Abstract
As a result of improving technologies and greatly increased sample sizes, the last 2 years has seen unprecedented advances in identification of specific genetic risk factors for psychiatric phenotypes. Strong genetic associations have been reported at common polymorphisms within ANK3 and CACNA1C in bipolar disorder and ZNF804A in schizophrenia and a relatively specific association between common variation in GABAA receptor genes and cases with features of both bipolar disorder and schizophrenia. Further, the occurrence of rare copy number variants (CNVs) has been shown to be increased in schizophrenia compared with controls. These emerging data provide a powerful resource for exploring the relationship between psychiatric phenotypes and can, and should, be used to inform conceptualization, classification, and diagnosis in psychiatry. It is already clear that, in general, genetic associations are not specific to one of the traditional diagnostic categories. For example, variation at ZNF804A is associated with risk of both bipolar disorder and schizophrenia, and some rare CNVs are associated with risk of autism and epilepsy as well as schizophrenia. These data are not consistent with a simple dichotomous model of functional psychosis and indicate the urgent need for moves toward approaches that (a) better represent the range of phenotypic variation seen in the clinical population and (b) reflect the underlying biological variation that gives rise to the phenotypes. We consider the implications for models of psychosis and the importance of recognizing and studying illness that has prominent affective and psychotic features. We conclude that if psychiatry is to translate the opportunities offered by new research methodologies, we must finally abandon a 19th-century dichotomy and move to a classificatory approach that is worthy of the 21st century.
Keywords: schizophrenia, bipolar disorder, schizoaffective disorder, psychosis, nosology, diagnosis, classification, DSM-V, ICD-11, categories, dimensions, genetics, dichotomy, unitary psychosis
Background
Recent findings in molecular genetics are providing information about etiology that can, and should, shape our thinking about psychiatric illness and the relationship between diagnostic entities. We have previously reviewed classical and molecular genetic literature relating to the traditional dichotomous diagnostic categories of schizophrenia and bipolar disorder and considered the implications for psychiatric nosology. We concluded that (a) the traditional dichotomy is not supported by the data, (b) some findings are consistent with relatively specific genetic susceptibility for illness with prominent features of both schizophrenia and bipolar illness (ie, a form of mixed psychosis or broadly defined “schizoaffective” illness), and (c) more genetic and clinical data were needed.1,2 Since then, genetic technologies have improved, sample sizes have increased dramatically (from hundreds to thousands of individuals), and strong findings have been published that are relevant to the biological relationship between mood and psychotic disorders. In this article, we consider the implications of those findings for models of the relationship between bipolar and psychotic illness.
Important Recent Genetic Findings
Genetic Epidemiology
One of the key scientific justifications for continued adherence to the Kraepelinian dichotomy has been the various studies showing that the prototypical disorders tend to “breed true.” However, the recent publication of the largest family study of schizophrenia and bipolar disorder ever undertaken, including over 2 million nuclear families identified from Swedish population and hospital discharge registers, shows that there are increased risks of both schizophrenia and bipolar disorder to first-degree relatives of probands with either disorder. Moreover, there is evidence from half-sibs and adopted-away relatives that this is due substantially to genetic factors. In our view, this new work provides compelling support for the various accumulating lines of evidence that schizophrenia and bipolar disorder partially share a common genetic etiology.3,4 An alternative view is to dismiss the findings as an artifact of “diagnostic error.” This requires us to accept the validity of etiologically distinct categories that cannot be reliably recognized but whose number is nevertheless set at 2. In the absence of a strong basis for that hypothesis, adherence to a dichotomous model seems more an act of faith rather than of science and is a position that is increasingly untenable in the face of the data.
Common Single-Nucleotide Polymorphisms
Large-scale collaborative genome-wide association studies (GWASs; hundreds of thousands of single-nucleotide polymorphisms [SNPs] studied in large numbers of cases and controls) have started to deliver genome-wide significant genetic associations for bipolar disorder and schizophrenia. Studies of approximately 10 000 individuals have shown strong evidence for association with susceptibility to bipolar disorder at variants within 2 genes involved in ion channel function: ANK3 (encoding the protein ankyrin G) and CACNA1C (encoding the alpha-1C subunit of the L-type voltage-gated calcium channel).5 The SNP showing maximum association with susceptibility to bipolar disorder shows similar association in UK schizophrenia and unipolar depression samples, showing that variation at CACNA1C influences susceptibility across the mood-psychosis spectrum (E. K. Green, D. Grozeva, I. Jones, et al, unpublished data). A similar study in close to 20 000 individuals has shown strong evidence for association with susceptibility to schizophrenia at a variant within ZNF804A (encoding a protein of unknown function, but which based upon sequence similarity, may act as a transcription factor).6 Further, the SNP in ZNF804A showing the strongest association signal with schizophrenia also showed an association with bipolar disorder,6 demonstrating that variation at this locus also has an effect on illness susceptibility across the traditional diagnostic boundaries.
Genomic Structural Variation
It has long been known that chromosomal abnormalities increase the risk for mood and psychotic illness,7,8 including the velocardiofacial syndrome (VCFS) arising from deletion on chromosome 22q119,10 and the Prader-Willi syndrome arising from maternal uniparental disomy on chromosome 15q11–q13.11 It has recently been recognized that structural genomic variants of small or modest size (100 base pairs–500 000 base pairs) are a common cause of genetic variation in humans,12 and such variants have been reported in neuropsychiatric phenotypes including autism, mental retardation, and schizophrenia.13–18 The overall load of copy number variants (CNVs) has been shown to be greater in individuals with schizophrenia compared with controls, and additionally, there is convincing evidence for association to a number of specific rare CNVs (<1% population minor allele frequency), particularly those at 22q11 (the VCFS deletion), 1q21.1, and 15q13.3.15,16 Further, some specific CNVs associated with risk of schizophrenia confer risk to multiple neuropsychiatric phenotypes including also autism.19 The estimated effect sizes for these rare variants are substantially larger than for the SNP associations discussed earlier. However, the typical effect sizes and population frequencies of the pathogenically relevant CNVs are not yet known nor is the extent to which CNVs contribute to the total population variance in risk of schizophrenia. The only study to date for bipolar disorder did not find any increase in overall CNV load, although there was a significant increase of “singleton” CNVs in cases compared with controls.20 Much more work is needed, particularly in cases with affective disorders and with mixed psychotic and affective phenotypes. It is possible that the CNV contribution is substantially less in cases with predominantly affective features, and this might help explain their generally higher level of cognitive functioning and less persistence of impairment.
