Abstract
Malignant gliomas represent one of the most aggressive forms of brain cancer. Recent advances in the understanding of the deregulated molecular pathways of gliomas have brought about targeted therapies that have the ability to increase therapeutic efficacy in tumors while decreasing toxicity. Multi-targeted kinase inhibitors, novel monoclonal antibodies, and new vaccines have been developed. Standard treatments and current development of new therapies for malignant gliomas are reviewed, focusing specifically on growth factors and their receptors (e.g. epidermal growth factor receptor, vascular endothelial growth factor receptor, and platelet-derived growth factor receptor), as well as the intracellular effector molecules that are downstream of these growth factors (e.g. Ras/Raf/mitogen-activated protein kinase, phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin, and protein kinase C). The efficacies of other novel targeted inhibitors such as deacetylase inhibitors and heat shock protein 90 inhibitors in the treatment of gliomas are also discussed, as well as new combination therapies. In order for new agents to increase treatment efficacy, new targets need to be developed, drug delivery efficiency needs to be improved, and new biomarkers need to be discovered. All of these goals can be accomplished with time through innovative experimental designs.
According to the WHO classification of brain tumors, astrocytomas have been categorized into four grades, determined by the underlying pathology.[1,2] The characteristics that are used to classify gliomas include mitoses, cellular or nuclear atypia, and vascular proliferation and necrosis with pseudopalisading features. Malignant (or high-grade) gliomas include anaplastic glioma (WHO grade III) as well as glioblastoma multiforme (GBM; WHO grade IV). These are the most aggressive brain tumors with the worst prognosis. The goal of this review is to discuss novel molecular targets (including growth factors and their receptors) and trials with agents that target these pathways in malignant gliomas. Recent attention has focused on pathways that are associated with epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and the intracellular effector molecules that are commonly associated with these receptors.
1. Standard Therapy for Malignant Glioma
The primary treatment for patients with high-grade gliomas is multi-modal, including surgical removal of the tumor, radiation, and chemotherapy. With radiation treatment, the median survival of a patient with GBM, the most aggressive and most common glioma, is 12 months. Westphal et al.[3] found that the median survival of patients with GBM could be extended to 13.9 months by using local chemotherapy with carmustine polifeprosan 20 wafers (Gliadel® wafers). Stupp et al.[4] further demonstrated that daily temozolomide combined with radiation increased the median survival rate of patients with glioblastoma by 3 months when compared with radiotherapy alone and increased the 2-year survival rate from 10% to 26%. Also, epigenetic silencing of the O6-methylguanine-DNA methyltransferase (MGMT) DNA repair gene by methylation causes DNA repair to be compromised and has been associated with increased patient survival. One study showed that patients with glioblastoma treated with a methylated MGMT promoter together with temozolomide and radiotherapy resulted in a median survival of 21.7 months.[5] Lastly, trials have begun using the abovementioned methods of therapy in combination with other chemotherapies, for example 06-benzylguanine, which may increase median patient survival when used in concert with standard interventions. These improvements are encouraging and further suggest that the discovery of novel, molecularly targeted therapies may one day improve the treatment of patients with high-grade gliomas.
2. Molecular and Genetic Alterations
Several genetic alterations have been shown to take place in gliomas, which affect pathways that control cell proliferation, growth, apoptosis, and invasion. Growth factors (i.e. epidermal growth factor [EGF], platelet-derived growth factor [PDGF] and their receptors [i.e. EGFR and PDGFR]) have been thought to play a role in the progression and recurrence of gliomas.[6] Growth factor stimulation causes downstream effector molecules to be activated (e.g. Ras/Raf/mitogen-activated protein kinase [MAPK]), which then take part in the transformation of the phenotype resulting from the mediation of transduction by these molecules. Targeting of these pathways has the potential to improve treatment of patients with malignant gliomas.
3. Molecularly Targeted Therapies
Despite the molecular heterogeneity of malignant gliomas, there exist common signal transduction pathways that are altered in many of these tumors. Homeostasis of these pathways is maintained in a normal state through cytokines, growth factors, and hormones; however, in malignancies, mutation or over-expression can occur in growth factor ligands and their receptors (e.g. EGF and EGFR), as well as in intracellular effector molecules (e.g. phosphatase and tensin homologue deleted on chromosome 10 [PTEN] and phosphoinositide-3-kinase [PI3K]/AKT). AKT is a serine/threonine protein kinase (also known as protein kinase B [PKB]) with pleiotropic effects on cell survival and growth. Over-expression or mutations leading to constitutive activation of these growth factors and their downstream effector molecules can result in uncontrolled cell proliferation, survival, and invasion. The sections below focus on the inhibition of these growth factors and their receptors as a possible form of glioma therapy.
4. Targeting Growth Factors and their Receptors
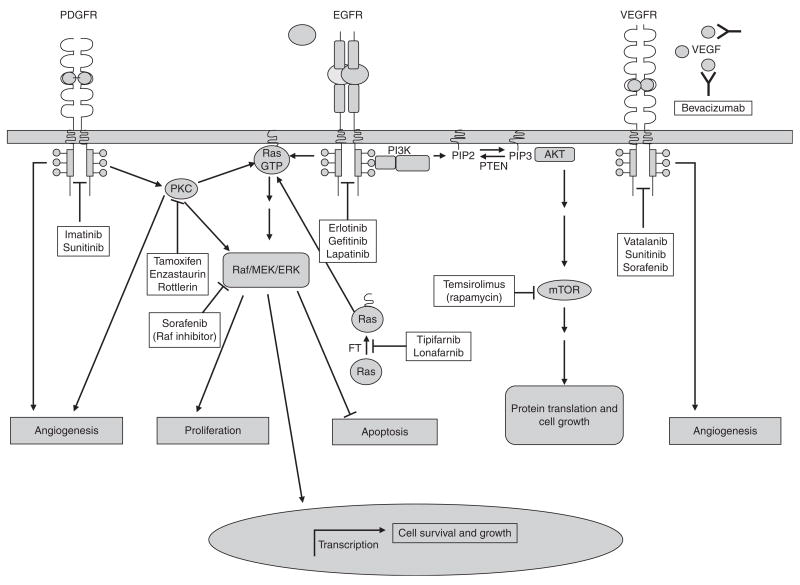
Growth factors bind to their receptors extracellularly and initiate an intracellular signal transduction cascade. Recent research has resulted in many different monoclonal antibodies and small-molecule inhibitors with the ability to specifically target these ligands and receptors (figure 1).
