Abstract
Previous surveys found a large (>10-fold) variation in schizophrenia prevalence at different geographic sites and a tendency for prevalence to increase with latitude. We conducted meta-analyses of prevalence studies to investigate whether these findings pointed to underlying etiologic factors in schizophrenia or were the result of methodological artifacts or the confounding of sites’ latitude with level of healthcare at those sites. We found that these patterns were still present after controlling for an index of healthcare—infant mortality—and focusing on 49 studies that used similar diagnostic and ascertainment methods. The tendencies for schizophrenia prevalence to increase with both latitude and colder climate were still large and significant and present on several continents. The increase in prevalence with latitude was greater for groups with low fish consumption, darker skin, and higher infant mortality—consistent with a role of prenatal vitamin D deficiency in schizophrenia. Previous research indicates that poor prenatal healthcare and nutrition increase risk for schizophrenia within the same region. These adverse conditions are more prevalent in developing countries concentrated near the equator, but schizophrenia prevalence is lowest at sites near the equator. This suggests that schizophrenia-producing environmental factors associated with higher latitude may be so powerful they overwhelm protective effects of better healthcare in industrialized countries. The observed patterns of correlations of risk factors with prevalence are consistent with an etiologic role for prenatal vitamin D deficiency and exposure to certain infectious diseases. Research to elucidate environmental factors that underlie variations in schizophrenia prevalence deserves high priority.
Keywords: epidemiology, etiology, immune function, prenatal, geography, risk factor
Introduction
Schizophrenia is an unusually burdensome disorder because of the great economic costs of extensive care and loss of economic productivity, as well as the personal suffering and stigma, which often affect a patient and his or her family for most of the patient's life. Moreover, for most patients there is still no cure or even an effective way of treating many of the most disabling, “negative” symptoms of the disorder. Therefore, a key goal of schizophrenia research is elucidation of etiologic factors, particularly environmental ones that could be readily avoided and used in effective, inexpensive, and ethically sound primary prevention programs.
In a comprehensive survey of schizophrenia prevalence studies around the world that were published in English over a period of 4 decades, Torrey1 noted 2 important patterns. First, prevalence rates varied widely at different geographic sites, with the highest rate being more than 10 times greater than the lowest. Second, there was a strong tendency for schizophrenia prevalence to increase with increasing latitude; ie, prevalence rates tended to be very low near the equator and to increase as one moved toward the poles. Both of Torrey's conclusions were also reached in a survey and meta-analysis by Saha et al,2 which included more recent studies as well as ones published in languages other than English. Several other surveys have also concluded that schizophrenia rates vary at least 10-fold around the world, including studies using measures of point prevalence, lifetime prevalence, and incidence.1,3–10
A number of explanations have been proposed for this variability in prevalence. Several complementary lines of research suggest that the tendency for schizophrenia prevalence to increase with latitude and cold climate may be due, at least in part, to some underlying pre- or perinatal environmental influences. For example, several studies have reported extremely high schizophrenia prevalence rates in populations that have recently immigrated to northern Europe from Africa or the Caribbean.11,12. Moreover, these rates appear to be especially high in groups of African and Afro-Caribbean ancestry who were born in northern Europe, rather than groups born in Africa or the Caribbean.11–14 These findings suggest that the risk of developing schizophrenia in these groups may be greatly increased by pre- or perinatal exposure to some adverse environmental factors that are associated with higher latitudes.
In addition, a widely replicated risk factor for schizophrenia is birth in the winter.15 The tendency for schizophrenia patients to be more likely than controls to be born in the winter, rather than other seasons, increases with latitude16 and severity of winter climate.17 This season-of-birth effect is smaller or even absent at sites near to the equator.18 Moreover, the season-of-birth effect tends to be stronger in cases of schizophrenia that lack either (a) a family history of the disorder19,20 or (b) a dysfunction in smooth pursuit eye movements, which several lines of evidence suggest is a behavioral indicator of a major susceptibility gene.21 Thus, pre- or postnatal exposure to seasonally variable environmental factors associated with latitude may contribute to schizophrenia risk.
These findings raise several important questions. One set of questions is concerned with how robust is the correlation of schizophrenia prevalence with latitude and cold climate. First, is the correlation still significant if one controls more carefully for differences among prevalence studies in diagnostic and ascertainment methods? Second, is the correlation found consistently for different continental regions? Third, do prevalence rates in different regions converge on low rates as the latitude of geographic sites approaches the equator?
A second set of questions concerns the fact that latitude and average income are highly correlated across sites because the developing countries with poorer economies and healthcare tend to be concentrated near the equator, whereas developed ones are concentrated at higher latitudes.1,2 Given that latitude, level of income, and access to healthcare are highly correlated with one another, can one discriminate their effects on schizophrenia prevalence? In other words, does the positive correlation of schizophrenia prevalence with latitude result because of the association of higher latitude with higher socioeconomic status and better healthcare—or despite it?
A third set of questions is concerned with what underlying adverse environmental variables correlated with latitude or general levels of economic development and healthcare may produce the observed correlation of schizophrenia prevalence with latitude. A large body of evidence indicates that, within the same geographic region, risk for schizophrenia is increased by pre- or perinatal exposure to a number of adverse environmental factors, including certain infectious diseases such as influenza and toxoplasmosis,22–25 psychosocial stress,26–28 malnutrition,29,30 and maternal obstetrical complications.31,32 Increased risk of schizophrenia is also associated with higher stillbirth and infant mortality rates that are in turn associated with poorer pre- and perinatal environments.33,34 Most of these adverse pre- and perinatal factors, as well as high infant mortality rates, are more prevalent in developing than industrialized countries. Because of that one might expect that schizophrenia prevalence rates would be higher in developing countries than in more developed ones. However, Torrey1 and Saha et al2 both found that schizophrenia prevalence rates tend to be lower in developing countries, which are concentrated at lower latitudes. One possible explanation is that there are environmental factors associated with living in a developing country that somehow protect against the development of schizophrenia.
An alternative explanation for why schizophrenia prevalence tends to be higher in industrialized countries is that 2 different sets of environmental factors contribute to schizophrenia, with one set being associated with high infant mortality and certain kinds of adverse pre- and perinatal factors, whereas the second set is associated with higher latitudes and colder climates. Both sets of adverse factors could contribute to schizophrenia, but the second set would be so powerful that their effects overwhelm the first set, so that prevalence rates are higher in developed countries at higher latitudes. If this second hypothesis is correct, then statistical analyses should reveal that latitude is a particularly strong predictor of schizophrenia prevalence. However, if one controls for latitude, then schizophrenia prevalence should tend to be higher for disadvantaged than advantaged groups.
There are a number of variables that are correlated with latitude for which prenatal exposure has been reported to be associated with higher schizophrenia prevalence. These variables include climactic variables such as mean low environmental temperature,17,35 diet,36 exposure to certain infectious diseases such as influenza and toxoplasmosis,22,25 and vitamin D deficiency.37
Influenza and toxoplasmosis are examples of infections for which prenatal exposure also appears to be more prevalent at higher latitudes. The influenza virus is known to be a central nervous system teratogen, and the virus is more contagious in cold, dry air.38 Prenatal exposure to toxoplasmosis is also an established teratogen with an affinity for the central nervous system, and prenatal exposure may be more common in colder climates because pregnant women usually become infected by contact with fecal material from house cats, which are the major vector of transmission and spend more time indoors in cold weather.25 If prenatal exposure to influenza and toxoplasmosis helps explain the high rates of schizophrenia at higher latitudes, then the effect of increasing latitude and cold on schizophrenia prevalence should also be greater for disadvantaged groups, who are more likely to live in housing that is crowded and unsanitary, as well as poorly insulated, heated, ventilated, and humidified. Disadvantaged pregnant mothers may also tend to be less likely to have the educational and economic resources to follow the recommended procedures to reduce the risk of becoming infected with influenza virus and toxoplasmosis.
Finally, McGrath37 proposed that prenatal vitamin D deficiency is an important etiologic factor in schizophrenia. In support of this prenatal “vitamin D deficiency” hypothesis, McGrath noted that this deficiency is more common at higher latitudes, particularly in winter (a season, as noted earlier, when birth is associated with an increased risk that a child will later develop schizophrenia; see Torrey et al15,34). The prevalence of vitamin D deficiency increases with latitude and cold climate because exposure of the skin to UVB radiation in sunlight is the major natural source of vitamin D, and the reduced hours and intensity of sunlight at higher latitudes make it difficult for people to generate enough vitamin D, especially in winter months.39 Cold climate is an additional risk factor for vitamin D deficiency because cold weather encourages people to spend more time indoors and wear heavier clothing, reducing both the duration and extent of skin exposure to sunlight. Regular daily exposure to direct sunlight at lower latitudes is sufficient for the generation of ample vitamin D, even for people with dark skin.39 If vitamin D deficiency is indeed an important contributor to schizophrenia, then factors other than sunshine that influence levels of vitamin D should contribute to schizophrenia prevalence primarily at sites that are at higher latitudes and/or have colder climates, where, for at least part of the year, many people do not receive enough sunlight exposure to produce adequate vitamin D.