Putting Psychiatric Genetics Into Context: Genetic Progress in Nonpsychiatric Illness
Over the last 2 years, GWASs have made major contributions to advancing our understanding of many common diseases including diabetes, heart disease, inflammatory bowel disease, various cancers, and rheumatoid arthritis.21 For example, in Crohn disease, 30 different genes have already been robustly shown to influence risk, and this has pointed to novel biological pathways involved in illness pathogenesis, including autophagy, the innate immune response, and regulation of the IL23 pathway.22 A similar pattern of multiple susceptibility genes distributed over multiple distinct pathways is also being found in other diseases, and a consistent finding is that effect sizes of common susceptibility variants are small and those detected to date explain at best a modest or small proportion of the overall population genetic variance for the phenotype. The remaining genetic variance may be explained by as yet undetected common variants of small effect (“polygenes”), rare variants (of large, small, or intermediate effect sizes), structural variation, or some combination of these.
GWASs in psychiatry are still in their infancy, and the total sample sizes available to date have generally been substantially smaller than those available for diseases such as type 1 and type 2 diabetes or Crohn disease for which many loci have been robustly implicated. Experience in other diseases suggests that to reveal the genetic contributions to psychiatric disease, we will need to use samples of tens of thousands rather than thousands traditionally used.23
How Can We Expect Genetics to Help Improve Understanding of Psychiatric Nosology and Classification?
As Kendler24 has clearly articulated, molecular genetics will not provide a simple, gene-based classification of psychiatric illness (as it will not for other common familial illnesses). The notion that there is a “gene for …” one or more psychiatric disorders is inappropriate and unhelpful.25 Rather, there is a complex relationship between genotype and phenotype that involves multiple genes and environmental factors, together with stochastic variation. Nonetheless, we can expect molecular genetic findings to play an important role in helping to delineate the relationship between specific biological pathways/systems/networks and broad patterns, or domains, of psychopathology.26 A precedent for such insights from genetic studies is already emerging from GWASs in other areas of medicine that have revealed unforeseen biological relationships among different autoimmune diseases.27 Genetic findings, which cannot be expected to map cleanly onto current descriptive psychiatric diagnostic categories, will be a guide to the biological processes and systems that are most important in the expression of the clinical phenotypes of psychiatry.28 In addition to pinpointing genes, and hence proteins and pathways involved in susceptibility to particular phenotypes, genetic findings can provide general insights into the sorts of nosological models that may be most useful in psychiatry.
Simple Models of the Genetic-Biological Relationship Between Schizophrenia, Bipolar Disorder, and Mixed (“Schizoaffective”) Psychoses
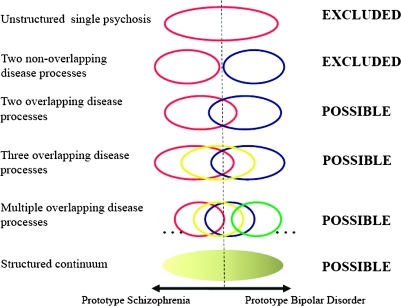
The nosological relationships between schizophrenia, bipolar disorder, and mixed forms of illness have been the subject of substantial interest and debate since Kraepelin proposed his dichotomy.29–39 One simple way of thinking about the issue is to consider a set of possible etiological relationships of increasing complexity along a single clinical schizophrenia-bipolar disorder axis (figure 1). Using this approach, the simplest model is one in which there is a single disorder in which there may be many genes influencing risk but in which there is no degree of specificity between etiology (in this case genotype) and phenotype. We can confidently reject this model on the grounds that family and twin studies show that there is some degree of specificity of genetic risk for prototypical schizophrenia and prototypical bipolar disorder. (It should be obvious from the discussion to date, but for avoidance of doubt, that any model that invokes only a single psychosis gene40 is incompatible with the large body of data from genetic epidemiology, and now the molecular genetic data,41–43 and must also be rejected). The next simplest model is that of 2 disease processes without phenotypic overlap. This can be confidently rejected on the grounds of the data from genetic epidemiology and molecular genetics discussed earlier. The next simplest model is that of 2 disease processes that have etiological overlap. We do not regard the weight of evidence as sufficient to confidently reject this model but note that it is not consistent with the analyses that show a genetic specificity for phenotypes with mixed features of schizophrenia and bipolar disorder.44–46 The remaining models cannot be rejected at present. We note that progressively larger datasets will be necessary to differentiate more complex models.
Fig. 1.
Models of the Possible Biological-Genetic Relationships Between Clinical Phenotypes on a 1-Dimensional Schizophrenia-Bipolar Disorder Clinical Spectrum. Each circle/ellipse denotes that a particular set of genes (which index associated proteins and biological pathways involved in that phenotype—“disease processes”) influence a range of clinical phenotypes within that part of the clinical spectrum and that further differentiation of the disease process-clinical phenotype relationship is not possible. The models are presented in order of their complexity from a single psychosis disease entity without any phenotypic structure through models with increasing numbers of biologically distinct clinical entities to a phenotypically structured continuum that represents the limit of an increasing number of biologically distinct clinical entities. Current data allow rejection of the first 2 models. The other models are possible and require testing against empirical data.