Fig. 1.
A generalized schematic presentation of signaling pathways targeted by molecular therapies in malignant glioma. Receptor tyrosine kinases (RTKs) are shown in their activated (dimerized) form, binding ligand. The diagram illustrates pathways that are activated upon receptor activation. Many receptors share common intracellular effectors (i.e. Ras, AKT, protein kinase C [PKC], etc.) and no single intracellular pathway is exclusive to a particular receptor. Arrowheads indicate the activation of an intracellular molecule and/or a cellular response. A line with a blunted end corresponds to the inhibition of a cellular molecule and/or signaling pathway. As shown, molecular therapies have been designed to inhibit signaling pathways at different stages of the cellular response. Some therapies, such as bevacizumab, target angiogenesis at the level of the receptor by antagonizing VEGF-VEGFR interactions. Others, such as temsirolimus, target effectors (mTOR) that are downstream in a cellular response. AKT is a serine/threonine protein kinase (also known as protein kinase B [PKB]) with pleiotropic effects on cell survival and growth. Raf is a serine/threonine protein kinase with pleiotropic effects on cell survival, differentiation, and proliferation. Ras is a small G protein, ‘a GTPase’, which acts as a second messenger by catalyzing the hydrolysis of GTP to guanosine diphosphate. EGFR =epidermal growth factor receptor; ERK =extracellular regulated kinase (also known as mitogen activated protein kinase [MAPK]); FT =farnesyltransferase; GTP =guanosine tripho-sphate; MEK =MAP-ERK kinase; mTOR =mammalian target of rapamycin; PDGFR =platelet-derived growth factor receptor; PI3K =phosphoinositide-3-kinase; PIP2 =phosphatidylinositol-4,5-biphosphate; PIP3 =phosphatidylinositol-3,4,5-triphosphate; PTEN =phosphatase and tensin homologue deleted on chromosome 10; VEGF =vascular endothelial growth factor; VEGFR =vascular endothelial growth factor receptor.
4.1. Epidermal Growth Factor Receptor (EGFR)
4.1.1 EGFR Inhibitors
EGFR is a receptor tyrosine kinase (RTK), a member of the Her/Erb family of proteins (EGFR is also known as Erb1 or Her1[7]). EGFR is amplified in 40–60% of glioblastomas, and approximately 30–50% of these tumors also possess the EGFR deletion mutant variant III (EGFRvIII), in which coding regions in the extracellular ligand domain (exons 2–7) are absent.[8,9] EGFR plays an integral role in the malignant phenotype of malignant brain tumors, and it is a common hope among researchers and clinicians that functional inhibition of EGFR-mediated signaling will slow tumor growth.[10]
A randomized phase II trial compared the efficacy of temozolomide or carmustine with the small-molecule EGFR tyrosine kinase inhibitor (TKI), erlotinib, in patients with recurrent GBM.[11] The 6-month progression-free survival (PFS-6) was 12% in patients treated with erlotinib and 24% in those treated with temozolomide or carmustine. Furthermore, the response to erlotinib could not be correlated to EGFR expression, amplification, or EGFRvIII mutation.[11] Nonetheless, responses were noted and studies with EGFR RTK inhibitors in malignant glioma continue to be carried out to seek to define a correlation between the patient response and EGFR-associated molecular aberrations. A molecular analysis of malignant gliomas treated with EGFR inhibitors was performed in tissue sets from patients in a multicenter phase I/II trial performed through the North American Brain Tumor Consortium.[12] The authors showed that erlotinib/gefitinib did not affect EGFR signaling (phosphorylation) in vivo, and that there was no correlation between deletion/amplification of EGFR and sensitivity to these EGFR inhibitors. However, the authors hinted at an association between decreased AKT/PI3K activity (phosphorylation) and the response to erlotinib and gefitinib in two patients. Of note, there were no tissue sets that demonstrated the EGFRvIII mutation.
In another study, Mellinghoff et al.[13] performed a molecular analysis in tissue sets from patients with glioblastoma treated with gefitinib or erlotinib. Their results supported previous reports that had failed to find a correlation between EGFR amplification and polysomy and a response to EGFR inhibitors. However, in this study by Mellinghoff et al.,[13] the authors were able to draw a strong correlation between coexpression of EGFRvIII and PTEN, and tumor sensitivity to EGFR kinase inhibitors. The authors found that 6 of 12 tumors (50%) expressing EGFRvIII responded to EGFR kinase inhibitors; only 1 of 14 patients who did not respond to therapy expressed EGFRvIII (p =0.03). The authors also observed that 7 of 13 patients who responded to treatment had tumors that were PTEN positive, while none of the 13 patients whose tumors lacked PTEN had a response to EGFR inhibitors (p =0.005). The greatest predictor of the response to EGFR kinase inhibitors in this tissue set was co-expression of EGFRvIII and PTEN (p <0.001). These findings indicate a molecular basis for patient response to such therapies and will most likely have an impact on whether or not EGFR kinase inhibitors are for patient-specific treatment. Moreover, while these results suggest the existence of molecular determinants involved in EGFR RTK inhibitor activity in glioblastoma, other researchers[14] have questioned the design of the study by Mellinghoff et al.[13] For instance, the tissue sets were analyzed at the time of tumor removal, prior to the administration of the experimental therapy. Also, there were tumors that exhibited PTEN loss, yet demonstrated a response to EGFR RTK inhibitors. These findings indicate a need to conduct molecular analysis of tumor specimens during treatment with novel RTK inhibitors and to identify other downstream signaling networks and molecular tests that will more definitively prove the efficacy of RTK inhibitor therapies.
4.1.2 EGFR Peptide Vaccines
Another promising approach to tumor treatment using EGFR has been the use of peptide vaccines derived from tumor antigens to prime the immune system to respond to the tumor. Just as vaccines are engineered to generate immune protection in anticipation of pathogen infection, so they are also generated to combat tumor recurrence and progression. Potential tumor antigens fall into two categories consisting of ‘tumor-associated’ antigens, which are expressed in other tissues but over-expressed within the tumors, and ‘tumor-specific’ antigens, which are composed of antigens that are only found within the tumor. These are antigens generated by somatic mutation of normal proteins that would otherwise be considered ‘tumor associated’ if it were not for the existence of a somatic mutation in clonal populations of tumor cells. Tumor-specific antigens are greatly preferred because the consequences of collateral damage within the brain are particularly grave, given its vulnerability to inflammation and inability to regenerate.
