Summary
Early stressful events can increase vulnerability for psychopathology, although knowledge on the effectors is still limited. Here we tested the hypothesis that peripheral levels of Brain-Derived Neurotrophic Factor (BDNF) and Nerve Growth Factor (NGF), which are involved in the response to stress and in the pathophysiology of anxiety and depression, might be affected in a non-human primate model of adverse rearing. Males and females rhesus macaques reared with their mothers (MR) or in peer-only groups (PR) were used as experimental subjects. BDNF, NGF, adrenocorticotropic hormone (ACTH), cortisol and growth hormone (GH) were determined at baseline on postnatal days (PND) 14, 30 and 60 by means of specific ELISA and RIA procedures. In addition, behavior was assessed on PND 7, 14, 21, 30 (Brazelton test) and 60 (home cage observation). Data indicate gender differences in basal levels of BDNF throughout development. Peer-rearing increased significantly BDNF levels only in females. In addition, while all peer-reared subjects showed high levels of stereotypies and self-directed behaviors, behavioral passivity was selectively increased in females. By contrast, NGF levels were increased in response to peer rearing only in males, and correlated positively with other “classic” endocrine responses to stress, such as cortisol and growth hormone. Our data identify BDNF and NGF as neuroendocrine markers underlying differential responses to maternal deprivation in males and females rhesus macaques. The selective changes in BDNF levels in females could help explain the greater vulnerability to mood disorders of this gender reported in humans.
Keywords: Nerve growth factor (NGF), Brain-derived neurotrophic factor (BDNF), depression, vulnerability, gender, non-human primates
1. Introduction
Early adverse experiences in humans are associated with an increased risk for developing psychiatric disorders such as anxiety and major depression, although little is known of the neurobiological mediators (Kaufman et al., 2000; McEwen, 2000; Heim and Nemeroff, 2001). Neurotrophins, such as Brain-Derived Neurotrophic Factor (BDNF) and Nerve Growth Factor (NGF), which play a fundamental role in brain function and neuroprotection, and are affected by stress, are good candidates for transducing the effects of adverse events in changes in brain function (Smith et al., 1995; Thoenen, 1995; Duman et al., 1997). Neurotrophins are also produced by cells outside the nervous system, thus being in a position to integrate neural, immune and endocrine responses to stress (Aloe et al., 1986; Nisoli et al., 1996; Nockher and Renz, 2005). Changes in peripheral levels of neurotrophins, as well as selected gene variants, have been associated with mood disorders, also in interaction with early trauma (Aloe et al., 1994; Hadjiconstantinou et al., 2001; Karege et al., 2002b; Karege et al., 2005; Kaufman et al., 2006; Castren et al., 2007; Kauer-Sant'Anna et al., 2007). NGF is increased in anxiety-loaded situations, such as in male soldiers experiencing their first parachute jump (Aloe et al., 1994), in spouses caring for Alzheimer’s patients (Hadjiconstantinou et al., 2001) or following smoking cessation (Lang et al., 2002). By contrast, decreased blood levels of BDNF characterize subjects diagnosed as major depressives, antidepressants reverting this change (Shimizu et al., 2003; Karege et al., 2005). Although a dimorphism in peripheral BDNF levels has been reported in humans, women showing higher levels of this neurotrophin than men (Lommatzsch et al., 2005), information on sex differences related to stress susceptibility are still lacking (Becker et al., 2007).
In preclinical studies modeling early adversity, maternal separation stress has been shown to affect NGF and BDNF levels in limbic areas as well as to produce long-lasting changes on emotional behavior and impaired responses to stress, suggesting that these neurotrophins might modulate mechanisms underlying social bonding (Plotsky and Meaney, 1993; Cirulli et al., 2000; Meaney, 2001; Cirulli, 2003a; Roceri et al., 2004; Cirulli et al., 2007). In non-human primate models, maternal deprivation with some form of social contact, such as access to peers, leads to important emotional and social disturbances and behavioral abnormalities, such as motor stereotypies (Suomi, 1991; Champoux et al., 2002; Barr et al., 2003). Peer-reared macaques develop inadequate social skills, are highly reactive and aggressive and, as adults, show increased voluntary alcohol consumption and rank at the bottom of the dominance hierarchy (Suomi, 1991; Barr et al., 2003).
The hypothesis tested in the present study is that early maternal deprivation would affect peripheral levels of neurobiological mediators of adult affective disorders, which may be related to early life stress. Preclinical studies performed in rodents have shown that neurotrophins are sensitive to manipulations of the mother-infant relationship and, more in general, of the rearing environment (Cirulli et al., 1998; Cirulli et al., 2000; Roceri et al., 2004; Branchi et al., 2006b). In particular, NGF and BDNF levels are increased in an age- and time-dependent fashion following maternal separation in young rodents (Cirulli et al., 1998; Cirulli et al., 2000; Roceri et al., 2004; Nair et al., 2007). These changes may program lasting alterations in the circuits regulating the expression of these neurotrophins, leading to the establishment of individual variability in vulnerability to stress-related psychopathology. To this purpose, BDNF and NGF were assessed in the peripheral circulation in rhesus macaques, and related to more common neuroendocrine effectors, such as cortisol and growth hormone (GH). Since there is a strong need for preclinical studies to model sex differences involved in individual susceptibility to psychiatric disorders, aim of this work was to verify differential responses to early adversity in the two genders, particularly in females (Thoenen, 1995; Becker et al., 2007; McEwen, 2007).
2. Methods
2.1. Animals and rearing procedures
Subjects of these studies were 16 males and 17 females rhesus monkey infants (Macaca mulatta) born between 2003 and 2004 in the NICHD breeding facility of the Animal Centre in Poolesville (MD, USA). Some subjects were “mother-reared” (MR; 10 males and 9 females), raised in social groups either by their biological mothers or by an unrelated, multiparous foster mother. Some infants were reared without adults, but with constant access to age-mate peers in a “peer-only reared” (PR; 6 males and 8 females) condition. Rearing conditions were randomly assigned at the time of birth balancing the number of males and females in each rearing condition.