Mixed Psychoses: “Schizoaffective Disorder” and the Importance of Recognizing Illness With a Prominent Mixture of Both Mood and Schizophrenia-Like Features
Mood disturbances are common in schizophrenia, and psychotic symptoms are common in bipolar disorder.47,48 Nevertheless, although clinical pictures with a mix of mood and psychotic symptoms are common, perhaps even the rule, psychiatrists feel constrained to assign most of them to one or other of the dichotomous categories that form the basis for our classification. The term “schizoaffective disorder” is available for application to cases with a mix of the clinical features associated with each prototypical disorder. However, definitions have varied substantially, and this has impacted on the proportion of cases with severe psychiatric disorder to which this term is applied in practice.49–51 Within the context of neo-Kraepelinian operational classifications such as the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV)52 and International Classification of Diseases(ICD), Tenth Revision,53 schizoaffective disorder has a very narrow interpretation and is used only when cases cannot be fitted to definitions of schizophrenia or bipolar disorder. Thus, in clinical practice and the vast majority of research, the diagnosis is treated as a diagnosis of exclusion that represents supposedly atypical cases. As a result, although some excellent work has been undertaken, cases with prominent mood and psychotic features have not received the same attention as schizophrenia and bipolar disorder with respect to research into treatment and pathogenesis. Indeed, the approach has often been to regard schizoaffective cases as a “nuisance” and to either exclude them from analysis or combine them with one or other of the dichotomous categories.
This approach to these common mixed or “schizoaffective-spectrum” psychoses (we use this latter term to refer to a broader group of cases with a mixture of psychotic and affective symptoms than is defined by current DSM and ICD criteria) cases is highly problematic if such cases are, in fact, more typical and representative of psychosis than are the dichotomous prototypes and/or actually reflect the expression of one or more relatively specific disease processes. Some clinicians and researchers have certainly believed that at least some schizoaffective cases represent distinct clinical entities and have continued to apply minority diagnostic traditions, such as “bouffée délirante” (France; eg, Picot54), psychogenic psychoses (Scandinavia; eg, McCabe and Stromgren55), and cycloid psychoses (eg, Jabs et al56). Further, the existence of one or more relatively discrete nosological entities with mixed features is supported by latent class analyses (eg, Kendler et al,36 Kendler et al,57 McGrath et al,58 and Sham et al59). Genetic epidemiology supports a strong genetic component to schizoaffective illness60–65 with similar heritabilities to schizophrenia and bipolar disorder. Recent analyses of the Wellcome Trust Case Control Consortium66 bipolar disorder sample have demonstrated that those bipolar cases meeting research diagnostic criteria67 for schizoaffective disorder, bipolar type (a substantially broader, more inclusive definition of schizoaffective than that in DSM-IV), showed a greater number of strong genetic signals than did the bipolar I or bipolar II disorder cases.68 Moreover, suggesting a degree of specificity between pathophysiology and phenotype, polymorphism within genes encoding γ-amino butyric acid A (GABAA) receptor subunits was associated en masse with this schizoaffective phenotype, the effect not being present for cases meeting diagnostic criteria for either bipolar I disorder or schizophrenia.46 These findings are consistent with other molecular genetic evidence for the existence of relatively specific genetic susceptibility for a form of major psychiatric illness that has features of both bipolar disorder and prominent psychosis.44,45,69,70 This clinical entity merits explicit recognition in order to explore this possibility further.
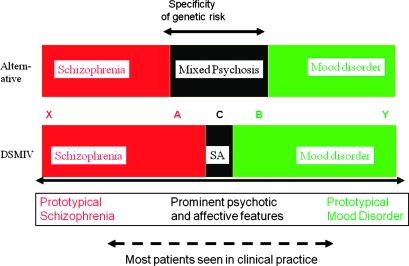
One of the major criticisms that has been leveled at the use of schizoaffective disorder as a diagnostic category in both clinical practice and research is a lack of reliability and temporal stability.71 However, this is an almost inevitable consequence of the overly restrictive nature of current definitions (figure 2), together with the tendency of clinicians to make diagnoses cross-sectionally rather than longitudinally. All clinicians know that the precise clinical presentation of any individual with psychosis varies over time. It follows that the clinical picture will fluctuate across the diagnostic boundaries of any category with narrow limits more frequently than a category with broad limits. Given the very restrictive definition of the schizoaffective category compared with the much broader definitions of schizophrenia and mood disorder, it is inevitable that the latter categories will seem more reliable and stable.
Fig. 2.
Simplified Representation of the Clinical Functional Psychosis Spectrum to Demonstrate the Problems if a Classification Fails to Facilitate Grouping Together Cases With Similar Clinical Features and Biological Predisposition. Below the solid double-headed arrow is a notional representation of a 1-dimensional spectrum of clinical features from “prototypical schizophrenia” on the left through “schizoaffective” to “prototypical mood disorder” on the right. Between the 2 sets of colored boxes, we show diagnostic categories and the locations of 5 individuals, A, B, C, X, and Y, on the clinical spectrum. The lower set of colored boxes correspond to current Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) diagnostic concepts; SA designates DSM-IV schizoaffective disorder. In the upper set of boxes, diagnostic categories correspond to an alternative scheme in which the commonly occurring “middle ground,” “mixed,” or “broad schizoaffective” category is accorded greater status and extends over a wider part of the middle of the spectrum than does the very restrictive DSM-IV definition. Individuals A, B, and C have similar clinical features but under DSM-IV are assigned to different categories. Further, individuals X and A are placed in the same category even though A shares much less in common with X than with B. (A similar argument holds for B and Y.) Given that there is evidence to support the existence of some degree of genetic specificity toward the phenotypes expressed by cases A and B, the current situation is extremely unhelpful to research and practice. It can be seen that broadening the concept of “schizoaffective” is one simple way of improving the appropriate recognition of the similarity of these cases. Of course, such a “trichotomy,” while an improvement over the dichotomy, is still associated with the problems inherent in setting boundaries between categories. Approaches involving dimensional measures may be preferable, but the key conceptual point is facilitating recognition and grouping together of such cases and making clinicians and researchers abandon dichotomous thinking.