Vaccines can be screened using techniques such as ‘reverse immunogenetics’. With reverse immunogenetics, peptides –whether endogenous or genetically engineered – can be eluted from MHC-class peptide clefts and screened for their ability to generate classic immune responses in vitro, such as T-cell proliferation, cytokine production, humoral immune response (antibody production), and others.[15] Vaccines with high immunogenicity are used in vivo alone, or are co-administered with cells, such as dendritic cells. Next, we discuss the efficacy of a peptide vaccine generated to mount an immune response against the EGFRvIII peptide.
As mentioned previously, EGFRvIII is an attractive target because it is not expressed in normal tissues but is expressed in a wide number of malignancies, including breast, lung, colon, and ovarian cancers,[16,17] as well as in 31–50% of malignant gliomas.[18,19] Initial preclinical studies targeting EGFRvIII in rodents were promising – a peptide vaccine developed for targeting the novel EGFRvIII epitope was successful in raising antibodies and stimulating a dose-dependent cytotoxic T lymphocyte response in half of the targeted cells (HC2 20d2/c cells but not C012 20c21b cells) and extending the survival rate in vaccinated rats by 72%.[20,21] The success of these preclinical studies led to several human trials, which have also produced promising results.
The first phase I human trial involving the EGFRvIII keyhole limpet vaccine (PEPvIII-KLH) showed that the vaccine was well tolerated and showed promise in patients with malignant glioma.[22] This study, based at Duke University (Durham, North Carolina, USA), enrolled 20 patients, with a WHO grade III or IV glioma, who had undergone gross resection and radiotherapy. Qualified patients (n =16) received three intradermal administrations of the vaccine at 2-week intervals via PEPvIII-KLH-pulsed autologous dendritic cells. The vaccine was successful in stimulating cellular immunity; post-vaccination re-stimulation with the peptide alone gave rise to a delayed hypersensitivity response in 5 of 13 patients and a positive in vitro proliferation response in 10 of 13 patients. The clinical results were also promising. There were no major adverse effects reported, and among patients with grade III tumors, two of three patients had stable disease at 66 and 123 months, respectively. Among the 13 patients with GBM, the mean time to disease progression was 46.9 weeks and median survival was 110.8 weeks. For the phase II trial, the study was expanded to include the MD Anderson Cancer Center (Houston, Texas USA) and the protocol was simplified to eliminate autologous dendritic cells and instead deliver the PEPvIII-KLH peptide vaccine along with granulocyte macrophage colony-stimulating factor (GM-CSF) to stimulate dendritic cell maturation in situ. Preliminary results have indicated that the vaccine is well tolerated and ex vivo studies have demonstrated humoral and cellular immunity; the final results have not yet been reported.[22] A phase III trial, comparing EGFRvIII vaccine with the alkylating agent, TMZ, on the basis of progression-free survival for 6 months, overall survival, and immune response, is underway and is expected to be completed in 2009 (NCT00458601).
One of the potential problems facing tumor vaccine researchers is the possibility of immunoediting, in which targeting of a specific peptide merely selects for variants that lack that peptide. This was observed in the original murine experiments investigating the effects of the EGFRvIII peptide vaccine,[20] where recurrent tumors that were EGFRvIII negative developed in 15% of the surviving mice. One approach to avoid immunoediting is to target multiple peptides in a tumor, either through autologous whole tumor vaccines or through personalized peptide vaccines. The personal peptide vaccine approach screens patient peripheral blood mononuclear cell samples for reactivity against a panel of tumor-associated and specific peptides, and then inoculates them with the best matches. In a 2005 phase I trial, 25 patients (8 with anaplastic astrocytoma [AA] and 17 with GBM) were screened and treated with up to four peptide vaccines.[23] The vaccine was well tolerated and the majority showed increased cellular and humoral responses. Clinical results were moderately favorable; 5 of 21 evaluable patients showed a partial radiographic response, 8 of 21 had stable disease, and 8 of 21 showed progressive disease. The median survival among patients with GBM was 622 days. Other tumor vaccine targets under investigation include cytochrome p450,[24] telomerase,[25] GALT3 (βGlcNAcβ1, 3-galactosyltransferase, polypeptide 3),[26] survivin,[27] tenascin,[28] and glycoprotein 240.[29]
4.2 Vascular Endothelial Growth Factor Receptor
Vascular proliferation is a hallmark of tumor survival and growth. It was suggested in one study that patients with highly vascularized tumors would have a poorer prognosis than patients with less neovascularization; however, this conjecture requires further validation.[6] Glioma cells produce many different proangiogenic factors, including VEGF. For these reasons, VEGF and VEGFR have been targeted for potential treatment of gliomas. Strategies for targeting this ligand and its receptor include using VEGFR TKIs, VEGF antibodies, and protein kinase C (PKC)-β inhibitors.
To date, the most promising VEGF inhibitor has been the monoclonal antibody bevacizumab. Bevacizumab targets the VEGF ligand with the goal of interfering with ligand-receptor signaling. Although at first there was hesitation about using bevacizumab for the treatment of patients with brain tumors, because of its known history of intra-tumoral hemorrhaging in patients with colorectal carcinoma,[30,31] Vredenburgh et al.[32] began a formal phase II trial to evaluate bevacizumab in combination with chemotherapy. They administered bevacizumab and irinotecan (CPT-11) to 32 patients with recurrent high-grade gliomas (23 had glioblastomas). The radiographic response rate was positive and 14 of 23 patients (61%) responded to therapy. The median progression-free survival (PFS) for treated patients was 20 weeks, the PFS-6 was 30%, and the overall median survival time was 40 weeks.