An additional group of 12 MR males born in 1999 were included in this study to assess longitudinal changes in basal neurotrophins levels in both plasma and cerebrospinal fluid (1 month, 1 year and 7 years of age, see below).
MR infants remained with their mothers in a stable social group of 8–10 adults and peers. Infants assigned to the PR condition were separated from their mothers following birth, and were subsequently hand-reared in a neonatal nursery. For the first 14 days, PR subjects were kept in an incubator and hand-fed. Each cage contained a blanket and a terrycloth-covered rocking “surrogate” covered by a heating pad. The infant could see and hear, but did not experience any physical contact with other infants. From day 15 until day 38, infants were moved with their surrogate in individual nursery cages. At 38 days of age all nursery-reared infants were placed into their final condition. PR infants were placed in permanent social groups of 3–4 age-mates, similarly-reared peers, with whom they had continuous contact.
Protocols for the use of experimental animals were approved by the Institutional Animal Care and Use Committee of the NICHD. All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Behavioral scoring
2.2.1. Neonatal assessment
A 30-min battery was administered on days 7, 14, 21 and 30 of life. This test, derived from the Brazelton Neonatal Assessment scale was administered between 1100h and 1300h (Champoux et al., 1994). Temperament characteristics were rated after administration of other items, based on the infant’s behavior throughout the test period. These measures included the tester’s impressions of the animal’s overall state of arousal (Predominant State). In addition, the amount of time spent active during the 5-min observation period was also scored (Active). All items were scored on a scale of 0–2, with scores of 0.5 and 1.5 allowable (Champoux et al., 1994). Raters were trained to a reliability criterion of 0.90 before collecting data (Pearson product-moment correlation) according to a rigorous training protocol.
2.2.2. Social behavior
Monkey infants were scored twice in their home cage on PND 60. Two 5-min observations (morning and afternoon) were performed and averaged across each individual to obtain a single value. The durations (sec) of the following behaviors were scored: Environmental explore, Play, Passive, Self-direct, Stereo/stypic (Table 1).
Table 1.
Definitions for behavioral observations scored on postnatal day 60
| BEHAVIORAL ITEM | DEFINITION |
|---|---|
| Environmental explore | Any manual, oral, or pedal examination, exploration, or manipulation of the physical environment, or the attempt to do the same. Includes manipulating or playing with chow while eating, or drinking |
| Play | Performance of any play behaviors including attempts to initiate play with neighbor via “play face”, wrestling (rough and tumble), chasing, tagging, swatting, bobbing, biting, pulling, lunging, mouthing, and responding positively to play from another animal |
| Passive | Absence of directed movement, social behaviors, and environmental manipulation (i.e., no simultaneously scoreable social or nonsocial behaviors, except self-directed behaviors, or vocalizations). Includes bouncing in place |
| Self-direct | Includes firm manual or pedal gripping of self, which is not a component of an ongoing behavior. Also includes self-grooming, self- scratching, self-mouth, self-clasp (must be firm grip) and sucking (not biting) at any bodily appendage as well |
| Stereo/stypic | Any repetitive, patterned, and rhythmic locomotive movement (stereotypy; such as changes in location through walking, running, dropping from ceiling to floor, rolling, hopping on all fours, bouncing around the cage, and “displays”) or idiosyncratic non locomotive stereotyped actions (stypic; such as repetitively saluting, picking the teeth or strumming the mesh). Stereo/stypic is only scored after three repetitive acts |
2.3. Blood and cerebrospinal fluid (CSF) samples
On days 14 and 30, after the test, the animals were anesthetized with ketamine hydrochloride (intramuscularly, 10 mg/kg), blood drawn from the femoral vein in EDTA-coated tubes. After centrifugation, a 500 μl aliquot of plasma was placed into a plastic vial and stored at −70°C until assay. On PND 60 another baseline measure was taken. For CSF sampling, following anesthesia with ketamine, one milliliter of CFS was removed from the cisterna magna using a 5-cc syringe. CSF samples were immediately placed on dry ice and stored at −70°C. The same sampling and processing procedures were used for all groups.
2.3.1. Neuroendocrine measures
Plasma was assayed for cortisol, adrenocorticotropic hormone (ACTH) and growth hormone (GH) using standard radioimmunoassays (Scheinin et al., 1983). Plasma and CSF levels of BDNF and NGF were measured by highly sensitive immunoenzymatic assays (Promega, Madison, WI, USA; (Aloe et al., 1994). The exogenous NGF yield was calculated by subtracting the amount of this NGF from the endogenous variety. Under these conditions the recovery of NGF on our assay ranged from 80 to 90% (Bracci-Laudiero et al., 1993; Aloe et al., 1994).
Plasma levels of BDNF were measured following the procedure suggested by the manufacturer (Emaxtm ImmunoAssay System number G6891 by Promega, Madison, WI, USA) using a monoclonal anti-mouse-BDNF antibody and a polyclonal anti-human-BDNF antibody. BDNF concentration was determined from the regression line for the BDNF standard curve (ranging from 7.8 to 500 pg/ml-purified mouse BDNF) incubated under similar conditions in each assay. The sensitivity of the assay is about 15 pg/ml of BDNF and the cross-reactivity with other related neurotrophic factors (NGF, NT-3, and NT-4) is considered nil.
Although BDNF is highly concentrated in the nervous system, it is also found in the plasma and serum of humans and other mammals (Fujimura et al., 2002). It has been shown that BDNF in the blood is stored and released by platelets. Alternative sources of blood BDNF have been identified in endothelial cells and lymphocytes, but their contribution is believed to be marginal compared to the bulk release from platelets (Noga et al., 2003; Karege et al., 2005). Previous work has shown that alterations in serum and plasma levels in BDNF found in depressed patients (reduced BDNF levels) are comparable and are not dependent upon the overall reactivity of platelets (Karege et al., 2002b; Karege et al., 2005). Actually, platelet reactivity has been shown to be increased in depressed patients, in the face of reduced plasma and serum levels of BDNF. Thus, our sampling methodology (which was the same for all experimental groups) is not biased by platelet reactivity and reflects circulating levels of BDNF.