The prenatal vitamin D hypothesis thus makes several predictions that the other hypotheses do not. In particular, it predicts (a) that schizophrenia prevalence will be associated not only with latitude, which is associated with level of exposure to sunlight, but also with several other factors that influence vitamin D levels and (b) that latitude may statistically interact with these other factors. These other factors include the following: (a) infant mortality (an index of pre- and postnatal care that is correlated with access to vitamin D in vitamin supplements and fortified foods); (b) skin color, which affects absorption of UV radiation in sunlight and the ability to synthesize vitamin D40 (ie, the darker the skin, the less vitamin D is synthesized for the same amount of sun exposure); and (c) consumption of fish, the major natural dietary source of vitamin D.
To test these hypotheses, we used data from a very large set of schizophrenia prevalence studies systematically identified by Saha et al,2 so that we could investigate several questions not examined in previous papers on schizophrenia prevalence. To reduce possible artifactual sources of differences in prevalence rates between studies, we focused our analyses on the subset of studies that used similar diagnostic and ascertainment methods.
Methods
Samples
The samples analyzed for this article were chosen from the larger list of 188 studies included in the Saha et al2 review of schizophrenia prevalence. Saha et al identified all studies that met their criteria of (a) reporting primary data on schizophrenia prevalence and (b) being published between 1965 and 2002. In order to make the prevalence rates obtained by different studies more comparable for our meta-analyses, we included only those studies that met each of 3 additional methodological criteria. The first inclusion criterion was that the prevalence rate was based on case ascertainment through community surveys. The second criterion was that the schizophrenia diagnosis was based on 1 of the 3 most widely used diagnostic systems with operationally defined criteria (DSM, ICD, or CATEGO systems). The third criterion was that the reported prevalence rate included only a diagnosis of schizophrenia proper (excluding other psychotic disorders). We focused on prevalence rates based on community surveys because these rates are likely to be more complete and comparable across studies than rates based only on hospitalization, which may be affected by a population's access to treatment. We focused on diagnoses of schizophrenia proper made using standard criteria to reduce artifactual variation in prevalence rates due to differences in investigators’ definitions of schizophrenia.
We also excluded some studies for other reasons. As discussed earlier, considerable evidence indicated that exposure to adverse environmental factors is particularly likely to increase risk for schizophrenia if exposure occurs prenatally or perinatally. Our analyses were therefore focused on investigating prenatal factors that might affect the prevalence rate in people born in each location. Consequently, we also excluded samples where it was likely that a high percentage of individuals were born outside of the study region. For this reason, in the US studies, prevalence rates for Hispanic- and Asian-American samples were excluded because in the samples studied these ethnic groups had relatively high numbers of individuals born outside the United States. A study from South Africa41 was excluded because that was the one study whose own investigators strongly indicated that the diagnoses of schizophrenia were most likely not valid; the investigators explained that after reviewing the diagnoses given by the CATEGO program, an experienced clinician concluded that the diagnoses of schizophrenia were not valid because the program did not take into account the fact that some apparent hallucinations and delusions were actually considered culturally appropriate in the population studied. When a study in a given country had published data separately for subsamples that were from sites with different latitudes and/or involved racially homogeneous subgroups, our analyses kept data on these subgroups separate, rather than using a country-wide figure that averaged across subsamples with potentially informative differences. Our analyses thus included the data for several different subsamples within the United States, as well as several different samples studied by Lehtinen et al.42 These latter samples were of particular relevance because the Lehtinen et al study was special in that the same investigative team had used the same diagnostic and ascertainment methods to obtain schizophrenia prevalence rates at 5 different sites in Finland that varied considerably in latitude.
These procedures yielded the 49 prevalence study samples that were used for most of our analyses. These 49 study samples, together with data on key study variables, are presented in table 1. We also conducted a supplementary analysis of the effect of medical and socioeconomic advantage on relative risk for schizophrenia. For this analysis, we included data from an additional 4 samples at 2 sites, as shown in table 2. All prevalence data used in our analyses were obtained from the primary source papers for the original studies.
Table 1.
Schizophrenia Prevalence and Respective Data, By Continent and Prevalence
| Study | Location | Schizophrenia Prevalencea | Persons Surveyed | Absolute Latitudeb | Mean Low Temperaturec | Infant Mortalityd | Fish Intakee | Skin Color |
| Africa | ||||||||
| Awas et al43 | S. Ethiopia | 6.6 | 600 | 9 | 62 | 175 | 0.2 | Darker |
| Ben-Tovim and Cushnie44 | Botswana | 5.3 | 1133 | 20 | 47 | 119 | 0.7 | Darker |
| Kebede and Alem45 | Ethiopia (Addis Ababa) | 3 | 4000 | 9 | 62 | 175 | 0.3 | Darker |
| Sikanartey and Eaton46 | Accra, Ghana | 0.9 | 33 113 | 5.6 | 73 | 132 | 25.4 | Darker |
| East Asia | ||||||||
| Lee etal47 | South Korea; rural | 5.4 | 1966 | 37.5 | 25 | 85 | 17.5 | Intermediate |
| Ran et al48 | China, Xinjin | 4.1 | 123 572 | 39.3 | 38 | 150 | 4.2 | Intermediate |
| Jablensky et al.49 | Nagasaki | 3.7 | 267 149 | 32.5 | 38 | 30.7 | 49 | Intermediate |
| Lee et al47 | Seoul | 3.1 | 3134 | 37.5 | 25 | 85 | 17.5 | Intermediate |
| Hwu et al50 | Taiwan, Taipei | 3 | 5005 | 25 | 58 | 30.5 | 27.4 | Intermediate |
| Chen et al51 | Hong Kong | 1.3 | 7229 | 22 | 55 | 44 | 30.1 | Intermediate |
| South Asia | ||||||||
| Chattopadhyay et al52 | New Delhi | 6.2 | 485 | 28.5 | 46 | 165 | 1.9 | Intermediate |
| Jablensky et al49 | India, Chandigarh (rural) | 4.8 | 61 642 | 30.4 | 44 | 165 | 1.9 | Intermediate |
| Jablensky et al49 | India, Chandigarh (urban) | 3.1 | 205 786 | 30.4 | 44 | 165 | 1.9 | Intermediate |
| Nandi et al53 | India, West Bengal | 2.8 | 1060 | 22.5 | 55 | 165 | 1.9 | Intermediate |
| Verghese et al54 | India; Tamil Nadu, Vellore | 2.7 | 1887 | 12.5 | 69 | 165 | 1.9 | Darker |
| Padmavathi et al55 | India, Madras | 2.6 | 101 229 | 13 | 69 | 165 | 1.9 | Darker |
| Sachdeva et al56 | India, Punjab | 2 | 1989 | 31.5 | 46 | 165 | 1.9 | Intermediate |
| Thacore et al57 | India; Lucknow slum | 1.9 | 2696 | 26.5 | 47 | 165 | 1.9 | Intermediate |
| Salan58 | Indonesia; Jakarta slum | 0.9 | 100 707 | 6 | 73 | 139 | 9.5 | Intermediate |
| Europe | ||||||||
| Lehtinen et al42 | Finland; N | 21 | 371 | 68.5 | 5 | 22 | 17.5 | Lighter |
| Lehtinen et al42 | Finland; W | 19 | 517 | 65 | 10 | 22 | 17.5 | Lighter |
| Lehtinen et al42 | Finland; E | 16 | 561 | 65 | 12 | 22 | 14 | Lighter |
| Lehtinen et al42 | Finland; SW | 9 | 780 | 61 | 20 | 22 | 17.5 | Lighter |
| Lehtinen et al42 | Finland; S | 9 | 1093 | 60 | 21 | 22 | 17.5 | Lighter |
| Wittchen et al59 | Munich | 7.2 | 417 | 48 | 26 | 33 | 7.7 | Lighter |
| Hodiamont et al60 | The Netherlands; Nijmegen | 6 | 3232 | 51.5 | 33 | 17.9 | 8.6 | Lighter |
| Jablensky et al49 | UK; Nottingham | 5.4 | 202 214 | 52.5 | 35 | 23 | 16.6 | Lighter |
| Jeffreys et al61 | UK; Hampstead | 4.8 | 112 127 | 51.5 | 41 | 23 | 20.8 | Lighter |
| Jablensky et al49 | Moscow | 4.7 | 231 866 | 55.8 | 16 | 38 | 11.2 | Lighter |
| Dilling and Weyerer62 | Upper Bavaria | 3.9 | 1536 | 49 | 26 | 33 | 7.7 | Lighter |
| Stefansson et al63 | Iceland | 3.5 | 862 | 64 | 29 | 47 | 60.2 | Lighter |
| Astrup64 | Norway; fishing village | 3.4 | 3503 | 70.5 | 22 | 19 | 61.7 | Lighter |
| Bojholm and Stromgren65 | Denmark; Bornholm Isl. | 3.3 | 37 576 | 55 | 32 | 22 | 24.9 | Lighter |
| Harvey et al66 | UK; Camden | 3.3 | 161 099 | 51 | 41 | 23 | 20.2 | Lighter |
| Jablensky et al49 | Dublin | 3.2 | 149 879 | 53 | 38 | 31 | 10.7 | Lighter |
| Jablensky et al49 | Denmark, Aarhus | 2.7 | 314 000 | 56 | 29 | 22 | 24.9 | Lighter |
| Bijl et al67 | Netherlands | 2 | 7076 | 52 | 33 | 17.9 | 13 | Lighter |
| North America (higher infant mortality) | ||||||||
| Sampath68 | Canada, Oxford Bay | 28 | 214 | 71 | −26 | 65 | n/a | Intermediate |
| Zhang and Snowden69 | ECA African American | 16 | 4481 | 38.8 | 23.8 | 44.3 | 15 | Darker |
| Zhang and Snowden69 | L.A. African American | 6 | 157 | 33.7 | 48 | 37.3 | 19.5 | Darker |
| North America (lower infant mortality) | ||||||||
| Bland et al70 | Canada, Alberta | 9.3 | 2144 | 54 | 5 | 25.5 | 12.2 | Lighter |
| Zhang and Snowden 199969 | ECA white | 9 | 11 298 | 38.8 | 23.8 | 22.9 | 15 | Lighter |
| Bland et al71 | Canada, Edmonton | 6.8 | 3258 | 54 | 5 | 22.3 | 12.2 | Lighter |
| Von Korff et al72 | Baltimore | 6.4 | 810 | 39 | 29 | 25.7 | 19.5 | Lighter |
| Zhang and Snowden69 | L.A. white | 6 | 1646 | 33.7 | 48 | 22.5 | 19.5 | Lighter |
| Weissman et al73 | New Haven | 3.9 | 511 | 41 | 17 | 22.3 | 19.5 | Lighter |
| Jablensky et al49 | Honolulu | 2.6 | 210 020 | 21 | 62 | 21.7 | 26 | Lighter |
| Other | ||||||||
| Di Marco74 | Buenos Aires | 24 | 3411 | 34.5 | 47 | 61 | 4.2 | Intermediate |
| Oakley-Browne et al75 | New Zealand, Christchurch | 3.3 | 1498 | 43.5 | 33 | 23.4 | 17.5 | Lighter |
Number of cases per 1000 adults.