Although these data are impressive and show great promise for future treatments, there exists a lingering question as to whether irinotecan actually adds anything to treatment. The only reason it was included in these trials was because treatment with bevacizumab and irinotecan was shown to be effective in patients with colorectal cancer. The problem, though, is that irinotecan is effective as monotherapy in colorectal cancer, whereas it is essentially ineffective as mono-therapy in gliomas.[33,34] Furthermore, irinotecan was shown in the study by Vredenburgh et al.[32] to create treatment-related systemic toxicity in nine of 32 (28%) patients. Also, four patients had thromboembolic events that ended in two treatment-related deaths. Therefore, it was suggested that bevacizumab as monotherapy may be the best way to minimize systemic toxicity while maximizing the efficacy of treatment, and hence a phase II trial of bevacizumab alone was initiated at the US National Institutes of Health (NIH). The results of this study showed that approximately 60% of patients who were treated with bevacizumab alone had objective radiographic responses, as well as a median PFS of about 110 days and a PFS-6 of 30%.[74] Although these findings were similar to those of Vredenburgh et al., the toxicity of irinotecan observed in that study was not seen in this study. These studies seem to indicate that irinotecan adds no benefit in the treatment of patients with high-grade gliomas.
4.3 Platelet-Derived Growth Factor Receptor
PDGF and its receptors play an important role in tumor interstitial pressure, tumor growth, and angiogenesis.[35] As with other growth factors and their receptors, over-expression of PDGF and its cognate receptors in gliomas has caused PDGF and PDGFR to become targets for anti-tumor treatments.
The PDGFR inhibitor for which most data have been obtained is imatinib mesylate, a small-molecule inhibitor of PDGFR-α and β, c-kit, and the Bcl-Abl fusion protein. Even though imatinib mesylate showed some anti-tumor effects in preclinical studies, minimal clinical benefit was seen using imatinib as monotherapy, with a radiographic response rate of <6% and a PFS-6 of <16%.[36] However, imatinib mesylate and hydroxyurea as combination therapy did show promising results.[37] These initial results were supported in a phase II trial in patients with recurrent GBM, which reported a PFS-6 of 27% and a median PFS of 14 weeks, and 42% of patients had stable radiologic disease at a median follow-up of 58 weeks.[38] The mechanism of action of this combination is still unknown, but it has been shown that imatinib does decrease the interstitial tumor pressure and may increase delivery of hydroxyurea, which gives a possible explanation for the initial success associated with this combination.[39] Unfortunately, a phase III trial did not provide further evidence of efficacy.
5. Targeting Downstream Intracellular Effector Molecules
Activation of the growth factor receptors discussed above leads to the recruitment of intracellular effector molecules to the cell membrane. Generally, gliomas are associated with either the activation of these molecules or the inactivation of their suppressive regulators (e.g. PTEN). This is because activation of second messenger proteins (e.g. PKC) stimulates cell proliferation, invasion, and growth. Overlapping and crosstalk between the pathways that involve these messengers is the cause of the great complexity of targeted therapies for malignant gliomas. Examples of crucial pathways in gliomas are Ras/MAPK, PI3K/AKT, and PKC.
5.1 Ras/Raf/Mitogen-Activated Protein Kinase Pathway
The Ras superfamily of genes regulates many important cellular functions, including cell proliferation and differentiation, protein trafficking, and cytoskeletal organization.[40] The Ras pathway is an important signal transduction effector of the aforementioned EGFR and PDGFR. Many glioblastomas have been shown to have hyperactive Ras due to mutation or amplification of their upstream growth factor receptors.[40] Overactivation of Ras is followed by the farnesylation of Ras, which catalyzes the recruitment of the Ras molecule to the plasma membrane. Then, downstream Ras and Raf molecules are activated, which triggers MAPKs, causing cytoskeletal organization, cell proliferation, and release of proangiogenic growth factors. In an attempt to target Ras, farnesyltransferase inhibitors have been used.
The two most prominent farnesyltransferase inhibitors used in an attempt to indirectly inhibit Ras are tipifarnib and lonafarnib. A phase II study of tipifarnib reported a PFS-6 of 12% in patients with GBM and 9% in patients with anaplastic glioma (AG).[41] Also, a phase I study with lonafarnib and temozolomide was performed in patients with recurrent glioblastoma in an attempt to overcome tumor resistance to temozolomide. Using this therapy, 27% of patients who were previously resistant to temozolomide treatment showed a partial response, and the PFS-6 was 33%.[42] These data show promising possibilities, especially the potential improvement of patient treatment using temozolomide in combination with lonafarnib.
5.2 Phosphatidylinositol 3-Kinase/AKT/Mammalian Target of Rapamycin Pathway
As with the Ras/MAPK pathway, overactivation of PI3K is a result of the initiation of receptor tyrosine kinases such as EGFR. PI3K is a serine/threonine kinase that controls several malignant characteristics consisting of avoidance of apoptosis, cell growth, and proliferation. This pathway is regulated by PTEN; when PTEN function is lost, constitutive activation of the PI3K pathway results. Activation of this pathway continues through a complex secondary messenger cascade that results in the activation of many downstream molecules including AKT, and is generally associated with negative prognosis in patients with GBM.[43] AKT is also a serine/threonine kinase that down-regulates apoptosis and therefore up-regulates cell growth and proliferation. Given the fact that AKT regulates many central biological processes, it is extremely difficult to target directly, and hence the inhibition of upstream and downstream targets has been suggested.
One downstream target of AKT is the mammalian target of rapamycin (mTOR).[44] mTOR integrates important cellular functions, including modulation of cell growth. The inhibitors of mTOR that have been used in clinical trials are the synthetic analogs of rapamycin, e.g. temsirolimus. Recent phase II trials of temsirolimus in patients with GBM have not shown much efficacy.[45,46] Although a radiographic improvement was seen in 36% of patients, no real survival benefit was recorded, with a PFS-6 of just 7.8%. mTOR contains two distinct complexes named raptor/mTORC1 and rictor/mTORC2. Temsirolimus, however, only inhibits the mTORC1 complex, which actually increases the PI3K/AKT activity, thus negating any anti-tumor effect it may have.[47,48] The probable cause of this increase in activity is the activation of compensatory pathways that will cause therapies inhibiting mTOR pathways to be ineffective until tumor escape mechanisms are discovered and solved.
