Abstract
We transplanted undifferentiated embryonic stem (ES) cells into the spleens of carbon tetrachloride (CCl4)-treated mice to determine their effects on liver fibrosis. Carbon tetrachloride at 0.5 ml/kg of body weight was injected intraperitoneally into C57BL/6 mice twice weekly for up to 20 weeks. Four weeks after the first injection, the mice were divided into two groups and those in group 1 received 1 × 105 ES cells genetically labelled with enhanced green fluorescent protein (GFP) in the spleens, while group 2 mice received 0.1 ml of phosphate-buffered saline. In group 1, GFP-immunopositive cells were retained and found in areas of fibrosis in the liver, and reduced liver fibrosis was observed as compared with group 2. Secondary transplantation of ES cells at 12 weeks after the initial transplantation enhanced the reduction in liver fibrosis. No teratoma formation or uncontrolled growth of ES cells in organs, including the liver and spleen, was observed in any of the mice. In the livers of group 1 mice, metalloproteinase 9-immunopositive cells derived from ES cells as well as those from the recipient were observed. These cells were also found to be immunopositive for the hepatoblast marker Delta-like (DlK-1), a member of the DlK-1 family of transmembrane proteins. These results suggest that ES-based cell therapy is potentially useful for liver fibrosis treatment and that reduction in CCl4-induced liver fibrosis by transplantation of ES cells may be related closely to the emergence of metalloproteinase-producing hepatoblast-like cells.
Keywords: carbon tetrachloride, DlK-1, embryonic stem cells, hepatoblast, intrasplenic transplantation, liver fibrosis, metalloproteinase
Liver cirrhosis is one of the most representative forms of liver fibrosis and represents a serious health problem. Recently, transplantation of bone marrow-derived cells including mesenchymal stem cells was reported to reduce carbon tetrachloride (CCl4)-induced liver fibrosis (Fang et al. 2004; Sakaida et al. 2004; Wu et al. 2005; Zhao et al. 2005; Oyagi et al. 2006), while foetal liver epithelial progenitor cells have also been shown to ameliorate diethylnitrosamine-induced liver fibrosis (Zheng et al. 2006). Thus, transplantation of cells carrying the characteristics of stem cells or progenitor cells seems to improve liver fibrosis.
Embryonic stem (ES) cells are self-renewing and pluripotent cells derived from the inner cell masses of preimplantation blastocysts (Evans & Kaufman 1981; Martin 1981), and are considered to be an optimal source for cell-replacement therapy, because of their limitless growth and pluripotent differentiation capacity. We previously reported the differentiation of mouse ES cells into dopaminergic neurons (Nishimura et al. 2003), rhodopsin-immunopositive photoreceptor-like cells (Sugie et al. 2005), insulin-secreting cells (Shiroi et al. 2002), enteric smooth muscles (Yamada et al. 2002) and hepatocyte-like cells (Ishizaka et al. 2002) in vitro. Further, we recently found that ES cells developed into hepatocyte-like cells after intrasplenic transplantation in mice that received short-term treatment with CCl4 (Moriya et al. 2007). In that study, it was also shown that intrasplenic transplantation of ES cells reduced fibrosis of the liver at the end of the 4-week period of CCl4 treatment.
In this study, we transplanted ES cells genetically labelled with enhanced green fluorescent protein (GFP) into the spleens of mice under long-term treatment with CCl4 to examine their potential efficacy toward liver fibrosis. Green fluorescent protein-immunopositive cells were consistently found in the areas of fibrosis in the mouse livers and liver fibrosis was reduced in mice that received ES cells, as compared with those that received phosphate-buffered saline (PBS). A second intrasplenic transplantation of ES cells at 12 weeks after the initial transplantation enhanced the improvement of liver fibrosis. Further, metalloproteinase 9 (MMP9)-immunopositive cells derived from ES cells were found in the livers of group 1 mice, as well as those derived from the recipients themselves, which were immunopositive for DlK-1, a hepatoblast marker (Tanimizu et al. 2003). These results suggest the potential usefulness of ES-based cell therapy for liver fibrosis and that improvement of CCl4-induced liver fibrosis by transplantation of ES cells may be due to the emergence of metalloproteinase-producing hepatoblast-like cells.