Absolute latitude in degrees north or south of the equator.
Mean low temperature of the coldest month (°F).
Infant deaths under 12 months old/year/1000 live births.
Fish consumption (kg/year/person); data not available for 1 site.68
Table 2.
Schizophrenia Prevalence in Disadvantaged vs Advantaged Groups at Different Latitudes
| First Author | Study Site | Study Date | Schizophrenia Prevalencea (No. Surveyed) |
Relative Risk (with 95% CL) | Absolute Latitudeb | |||
| Disadvantaged Group | Relatively Advantaged Group | |||||||
| Roy | Canada | c.1970 | Native American | 5.7 (4273) | Whites | 1.6 (28 096) | 3.58 (2.2, 5.8)*** | 52.8 |
| Zhang | USA; ECA sites (except L.A.) | 1980 | African American | 16.3 (4481) | Whites | 9.4 (11 298) | 1.75 (1.3, 2.4)** | 38.8 |
| Zhang | USA; L.A. | 1980 | African Americanc | 6.4 (157) | Whites | 6.1 (1646) | 1.05 (0.1, 8.2) | 33.7 |
| Jablensky | India | 1978–1979 | Rurald | 4.8 (61 642) | Urban | 3.1 (205 786) | 1.55 (1.35,1.78)*** | 30.4 |
| Rin | Taiwan | c. 1962 | Aboriginese | 1.1 (5302) | Han Chinese | 2.1 (19 931) | 0.47 (0.2, 1.1) | 25.0 |
Note: The relative risk (in disadvantaged vs advantaged groups) rather than the absolute prevalence should be compared across different sites because in this table the studies at different sites did not all use the same methods.
Schizophrenia prevalence is the estimated number of cases per 1000 adults in the population surveyed.
Absolute latitude (in degrees north or south of the equator).
Data on Los Angeles were analyzed separately from the other US ECA sites because published data were available for Los Angeles as opposed to the other specific ECA sites, and Los Angeles is of particular interest for our analyses because its latitude is lower than that of the other ECA sites.
While subjects from both rural and urban Chandigarh were disadvantaged relative to Western countries, the investigators described their subjects from their rural sample as coming from poorer neighborhoods with a higher illiteracy rate than in the urban sample.
The investigators described the Ayatal aborigines as the least technologically advanced ethnic Taiwanese group; their main livelihood was from hunting and fishing.
**P < 0.005; ***P < 0.0005; all P values are 2 tailed.
Measures
We used the period, rather than the lifetime or point, prevalence rate whenever it was available because this rate was available for the most studies. As Saha et al (2005) note, the period prevalence referred to the proportion of individuals in a sample who present with a given disorder during a specified period, typically 1 year.2 Our index of cold climate was the daily average minimum temperature in the coldest month of the year at the site. These data were obtained from the same Web site.76 If weather data for the specific geographic site were not available, we used data from the closest geographic site with available data. Because the peak age of onset for schizophrenia is in the early to mid-20s, and our analyses were focused on environmental factors to which a population surveyed would have pre- or postnatal exposure, we used the infant mortality rates for the period 25 years before the study was conducted, or the closest available year. The data on fish consumption for a site77 were also taken for the date 25 years prior to the time the original source study was conducted or the next earliest year for which data were available. For some sites, data on fish consumption at that time period were available only for the country as a whole, rather than for specific regions within the country. When other information was available on regional differences in fish consumption, that information was used to proportionally adjust consumption figures to reflect those regional differences, such as the fact that research indicates that fish consumption is significantly higher at coastal than inland regions.78,79 For correlational and general linear model (GLM) data analyses, the quantitative score for fish consumption in each sample was used, but for clarity in graphic presentations, sites were divided into 3 groups: “low” fish consumption was considered to be <5 kg/person/year; “moderate” consumption to be 5–23 kg/person/year, and “high” consumption to be >23 kg/person/year (the latter sites were islands, seaports, or a fishing village).
For our index of socioeconomic and healthcare advantage, we chose a variable, the infant mortality rate, that (a) would not change as a result of the individual's developing psychiatric symptoms and (b) would tend to reflect the average health, nutritional, and economic conditions to which individuals in a population would have been exposed during pre- and perinatal development. For correlational and GLM data analyses, we used the continuous measure of infant mortality scores. However, for simplicity in graphic presentations and for delineating some subgroups, samples were considered to be relatively medically and economically disadvantaged if they were (a) from countries with a high infant mortality rate at the time most subjects in a study's sample were born or (b) from industrialized countries with low infant mortality but belonged to an ethnic minority group that had been for generations economically and medically relatively disadvantaged, with high infant mortality rates. Thus, all but one of the sites from East Asia (from Hong Kong, Japan, Korea, Taiwan, and Xinjin, China) were considered more medically advantaged than those from South Asia (from several sites in India as well as Jakarta, Indonesia), because of higher infant mortality rates at the South Asian sites. In North America, Native American and African American samples were considered more disadvantaged than other samples because those 2 minority groups had consistently high infant mortality rates over a period of many decades.80
Infant mortality rates were obtained from the United Nations81 or other government publications.80–86 Skin color was classified as dark for African Americans and groups from sub-Saharan Africa and southern India; light for groups of European ancestry; and intermediate for all other groups, such as East Asians.40
For tests of hypotheses, we calculated Pearson product-moment correlations between prevalence rates and various predictor variables, once for all samples, and again separately for major subgroups of samples. We used GLM procedures to test for main effects and hypothesized statistical interactions. The analyses did not weight samples by their size, as the size was so large for a few samples that weighting would have resulted in a small number of studies overly dominating the results. We used linear models for hypothesis tests because the hypotheses did not make strong predictions about nonlinear relationships. Statistical analyses were conducted with SAS, version 9.1 (Cary, NC).
Results
Schizophrenia Prevalence Varies Greatly (>10-fold) Across Different Sites
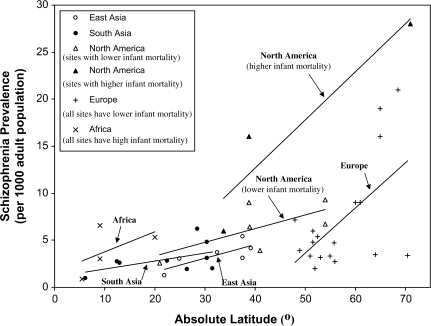
As was previously found by Torrey1 and Saha et al,2 schizophrenia prevalence across all 49 samples varied widely (>10-fold) across different sites, from a high of 28 cases per 1000 at Oxford Bay, Canada, near the Arctic Circle,68 to a low of 0.9 per 1000 at 2 sites near the equator: Accra, Ghana,46 and a slum in Jakarta, Indonesia.58 The tendency for schizophrenia prevalence to vary widely across different geographic sites was robust as schizophrenia prevalence also varied widely (>5-fold) across samples within each of the 4 continents for which data were available on multiple sites (Africa, Asia, Europe, and North America). It is notable that this wide range in prevalence occurred even though in our analyses we restricted the studies to those that had used similar methods for the ascertainment and diagnosis of schizophrenia. The prevalence rates for different sites are shown in table 1 and figure 1.