Multiple measures where derived from the same subject (behavioral scores and neuroendocrine measures), when available.
2.4. Statistical analysis
StatView 5.0.1 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. Mixed design, repeated measures ANOVAs were performed on behavioral data, neurotrophins and endocrine variables to assess the effect of age, sex and rearing condition, and their interactions. Tukey HSD tests were used for post-hoc comparisons. Correlation among neurotrophins and hormonal data were assessed by Pearson’s linear correlation coefficient r.
3. Results
3.1. Developmental changes in BDNF and NGF levels in rhesus monkeys: comparison between plasma and CSF
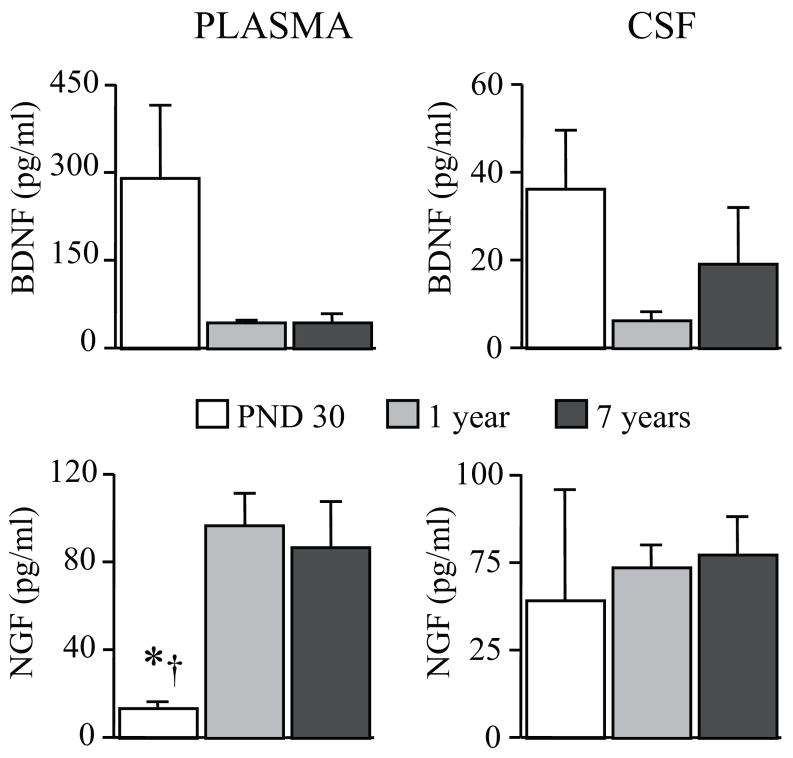
Levels of BDNF and NGF are detectable in the peripheral circulation and in CSF on rhesus macaques early after birth, showing important changes over the first 7 years of life (Figure 1).
Figure 1.
Developmental changes in BDNF and NGF levels in rhesus macaques. Plasma and CSF levels of BDNF and NGF in PND 30 and in 1 and 7 years-old mother-reared rhesus macaques (n = 4 males in each age group) showed important developmental changes. Compared to BDNF, NGF levels showed an opposite developmental curve, increasing from 1 month to 1 and 7 years of life (1 month vs * 1 year, or † 7 years; p < 0.05). CSF = cerebrospinal fluid; PND = Postnatal day. All data expressed as means (+SEM).
Independently from the rearing condition, plasma concentrations of NGF increased significantly with age (F (2,9) = 8.64, p = 0.0081), while an opposite trend was detected for BDNF, plasma concentrations of this neurotrophin being higher in 1-month than in 1-year or 7-years-old subjects (F (2,9) = 3.62, p = 0.0701; Figure 1) in agreement with rodent data (Bimonte-Nelson et al., 2008). Although a correlation between CSF and plasma levels for these neurotrophins was not found, CSF BDNF levels showed a similar age-dependent profile compared to plasma concentrations (Figure 1).
3.2. Effects of rearing condition on plasma BDNF and NGF levels in rhesus monkey infants
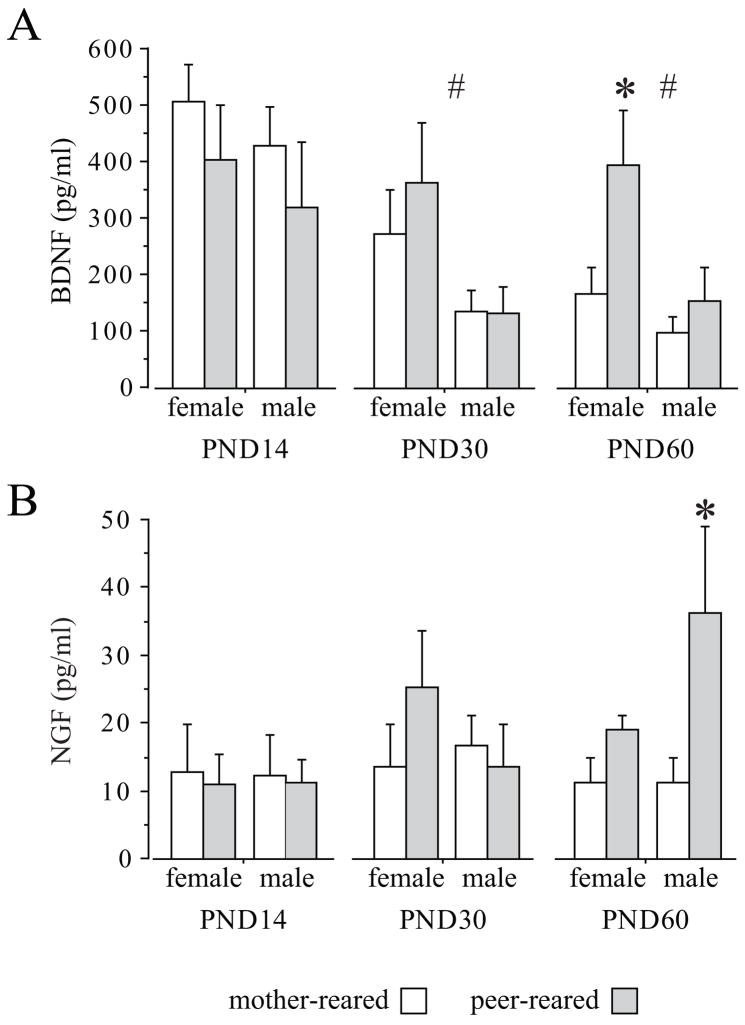
BDNF plasma concentrations decreased over the first weeks of postnatal life in all subjects (F (2,56) = 10.324, p = 0.002; Tukey HSD: postnatal day (PND) 14 vs PND 60, p < 0.01; Figure 2A). Peripheral levels of this neurotrophin were higher in females compared to males both on PND 30 (F (1,28) = 5.896, p = 0.0219) and on PND 60 (F (1,28) = 6.4, p = 0.0173). Peer rearing increased BDNF levels in females only on PND 60, preventing the normal developmental decline in BDNF (F (1,28) = 5.38, p = 0.0279); Tukey HSD, p < 0.05; Figure 2A). Rearing condition did not affect BDNF levels in males.