5.3 Protein Kinase C Pathways
PKC is a family of 14 protein kinases that regulate cell growth, proliferation, and angiogenesis.[49] PKC is downstream of growth factor receptors such as EGFR and PDGFR and is activated via signaling from the extracellular signal-regulated Ras/MAPK pathway.[50] Also, there have been some data to show that there is cross-talk between PKC and the PI3K/AKT pathway.[51]
Two PKC inhibitors for which significant data have been recorded are tamoxifen and enzastaurin. Tamoxifen inhibits PKC and has antiestrogenic effects.[52] In glioma xenografts, tamoxifen has shown some anti-tumor activity, but has shown only moderate efficacy in the clinical setting with no improvement in the median survival time (patients with GBM had a median survival time of 27 weeks and patients with AG had a median survival time of 57 weeks).[53,54] Enzastaurin is a PKC-β inhibitor that has also shown activity against glycogen synthase kinase.[55] As with tamoxifen, it has shown a radiographic anti-tumor response in 14 of 79 patients (18%).[56] Contrary to tamoxifen, enzastaurin has shown promising efficacy in the clinic, with a 29% radiographic response rate in patients with recurrent high-grade gliomas.[56] Progress with enzastaurin was brought to a halt when a phase III trial was stopped prematurely as significant survival benefits were not seen.
6. Miscellaneous
6.1 Deacetylase Inhibitors
Histone deacetylase (HDAC) inhibitors target gliomas by modifying epigenetic programming involved in the regulation of gene transcription in order to induce cell-cycle arrest via up-regulation of p21 and induction of apoptosis. It has been noted that the use of HDAC inhibitors in the treatment of glioma has potential benefit. For example, pretreatment of glioma cells with suberoylanilide hydroxamic acid (vorinostat) can make glioma cells more susceptible to chemotherapy and radiation.[57] A phase II trial of vorinostat in recurrent GBM was completed by the North Central Cancer Treatment Group,[58] and it was shown to be well tolerated in patients with recurrent GBM. Also, anti-tumor efficacy was noted in the interim analysis, as five of the first 22 patients treated (23%) were progression-free at 6 months. Vorinostat in combination with temozolomide is under investigation in a phase I/II trial conducted by the North American Brain Tumor Coalition; other deacetylase inhibitors (e.g. romidepsin [depsipeptide; FK288], LBH589, and valproic acid) are being developed clinically for the treatment of GBM.
6.2 Heat Shock Protein (Hsp) 90 Inhibitors
Hsp90 is a molecular chaperone that is ATP dependent and assists in protein folding, cell signaling, and tumor repression.[59] Targeting of this protein may therefore act either as a direct anti-tumor agent, or it may act to enhance the efficacy of chemotherapy and radiation. Hsp90 inhibition has been explored preclinically against glioma cells using geldanamycin, which disrupts Hsp90. Geldanamycin showed anti-tumor effects in vitro, and 17-allylamino-17-demethoxygeldanamycin (17-AAG), a less toxic derivative of geldanamycin, was shown to inhibit tumor growth in glioma xenografts in nude mice.[60] In this study, the tumor size in mice differed significantly (p ≤ 0.017) between the two treatment groups (i.e. mice treated with 17-AAG and those not treated with 17-AAG) from day 5 to day 22.
7. Challenges Associated with Targeted Therapies
There are many controversies surrounding molecularly targeted therapies. Drug delivery across the blood-brain barrier (BBB) is one of the vital problems in targeted therapy treatments. Recent studies have shown that some small molecules used in these therapies are substrates of P-glycoprotein, as well as other efflux pumps that will not allow drugs of this sort to reach the tumor. This information creates a dilemma as to what is the most efficient way to deliver drugs across the BBB. Response to treatment with targeted therapies is also complicated by the fact that tumor cells activate alternate pathways when necessary to enhance tumor survival.[47] For this reason, it is important to vigilantly assess the dynamic changes that take place in target inhibition in order to block as many pathways as possible with the greatest effectiveness. Lastly, higher efficiency of targeted therapies will require technological upgrades that will improve response evaluations. The advent of technology that is better than current MRIs will allow investigators to potentially see whether a treatment has true anti-tumor effects.
As mentioned above, drug delivery across the BBB remains a hurdle in the treatment of high-grade gliomas. One novel approach to the delivery of drugs across the BBB is convection-enhanced delivery (CED). This technique was created because of the dilemma that when a drug is delivered locally within the brain, the concentration of that drug traveling by diffusion drops exponentially from the site of administration. This becomes more of a burden with micrometastases and infiltrative satellites that are commonly seen in the most aggressive type of brain tumor – malignant glioma – where it is difficult for a drug to reach intraparenchymal regions of the brain beyond the resection cavity, especially in ‘eloquent’ areas of the brain. Therefore, researchers at the NIH developed CED as a novel way to deliver drugs over a large volume of tissue. With CED, a catheter(s) is placed in a predetermined area of the brain using standard imaging techniques (i.e. postoperative MRI scans and CT scans), and a drug is infused over a period of hours to days. A constant pressure gradient created by an infusion pump permits the drug infusate to be delivered homogeneously to circumscribed areas of brain tissue without causing significant tissue damage. This method of delivery has become especially relevant in drug delivery to brain tumors because it bypasses the BBB and reduces systemic toxicities. Moreover, it allows for local delivery of targeted macromolecules, such as viruses, liposomes, antibodies, and protein moieties to large areas of the brain parenchyma.
One of the first clinical trials to evaluate the application of CED in brain tumors involved studies with cintredekin besudotox (CB), a recombinant protein toxin consisting of the interleukin (IL)-13 ligand conjugated to a truncated form of Pseudomonas exotoxin. CB targets the IL-13 receptor α2, which is commonly over-expressed in malignant glioma. This recombinant cytotoxin demonstrated potent anti-tumor activity in vitro and in vivo, with 50% inhibitory concentrations of 0.1–30 ng/mL.[61,62,63] The first clinical studies assessing the CED-mediated delivery of CB were in patients with recurrent malignant glioma.[64] These studies established the dose-limiting toxicity (0.5 μg/mL) and infusion dose (6 days of infusion being well tolerated) as well as the importance of catheter placement in the outcome of efficacy. A follow-up study was conducted with CB in patients with newly diagnosed malignant gliomas. Twenty-two patients undergoing gross total resection of their glioma mass received 96-hour infusions of CB (0.25 or 0.5 μg/mL), followed by external beam radiation with or without temozolomide treatment.[65] The majority of treatment-associated adverse events recorded were either grade 1 or 2, and related to catheter placement and CB. A randomized, phase III study, termed PRECISE (Phase III Randomized Evaluation of Convection Enhanced Delivery of IL13-PE38QQR Compared to GLIADEL® Wafer With Survival Endpoint in Glio-blastoma Multiforme Patients at First Recurrence), recently compared the efficacies of CB therapy and carmustine polifeprosan 20 wafers in the treatment of malignant gliomas. While an official report has yet to be published, a press release from Neopharm, Inc., a pharmaceutical company sponsoring the trial, has reported a median survival time of 36.4 weeks in patients treated with CB versus 35.3 weeks in patients treated with carmustine wafers.[66] There was no significant difference in survival between the two treatment groups.