Fig. 1.
Schizophrenia Prevalence and Latitude by Continent and Infant Mortality.
Note: South Asia sites were from India and Indonesia; those sites had higher infant mortality rates than all but one of the East Asian sites. For the regression lines in North America, those with infant mortality rates above 30 per 1000 were grouped as having a higher rate, those below 30 as having a lower rate. The slopes of linear regression lines were 0.15 for the East Asian sites, 0.22 for the African sites, 0.086 for the South Asian sites, 0.14 for the North American sites with lower infant mortality, 0.51 for the North American sites with higher infant mortality, and 0.48 for the European sites.
Prevalence Increases With Latitude and Colder Climate
Also consistent with the conclusions of Torrey1 and Saha et al,2 our analyses shown in table 3 found a strong tendency for prevalence to increase with latitude. We also found a similar tendency for prevalence to increase with cold winter climate, which is correlated with latitude. These patterns are evident in figure 1 and table 3. For all 49 samples, the correlations of prevalence with latitude (r = .46) and mean low temperature (r = −.60) are both significant (P < .001 in each case). This tendency for prevalence to increase with latitude and cold climate is also robust: it is present in each of the 6 major continental and demographic groups for which there are at least 3 different geographic sites. As figure 1 shows, the slopes of the best-fit linear regression lines are positive for all 6 subgroups, and the slopes are steepest for subgroups that have more sites at higher latitudes. The 6 subgroup correlations with prevalence ranged from r = .51 to .94 for latitude and from r = −.51 to −.99 for mean low temperature. Thus, for each continent and demographic subgroup, the correlations are consistently in the same direction, and for several continents the correlations are significant, even though the number of samples within each continent is modest so that statistical power is relatively weak.
Table 3.
Correlations of Schizophrenia Prevalence with Predictor Variables
| Geographic Group of Samples | N | Absolute Latitudea | Temperatureb | Infant Mortalityc | Fish Intaked |
| All study samples | 49 | .46** | −.60*** | −.26† | −.10 |
| Europe | 18 | .58* | −.81*** | −.22 | −.21 |
| North America | |||||
| All samples | 10 | .75* | −.74* | .92*** | −.64† |
| High infant mortality | 3 | .94 | −.99* | .97 | n/a |
| Low infant mortality | 7 | .68† | −.62 | .58 | −.85* |
| Asia | |||||
| All samples | 15 | .58* | −.56* | .05 | −.05 |
| South Asia | 9 | .51† | −.51† | .49 | −.49 |
| East Asia | 6 | .78* | −.69 | .46 | −.20 |
| Africa | 4 | .53 | −.67 | .25 | −.81 |
| Othere | 2 | n/a | n/a | n/a | n/a |
n/a = data available on only 2 samples.
Absolute latitude in degrees north or south of the equator.
Mean low temperature for the coldest month of the year in degrees Fahrenheit.
Infant mortality rate is the number of cases per 1000 adult population.
Fish intake (in kg/person/year); n = 48 because data were not available for Oxford Bay.68
Includes Christchurch, New Zealand, and Buenos Aires, Argentina.
0.05 < P ≤ .10; *P < 0.05; **P < 0.001; ***P < 0.0005; all P values are two tailed, for Pearson product-moment correlations.
Prevalence Is More Strongly Associated With Latitude and Climate Than With Infant Mortality
While higher latitude and colder climate are both strongly associated with higher prevalence, infant mortality rates showed a much weaker association with prevalence. In a GLM analysis, the effect of latitude for all 49 sites was highly significant (for type I sum of squares, df = 48; F = 13.27; P = .0007), but neither the main effect of infant mortality nor its interaction with latitude was significant. In addition, table 3 shows that while, as just noted, for all 49 sites the correlations of schizophrenia prevalence with both latitude and low temperature are highly significant (P < .001 in each case), the correlation with infant mortality is not significant at the .05 level. Moreover, the direction (sign) of the respective correlations of prevalence with latitude and cold are consistent across all continents and subgroups. In contrast, the direction of the correlation of prevalence with infant mortality is inconsistent across different continents.
The best-fit regression lines in figure 1 also suggest that the increase in prevalence with increasing latitude occurs despite the fact that countries at higher latitudes tend to have lower infant mortality rates. This is indicated by the finding that, for sites with higher infant mortality rates, the best-fit regression lines are displaced upward compared with the regression line for sites in the same continent that have lower infant mortality rates. In other words, at the same latitude, prevalence tends to be higher for sites with higher infant mortality. It is also notable that prevalence rates near the equator— eg, in a particularly disadvantaged region of Jakarta,58 or in poor African cities such as Accra, Ghana,46 and Addis Ababa, Ethiopia45—are extremely low, even though these tropical sites have very high infant mortality rates.
The Increase in Prevalence With Latitude Is Greater for Disadvantaged Groups
A complementary analysis used a different approach to examine whether the tendency for prevalence to increase with latitude occurs because, or in spite of, the fact that sites at higher latitudes tend to be relatively advantaged and have lower infant mortality rates. To investigate this question, we examined how the ratio of schizophrenia prevalence in disadvantaged vs advantaged samples changed as latitude increased. For this analysis, we were able to identify 5 geographic sites (see table 2) for which comparable prevalence data based on the same methods were available on both disadvantaged and advantaged samples. That is, for each of these sites, prevalence rates had been assessed at the same site by the same investigators using the same diagnostic criteria and ascertainment methods in community surveys at a similar time period—once for a more advantaged sample and once for a less advantaged sample. At each site, we calculated the relative risk or ratio of the schizophrenia prevalence in the disadvantaged to that in the advantaged group. (That is, a relative risk greater than 1.0 indicates that the prevalence rate at a given site was higher in the disadvantaged than in the advantaged group.) For this analysis, we included 2 studies—by Roy et al87 and Rin et al88—because, even though they had not used one of the standard diagnostic systems used in the other 49 studies, they had used the same diagnostic methods in comparing prevalence rates for advantaged and disadvantaged groups at the same site. (It should be noted that for this one analysis, it is likely to be misleading to compare absolute prevalence rates between different sites because for this analysis we included some studies that had used different diagnostic methods at different sites).
For this one analysis, we wanted to examine whether or not schizophrenia prevalence would be higher in medically disadvantaged than advantaged groups when controlling for latitude, climate, and study methods. In fact, for 4 of the 5 sites, the relative risk was higher for the disadvantaged groups, significantly so at 3 of the sites (see table 2). Even more noteworthy was a strong tendency for the relative risk to increase with latitude. Although this tendency should be viewed with caution, because it only involves studies at 5 different geographic sites, the strength of this tendency is reflected in a linear correlation of latitude with relative risk for schizophrenia at the 5 sites that is large, positive, and significant (r = .98, df = 4, P < .01).
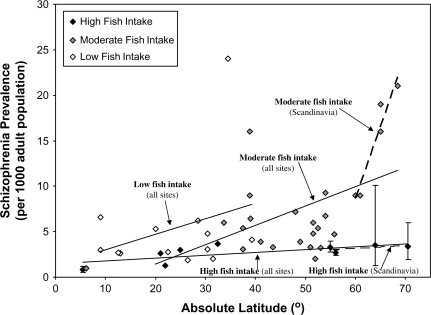
The Increase in Prevalence With Latitude Is Greater for Groups With Low Fish Consumption
A GLM analysis of data for the 48 samples with data available on fish consumption found that for type I sum of squares there were significant main effects on prevalence of both latitude (df = 47; F = 9.01; P = .004) and fish intake (F = 4.56; P = .038), though the interaction did not reach significance at the .05 level. However, a more sensitive test of an interaction was provided by the 9 samples from Scandinavia. The vitamin D hypothesis would predict that an interaction of latitude with fish consumption would be most evident in these Scandinavian samples, where vitamin D deficiency is a more severe problem because of the high latitude and reduced sunlight in winter, so that dietary vitamin D intake would be more likely to affect risk for schizophrenia. In fact, for the Scandinavian sample, there was a significant interaction of latitude with fish consumption (df = 8; F = 11.8; P = .02), as well as significant main effects for latitude (F = 35.0; P = .002) and fish intake (F = 107.0; P < .0001).
Figure 2 illustrates this tendency for fish consumption to interact with latitude, in that the best-fit regression lines have a steeper slope for the sites with lower fish intake. Figure 2 also shows that this effect is particularly pronounced for the Scandinavian samples, where the slope is much steeper for the sites with lower fish consumption.
Fig. 2.
Schizophrenia Prevalence and Latitude by Fish Intake.