Figure 2.
Rearing condition affects BDNF and NGF plasma levels in rhesus macaques. A. Effects of rearing condition on BDNF plasma levels in 14-, 30- and 60-day-old subjects (MR: 9 females and 9 males; PR: 8 females and 6 males). BDNF levels were higher in females compared to males starting on PND 30 (# p < 0.05). On PND 60 also a rearing effect was found, peer-rearing increasing levels of BDNF in females only (PR females vs MR females * p <0.05). B. Effects of rearing condition on NGF plasma levels in 14-, 30- and 60-day-old subjects (MR: 5 females and 7 males; PR: 4 females and 4 males). Levels of NGF where higher in PR compared to MR males only on PND 60 (* p <0.05). MR = Mother-reared; PR = Peer-only reared. All data expressed as means (+SEM); other abbreviations as in Figure 1.
Differently from BDNF, plasma NGF levels did not show meaningful developmental changes (F (2,28) =1.712, p = 0.1989). On PNDs 14 and 30 no main effects of sex, rearing, or any interaction effect, were found. However, on PND 60, PR males showed higher levels of NGF compared to MR subjects of the same sex (F (1,16) = 6.5358; p = 0.0211; Tukey HSD: p < 0.05; Figure 2B).
3.3. Relationship between NGF levels, HPA axis activity and GH levels in rhesus monkey infants
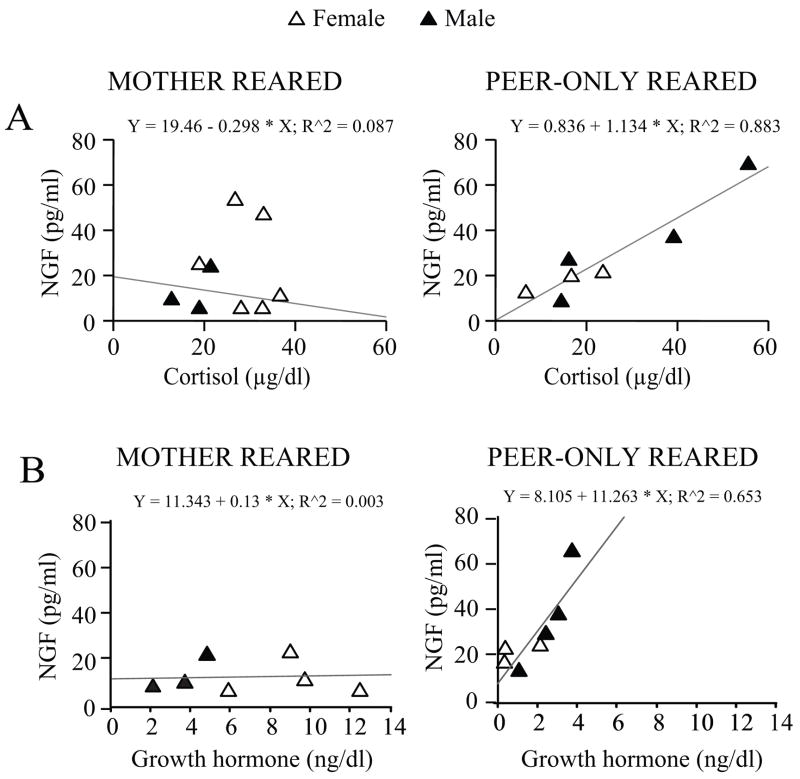
Basal levels of cortisol changed as a function of age (F (2,38) = 4.816, p = 0.0137) being higher at birth and then declining in all subjects. PR males tended to show higher levels of cortisol compared to MR subjects (sex by rearing interaction: F (1,19) = 3.581, p = 0.0738; data not shown). At two months of age, a positive correlation between cortisol and NGF levels was found in the PR group (Pearson’s correlation coefficient, r = 0.940, p = 0.0017; Figure 3A) although levels of cortisol did not differ significantly overall between the two groups (F (1,19) = 0.173, p = 0.0682) or in the two sexes (F (1,19) = 0.064, p = 0.8027; data not shown). These data indicate higher basal adrenocortical output as a consequence of adverse rearing (Champoux et al., 1989).
Figure 3.
Correlation between peripheral levels of NGF and neuroendocrine activity in rhesus monkeys. (A) NGF plasma levels correlated with cortisol in PR 60-days-old rhesus monkeys. For the PR monkey group a positive linear correlation between NGF and cortisol plasma levels was found (Pearson’s correlation coefficient, r = 0.940, p = 0.0017; MR: 6 females and 3 males; PR: 3 females and 4 males). (B) NGF plasma levels were found to correlate with GH in PR but not MR 60-days-old rhesus monkeys (Pearson’s correlation coefficient, r = 0.808, p < 0.05; MR: 4 females and 3 males; PR: 3 females and 4 males). All data expressed as means (+ SEM). GH = growth hormone; other abbreviations as in Figure 1 and 2.