8. The Future of Targeted Therapies for Gliomas
Through the study of the molecular mechanisms of gliomas, many potential resistance mechanisms against targeted therapies have been understood. Studies have shown that even tumors that have been treated with growth factor receptor inhibitors tend to recur. The tumors that progress after therapy usually exhibit increased expression of other mitogenic growth factors and little to no expression of the previously treated growth factors; in other words, tumor growth is selective for cells that do not express the targets of these novel therapies. For example, tumors that were treated with EGFR inhibitors were shown to recur with activation of the IGF1/PI3K/AKT pathway.[67,68] Thus, targeting of a single signaling pathway in cancer may not be sufficient to combat tumor progression, and it has been theorized that targeting multiple growth factors using either multiple single inhibitors or multi-targeted inhibitors may be beneficial. One multi-targeted approach that has been explored both preclinically and clinically is targeting both upstream EGFR and its associate downstream PI3K pathway.
A phase I study was recently completed using gefitinib and rapamycin in the treatment of recurrent malignant glioma in adult patients.[69] Encouraging anti-tumor activity was noted: 13 of the 32 patients (38%) achieved stable disease and two of the 32 (6%) showed a partial radiographic response. Another multi-targeted inhibitor is the EGFR and kinase domain region inhibitor AEE788, which has been combined with the mTOR inhibitor RAD001 in the treatment of malignant glioma. This combination treatment regimen induced greater tumor cell apoptosis and cell cycle arrest, and reduced proliferation compared with single-agent therapy.[70] A phase I/II trial of AEE788 plus RAD001 is ongoing.
There are many other combination therapy agents that are beginning to be explored further. For example, EGFR and the potent VEGFR-2 inhibitor vandetanib (ZD6474) have been shown in vitro to have anti-tumor effects.[71] Another example is sunitinib, which inhibits VEGFR, PDGFR, c-kit, and fetal liver tyrosine kinase. Sunitinib was shown in vivo to increase survival by 36% in mice with intracerebral U87MG GBM tumors.[72] Also, treatment with sunitinib increased tumor necrosis and reduced angiogenesis and caused a 74% reduction in microvessel density. Lastly, sorafenib is a Raf and VEGFR inhibitor that is being studied in the context of glioma.[73] Sorafenib in combination with the PKC-δ inhibitor rottlerin decreased proliferation in all cells lines, and the effects of these agents as a combination were greater than their effects as monotherapies. There will be a continuing effort to develop novel combinations of targeted therapies in the future using the estimated 214 possible drug combinations available at present.[6] With this many combinations, there is infinite potential in targeted therapies that remain to be explored.
9. Conclusions
Recent research has provided new insight into the molecular pathways involved in malignant gliomas. Although much progress has been made, there is a lack of knowledge as to which targets are the most favorable, as well as the optimal way to inhibit these targets. Also, the heterogeneity of brain tumors and patients has made unselected patient treatment difficult. As new approaches to molecularly targeted therapies are explored, and novel combination therapies are discovered, insights will be gained as to where further research is best directed in order to provide personalized therapy for patients with malignant gliomas.
Acknowledgments
This work was supported by the National Cancer Institute (R01-CA122930), the National Institute of Neurological Disorders and Stroke (K08-NS046430), the Alliance for Cancer Gene Therapy Young Investigator Award, and the American Cancer Society (RSG-07-276-01-MGO). Dr Maciej Lesniak has received grants from the National Institutes of Health.
References
- 1.Percy C. Diagnostic techniques and tumor classification [letter] Hum Pathol. 1984 Jun;15( 6):598. doi: 10.1016/s0046-8177(84)80018-0. [DOI] [PubMed] [Google Scholar]
- 2.Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993 Jul;3( 3):255–68. doi: 10.1111/j.1750-3639.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 3.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003 Apr;5( 2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10;352( 10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005 Mar 10;352( 10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 6.de Groot JF, Gilbert MR. New molecular targets in malignant gliomas. Curr Opin Neurol. 2007 Dec;20( 6):712–8. doi: 10.1097/WCO.0b013e3282f15650. [DOI] [PubMed] [Google Scholar]
- 7.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006 Jul;7( 7):505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 8.Humphrey PA, Wong AJ, Vogelstein B, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 1988 Apr 15;48( 8):2231–8. [PubMed] [Google Scholar]
- 9.Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007 Jun 1;25( 16):2288–94. doi: 10.1200/JCO.2006.08.0705. [DOI] [PubMed] [Google Scholar]
- 10.Sarkaria JN, Yang L, Grogan PT, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007 Mar;6( 3):1167–74. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Bent MJ, Brandes A, Rampling R, et al. Randomized phase II trial of erlotinib (E) versus temozolomide (TMZ) or BCNU in recurrent glioblastoma multiforme (GBM): EORTC 26034 [abstract no. 2005]. 2007 Annual Meeting, American Society of Clinical Oncology; Chicago (IL). 2007 Jun 1–5; [Accessed 2009 Feb 5]. [online]. Available from URL: http://meeting.ascopubs.org/cgi/content/abstract/25/18_suppl/2005. [Google Scholar]
- 12.Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01–03 and 00–01. Clin Cancer Res. 2005 Nov 1;11( 21):7841–50. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 13.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005 Nov 10;353( 19):2012–24. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 14.Lassman AB, Abrey LE, Gilbert MR. Response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2006 Feb 2;354(5):525–6. doi: 10.1056/NEJMc053459. author reply 526. [DOI] [PubMed] [Google Scholar]