Note: Error bars represent 95% confidence limits. The slopes of linear regression lines were 0.03 for sites with high fish intake, 0.21 for sites with moderate fish intake, and 0.17 for sites with low fish intake. For the subset of Scandinavian sites, the slope of linear regression for was 0.03 for sites with high fish intake and 1.57 for sites with moderate fish intake.
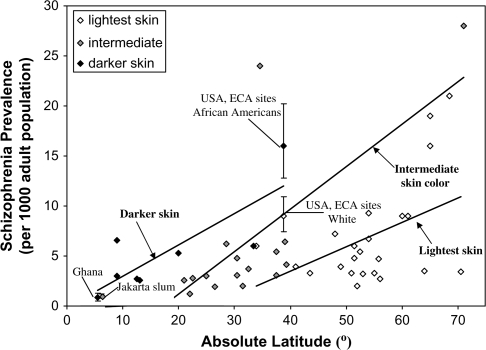
The Increase in Prevalence Is Greater for Groups With Dark Skin
A GLM analysis of latitude and skin color found that for all sites there were significant main effects for both latitude (df = 47; F = 15.74; P = .0003) and skin color (F = 13.70; P = .0006), but the interaction was not significant at the .05 level. Figure 3 displays visually how skin color combines with latitude in predicting schizophrenia prevalence. Prevalence increases with latitude for samples with darker skin color as well as those with intermediate and lighter skin. However, the regression lines are displaced upward for the samples with darker skin; that is, at the same latitude, prevalence tends to be higher for samples with darker skin. The figure and the data in table 1 also show that darker skin, by itself, is not sufficient to produce a high prevalence because groups in southern India54,55 as well as Africans living near the equator tend to have very low prevalences.
Fig. 3.
Schizophrenia Prevalence and Latitude by Skin Color.
Note: Error bars represent 95% confidence limits. The slopes of the linear regression lines were 0.24 for those with lightest skin, 0.43 for those with intermediate skin color, and 0.31 for those with darkest skin.
Discussion
Our results suggest that the key findings from comprehensive surveys of the prevalence of schizophrenia at different sites around the world1,2 were not simply artifacts of differences in diagnostic and ascertainment methods used by different studies. Even though our analyses restricted prevalence studies to those that used more similar research methods for diagnosis and ascertainment of schizophrenia cases, our analyses also found (a) that prevalence rates varied more than 10-fold across geographic sites and (b) that prevalence increased markedly with increasing latitude.
That the correlation of schizophrenia prevalence with latitude is both robust and large, and is not due simply to between-site differences in diagnostic and ascertainment methods, is further suggested by analysis of data collected by Lehtinen et al.42 Their study is particularly informative because it is the study that best controlled for possible methodological sources of differences in prevalence at different geographic sites; the same investigative personnel, as well as the same methods, were used to obtain prevalence rates at several different sites within the same country. Those investigators studied schizophrenia prevalence at 5 different Finnish sites in the same time period, using a consistent set of diagnostic criteria and ascertainment methods, and their data showed a very high correlation of prevalence with both latitude (r = .97, P < .01) and mean low temperature (r = −.99, P < .001).
At higher latitudes, there is an especially wide variation in prevalence rates. By contrast, near the equator, prevalence for all sites tends to be quite low. That is, for all groups and continental regions for which there are data from multiple sites, the best-fit (least squares) regression lines all have positive slopes, and the slopes tend to be steeper for sites at higher latitudes. This is evident by inspecting table 1, but is most readily visualized in figure 1.
The results suggest that etiologic factors closely associated with latitude and cold climate may be much more powerful contributors to risk for schizophrenia than is generally recognized. The general level of a population's economic status and healthcare, as indexed in our analysis by the infant mortality rate, appears to be a much weaker predictor of risk for schizophrenia than are latitude and winter temperature. A high infant mortality rate was strongly associated with increased risk for schizophrenia prevalence only at higher latitudes, suggesting that better incomes and access to healthcare may affect schizophrenia prevalence primarily by their effects in moderating exposure to underlying risk factors associated with latitude and climate.
A key question then is what those underlying risk factors might be. The prenatal vitamin D deficiency and certain infectious disease hypotheses both correctly predicted that schizophrenia prevalence should increase with latitude and cold climate. These hypotheses also make additional predictions about schizophrenia. The vitamin D hypothesis, eg, correctly predicts that the increase in schizophrenia risk with latitude is greater for groups that are economically disadvantaged. Groups with poorer prenatal care are less likely to get adequate vitamin D from sources other than sunlight, such as prenatal vitamins or vitamin D enriched food; dietary vitamin D intake is important for maternal vitamin D levels only when sun exposure is insufficient to produce healthy levels of the vitamin.
The results of our analyses fit particularly well with the predictions of the prenatal vitamin D deficiency hypothesis because, as was noted in the introduction, other research suggests that the effect of latitude on risk may involve prenatal exposure to environmental factors associated with higher latitudes. The prevalence of vitamin D deficiency increases markedly at higher latitudes with reduced exposure to sunlight, and cold weather will tend to cause people to spend more time indoors and wear clothing that covers more of their skin when they are outside, thereby further reducing sunlight exposure and vitamin D production. Thus, the strong correlation of prevalence with colder climate also fits well with the vitamin D hypothesis. Our findings also suggest that lighter skin may be a protective factor against etiologic factors in schizophrenia, such as vitamin D deficiency, that are associated with higher latitudes. Other epidemiologic research is consistent with this finding. For example, Bresnahan et al89 found that, even after controlling for measures of family socioeconomic status, the prevalence of hospitalization for schizophrenia in Alameda County, CA, was significantly higher for African Americans than for whites. A meta-analysis by Cantor-Graae and Selten90 found that relative risk for developing schizophrenia was 4.8 for migrants from areas where the majority of the population was black vs those from areas where most of the population was not black.
Our results do not appear to be consistent with a role for infectious diseases in general because pre- and perinatal morbidity for many infectious diseases tends to be relatively high in poorer countries, particularly in poor urban areas that are characterized by crowding and poor hygiene. Yet as we have noted, the schizophrenia prevalence rates were particularly low for disadvantaged urban sites near the equator. Our results may, however, be consistent with a role in schizophrenia of prenatal exposure to particular infectious diseases, such as influenza and toxoplasmosis, that appear to be more prevalent at sites with colder climates. Cold weather leads people to spend more time together in close proximity indoors, and there is evidence that the influenza virus is adapted to spread more readily in cold, dry air.38 More than a dozen epidemiologic studies have reported that increased risk of schizophrenia is associated with prenatal exposure to particular infectious diseases such as toxoplasmosis25 and influenza.22,24,91 The most direct study of prenatal influenza and schizophrenia risk92 found that exposure to influenza in the first half of pregnancy was associated with a 3-fold increase in schizophrenia risk, based on data from a follow-up study of individuals who were in utero when sera were obtained from their mothers and later assayed for levels of antibodies to influenza.
Christensen and Christensen93 found that in cross-national comparisons, high consumption of seafood (which is low in saturated fat and high in omega-3 fatty acids, as well as being the most important dietary source of vitamin D39) was associated with lower schizophrenia rates. Based on this and other evidence, Mahadik36 hypothesized that prenatal deficiency of omega-3 fatty acids is a contributing factor in the development of schizophrenia because these fatty acids are essential for brain and behavioral development. Prenatal deficiency of omega-3 fatty acids has been shown to lead to reduced body and brain weight, reduced head size, and increased cognitive deficits in children. These abnormalities are more common in children who later develop schizophrenia. However, our analyses appear to offer greater support for the role of vitamin D than for omega-3 fatty acids because the association of fish consumption with prevalence was most evident at high latitude sites where populations were most likely to get inadequate vitamin-D from sunlight alone. Samples with low fish consumption nonetheless tended to have low prevalence rates, so long as they were at low latitudes—a finding that would not be expected if omega-3 consumption rather than vitamin D consumption was more important for schizophrenia prevalence.
Most hypotheses about prenatal environmental factors in schizophrenia simply predict a significant main effect—ie, that the hypothesized predictor variable, such as infant mortality or fish consumption, will be significantly correlated with schizophrenia prevalence. In contrast, the vitamin D hypothesis correctly predicted a number of different associations that were found between prevalence and several different variables, including skin color, fish consumption, and infant mortality, as well as latitude and temperature. One possible explanation for the strong correlations between latitude and cold climate with schizophrenia prevalence, and the large (>10-fold) difference in prevalence between near-Arctic and equatorial sites, may be that vitamin D deficiency has several adverse effects, each of which contributes to increased risk for schizophrenia. These multiple adverse effects may include (1) disruptive effects on prenatal brain development94 as well as (2) harmful effects on maternal immune function that make the pregnant mother and her fetus or young infant more vulnerable to prenatal exposure to infections, thereby interacting synergistically with infections, such as influenza22,92 or toxoplasmosis,25,95,96 that previous research has linked to schizophrenia. Cannell et al97 reviewed evidence indicating that vitamin D is important for several aspects of immunological defense against infectious diseases and that seasonal variation in vitamin D deficiency may explain the marked seasonality of epidemic influenza. Moreover, as McGrath98 noted, both animal and in vitro experiments have demonstrated that administration of vitamin D can inhibit the intracellular growth of Toxoplasma gondii.