Levels of ACTH followed a similar pattern as cortisol, declining with age (F (2,38) = 9.342, p = 0.0005; data not shown). A main effect of rearing was found in 2 months-old subjects, ACTH levels being lower in PR compared to MR subjects (F (1,19) = 5.941, p = 0.0248), suggesting increased adrenal sensitivity in those subjects experiencing early stressful manipulations of the social environment (data not shown).
Non-human primates raised in the absence of the mother showed a meaningful decline in GH with age (F (2,36) = 15.564, p = 0.0001), an effect especially evident in PR females at 30 days (F (2,36) = 4.015, p = 0.0267; Tukey HSD test, p < 0.05; data not shown). At 60 days, a positive correlation between GH and NGF levels in the PR group was found, resembling the one described for cortisol (Pearson’s correlation coefficient, r = 0.808, p = 0.0278; Figure 3B), although overall GH levels did not differ as a function of rearing condition and sex (data not shown).
3.4. Effects of rearing conditions on behavioral development of rhesus monkey infants
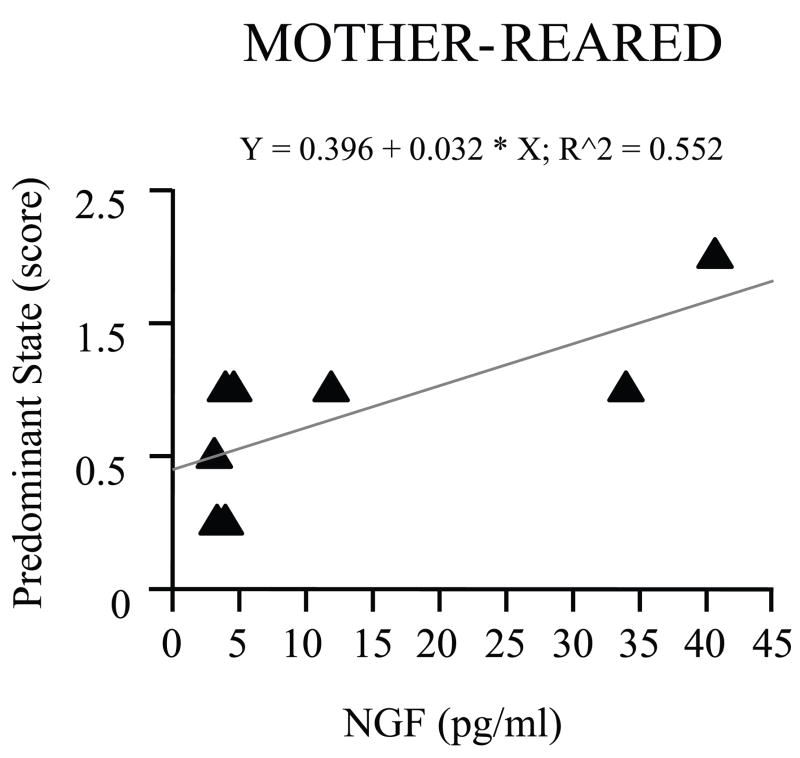
All monkeys were tested on PND 7, 14, 21 and 30 by means of a standardized battery of tests to assess their neurological development (Table 2). This test battery has been designed to assess a variety of abilities and characteristics that mature across the first month of life (Champoux et al., 1994). As far as behavioral activity is concerned, while overall scores increased with age (F (3,69) = 16.042, p = 0.0001), lower scores of “Activity” characterized PR infants raised in the nursery as they grew up (F (3,69) = 3.282, p = 0.0259; PND 30, Tukey HSD test, p < 0.05; Table 2). MR subjects showed a much more agitated behavior in response to the examination (scored as “Predominant state”) than PR infants (high scores of “Predominant state”; F (1,23) = 51.083, p = 0.0001), this difference becoming more pronounced with age (F (3,69) = 5.258, p = 0.0025). Moreover, among two-weeks old subjects “Predominant state” correlated with plasma levels of NGF only in MR subjects (r = 0.743, p = 0.0347; Figure 4). No further correlations were found in older subjects, possibly suggesting that the animals became habituated to the manipulations later on.
Table 2.
Neurobehavioral development assessed in differentially-reared rhesus macaques at different developmental ages
| Mother-reared (n=15) |
||||
|---|---|---|---|---|
| PND 7 | PND 14 | PND 21 | PND 30 | |
| Activity cluster | 0.9±0.1 | 1.2±0.1 | 1.5±0.02 | 1.5±0.03 |
| Predominant state | 0.5±0.1 | 0.8±0.2 | 1.0±0.1 | 1.5±0.1 ‡ |
|
| ||||
| Peer-reared (n=12) |
||||
| PND 7 | PND 14 | PND 21 | PND 30 | |
| Activity cluster | 1.0±0.1 | 1.4±0.1 | 1.3±0.1 | 1.2±0.1 * |
| Predominant state | 0.1±0.1 | 0.1±0.1 | 0.1±0.1 | 0.2±0.1 |
Peer-rearing affected neurological development of infants, differences becoming evident on PND 30. PR subjects did not display agitated behavior in response to the examination (scored as “Predominant state”) as MR infants did (‡ = age by rearing condition interaction; p = 0.0001). In addition, lower scores of “Activity” characterized PR infants (* p < 0.05). Abbreviations as in Figure 1 and 2
Figure 4.
Correlation between plasma levels of NGF and Predominant state score in mother-reared rhesus macaques. A positive correlation between NGF and agitated behavior (scored as “Predominant state”) was found on day 14 of postnatal life (Pearson’s correlation coefficient, r = 0.743, p < 0.05; 5 females and 3 males).