In summary, if cases with “schizophrenic” and affective symptoms do indeed represent a group with a particularly strong or relatively specific genetic loading, then the neo-Kraepelinian dichotomous approach, with its narrow definition of schizoaffective disorder, will serve to impede etiological research. Further, we note that the imminent revision of official diagnostic classifications (ie, Diagnostic and Statistical Manual of Mental Disorders [Fifth Edition] [DSM-V] and International Classification of Diseases, Eleventh Revision[ICD-11]) may be influenced by the opinions articulated in several recent articles that the concept of schizoaffective disorder is unreliable and unhelpful and should be abandoned.72–75 Contrasting with this view, current data suggest that what is needed is better recognition of such cases. Simply to abandon the category of schizoaffective disorder might not be the best way of achieving that goal.
The Need for More Complex Models
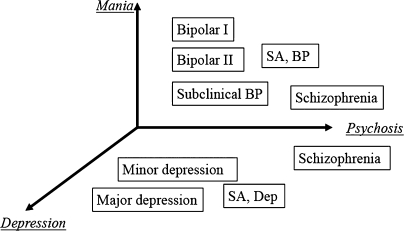
Traditionally, most interest in nosological relationships and clinical spectra of psychoses has focused on the schizophrenia-bipolar disorder axis. However, even though they do not appear in current operational diagnostic definitions, autistic features have long been proposed as a core feature of schizophrenia.76 Recent CNV findings discussed earlier provide a strong rationale for at least considering the validity of a schizophrenia-autism axis. When considering mood disorders, the bipolar-unipolar axis is of obvious importance, and we have already mentioned that variation at CACNA1C is associated with risk across bipolar and unipolar mood disorders. Such axes are clearly a simplification and reduce an enormous amount of clinical complexity to a single dimension based on high-level clinical entities (syndromes). In our current state of knowledge, they are probably useful to include within the set of clinical measures that are used as starting points for exploring the biological underpinnings of psychiatric phenotypes. An example of the type of approach that may be useful is shown in figure 3 that is based on a descriptive dimensional scale that we developed for research in bipolar spectrum illness.77 Here, we show just 3 key domains (or syndromes) of psychopathology: mania, depression, and psychosis (positive symptoms) that are all well recognized clinically and receive support from factor analyses of descriptive clinical data in individuals with functional psychosis.78 More dimensions are, of course, needed to capture the other important domains of psychopathology (eg, autistic traits, global intellectual functioning, …). A vital part of the iterative process of working toward more biologically valid classification approaches will be determining which of the descriptive domains/dimensions are usefully associated with variation in biological systems. This will then allow subsets (or mappings) of the domains/dimensions to be selected. One part of the work needed to make this possible is an increased understanding of how networks operate in the brain. Another is an improvement in understanding of the clinical phenotype. As we and others have argued, the development and application of tools to facilitate careful measurement and reappraisal of psychopathology, including using dimensional measures of key domains of psychopathology that can sit alongside the use of categories, are urgently required. This approach will allow us to work toward more complex but realistic models of the relationships between psychiatric phenotypes and biological systems and will pave the way toward more biologically valid and, it is to be hoped, clinically useful approaches to classification and diagnosis.
Fig. 3.
Example of a 3-Dimensional Representation of Some Key Clinical Domains of Functional Psychosis Showing How Current Diagnostic Categories Are Related to Dimensional Scores. This is illustrative, and we can expect that more dimensions will be necessary to capture the biologically relevant clinical variation. Determining the most useful dimensional measures will be an iterative process requiring substantial work on both the biological underpinnings of illness and the measurement of the phenotype (which will include clinical characteristics as well as measures of psychological functioning). This simple 3-dimensional representation is based on 3 dimensions from our descriptive scale, the Bipolar Affective Disorder Dimension Scale.77 It can be seen that current diagnostic categories map to different parts of the 3-dimensional space. Thus, all information contained within a current diagnostic category is included, but the approach provides substantial additional information that provides a better characterization of the individual's illness. BP: bipolar disorder; SA, BP: schizoaffective disorder, bipolar type; SA, Dep: schizoaffective disorder, depressive type.
In order to know what we may expect for psychiatry, it is useful to consider what has been learned about such models for nonpsychiatric diseases. There is evidence from a range of nonpsychiatric diseases for a relationship between the disease phenotype expressed and the biological similarity of the key proteins involved.79,80 In other words, when disease phenotypes are very similar, the key proteins associated with expression of those disease phenotypes tend to be biologically related, eg, interacting with each other or otherwise falling within the same or closely related biological pathways or networks.80 An important finding is that a relatively small proportion of proteins are network “hubs” and are involved in many pathways, whereas most proteins are involved in only one or a few pathways.79,80 The implication of this “small-world” architecture is that individual proteins, and pathways, are likely to be functionally related to many others by way of these hub proteins. We can expect that the pattern of biological relationships between disease phenotypes will be similar and that in general there will be groups of diseases related through a hub protein, with varying subgroupings of diseases depending upon proximity of the proteins and pathways involved in their expression. This model of common disease has, as its natural consequences, the features of illness with which psychiatrists are so familiar: high levels of pleiotropy leading to “comorbidity” between different phenotypes (eg, O'Donovan et al81), overlaps with normality, lack of clarity about whether dimensional rather than categorical approaches are better, and close interplay of genetic, environmental, and stochastic factors (because function and dysfunction of pathways reflect the dynamic response to environmental and stochastic change).