- 15.Tacken PJ, de Vries IJ, Torensma R, et al. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007 Oct;7( 10):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 16.Garcia de Palazzo IE, Adams GP, Sundareshan P, et al. Expression of mutated epidermal growth factor receptor by non-small cell lung carcinomas. Cancer Res. 1993 Jul 15;53( 14):3217–20. [PubMed] [Google Scholar]
- 17.Wikstrand CJ, Hale LP, Batra SK, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995 Jul 15;55( 14):3140–8. [PubMed] [Google Scholar]
- 18.Ekstrand AJ, James CD, Cavenee WK, et al. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991 Apr 15;51( 8):2164–72. [PubMed] [Google Scholar]
- 19.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005 Feb 15;11( 4):1462–6. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 20.Moscatello DK, Ramirez G, Wong AJ. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997 Apr 15;57( 8):1419–24. [PubMed] [Google Scholar]
- 21.Ciesielski MJ, Kazim AL, Barth RF, et al. Cellular anti-tumor immune response to a branched lysine multiple antigenic peptide containing epitopes of a common tumor-specific antigen in a rat glioma model. Cancer Immunol Immunother. 2005 Feb;54( 2):107–19. doi: 10.1007/s00262-004-0576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampson JH, Archer GE, Mitchell DA, et al. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008 Oct;20( 5):267–75. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yajima N, Yamanaka R, Mine T, et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res. 2005 Aug 15;11( 16):5900–11. doi: 10.1158/1078-0432.CCR-05-0559. [DOI] [PubMed] [Google Scholar]
- 24.Barnett JA, Urbauer DL, Murray GI, et al. Cytochrome P450 1B1 expression in glial cell tumors: an immunotherapeutic target. Clin Cancer Res. 2007 Jun 15;13( 12):3559–67. doi: 10.1158/1078-0432.CCR-06-2430. [DOI] [PubMed] [Google Scholar]
- 25.Komata T, Kanzawa T, Kondo Y, et al. Telomerase as a therapeutic target for malignant gliomas. Oncogene. 2002 Jan 21;21( 4):656–63. doi: 10.1038/sj.onc.1205072. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda N, Nonaka Y, Shichijo S, et al. UDP-Gal: betaGlcNAc beta1, 3-galacto-syltransferase, polypeptide 3 (GALT3) is a tumour antigen recognised by HLA-A2-restricted cytotoxic T lymphocytes from patients with brain tumour. Br J Cancer. 2002 Oct 21;87( 9):1006–12. doi: 10.1038/sj.bjc.6600593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada Y, Kuroiwa T, Nakagawa T, et al. Transcriptional expression of survivin and its splice variants in brain tumors in humans. J Neurosurg. 2003 Oct;99( 4):738–45. doi: 10.3171/jns.2003.99.4.0738. [DOI] [PubMed] [Google Scholar]
- 28.Ventimiglia JB, Wikstrand CJ, Ostrowski LE, et al. Tenascin expression in human glioma cell lines and normal tissues. J Neuroimmunol. 1992 Jan;36( 1):41–55. doi: 10.1016/0165-5728(92)90029-k. [DOI] [PubMed] [Google Scholar]
- 29.Kurpad SN, Zhao XG, Wikstrand CJ, et al. Tumor antigens in astrocytic gliomas. Glia. 1995 Nov;15( 3):244–56. doi: 10.1002/glia.440150306. [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350( 23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 31.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007 Apr 20;25( 12):1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 32.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007 Feb 15;13( 4):1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 33.Batchelor TT, Gilbert MR, Supko JG, et al. Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97-11. Neuro Oncol. 2004 Jan;6( 1):21–7. doi: 10.1215/S1152851703000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006 Apr;8( 2):189–93. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostman A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 2004 Aug;15( 4):275–86. doi: 10.1016/j.cytogfr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006 Aug 15;12( 16):4899–907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 37.Dresemann G. Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: a patient series. Ann Oncol. 2005 Oct;16( 10):1702–8. doi: 10.1093/annonc/mdi317. [DOI] [PubMed] [Google Scholar]
- 38.Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005 Dec 20;23( 36):9359–68. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 39.Bihorel S, Camenisch G, Gross G, et al. Influence of hydroxyurea on imatinib mesylate (Gleevec) transport at the mouse blood-brain barrier. Drug Metab Dispos. 2006 Dec;34( 12):1945–9. doi: 10.1124/dmd.106.010975. [DOI] [PubMed] [Google Scholar]
- 40.Knobbe CB, Reifenberger J, Reifenberger G. Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol. 2004 Dec;108( 6):467–70. doi: 10.1007/s00401-004-0929-9. [DOI] [PubMed] [Google Scholar]
- 41.Cloughesy TF, Wen PY, Robins HI, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006 Aug 1;24( 22):3651–6. doi: 10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert MR, Gaupp P, Liu V, et al. A phase I study of temozolomide (TMZ) and the farnesyltransferase inhibitor (FTI), lonafarnib (Sarasar, SCH66336) in recurrent glioblastoma [abstract no. 1556]. 2006 Annual Meeting, American Society of Clinical Oncology; Atlanta (GA). 2006 Jun 2–6; [Accessed 2009 Feb 5]. [online]. Available from URL: http://meeting.ascopubs.org/cgi/content/abstract/24/18_suppl/1556. [Google Scholar]
- 43.Chakravarti A, Zhai G, Suzuki Y, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004 May 15;22( 10):1926–33. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 44.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002 Jul;2( 7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 45.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005 Aug 10;23( 23):5294–304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 46.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005 Aug;23( 4):357–61. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 47.Sawyers CL. Will kinase inhibitors have a dark side? N Engl J Med. 2006 Jul 20;355( 3):313–5. doi: 10.1056/NEJMcibr062354. [DOI] [PubMed] [Google Scholar]