The prevalence of a disease reflects factors such as remission and chronicity as well as incidence, and latitude appears to be more strongly correlated with the prevalence than the incidence of schizophrenia.6 Might vitamin D deficiency contribute to schizophrenia prevalence by increasing the chronicity of the disorder as well as its incidence? It is possible that prenatal vitamin deficiency might disrupt prenatal brain development in a way that leads to more chronic forms of schizophrenia. In addition, it is noteworthy that vitamin D is crucial for immune function throughout postnatal life as well as during prenatal development, eg, by helping to promote innate immune responses and prevent autoimmune disorders.99 Thus, it is conceivable that postnatal vitamin D deficiency could increase the chronicity as well as the incidence of schizophrenia, by weakening the immune system and its ability to fight off any postnatal infections or immune disorders that may contribute to schizophrenia.
Our results complement several other lines of research on risk factors for schizophrenia. For example, in his survey of international prevalence studies, Torrey1 found several patterns that were consistent with those we found in our analyses, even for those studies that we excluded because of differences in diagnostic and ascertainment methods. Thus, Torrey also found extremely high prevalence rates at sites that are at high latitudes, such as those in Finland and other sites in northern Scandinavia, and extremely low rates at sites near the equator, such as Java and New Guinea.
Gupta100 undertook a preliminary investigation of environmental factors that may predict the observed patterns of schizophrenia prevalence. He analyzed data on samples that had been previously ascertained as part of a World Health Organization international program of research on schizophrenia prevalence.101 Gupta found that both schizophrenia risk and a more chronic course of illness were positively correlated with several variables, including mean daily environmental temperature, infant mortality rate, and high consumption of animal fat. However, as Gupta noted, data for his analyses were available for only a dozen or fewer sites, and the different predictor variables were themselves highly intercorrelated and confounded, making it difficult to discriminate which variables might actually be contributing to increased risk for schizophrenia. In the present article's analyses, we were better able to separate the effects of these different variables because we examined data on a much larger set of schizophrenia prevalence studies than Gupta was able to.
The tendency for schizophrenia patients to be more likely than people in the general population to have been born in winter months appears to have declined in recent decades in some industrialized countries.102 Overall incidence rates for schizophrenia in northern Europe may also have declined over recent decades.103,104 This decline in schizophrenia incidence has paralleled declines in infant mortality rates, as would be expected if improved standards of nutrition and healthcare protected mothers and young infants against adverse pre- and perinatal environmental hazards, such as vitamin D deficiency, that may contribute to schizophrenia. A possible etiologic role of temperature extremes is suggested by associations of high infant mortality rates with low winter temperature and by correlations of infant mortality and stillbirth rates with schizophrenia prevalence rates at the same site.34 If winter weather near birth is in fact a risk factor for schizophrenia, then overall schizophrenia prevalence rates should be especially high in regions with higher latitudes and more severe winters, as our analyses indicate is the case.
An important caveat in interpreting the results of our analyses is that the number of studies available was often modest, especially for analyses that involved studies within the same continent, where the correlations observed sometimes depended on extreme values at only a few sites. Our results should thus be viewed with caution until they can be replicated on new and larger international epidemiological studies. While such studies may be costly and time consuming to conduct, the large effect sizes found in our analyses suggest that these could be highly cost effective because the underlying causal variables could be important contributors to schizophrenia.
Another limitation of the present study was its use of ecologic analyses. That is, the analyses involve comparisons of characteristics of groups rather than individuals. The use of data aggregated over groups and the associated loss of information about within-group variability can potentially result in important biases.105 Research is therefore needed that contains data on key variables such as prenatal vitamin D at the individual level in people with schizophrenia and in control samples. Of particular interest, eg, would be studies of whether serum levels of vitamin D in women at different periods of their pregnancies are associated with their offspring's risk for schizophrenia proper and/or with risk for traits that are known to be associated with risk for schizophrenia. A pioneering study by McGrath et al106 using this research design with samples of modest size found evidence that increased risk for schizophrenia in offspring was associated with prenatal exposure to particularly low serum levels of maternal vitamin D in African Americans. More research of this kind is needed, using samples that are larger, come from diverse geographic regions, and contain data on other potential etiologic factors such as prenatal exposure to influenza and toxoplasmosis, as well as to maternal vitamin D levels. Analyses of this kind should be considered as part of future prospective longitudinal studies of large cohorts.
In summary, our results suggest that underlying environmental factors associated with higher latitudes and colder climate may be powerful contributors to schizophrenia—so powerful that they overwhelm the protective effects of better prenatal healthcare and nutrition found in more developed countries that are concentrated at higher latitudes. One promising candidate for such a factor is vitamin D deficiency, acting either alone or in synergism with certain infectious diseases such as influenza and toxoplasmosis. Further, more definitive research to elucidate the possible underlying environmental factors deserves high priority because these underlying factors could potentially be modified and prove valuable in programs for primary prevention of schizophrenia.
Funding
Stanley Medical Research Foundation, the Michael Braman Pomeroy Memorial Fund, and the James Leach Memorial Fund at McLean Hospital.
Acknowledgments
We thank Dan Barch and Sharon Tramer for their help in preparing the manuscript.
References
- 1.Torrey EF. Prevalence studies in schizophrenia. Br J Psychiatry. 1987;150:598–608. doi: 10.1192/bjp.150.5.598. [DOI] [PubMed] [Google Scholar]
- 2.Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:413–433. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton WW. Epidemiology of schizophrenia. Epidemiol Rev. 1985;7:320–328. doi: 10.1093/oxfordjournals.epirev.a036278. [DOI] [PubMed] [Google Scholar]
- 4.Eaton WW. Update on the epidemiology of schizophrenia. Epidemiol Rev. 1991;13:320–328. doi: 10.1093/oxfordjournals.epirev.a036075. [DOI] [PubMed] [Google Scholar]
- 5.Eaton WW, Byrne M, Ewald H, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163:521–528. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- 6.McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008 doi: 10.1093/epirev/mxn001. doi:10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 7.Goldner EM, Hsu L, Waraich P, Somers JM. Prevalence and incidence studies of schizophrenic disorders: a systematic review of the literature. Can J Psychiatry. 2002;47:833–843. doi: 10.1177/070674370204700904. [DOI] [PubMed] [Google Scholar]
- 8.Warner R, de Girolamo G. Schizophrenia. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]
- 9.Fahy TA, Jones PB, Sham PC, Takei N, Murray RM. Schizophrenia in Afro-Carribeans in the UK following prenatal exposure to the 1957 A2 influenza pandemic (abstract) Schizophr Res. 1993;9:132. [Google Scholar]
- 10.Fahy T, Jones PB, Sham P, Murray RM. A family history study in British Afro-Caribbean schizophrenic patients (abstract) Schizophr Res. 1993;9:132. [Google Scholar]
- 11.Sugarman PA, Craufurd D. Schizophrenia in the Afro-Caribbean community. Br J Psychiatry. 1994;164:474–480. doi: 10.1192/bjp.164.4.474. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson G, Takei N, Fahy TA, et al. Morbid risk of schizophrenia in first-degree relatives of white and African-Caribbean patients with psychosis. Br J Psychiatry. 1996;169:776–780. doi: 10.1192/bjp.169.6.776. [DOI] [PubMed] [Google Scholar]