There is increasing evidence that the brain's structural and functional systems also have features of complex networks, such as small-world topology, highly connected hubs, and modularity, though attempts to apply these concepts to psychiatric disorders are in their infancy.82 However, it is of interest that a recent analysis of co-occurrence of psychiatric and nonpsychiatric diagnoses in individuals attending a large US hospital demonstrated positive relationships between schizophrenia, bipolar disorder, and autism diagnoses and a strong negative relationship between bipolar disorder and breast cancer.83 The findings, which need to be replicated and explored further, were interpreted as reflecting the underlying biological (genetic) relationships between the phenotypes. As we have discussed, there is substantial molecular genetic support for relationships between bipolar disorder and schizophrenia. We have also pointed out that the recent CNV data show that the same CNVs may predispose to schizophrenia, autism, and attention deficit/hyperactivity disorder.81 Further, it is known that rare mutations in the gene CACNA1C, which was implicated by association of a common polymorphism with bipolar disorder, can cause autistic traits.84 Thus, evidence is accumulating that suggests we should be rethinking the relevance and implications of the frequent similarities of clinical features across current diagnostic categories and the common co-occurrence of diagnostic assignments to individuals. Further, we have the potential to move toward an understanding of the observed common clinical co-occurrence of psychiatric and nonpsychiatric disease. For example, an etiological relationship between epilepsy and psychosis is supported by recent CNV studies85 and could in part be explained by ion channel dysfunction.86 Another important example is the relationship between mood disorders and cardiovascular disease including sudden death.87 In part, this could be related to ion channel dysfunction influencing both mood regulation and cardiac function.86,88
Implications for DSM-V and ICD-11
There has probably never been a more difficult time to be revising the descriptive psychiatric classifications to be used throughout the world. Robust evidence is rapidly emerging that demonstrate the shortcomings of the current approach and the associated conventional diagnostic categories, but we do not yet have sufficient information on which to make biologically valid, evidence-based changes. The medical principle of “first do no harm” is important. Any changes should not make research or clinical work more difficult or otherwise impede progress. The real need is to improve attention to clinical symptoms and course variables and consider the full richness of the clinical picture within individual patients rather than persisting in using largely arbitrary diagnostic “boxes” that, though never intended by those who developed the current approach, are in the minds of many, reified, and act as a major impediment to thinking and clinical description. For the moment, it would be helpful to make changes that (a) encourage more detailed clinical description and characterization, (b) encourage conceptualization of psychiatric illness in ways that are consistent with plausible biological models, and (c) put an end to the simple bipolar disorder-schizophrenia dichotomy. This latter issue is important both practically and theoretically because it will signal the willingness to move to a more mature approach to the complexity of functional psychosis. The dichotomous classification approach has pushed clinicians and researchers away from explicitly recognizing such cases. Given that they are clinically common, it is possible that focused study of such cases may be most productive for understanding the mood-psychosis axis. A classification sets a tone and provides a context for both clinical and research thinking. With the weight of evidence against a dichotomy, what is needed is a high-level signal that we are finally willing to rethink psychosis rather than cling to the familiar, simple ideas handed down by the 19th-century father of psychiatric nosology. There is evidence (reviewed above) that it may be particularly important to recognize illness in which there is a prominent mix of the clinical features of both bipolar disorder and schizophrenia. There is a real danger that the recent calls to abolish the diagnostic entity of schizoaffective disorder will result in it being more difficult to recognize schizoaffective-spectrum cases, just at the time when evidence is accumulating that such cases might result from specific underlying pathogenic mechanisms. Careful thought needs to be given to the best ways of delineating and describing cases in which prominent mood and psychotic symptoms occur that recognize the various patterns of temporal relationship that exist in different patients.
Likely Convergence of Approaches to Understanding, Classifying, and Diagnosing Psychiatric and Nonpsychiatric Illness
Throughout its history, psychiatry has grappled with issues of comorbidity of syndromes, clinical and subclinical spectra, interface with normality, and prominent importance of both genetic and environmental factors and considered the relative merits of widely differing models to understand and describe illness, including, eg, categorical or dimensional approaches. For such reasons, and also in part perpetuating the thinking of Cartesian duality, “mental” illness has often been thought of as qualitatively distinct from “physical” illness. However, when the eminent British nosologist, Robert Kendell, considered the question, “What is the difference between psychiatric and non-psychiatric illness?,” he concluded that there is no theoretical difference but just the arbitrary practical issue that psychiatric illness is the specialist domain of psychiatrists, whereas nonpsychiatric illness is not.89
It is of great interest, therefore, to note that, at the time when molecular genetics is providing psychiatry with powerful tools to explore the biological relationships between different diagnostic entities, those studying nonpsychiatric diseases are discovering that molecular genetic findings require them to move away from their traditional understanding and approaches and consider and embrace many of the complexities with which psychiatry is familiar. It seems likely that, over the coming years and decades, conceptualization, classification, and diagnosis will become progressively closer in psychiatry and other branches of medicine. This is likely to benefit patients through improved diagnosis and treatment, improve public understanding of psychiatric illness, and help to reduce stigma. It should also facilitate understanding of the common co-occurrence of psychiatric and nonpsychiatric illness.
Conclusion
Our key message is that we need to prepare ourselves to move toward more complex and biologically plausible models than the rather simple clinically driven, biology-free models that have been the tradition of psychiatry.