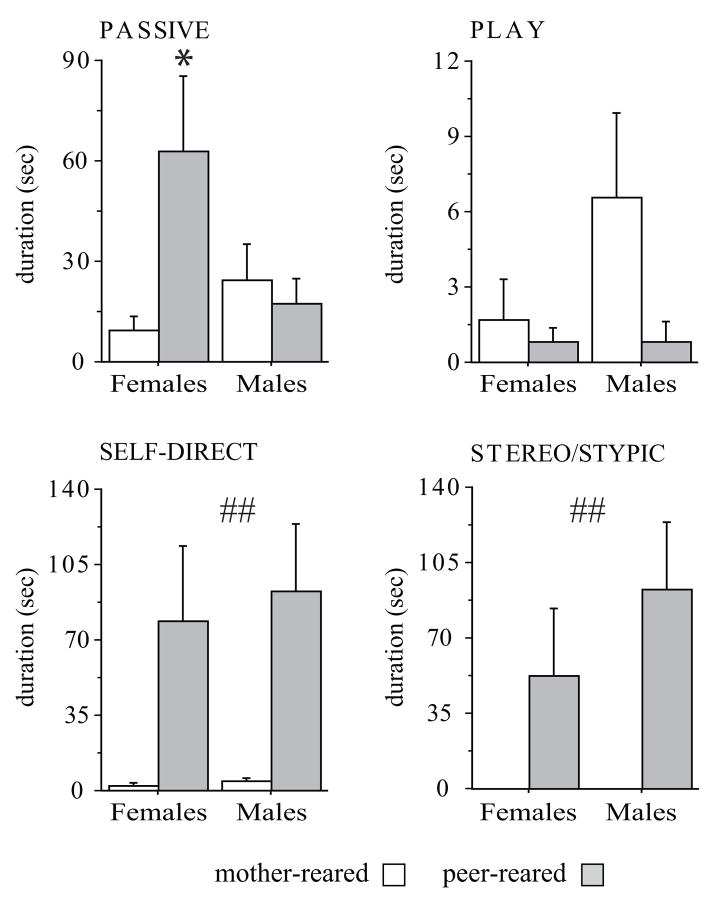
On PND 60, behavior of a group of infants was observed while in their home cage. Passive and stereotyped behavior characterized infants after they were removed from the nursery and reared with peers. In particular, as reported in Figure 5, 2-months-old PR female monkeys showed significantly more Passive behavior than MR females (F (1,23) = 3.844, p = 0.0621; Tukey HSD test of the interaction effect, p < 0.05). PR monkeys performed high levels of Stereo/Stypic (F (1,23) = 12,400, p = 0.0018) as well as Self-direct behaviors (F (1,23) = 13,714, p = 0.0012), which were rarely seen in MR subjects. By contrast, Environmental Explore did not differ significantly in the two groups (F (1,23) = 3,211, p = 0.0863; data not shown) nor did Play behavior (F (1,23) = 1,377 p = 0.2526; Figure 5), although MR males tended to show more of this latter behavior than MR females and peer-rearing completely abated this difference.
Figure 5.
Behavior assessed in 60-days-old rhesus macaques in their home cage. Only peer-reared infants showed high levels of abnormal behaviors such as Self-direct and Stero/Stypic behaviors (## = main effect of rearing condition; p < 0.01). PR females showed higher levels of passive behavior compared to MR subjects (* = interaction between sex and rearing condition; p < 0.05). MR: 5 females and 10 males; PR: 7 females and 5 males. All data expressed as means (+ SEM). Abbreviations as in Figure 2.
4. Discussion
Results from this study indicate selective increases in BDNF and NGF peripheral levels, respectively in females and males rhesus macaques, in response to early adversity caused by peer-rearing. Higher levels of BDNF in PR females were associated with greater behavioral passivity. NGF levels were selectively increased in PR males and correlated positively with other “classic” hormonal responses to stress, such as cortisol and growth hormone, in both sexes. These changes in neurotrophin levels reveal different responses to early stress in the two genders and point to a greater vulnerability in females.
One of the most important findings here reported is that BDNF levels were affected by early trauma selectively in females. Studies performed in rodents have shown that neurotrophins are sensitive to manipulations of the mother-infant relationship and, more in general, of the rearing environment (Cirulli et al., 1998; Cirulli, 2003a; Branchi et al., 2006a; Branchi et al., 2006b; Cirulli et al., 2007). In particular, multiple separations increase levels of BDNF in young subjects in the hippocampus and in prefrontal cortical areas (Roceri et al., 2004). In line with the rodent model, in the present study, BDNF levels measured in the peripheral circulation were increased in response to the stress provided by maternal deprivation. However, differently from rodents, BDNF changes were found only in females, independently from HPA axis activity, and were accompanied by increased behavioral passivity. This piece of data is especially interesting since, so far, only very few animal models have been sensitive enough to discriminate gender differences in the response to stress before puberty (Barr et al., 2004; Becker et al., 2007), and none reporting changes in BDNF, underlying the potency of this non-human primate model.
In males, peer-rearing led to increased NGF levels in association with a more active coping response, without affecting BDNF. It has been previously detailed that, despite many normal patterns of social behavior, peer-reared monkeys are unable to regulate their fearfulness (Novak and Suomi, in press). These behavioral differences have been associated with alterations in monoamine, neuroendocrine and immune function (Champoux et al., 1989). Although on average peer-rearing did not affect basal adrenal activity, as previously reported (Clarke, 1993), males presenting high levels of NGF also showed high levels of cortisol and GH. These data are in line with reports showing that peripheral administration of NGF stimulates HPA axis activity and corroborate previous data indicating NGF as one of the key effectors of peripheral neuroendocrine responses to stress (Otten et al., 1979; Aloe et al., 1986; Alleva and Aloe, 1989; Aloe et al., 1994). In agreement with findings obtained both in animal models and in humans, behaviors indicative of distress, such as agitation during the neurological examination (Predominant state), correlated positively with NGF levels, suggesting that this neurotrophin plays a role in the physiological response to aversive events and, differently from BDNF, indicates a more active coping in response to stressful conditions (Aloe et al., 1986; Aloe et al., 1994; Hadjiconstantinou et al., 2001). Interestingly, stressful situations of a social nature, characterized by high levels of arousal, such as establishing a strong social bond, can selectively elicit an increase in NGF levels in the bloodstream of humans (Alleva and Branchi, 2006; Emanuele et al., 2006). More in detail, a positive correlation between peripheral NGF levels and the intensity of the bond established with the other partner has been reported. Although nursery rearing results in the inability of young subjects to establish an attachment relationship with the mother, PR subjects interact and do attempt to establish primary attachment relationships with peers (Novak and Suomi, in press). It is possible to hypothesize that high levels of NGF characterizing peer reared subjects could reflect these efforts, suggesting that NGF could be involved in the establishment of early social relationships (Alleva and Branchi, 2006).
