Moreover, α6β4 integrin seems to be necessary to drive tumorigenesis in the mouse mammary tumor virus–Neu mouse model of breast cancer. Mice with mammary gland–specific constitutive activation of HER2 develop breast cancers, and when these mice are crossed with mice that express a signaling-deficient β4 subunit, tumorigenesis and invasive growth are inhibited (29).
Although we observed a statistically significant association between α6β4 integrin and HER2 in this patient cohort, it is important to emphasize that many HER2-positive patients did not express α6β4 and NET1 coexpression. The proportion of HER2-positive patients with α6β4 and NET1 coexpression was 35%, indicating that only a subset of HER2-positive patients seemed to have this functional integrin signaling pathway. Moreover, the data from Lu et al. (9) suggest that the association we observed between α6β4 and HER2 may not be applicable to breast cancer patients in general. However, our data indicate that α6β4 and HER2 expression are not mutually exclusive and suggest that the α6β4 integrin–RhoA signaling pathway might be important for subsets of HER2-positive and hormone receptor–positive breast cancers.
Our study had a higher percentage of α6β4-positive cases than that reported by Lu et al. (48% versus 32%, respectively; ref. 9). All of the patients in our study were lymph node positive, whereas only 59% of the 105 patients evaluated by immunohistochemistry in the study by Lu et al. (9) had positive lymph nodes. Moreover, we used a modified antigen retrieval method (a series of short bursts of microwave treatment in citrate buffer compared with the usual longer single treatment) to improve detection of the β4 integrin subunit in archival paraffin-embedded tissues. These differences could account, in part, for conflicting results between our study and that of Lu et al. (9).
The analysis by Lu et al. (9) revealed an association between α6β4 expression and triple-negative breast cancers (breast cancers negative for estrogen receptor, progesterone receptor, and HER2). The α6β4 integrin is normally expressed in the myoepithelial cell layer of breast ductal epithelium (9), and the β4 integrin subunit was one of the genes in the initial molecular profiling study on breast cancer that clustered with the gene set that identified the basal-like group of breast cancers (10), which are generally triple-negative cancers. We did not observe an association between α6β4 expression and triple-negative cancers in our patient cohort. However, this should not be surprising because individual genes within the basal-like subgroup are not as robust at classifying the basal-like subtype as an entire gene transcription profile (10, 30, 31). Many estrogen receptor–positive or HER2-positive breast cancers may express individual proteins that have been identified in the basal-like subgroup, and some of these might reflect important biological subtypes of estrogen receptor–positive or HER2-positive breast cancer.
It is important to acknowledge that the number of patients included in the multivariate analysis in this study was relatively small for a model with several factors, so this analysis is best considered hypothesis generating. It is also important to emphasize that patients in this cohort did not receive tamoxifen or trastuzamab, and it is unclear whether an adverse outcome for patients with α6β4 and Net1 coexpression is maintained with these targeted therapies. Nevertheless, we observed that α6β4 integrin and Net1 coexpression seems to select patients with a high risk for distant metastasis. Future studies should assess whether this prognostic value is maintained with current targeted therapies. If so, α6β4 and Net1 might be used to select patients for alternative therapies, including perhaps therapies targeting the α6β4 integrin–RhoA signaling pathway.
Acknowledgments
Grant support: Susan G. Komen Breast Cancer Foundation grants BCTR022043 (M.Z. Gilcrease) and BCTR123806 (J.A. Frost) and Cancer Center Support grant CA16672 from the National Cancer Institute.
Footnotes
H.S. Carr, J.A. Frost. Unpublished observation.
This study is an original work and has not been presented previously.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol Cell Biol. 2001;21:5082–93. doi: 10.1128/MCB.21.15.5082-5093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–60. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–84. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovitz I, Toker A, Mercurio AM. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol. 1999;146:1147–60. doi: 10.1083/jcb.146.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabinovitz I, Gipson IK, Mercurio AM. Traction forces mediated by alpha6beta4 integrin: implications for basement membrane organization and tumor invasion. Mol Biol Cell. 2001;12:4030–43. doi: 10.1091/mbc.12.12.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM. Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J Cell Biol. 2002;158:165–74. doi: 10.1083/jcb.200112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Connor KL, Nguyen BK, Mercurio AM. RhoA function in lamellae formation and migration is regulated by the alpha6beta4 integrin and cAMP metabolism. J Cell Biol. 2000;148:253–8. doi: 10.1083/jcb.148.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 9.Lu S, Simin K, Khan A, Mercurio AM. Analysis of integrin {beta}4 expression in human breast cancer: association with basal-like tumors and prognostic significance. Clin Cancer Res. 2008;14:1050–8. doi: 10.1158/1078-0432.CCR-07-4116. [DOI] [PubMed] [Google Scholar]
- 10.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 11.Gambaletta D, Marchetti A, Benedetti L, Mercurio AM, Sacchi A, Falcioni R. Cooperative signaling between alpha(6)beta(4) integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J Biol Chem. 2000;275:10604–10. doi: 10.1074/jbc.275.14.10604. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay R, Theriault RL, Price JE. Increased levels of alpha6 integrins are associated with the metastatic phenotype of human breast cancer cells. Clin Exp Met. 1999;17:325–32. doi: 10.1023/a:1006659230585. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor KL, Shaw LM, Mercurio AM. Release of cAMP gating by the alpha6beta4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J Cell Biol. 1998;143:1749–60. doi: 10.1083/jcb.143.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 15.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–10. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 16.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–7. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 18.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt A, Hall A. The Rho exchange factor Net1 is regulated by nuclear sequestration. J Biol Chem. 2002;277:14581–8. doi: 10.1074/jbc.M111108200. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Mata R, Dubash AD, Sharek L, Carr HS, Frost JA, Burridge K. The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization, and influences their tumor suppressor activity. Mol Cell Biol. 2007;27:8683–97. doi: 10.1128/MCB.00157-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005;280:12152–61. doi: 10.1074/jbc.M405073200. [DOI] [PubMed] [Google Scholar]
- 23.Qin H, Carr HS, Wu X, Muallem D, Tran NH, Frost JA. Characterization of the biochemical and transforming properties of the neuroepithelial transforming protein 1. J Biol Chem. 2005;280:7603–13. doi: 10.1074/jbc.M412141200. [DOI] [PubMed] [Google Scholar]
- 24.Cattoretti G, Pileri S, Parravicini C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 25.Liapis H, Hutton K. Detection of integrins in formalin-fixed, paraffin-embedded tissues. J Histochem Cytochem. 1997;45:737–41. doi: 10.1177/002215549704500512. [DOI] [PubMed] [Google Scholar]
- 26.Assikis V, Buzdar A, Yang Y, et al. A phase III trial of sequential adjuvant chemotherapy for operable breast carcinoma: final analysis with 10-year follow-up. Cancer. 2003;97:2716–23. doi: 10.1002/cncr.11396. [DOI] [PubMed] [Google Scholar]
- 27.Diaz LK, Zhou X, Welch K, Sahin A, Gilcrease MZ. Chromogenic in situ hybridization for α6β4 integrin in breast cancer. Correlation with protein expression. J Mol Diagn. 2004;6:10–5. doi: 10.1016/s1525-1578(10)60485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcioni R, Antonini A, Nistico P, et al. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res. 1997;236:76–85. doi: 10.1006/excr.1997.3695. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, Pylayeva Y, Pepe A, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 30.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]