Emil Kraepelin based his original 19th-century dichotomous distinction of manic depressive insanity and dementia praecox largely on the basis of differing longitudinal outcome. At that time, there were no effective treatments, and it was logical to use a simple diagnostic approach that had as its modest goal prognostic validity. However, in the 21st century, it is time to be more ambitious.
Funding
The authors' work on the genetics of psychosis and mood disorders is funded through grants from the Wellcome Trust, Medical Research Council National Institutes of Health, and Stanley Medical Research Institute.
Acknowledgments
We are members of the MRC Centre for Neuropsychiatric Genetics and Genomics in Cardiff University. We are indebted to all the participants in our studies.
References
- 1.Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- 2.Craddock N, O'Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen MJ, Craddock N. Diagnosis of functional psychoses: time to face the future. Lancet. 2009;373:190–191. doi: 10.1016/S0140-6736(09)60053-2. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira MA, O'Donovan MC, Meng YA, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Donovan MC, Craddock N, Norton N, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- 7.Blackwood DH, Thiagarajah T, Malloy P, Pickard BS, Muir WJ. Chromosome abnormalities, mental retardation and the search for genes in bipolar disorder and schizophrenia. Neurotox Res. 2008;14:113–120. doi: 10.1007/BF03033803. [DOI] [PubMed] [Google Scholar]
- 8.Craddock N, Owen M. Chromosomal aberrations and bipolar affective disorder. Br J Psychiatry. 1994;164:507–512. doi: 10.1192/bjp.164.4.507. [DOI] [PubMed] [Google Scholar]
- 9.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 10.Kates WR, Antshel KM, Fremont WP, et al Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. Am J Med Genet A. 2007;143A:2642–2650. doi: 10.1002/ajmg.a.32012. [DOI] [PubMed] [Google Scholar]
- 11.Soni S, Whittington J, Holland AJ, et al. The phenomenology and diagnosis of psychiatric illness in people with Prader-Willi syndrome. Psychol Med. 2008;38:1505–1514. doi: 10.1017/S0033291707002504. [DOI] [PubMed] [Google Scholar]
- 12.McCarroll SA, Altshuler DM. Copy-number variation and association studies of human disease. Nat Genet. 2007;39(suppl):S37–S42. doi: 10.1038/ng2080. [DOI] [PubMed] [Google Scholar]
- 13.Weiss LA, Shen Y, Korn JM, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 14.Walsh T, McClellan JM, McCarthy SE, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 15.Stefansson H, Rujescu D, Cichon S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirov G, Grozeva D, Norton N, et al. Support for the involvement of large cnvs in the pathogenesis of schizophrenia [published online ahead of print January 29, 2009] Hum Mol Genet. doi: 10.1093/hmg/ddp043. doi:10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagnamenta AT, Wing K, Akha ES, et al. A 15q13.3 microdeletion segregating with autism [published online ahead of print December 03, 2008] Eur J Hum Genet. doi: 10.1038/ejhg.2008.228. doi:10.1038/ejhg.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burbach JP, van der Zwaag B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. 2009;32:69–72. doi: 10.1016/j.tins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D, Cheng L, Qian Y, et al. Singleton deletions throughout the genome increase risk of bipolar disorder [published online ahead of print December 30, 2008] Mol Psychiatry. doi: 10.1038/mp.2008.144. doi:10.1038/mp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manolio TA, Brooks LD, Collins FS. A HapMap harvest of insights into the genetics of common disease. J Clin Invest. 2008;118:1590–1605. doi: 10.1172/JCI34772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew CG. New links to the pathogenesis of Crohn disease provided by genome-wide association scans. Nat Rev Genet. 2008;9:9–14. doi: 10.1038/nrg2203. [DOI] [PubMed] [Google Scholar]
- 23.Craddock N, O'Donovan MC, Owen MJ. Genome-wide association studies in psychiatry: lessons from early studies of non-psychiatric and psychiatric phenotypes. Mol Psychiatry. 2008;13:649–653. doi: 10.1038/mp.2008.45. [DOI] [PubMed] [Google Scholar]
- 24.Kendler KS. Reflections on the relationship between psychiatric genetics and psychiatric nosology. Am J Psychiatry. 2006;163:1138–1146. doi: 10.1176/ajp.2006.163.7.1138. [DOI] [PubMed] [Google Scholar]
- 25.Kendler KS. “A gene for …”: the nature of gene action in psychiatric disorders. Am J Psychiatry. 2005;162:1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- 26.Craddock N, Owen MJ. Rethinking psychosis: the disadvantages of a dichotomous classification now outweigh the advantages. World Psychiatry. 2007;6:84–91. [PMC free article] [PubMed] [Google Scholar]
- 27.Lettre G, Rioux JD. Autoimmune diseases: insights from genome-wide association studies. Hum Mol Genet. 2008;17:R116–R121. doi: 10.1093/hmg/ddn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross Disorder Phenotype Group of the Psychiatric GWAS Consortium. Dissecting the phenotype in genome-wide association studies of psychiatric illness. Br J Psychiatry. doi: 10.1192/bjp.bp.108.063156. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marneros A, Akiskal HS. The Overlap of Affective and Schizophrenic Spectra. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 30.Crow TJ. The continuum of psychosis and its genetic origins. Br J Psychiatry. 1990;156:788–797. doi: 10.1192/bjp.156.6.788. [DOI] [PubMed] [Google Scholar]