Changes in NGF and BDNF levels following stress indicate “allostatic” processes activated to coordinate brain and body responses to specific external challenges (Chaldakov et al., 2004; McEwen, 2007). Neurotrophins could counteract the negative impact of stress hormones on selected brain regions, such as the hippocampus, as well as on other body organs (Thoenen, 1995). Indeed, in addition to neurons, both NGF and BDNF are produced by a variety of cell types, including immune cells, adipocytes, endocrine and endothelial cells, thus being in a position to affect maturation and activity of selected tissues involved in the response to stress (Aloe et al., 1986). For instance, NGF stimulates the sympathetic nervous system and adrenal function following social competition in rodents, leading to male subordination (Alleva and Aloe, 1989; Chaldakov et al., 2004). Since peer-reared monkeys are highly reactive and aggressive, ranking at the bottom of the dominance hierarchy as adults, NGF could be involved in shaping social functioning in a competitive setting early on (Novak and Suomi, in press). As for peripheral BDNF, it could mediate the response to stress through activation of peripheral tissues (Chaldakov et al., 2004). However, an effect of peripheral BDNF on the central nervous system cannot be excluded since peripheral and central BDNF levels are closely related (Karege et al., 2002a) and a correlation between serum BDNF levels and an in vivo marker of cortical integrity has been recently shown (Lommatzsch et al., 2005; Lang et al., 2007). In addition, the ability of BDNF to cross the blood-brain barrier has been demonstrated (Pan et al., 1998).
Anxiety and mood disorders are complex, heritable conditions. Although genetic differences contribute to the vulnerability and progression of stress-related neuropsychiatric disorders, environmental factors are also important and there is evidence in humans showing that vulnerability to depression and anxiety disorders are markedly increased by early trauma (Heim and Nemeroff, 2001). While depression is reportedly more prevalent in women than men (Grigoriadis and Robinson, 2007), the underlying mechanisms have been elucidated yet. A first hint is provided by BDNF, a central molecule in the neurotrophic theory of depression: reduced levels of this neurotrophin have been reported among depressed patients, a history of trauma further reducing its levels (Duman et al., 1997; Kauer-Sant'Anna et al., 2007). Accordingly, gender dependent differences in BDNF levels in healthy subjects (Lommatzsch et al., 2005), and in relation to vulnerability to depression (Karege et al., 2002b) are being reported (Aydemir et al., 2006). In particular, BDNF levels were found to be lower in a population of female depressed patients compared to healthy controls suggesting that BDNF is an important factor in the etiopathogenesis of depression (Aydemir et al., 2006).
However, to our knowledge, a differential activation in BDNF levels in females, also in relationship to early trauma, has not been reproduced in animal models during development. Here we show, in a primate model, that BDNF is a selective marker of early adversity in the female gender and is associated with increased levels of abnormal responses indicative of a depression-like state. Changes in BDNF expression have been shown to be age-, time- and stimulus-specific. In humans, both positive and negative correlations between age and BDNF levels have been reported, possibly reflecting the fact that environmental variables may play an important role in modulating peripheral levels of this neurotrophin (Lang et al., 2004; Lommatzsch et al., 2005). Indeed, increased levels of BDNF characterize acute responses to stressful events -including maternal separation- early during postnatal life, decreased expression being more reliably found at adulthood in response to a wide array of stressful stimuli (Nair et al., 2007). Although lower levels of BDNF characterize depressed patients, an increase in BDNF levels early on may contribute to the generation of individual differences in stress neurocircuitry, providing a substrate for altered vulnerability to depressive disorders at adulthood (Nair et al., 2007).
Not only was BDNF affected only in females, but higher basal levels of this neurotrophin characterized female subjects suggestive of gender-related neurobiological differences already before puberty. By contrast, NGF levels were increased in response to peer-rearing only in male subjects.
The comparison of results obtained in this paper with data collected in humans is of interest and suggests that changes in BDNF and NGF level might be associated with different emotional states. Numerous lines of evidence implicate BDNF in the pathophysiology of depression (Sen et al., 2008). By contrast, while no main changes in NGF levels accompany depressive states (Hellweg et al., 2008), peripheral levels of this neurotrophin are increased in subjects experiencing arousing or anxiety states, thus representing an active coping response to adverse situations (Aloe et al., 1994; Hadjiconstantinou et al., 2001).