- 13.Harrison G, Cooper JE. Why isn't schizophrenia disappearing in Nottingham? Schizophr Res. 1991;4:259. [Google Scholar]
- 14.Veling W, Selten J, Veen N, Laan W, Blom JD, Hoek HW. Incidence of schizophrenia among ethnic minorities in the Netherlands: a four-year first-contact study. Schizophr Res. 2006;86:189–193. doi: 10.1016/j.schres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28:1–38. doi: 10.1016/s0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 16.Davies G, Welhan J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of northern hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29:587–593. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- 17.DuMouchel R, Waternaux C, Kinney D. Hierarchical Bayesian linear models for assessing the effect of extreme cold weather on schizophrenic births. In: Berry D, Stangl D, editors. Bayesian Biostatistics. New York: Marcel Dekker; 1996. pp. 451–465. [Google Scholar]
- 18.Parker G, Mahendran R, Machin D. Season of birth in schizophrenia: no latitude at the equator. Br J Psychiatry. 2000;176:68–71. doi: 10.1192/bjp.176.1.68. [DOI] [PubMed] [Google Scholar]
- 19.Kinney DK, Jacobsen B, Jansson L, Faber B, Tramer SJ, Suozzo M. Winter birth and biological family history in adopted schizophrenics. Schizophr Res. 2000;44(2):95–103. doi: 10.1016/S0920-9964(99)00162-0. [DOI] [PubMed] [Google Scholar]
- 20.O'Callaghan E, Gibson T, Colohan HA, Buckley P, Larkin C, Waddington JL. Season of birth in schizophrenia: evidence for confinement of an excess of winter births to patients without a family history of mental disorder. Br J Psychiatry. 1991;158:764–769. doi: 10.1192/bjp.158.6.764. [DOI] [PubMed] [Google Scholar]
- 21.Kinney DK, Levy DL, Yurgelun-Todd DA, Lajonchere CM, Holzman P. Eye tracking dysfunction and birth-month weather in schizophrenia. J Abnorm Psychol. 1999;108:359–362. doi: 10.1037//0021-843x.108.2.359. [DOI] [PubMed] [Google Scholar]
- 22.Mednick SA, Huttunen MO, Machon RA. Prenatal influenza infections and adult schizophrenia. Schizophr Bull. 1994;20:263–267. doi: 10.1093/schbul/20.2.263. [DOI] [PubMed] [Google Scholar]
- 23.Zammit S, Lewis S, Gunnell D, Smith GD. Schizophrenia and neural tube defects: comparisons from an epidemiological perspective. Schizophr Bull. 2007;33:853–858. doi: 10.1093/schbul/sbl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32(2):200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerg Infect Dis. 2003;9(11):1375–1380. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttunen MO, Niskanen P. Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry. 1978;35:429. doi: 10.1001/archpsyc.1978.01770280039004. [DOI] [PubMed] [Google Scholar]
- 27.Kinney DK. Prenatal stress and risk for schizophrenia. Int J Ment Health. 2001;29:62–72. [Google Scholar]
- 28.van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of the Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 29.Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: the story of the Dutch famine study. Am J Epidemiol. 1998;147:213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- 30.Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. Obstet Gynecol Surv. 2006;61(1):2–3. [Google Scholar]
- 31.McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–212. doi: 10.1176/appi.ajp.157.2.203. [DOI] [PubMed] [Google Scholar]
- 32.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2003;160:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Murray RM. The relationship of environmental temperature to the incidence and outcome of schizophrenia. Br J Psychiatry. 1992;160:788–792. doi: 10.1192/bjp.160.6.788. [DOI] [PubMed] [Google Scholar]
- 34.Torrey EF, Rawlings RR, Ennis JM, Merrill DD, Flores DS. Birth seasonality in bipolar disorder, schizophrenia, schizoaffective disorder, and stillbirths. Schizophr Res. 1996;21:141–149. doi: 10.1016/0920-9964(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 35.Welham J, Davies G, Auliciems A, McGrath J. Climate, geography and the search for candidate, nongenetic, risk factors for schizophrenia. Int J Ment Health. 2000;29(3):79–100. [Google Scholar]
- 36.Mahadik SP. Nutritional factors and schizophrenia. In: Keshavan M, Kennedy J, Murray R, editors. Neurodevelopment and Schizophrenia. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- 37.McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res. 1999;40(3):173–177. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 38.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 40.Jablonsky NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39(1):57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 41.Rumble S, Swartz L, Parry C, Zwarenstein M. Prevalence of psychiatric morbidity in the adult population of a rural South African village. Psychol Med. 1996;26:997–1007. doi: 10.1017/s0033291700035327. [DOI] [PubMed] [Google Scholar]
- 42.Lehtinen V, Joukamaa M, Lahtela K, et al. Prevalence of mental disorders among adults in Finland: basic results from the mini Finland health survey. Acta Psychiatr Scand. 1990;81:418–425. doi: 10.1111/j.1600-0447.1990.tb05474.x. [DOI] [PubMed] [Google Scholar]
- 43.Awas M, Kebede D, Alem A. Major mental disorders in Butajira, Southern Ethiopia. Acta Psychiatr Scand Suppl. 1999;397:56–64. doi: 10.1111/j.1600-0447.1999.tb10695.x. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Tovim DI, Cushnie JM. The prevalence of schizophrenia in a remote area of Botswana. Br J Psychiatry. 1986;148:576–580. doi: 10.1192/bjp.148.5.576. [DOI] [PubMed] [Google Scholar]
- 45.Kebede D, Alem A. Major mental disorders in Addis Ababa, Ethiopia. I. schizophrenia, schizoaffective and cognitive disorders. Acta Psychiatr Scand Suppl. 1999;397:11–17. doi: 10.1111/j.1600-0447.1999.tb10688.x. [DOI] [PubMed] [Google Scholar]
- 46.Sikanartey T, Eaton WW. Prevalence of schizophrenia in the Labadi district of Ghana. Acta Psychiatr Scand. 1984;69:156–161. doi: 10.1111/j.1600-0447.1984.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee CK, Kwak YS, Yamamoto J, et al. Psychiatric epidemiology in Korea. Part II: urban and rural differences. J Nerv Ment Dis. 1990;178:247–252. doi: 10.1097/00005053-199004000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Ran M, Xiang M, Li SX, Shan Y, Huang M. Prevalence and outcome of schizophrenia in a Chinese rural area: an epidemiological study. Aust N Z J Psychiatry. 2003;37:452–457. doi: 10.1046/j.1440-1614.2003.01203.x. [DOI] [PubMed] [Google Scholar]
- 49.Jablensky A, Santorius N, Ernbeg G, et al. Schizophrenia: manifestations, incidence, and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 50.Hwu HG, Yeh EK, Chang LY. Prevalence of psychiatric disorders in Taiwan defined by the Chinese diagnostic interview schedule. Acta Psychiatr Scand. 1989;79:136–147. doi: 10.1111/j.1600-0447.1989.tb08581.x. [DOI] [PubMed] [Google Scholar]
- 51.Chen CN, Wong J, Lee N, et al. The Shatin community mental health survey in Hong Kong. II. Major findings. Arch Gen Psychiatry. 1993;50:125–133. doi: 10.1001/archpsyc.1993.01820140051005. [DOI] [PubMed] [Google Scholar]
- 52.Chattopadhyay O, Gill JS, Bali P, Wig NN. Psychotic disorders in the adult population of an urban slum. Indian J Public Health. 1989;33:37. [PubMed] [Google Scholar]
- 53.Nandi DN, Ajmany S, Ganguli H, Banerjee G, Boral GC. Psychiatric disorders in a rural community in West Bengal—an epidemiological study. Indian J Psychiatry. 1975;17:87–89. [Google Scholar]
- 54.Verghese A, Beig A, Senseman LA, Rao SS, Benjamin V. A social and psychiatric study of a representative group of families in Vellore Town. Indian J Med Res. 1973;61:608–620. [PubMed] [Google Scholar]
- 55.Padmavathi R, Rajkumar S, Srinivasan TN. Schizophrenic patients who were never treated—a study in an Indian urban community. Psychol Med. 1998;28:1113–1117. doi: 10.1017/s0033291798007077. [DOI] [PubMed] [Google Scholar]
- 56.Sachdeva J, Singh S, Sidhu BS, Goyal RKD, Sing J. An epidemiological study of psychiatric disorders in rural Faridkot. Indian J Psychiatry. 1986;28:317–323. [PMC free article] [PubMed] [Google Scholar]
- 57.Thacore VR, Gupta SC, Suraiya M. Psychiatric morbidity in a North Indian community. Br J Psychiatry. 1975;126:365–369. doi: 10.1192/bjp.126.4.364. [DOI] [PubMed] [Google Scholar]
- 58.Salan R. Epidemiology of schizophrenia in Indonesia (the Tambora I study) ASEAN J Psychiatry. 1992;2:52–57. [Google Scholar]
- 59.Wittchen HU, Essau CA, von Zerssen D, Krieg JC, Zaudig M. Lifetime and six-month prevalence of mental disorders in the Munich follow-up study. Eur Arch Psychiatry Clin Neurosci. 1992;241:247–258. doi: 10.1007/BF02190261. [DOI] [PubMed] [Google Scholar]
- 60.Hodiamont P, Peer N, Syben N. Epidemiological aspects of psychiatric disorder in a Dutch health area. Psychol Med. 1987;17:495–505. doi: 10.1017/s0033291700025058. [DOI] [PubMed] [Google Scholar]