- 31.Kendell RE. Diagnosis and classification of functional psychoses. Br Med Bull. 1987;43:499–513. doi: 10.1093/oxfordjournals.bmb.a072198. [DOI] [PubMed] [Google Scholar]
- 32.Kendell RE, Brockington IF. The identification of disease entities and the relationship between schizophrenic and affective psychoses. Br J Psychiatry. 1980;137:324–331. doi: 10.1192/bjp.137.4.324. [DOI] [PubMed] [Google Scholar]
- 33.Brockington IF, Kendell RE, Wainwright S, Hillier VF, Walker J. The distinction between the affective psychoses and schizophrenia. Br J Psychiatry. 1979;135:243–248. doi: 10.1192/bjp.135.3.243. [DOI] [PubMed] [Google Scholar]
- 34.Taylor MA. Are schizophrenia and affective disorder related? A selective literature review. Am J Psychiatry. 1992;149:22–32. doi: 10.1176/ajp.149.1.22. [DOI] [PubMed] [Google Scholar]
- 35.Van Os J, Gilvarry C, Bale R, et al. A comparison of the utility of dimensional and categorical representations of psychosis. UK700 Group. Psychol Med. 1999;29:595–606. doi: 10.1017/s0033291798008162. [DOI] [PubMed] [Google Scholar]
- 36.Kendler KS, Karkowski LM, Walsh D. The structure of psychosis: latent class analysis of probands from the Roscommon Family Study. Arch Gen Psychiatry. 1998;55:492–499. doi: 10.1001/archpsyc.55.6.492. [DOI] [PubMed] [Google Scholar]
- 37.Murray RM, Sham P, Van Os J, et al. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Angst J, Scharfetter C, Stassen HH. Classification of schizo-affective patients by multidimensional scaling and cluster analysis. Psychiatr Clin (Basel) 1983;16:254–264. doi: 10.1159/000283974. [DOI] [PubMed] [Google Scholar]
- 39.Brockington IF, Meltzer HY. The nosology of schizoaffective psychosis. Psychiatr Dev. 1983;1:317–338. [PubMed] [Google Scholar]
- 40.Crow TJ. A continuum of psychosis, one human gene, and not much else–the case for homogeneity. Schizophr Res. 1995;17:135–145. doi: 10.1016/0920-9964(95)00059-u. [DOI] [PubMed] [Google Scholar]
- 41.Risch N. Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet. 1990;46:222–228. [PMC free article] [PubMed] [Google Scholar]
- 42.Craddock N, Khodel V, Van Eerdewegh P, Reich T. Mathematical limits of multilocus models: the genetic transmission of bipolar disorder. Am J Hum Genet. 1995;57:690–702. [PMC free article] [PubMed] [Google Scholar]
- 43.Craddock N, O'Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genet. 2005;42:193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green E, Raybould R, Macgregor S, et al. Operation of the schizophrenia susceptibility gene, neuregulin 1, across traditional diagnostic boundaries to increase risk for bipolar disorder. Arch Gen Psychiatry. 2005;62:642–648. doi: 10.1001/archpsyc.62.6.642. [DOI] [PubMed] [Google Scholar]
- 45.Hamshere ML, Bennett P, Williams N, et al. Genome-wide linkage scan in schizoaffective disorder: significant evidence for linkage (LOD = 3.54) at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19q13. Arch Gen Psychiatry. 2005;62:1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- 46.Craddock N, Jones L, Jones IR, et al. Strong genetic evidence for a selective influence of GABA(A) receptors on a component of the bipolar disorder phenotype [published online ahead of print July 01, 2008] Mol Psychiatry. doi:10.1038/mp.2008.66. [Google Scholar]
- 47.Kraepelin E. Manic-Depressive Insanity and Paranoia. Edinburgh, UK: Livingstone; 1919. [Google Scholar]
- 48.Kraepelin E. Dementia Praecox. Edinburgh, UK: Livingstone; 1919. [Google Scholar]
- 49.Brockington IF, Leff JP. Schizo-affective psychosis: definitions and incidence. Psychol Med. 1979;9:91–99. doi: 10.1017/s0033291700021590. [DOI] [PubMed] [Google Scholar]
- 50.Maj M. Evolution of the American concept of schizoaffective psychosis. Neuropsychobiology. 1984;11:7–13. doi: 10.1159/000118042. [DOI] [PubMed] [Google Scholar]
- 51.Maj M. The evolution of some European diagnostic concepts relevant to the category of schizoaffective psychoses. Psychopathology. 1984;17:158–167. doi: 10.1159/000284050. [DOI] [PubMed] [Google Scholar]
- 52.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 53.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Diagnostic Criteria for Research. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 54.Pichot P. The concept of psychiatric nosology. In: Schramme Thomas, Thome Johannes., editors. Philosophy and Psychiatry. Berlin: Walter de Gruyter; 2004. pp. 83–93. [Google Scholar]
- 55.McCabe MS, Stromgren E. Reactive psychoses. A family study. Arch Gen Psychiatry. 1975;32:447–454. doi: 10.1001/archpsyc.1975.01760220059007. [DOI] [PubMed] [Google Scholar]
- 56.Jabs BE, Pfuhlmann B, Bartsch AJ, et al. Cycloid psychoses—from clinical concepts to biological foundations. J Neural Transm. 2002;109:907–919. doi: 10.1007/s007020200074. [DOI] [PubMed] [Google Scholar]
- 57.Kendler KS, Karkowski-Shuman L, O'Neill FA, et al. Resemblance of psychotic symptoms and syndromes in affected sibling pairs from the Irish Study of High-Density Schizophrenia Families: evidence for possible etiologic heterogeneity. Am J Psychiatry. 1997;154:191–198. doi: 10.1176/ajp.154.2.191. [DOI] [PubMed] [Google Scholar]
- 58.McGrath JA, Nestadt G, Liang KY, et al. Five latent factors underlying schizophrenia: analysis and relationship to illnesses in relatives. Schizophr Bull. 2004;30:855–873. doi: 10.1093/oxfordjournals.schbul.a007138. [DOI] [PubMed] [Google Scholar]