- 48.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006 Sep;6( 9):729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 49.Couldwell WT, Uhm JH, Antel JP, et al. Enhanced protein kinase C activity correlates with the growth rate of malignant gliomas in vitro. Neurosurgery. 1991 Dec;29(6):880–86. doi: 10.1097/00006123-199112000-00013. discussion 886–7. [DOI] [PubMed] [Google Scholar]
- 50.da Rocha AB, Mans DR, Regner A, et al. Targeting protein kinase C: new therapeutic opportunities against high-grade malignant gliomas? Oncologist. 2002;7( 1):17–33. doi: 10.1634/theoncologist.7-1-17. [DOI] [PubMed] [Google Scholar]
- 51.Balendran A, Hare GR, Kieloch A, et al. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000 Nov 10;484( 3):217–23. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- 52.Hui AM, Zhang W, Chen W, et al. Agents with selective estrogen receptor (ER) modulator activity induce apoptosis in vitro and in vivo in ER-negative glioma cells. Cancer Res. 2004 Dec 15;64( 24):9115–23. doi: 10.1158/0008-5472.CAN-04-2740. [DOI] [PubMed] [Google Scholar]
- 53.Brandes AA, Ermani M, Turazzi S, et al. Procarbazine and high-dose tamoxifen as a second-line regimen in recurrent high-grade gliomas: a phase II study. J Clin Oncol. 1999 Feb;17( 2):645–50. doi: 10.1200/JCO.1999.17.2.645. [DOI] [PubMed] [Google Scholar]
- 54.Spence AM, Peterson RA, Scharnhorst JD, et al. Phase II study of concurrent continuous temozolomide (TMZ) and tamoxifen (TMX) for recurrent malignant astrocytic gliomas. J Neurooncol. 2004 Oct;70( 1):91–5. doi: 10.1023/b:neon.0000040837.68411.97. [DOI] [PubMed] [Google Scholar]
- 55.Graff JR, McNulty AM, Hanna KR, et al. The protein kinase Cbeta-selective inhibitor, enzastaurin ( LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005 Aug 15;65( 16):7462–9. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 56.Fine HA, Kim L, Royce C, et al. Results from phase II trial of enzastaurin ( LY317615) in patients with recurrent high grade gliomas [abstract no. 1504]. 2005 Annual Meeting, American Society of Clinical Oncology; Orlando (FL). 2005 May 13–17; [Accessed 2009 Feb 5]. [online]. Available from URL: http://meeting.ascopubs.org/cgi/content/abstract/23/16_suppl/1504. [Google Scholar]
- 57.Chinnaiyan P, Vallabhaneni G, Armstrong E, et al. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005 May 1;62( 1):223–9. doi: 10.1016/j.ijrobp.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 58.Galanis E, Jaeckle KA, Maurer MJ, et al. N047B: NCCTG phase II trial of vorinostat (suberoylanilide hydroxamic acid) in recurrent glioblastoma multiforme (GBM) [abstract no. 2004]. 2007 Annual Meeting, American Society of Clinical Oncology; Chicago (IL). 2007 Jun 1–5; [Accessed 2009 Feb 5]. [online]. Available from URL: http://meeting.ascopubs.org/cgi/content/abstract/25/18_suppl/2004. [Google Scholar]
- 59.Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001 Sep;188( 3):281–90. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 60.Yang J, Yang JM, Iannone M, et al. Disruption of the EF-2 kinase/Hsp90 protein complex: a possible mechanism to inhibit glioblastoma by geldanamycin. Cancer Res. 2001 May 15;61( 10):4010–6. [PubMed] [Google Scholar]
- 61.Debinski W, Obiri NI, Powers SK, et al. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin. Clin Cancer Res. 1995 Nov;1( 11):1253–8. [PubMed] [Google Scholar]
- 62.Husain SR, Puri RK. Interleukin-13 receptor-directed cytotoxin for malignant glioma therapy: from bench to bedside. J Neurooncol. 2003 Oct;65( 1):37–48. doi: 10.1023/a:1026242432647. [DOI] [PubMed] [Google Scholar]
- 63.Kioi M, Husain SR, Croteau D, et al. Convection-enhanced delivery of interleukin-13 receptor-directed cytotoxin for malignant glioma therapy. Technol Cancer Res Treat. 2006 Jun;5( 3):239–50. doi: 10.1177/153303460600500307. [DOI] [PubMed] [Google Scholar]
- 64.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007 Mar 1;25( 7):837–44. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 65.Vogelbaum MA, Sampson JH, Kunwar S, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007 Nov;61(5):1031–7. doi: 10.1227/01.neu.0000303199.77370.9e. discussion 1037–8. [DOI] [PubMed] [Google Scholar]
- 66.Neopharm, Inc. NeoPharm (NEOL) announces efficacy results for phase 3 PRECISE trial; drug misses goal in brain-cancer trial; stock plunges [media release] 2006 Dec 11 [online]. Available from URL: http://www.biospace.com/news_story.aspx?NewsEntityId=39560.
- 67.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002 Jan 1;62( 1):200–7. [PubMed] [Google Scholar]
- 68.Li B, Chang CM, Yuan M, et al. Resistance to small molecule inhibitors of epidermal growth factor receptor in malignant gliomas. Cancer Res. 2003 Nov 1;63( 21):7443–50. [PubMed] [Google Scholar]
- 69.Reardon DA, Quinn JA, Vredenburgh JJ, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006 Feb 1;12( 3 Pt 1):860–8. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- 70.Goudar RK, Shi Q, Hjelmeland MD, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005 Jan;4( 1):101–2. [PubMed] [Google Scholar]
- 71.Sandstrom M, Johansson M, Bergstrom P, et al. Effects of the VEGFR inhibitor ZD6474 in combination with radiotherapy and temozolomide in an orthotopic glioma model. J Neurooncol. 2008 May;88( 1):1–9. doi: 10.1007/s11060-008-9527-3. [DOI] [PubMed] [Google Scholar]
- 72.de Bouard S, Herlin P, Christensen JG, et al. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007 Oct;9( 4):412–23. doi: 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jane EP, Premkumar DR, Pollack IF. Coadministration of sorafenib with rottlerin potently inhibits cell proliferation and migration in human malignant glioma cells. J Pharmacol Exp Ther. 2006 Dec;319( 3):1070–80. doi: 10.1124/jpet.106.108621. [DOI] [PubMed] [Google Scholar]
- 74.Fine HA. Promising new therapies for malignant gliomas. Cancer J. 2007 Nov–Dec;13(6):349–54. doi: 10.1097/PPO.0b013e31815b18db. [DOI] [PubMed] [Google Scholar]