- 61.Jeffreys SE, Harvey CA, McNaught AS, et al. The Hampstead schizophrenia survey 1991. I: prevalence and service use comparisons in an inner London health authority, 1986–1991. Br J Psychiatry. 1997;170:301–306. doi: 10.1192/bjp.170.4.301. [DOI] [PubMed] [Google Scholar]
- 62.Dilling H, Weyerer S. Prevalence of mental disorders in the smalltown-rural region of Traunstein (Upper Bavaria) Acta Psychiatr Scand. 1984;69:60–79. doi: 10.1111/j.1600-0447.1984.tb04517.x. [DOI] [PubMed] [Google Scholar]
- 63.Stefansson JG, Lindal E, Bjornsson JK, Guomundsdottir A. Lifetime prevalence of specific mental disorders among people born in Iceland in 1931. Acta Psychiatr Scand. 1991;84:142–149. doi: 10.1111/j.1600-0447.1991.tb03118.x. [DOI] [PubMed] [Google Scholar]
- 64.Astrup C. The Berlevag project from 1939 through 1976. Acta Psychiatr Scand Suppl. 1989;348:79–83. doi: 10.1111/j.1600-0447.1989.tb05217.x. [DOI] [PubMed] [Google Scholar]
- 65.Bojholm S, Stromgren E. Prevalence of schizophrenia on the island of Bornholm in 1935 and in 1983. Acta Psychiatr Scand Suppl. 1989;348:157–166. doi: 10.1111/j.1600-0447.1989.tb05223.x. [DOI] [PubMed] [Google Scholar]
- 66.Harvey CA, Pantelis C, Taylor J, et al. The Camden schizophrenic surveys. II. High prevalence of schizophrenia in an inner London borough and its relationship to socio-demographic factors. Br J Psychiatry. 1996;168:418–426. doi: 10.1192/bjp.168.4.418. [DOI] [PubMed] [Google Scholar]
- 67.Bijl RV, Ravelli A, van Zessen G. Prevalence of psychiatric disorder in the general population: results of the Netherlands mental health survey and incidence study (NEMESIS) Soc Psychiatry Epidemiol. 1998;33:587–595. doi: 10.1007/s001270050098. [DOI] [PubMed] [Google Scholar]
- 68.Sampath HM. Prevalence of psychiatric disorders in a southern Baffin Island Eskimo settlement. Can Psychiatr Assoc J. 1974;19:363–367. doi: 10.1177/070674377401900406. [DOI] [PubMed] [Google Scholar]
- 69.Zhang AY, Snowden LR. Ethnic characteristics of mental disorders in five U.S. communities. Cultur Divers Ethnic Minor Psychol. 1999;5(2):134–146. doi: 10.1037/1099-9809.5.2.134. [DOI] [PubMed] [Google Scholar]
- 70.Bland RC, Orn H, Newman SC. Schizophrenia: lifetime comorbidity in a community sample. Acta Psychiatr Scand. 1987;75:383–391. doi: 10.1111/j.1600-0447.1987.tb02806.x. [DOI] [PubMed] [Google Scholar]
- 71.Bland RC, Orn H, Newman SC. Lifetime prevalence of psychiatric disorders in Edmonton. Acta Psychiatr Scand Suppl. 1988;338:24–32. doi: 10.1111/j.1600-0447.1988.tb08544.x. [DOI] [PubMed] [Google Scholar]
- 72.Von Korff M, Nestadt G, Romanoski A, et al. Prevalence of treated and untreated DSM-III schizophrenia. Results of a two-stage community survey. J Nerv Ment Dis. 1985;173:577–581. doi: 10.1097/00005053-198510000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Weissman MM, Myers JK, Harding PS. Psychiatric disorders in a U.S. urban community: 1975–1976. Am J Psychiatry. 1978;135:459–462. doi: 10.1176/ajp.135.4.459. [DOI] [PubMed] [Google Scholar]
- 74.Di Marco G. Prevalencia de desordenes mentales en el area metropolitan de la Republica Argentina. Acta Psychiatr Psicol Amer Lat. 1982;28:93–102. [PubMed] [Google Scholar]
- 75.Oakley-Browne MA, Joyce PR, Wells JE, Bushnell JA, Hornblow AR. Christchurch psychiatric epidemiology study, part II: six month and other period prevalence of specific psychiatric disorders. Aust N Z J Psychiatry. 1989;23:327–240. doi: 10.3109/00048678909068290. [DOI] [PubMed] [Google Scholar]
- 76.MSN Weather. http://weather.msn.com. 2008. Last accessed October 14, 2008. [Google Scholar]
- 77.Laurenti G. 1961–2001, Fish and Fishery Products. [Google Scholar]
- 78.Mahaffey KR, Clickner RP, Jeffries RA. Adult women's blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999–2004) Environ Health Perspect. 2009;117(1):47–53. doi: 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.World Health Organization. Global and Regional Food Consumption Patterns and Trends: Availability and Consumption of Fish. http://www.who.int/nutrition/topics/3_foodconsumption/en/index5.html. Accessed February 28, 2009. [Google Scholar]
- 80.Miniño AM, Arias E, Kochanek KD, Murphy SL, Smith BL. Table 34. infant, neonatal, and postneonatal mortality rates by race and sex: United States, 1940, 1950, 1960, 1970, and 1975–2000. Natl Vital Statist Report. 2002;50(15):100. [PubMed] [Google Scholar]
- 81.18th ed. New York: Statistical Office of the United Nations, Department of Economical and Social Affairs; 1967. United Nations Statistical Yearbook 1966. [Google Scholar]
- 82.Atlas of Canada. Births, Marriages, and Deaths. http://atlas.nrcan.gc.ca/site/english/maps/archives/3rdedition/peopleandsociety/population/050?w = 4&h = 4&1 = 2&r = 0&c = 0. 2004. Updated 2004, 2008. Last accessed October 14, 2008. [Google Scholar]
- 83.Canada Year Book: Official Statistical Annual of the Resources, History, Institutions and Social and Economic Conditions of Canada. Ottawa, Ontario: Dominion Bureau of Statistics: Canada Year Book Division. Authority of the Minister of Trade and Commerce; 1966. [Google Scholar]
- 84.Natality. Vol 1: Washington, DC: US Department of Health, Education, and Welfare Public Health Service, National Vital Statistics Division; 1961. Vital Statistics of the United States. [Google Scholar]
- 85.UNICEF. The State of the World's Children. Oxford: Oxford UP for UNICEF; 1989. [Google Scholar]
- 86.Dzakpasu S, Joseph KS, Kramer MS, Allen AC. The Matthew effect: infant mortality in Canada and internationally. Pediatrics. 2000;106:e5. doi: 10.1542/peds.106.1.e5. [DOI] [PubMed] [Google Scholar]
- 87.Roy C, Choudhuri A, Irvine D. The prevalence of mental disorders among Saskatchewan Indians. J Cross Cult Psychol. 1970;1:383–392. [Google Scholar]
- 88.Rin H, Lin TY. Mental illness in Formosan aborigines as compared with the Chinese in Taiwan. J Ment Sci. 1962;108:134–146. doi: 10.1192/bjp.108.453.134. [DOI] [PubMed] [Google Scholar]
- 89.Bresnahan M, Begg MD, Brown A, et al. Race and risk of schizophrenia in a US birth cohort: another example of health disparity? Int J Epidemiol. 2007;36:751–758. doi: 10.1093/ije/dym041. [DOI] [PubMed] [Google Scholar]
- 90.Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 91.Brown AS, Bottiglieri T, Schaefer CA, et al. Elevated prenatal homocystine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007;64(1):31–39. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- 92.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of parental influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 93.Christensen O, Christensen E. Fat consumption and schizophrenia. Acta Psychiatr Scand. 1988;78:587–591. doi: 10.1111/j.1600-0447.1988.tb06388.x. [DOI] [PubMed] [Google Scholar]
- 94.Becker A, Eyles DW, McGrath JJ, Grecksh G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res. 2005;161:306–312. doi: 10.1016/j.bbr.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 95.Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Yolken RH. Early infections of toxoplasma gondii and the later development of schizophrenia. Schizophr Bull. 2007;33:741–744. doi: 10.1093/schbul/sbm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hinze-Selch D, Daubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. A controlled prospective study of toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophr Bull. 2007;33:782–788. doi: 10.1093/schbul/sbm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McGrath J. Comment on Antibodies to Toxoplasma gondii in Patients with Schizophrenia: A Meta-analysis. 2007. http://schizophreniaforum.org/for/live/detailprint.asp?liveID = 56. Accessed February 27, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Arch Dis Child. 2007;92:737–740. doi: 10.1136/adc.2007.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta S. Can environmental factors explain the epidemiology of schizophrenia in immigrant groups? Soc Psychiatry Epidemiol. 1993;28:263–266. doi: 10.1007/BF00795905. [DOI] [PubMed] [Google Scholar]
- 101.World Health Organization. Schizophrenia: An International Follow-Up Study. Chichester, UK: Wiley; 1979. [Google Scholar]
- 102.Suvisaari JM, Haukka JK, Tanskanen AJ, Lönnqvist JK. Decreasing seasonal variation of births in schizophrenia. Psychol Med. 2000;30:315–324. doi: 10.1017/s0033291700001756. [DOI] [PubMed] [Google Scholar]
- 103.Der G, Gupta S, Murray RM. Is schizophrenia disappearing? Lancet. 1990;335:513–516. doi: 10.1016/0140-6736(90)90745-q. [DOI] [PubMed] [Google Scholar]
- 104.Haukka J, Suvisaari J, Varilo T, Lönnqvist J. Regional variation in the incidence of schizophrenia in Finland: a study of birth cohorts born from 1950 to 1969. Psychol Med. 2001;31:1045–1053. doi: 10.1017/s0033291701004299. [DOI] [PubMed] [Google Scholar]
- 105.Wakefield J. Ecologic studies revisited. Annu Rev Public Health. 2008;29:75–90. doi: 10.1146/annurev.publhealth.29.020907.090821. [DOI] [PubMed] [Google Scholar]
- 106.McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63(1–2):73–78. doi: 10.1016/s0920-9964(02)00435-8. [DOI] [PubMed] [Google Scholar]