- 59.Sham PC, Castle DJ, Wessely S, et al. Further exploration of a latent class typology of schizophrenia. Schizophr Res. 1996;20:105–115. doi: 10.1016/0920-9964(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 60.Andreasen NC, Rice J, Endicott J, et al. Familial rates of affective disorder. A report from the National Institute of Mental Health Collaborative Study. Arch Gen Psychiatry. 1987;44:461–469. doi: 10.1001/archpsyc.1987.01800170083011. [DOI] [PubMed] [Google Scholar]
- 61.Bertelsen A, Gottesman II. Schizoaffective psychoses: genetical clues to classification. Am J Med Genet. 1995;60:7–11. doi: 10.1002/ajmg.1320600103. [DOI] [PubMed] [Google Scholar]
- 62.Farmer AE, McGuffin P, Gottesman II. Twin concordance for DSM-III schizophrenia. Scrutinizing the validity of the definition. Arch Gen Psychiatry. 1987;44:634–641. doi: 10.1001/archpsyc.1987.01800190054009. [DOI] [PubMed] [Google Scholar]
- 63.Gershon ES, Hamovit J, Guroff JJ, et al. A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry. 1982;39:1157–1167. doi: 10.1001/archpsyc.1982.04290100031006. [DOI] [PubMed] [Google Scholar]
- 64.Maier W, Lichtermann D, Minges J, et al. Continuity and discontinuity of affective disorders and schizophrenia. Results of a controlled family study. Arch Gen Psychiatry. 1993;50:871–883. doi: 10.1001/archpsyc.1993.01820230041004. [DOI] [PubMed] [Google Scholar]
- 65.Slater E, Cowie C. The Genetics of Mental Disorders. London, UK: Oxford University Press; 1971. [Google Scholar]
- 66.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 68.Hamshere ML, Green EK, Jones IR, et al. Strong genetic support for broadly defined bipolar schizoaffective disorder as a useful diagnostic concept. Br J Psychiatry. doi: 10.1192/bjp.bp.108.061424. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Potash JB, Zandi PP, Willour VL, et al. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am J Psychiatry. 2003;160:680–686. doi: 10.1176/appi.ajp.160.4.680. [DOI] [PubMed] [Google Scholar]
- 70.Park N, Juo SH, Cheng R, et al. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9:1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- 71.Maj M, Pirozzi R, Formicola AM, et al. Reliability and validity of the DSM-IV diagnostic category of schizoaffective disorder: preliminary data. J Affect Disord. 2000;57:95–98. doi: 10.1016/s0165-0327(99)00059-2. [DOI] [PubMed] [Google Scholar]
- 72.Maier W. Do schizoaffective disorders exist at all? Acta Psychiatr Scand. 2006;113:369–371. doi: 10.1111/j.1600-0447.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 73.Vollmer-Larsen A, Jacobsen TB, Hemmingsen R, Parnas J. Schizoaffective disorder—the reliability of its clinical diagnostic use. Acta Psychiatr Scand. 2006;113:402–407. doi: 10.1111/j.1600-0447.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 74.Lake CR, Hurwitz N. Schizoaffective disorder merges schizophrenia and bipolar disorders as one disease–there is no schizoaffective disorder. Curr Opin Psychiatry. 2007;20:365–379. doi: 10.1097/YCO.0b013e3281a305ab. [DOI] [PubMed] [Google Scholar]
- 75.Malhi GS, Green M, Fagiolini A, Peselow ED, Kumari V. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10(pt 2):215–230. doi: 10.1111/j.1399-5618.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 76.Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York, NY: International Universities Press; 1950. [Google Scholar]
- 77.Craddock N, Jones I, Kirov G, et al. The Bipolar Affective Disorder Dimension Scale (BADDS)—a dimensional scale for rating lifetime psychopathology in bipolar spectrum disorder. BMC Psychiatry. 2004;4:19. doi: 10.1186/1471-244X-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peralta V, Cuesta MJ. How many and which are the psychopathological dimensions in schizophrenia? Issues influencing their ascertainment. Schizophr Res. 2001;49:269–285. doi: 10.1016/s0920-9964(00)00071-2. [DOI] [PubMed] [Google Scholar]
- 79.Ideker T, Sharan R. Protein networks in disease. Genome Res. 2008;18:644–652. doi: 10.1101/gr.071852.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oti M, Brunner HG. The modular nature of genetic diseases. Clin Genet. 2007;71:1–11. doi: 10.1111/j.1399-0004.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 81.O'Donovan MC, Kirov G, Owen MJ. Phenotypic variations on the theme of CNVs. Nat Genet. 2008;40:1392–1393. doi: 10.1038/ng1208-1392. [DOI] [PubMed] [Google Scholar]
- 82.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems [published online ahead of print February 04, 2009] Nat Rev Neurosci. doi: 10.1038/nrn2575. doi:10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 83.Rzhetsky A, Wajngurt D, Park N, Zheng T. Probing genetic overlap among complex human phenotypes. Proc Natl Acad Sci U S A. 2007;104:11694–11699. doi: 10.1073/pnas.0704820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Splawski I, Timothy KW, Sharpe LM, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Helbig I, Mefford HC, Sharp AJ, et al. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41:160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60:177–185. doi: 10.1016/j.biopsych.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 87.Glassman A. Depression and cardiovascular disease. Pharmacopsychiatry. 2008;41:221–225. doi: 10.1055/s-2008-1058108. [DOI] [PubMed] [Google Scholar]
- 88.Viswanathan PC, Balser JR. Inherited sodium channelopathies: a continuum of channel dysfunction. Trends Cardiovasc Med. 2004;14:28–35. doi: 10.1016/j.tcm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Kendell RE. The distinction between mental and physical illness. Br J Psychiatry. 2001;178:490–493. doi: 10.1192/bjp.178.6.490. [DOI] [PubMed] [Google Scholar]