Maladaptive or repeated activation of stress responsive biological mediators, such as neurotrophins, may have long-term influences on stress sensitivity at adulthood and increase vulnerability for stress-related illnesses, including psychopathology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alleva E, Aloe L. Physiological roles of nerve growth factor in adult rodents: a biobehavioral perspective. Int J Comp Psychol. 1989;2:147–163. [Google Scholar]
- Alleva E, Branchi I. NGF: a social molecule. Psychoneuroendocrinology. 2006;31:295–296. doi: 10.1016/j.psyneuen.2005.12.003. author reply 297–298. [DOI] [PubMed] [Google Scholar]
- Aloe L, Alleva E, Bohm A, Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci U S A. 1986;83:6184–6187. doi: 10.1073/pnas.83.16.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloe L, Bracci-Laudiero L, Alleva E, Lambiase A, Micera A, Tirassa P. Emotional stress induced by parachute jumping enhances blood nerve growth factor levels and the distribution of nerve growth factor receptors in lymphocytes. Proc Natl Acad Sci U S A. 1994;91:10440–10444. doi: 10.1073/pnas.91.22.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, Goka E. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1256–1260. doi: 10.1016/j.pnpbp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, Bale TL. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Granholm AC, Nelson ME, Moore AB. Patterns of neurotrophin protein levels in male and female Fischer 344 rats from adulthood to senescence: how young is “young” and how old is “old”? Exp Aging Res. 2008;34:13–26. doi: 10.1080/03610730701761908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci-Laudiero L, Aloe L, Levi-Montalcini R, Galeazzi M, Schilter D, Scully JL, Otten U. Increased levels of NGF in sera of systemic lupus erythematosus patients. Neuroreport. 1993;4:563–565. doi: 10.1097/00001756-199305000-00025. [DOI] [PubMed] [Google Scholar]
- Branchi I, D'Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006a;60:690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Branchi I, D'Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and “depression”-like behavior. J Neurosci Res. 2006b;83:965–973. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chaldakov GN, Fiore M, Stankulov IS, Manni L, Hristova MG, Antonelli A, Ghenev PI, Aloe L. Neurotrophin presence in human coronary atherosclerosis and metabolic syndrome: a role for NGF and BDNF in cardiovascular disease? Prog Brain Res. 2004;146:279–289. doi: 10.1016/S0079-6123(03)46018-4. [DOI] [PubMed] [Google Scholar]
- Champoux M, Suomi SJ, Schneider ML. Temperament differences between captive Indian and Chinese-Indian hybrid rhesus macaque neonates. Lab Anim Sci. 1994;44:351–357. [PubMed] [Google Scholar]
- Champoux M, Coe C, Schanberg S, Kuhn CM, Suomi SJ. Hormonal effects of early rearing conditions in the infant rhesus monkey. American Journal of Primatology. 1989;19:111–117. doi: 10.1002/ajp.1350190204. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Micera A, Alleva E, Aloe L. Early maternal separation increases NGF expression in the developing rat hippocampus. Pharmacol Biochem Behav. 1998;59:853–858. doi: 10.1016/s0091-3057(97)00512-1. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Alleva E, Antonelli A, Aloe L. NGF expression in the developing rat brain: effects of maternal separation. Brain Res Dev Brain Res. 2000;123:129–134. doi: 10.1016/s0006-8993(00)02844-4. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Capone F, Bonsignore LT, Aloe L, Alleva E. Early behavioural enrichment in the form of handling renders mouse pups unresponsive to anxiolytic drugs and increases NGF levels in the hippocampus. Behav Brain Res. 2007;178:208–215. doi: 10.1016/j.bbr.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Alleva E. Early disruption of the mother-infant relationship: effects on brain plasticity and implications for psychopathology. Neurosci Biobehav Rev. 2003a;27:73–82. doi: 10.1016/s0149-7634(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Emanuele E, Politi P, Bianchi M, Minoretti P, Bertona M, Geroldi D. Raised plasma nerve growth factor levels associated with early-stage romantic love. Psychoneuroendocrinology. 2006;31:288–294. doi: 10.1016/j.psyneuen.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, McGuire L, Duchemin AM, Laskowski B, Kiecolt-Glaser J, Glaser R. Changes in plasma nerve growth factor levels in older adults associated with chronic stress. J Neuroimmunol. 2001;116:102–106. doi: 10.1016/s0165-5728(01)00278-8. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hellweg R, Ziegenhorn A, Heuser I, Deuschle M. Serum concentrations of nerve growth factor and brain-derived neurotrophic factor in depressed patients before and after antidepressant treatment. Pharmacopsychiatry. 2008;41:66–71. doi: 10.1055/s-2007-1004594. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002a;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002b;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Kauer-Sant'Anna M, Tramontina J, Andreazza AC, Cereser K, da Costa S, Santin A, Yatham LN, Kapczinski F. Traumatic life events in bipolar disorder: impact on BDNF levels and psychopathology. Bipolar Disord. 2007;9 Suppl 1:128–135. doi: 10.1111/j.1399-5618.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Gallinat J. BDNF serum concentrations in healthy volunteers are associated with depression-related personality traits. Neuropsychopharmacology. 2004;29:795–798. doi: 10.1038/sj.npp.1300382. [DOI] [PubMed] [Google Scholar]
- Lang UE, Gallinat J, Kuhn S, Jockers-Scherubl C, Hellweg R. Nerve growth factor and smoking cessation. Am J Psychiatry. 2002;159:674–675. doi: 10.1176/appi.ajp.159.4.674-a. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol Psychiatry. 2007;62:530–535. doi: 10.1016/j.biopsych.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, Vaidya VA. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32:1504–1519. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Benarese M, Liberini P, Carruba MO. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology. 1996;137:495–503. doi: 10.1210/endo.137.2.8593794. [DOI] [PubMed] [Google Scholar]
- Nockher WA, Renz H. Neurotrophins in clinical diagnostics: pathophysiology and laboratory investigation. Clin Chim Acta. 2005;352:49–74. doi: 10.1016/j.cccn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Noga O, Englmann C, Hanf G, Grutzkau A, Seybold J, Kunkel G. The production, storage and release of the neurotrophins nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 by human peripheral eosinophils in allergics and non-allergics. Clin Exp Allergy. 2003;33:649–654. doi: 10.1046/j.1365-2222.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- Novak MA, Suomi SJ. Abnormal behavior in nonhuman primates and models of development. In: Burbacher TMK, Sackett GP, Grant KS, editors. Nonhuman primate models in research on developmental disabilities. Elsevier; New York: in press. [Google Scholar]
- Otten U, Baumann JB, Girard J. Stimulation of the pituitary-adrenocortical axis by nerve growth factor. Nature. 1979;282:413–414. doi: 10.1038/282413a0. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry. 2004;55:708–714. doi: 10.1016/j.biopsych.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Chang WH, Kirk KL, Linnoila M. Simultaneous determination of 3-methoxy-4-hydroxyphenylglycol, 5-hydroxyindoleacetic acid, and homovanillic acid in cerebrospinal fluid with high-performance liquid chromatography using electrochemical detection. Anal Biochem. 1983;131:246–253. doi: 10.1016/0003-2697(83)90162-8. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Effects of stress on neurotrophic factor expression in the rat brain. Ann N Y Acad Sci. 1995;771:234–239. doi: 10.1111/j.1749-6632.1995.tb44684.x. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Found Symp. 1991;156:171–183. doi: 10.1002/9780470514047.ch11. discussion 183–178. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]