Materials and methods
Murine ES cell line
We utilized G4-2 cells from a mouse ES cell line (129/SvJ mouse ES cells, a kind gift from Dr Hitoshi Niwa, RIKEN Center for Developmental Biology, Kobe, Japan), which carry an enhanced GFP gene under the control of the CAG expression unit (Niwa et al. 2000). For this study, undifferentiated G4-2 cells were maintained on gelatin-coated dishes without feeder cells in Dulbecco’s modified Eagle’s medium (Sigma, St Louis, MO, USA) supplemented with 10% foetal bovine serum (FBS; GIBCO/BRL, Grand Island, NY, USA), 0.1 mM 2-mercaptoethanol (Sigma), 10 mM non-essential amino acids (GIBCO/BRL), 1 mM sodium pyruvate (Sigma) and 1400 U/ml of leukaemia inhibitory factor (GIBCO/BRL). The G4-2 cells carried the blasticidin S-resistant selection marker gene driven by the Oct-3/4 promoter (active under undifferentiated status) and were occasionally cultured in medium containing 10 μg/ml blasticidin S to eliminate differentiated cells.
Preparation of graft cells and transplantation
Culture dishes (9 cm in diameter) were used to maintain the undifferentiated ES colonies, then washed with 8 ml of ice-cold PBS (pH 7.4) thrice and treated with 1.0 ml of 0.025% trypsin/PBS for 2 min at 37 °C. Five millilitres of ES maintenance medium containing 10% FBS was added to each dish to stop trypsin activity. Single cell solutions were easily obtained by repeated pipetting. Cells were washed with ice-cold PBS thrice and finally prepared for transplantation in a PBS solution at a cell concentration of 1 × 106/ml.
Green fluorescent protein-positive undifferentiated ES cells at 1 × 105 (0.1 ml of 1 × 106/ml solution) were transplanted into the spleen, using an intrasplenic transplantation procedure previously described (Moriya et al. 2007). Briefly, a 1-cm incision was made on the left flank and the spleen was extracted through the incision. Then, 0.1 ml of the graft cell solution (1 × 106 cells/ml) was slowly infused into the inferior pole of the spleen using a 31-gauge needle and a Hamilton syringe. Once the infusion was complete, the syringe was left in place for 1 min.
CCl4 treatment
To induce liver damage, CCl4 at 0.5 ml/kg of body weight was injected into the peritoneum twice a week throughout the treatment period.
Grouping of animals and outline of experiments
Female C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan) at 6 weeks of age and used as experimental animals. All procedures including the surgical steps were performed in accordance with the Guidelines of Nara Medical University for experiments involving animals and recombinant DNA.
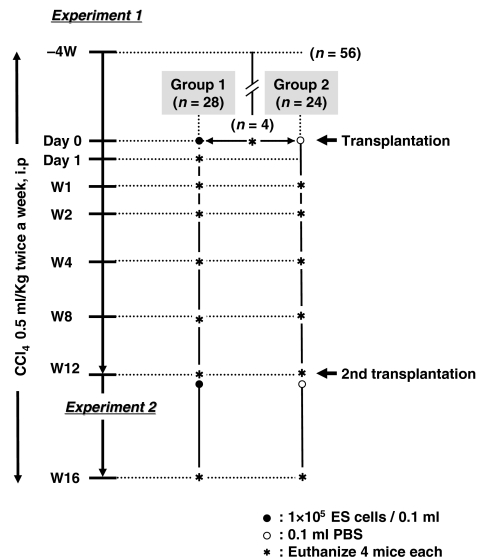
In experiment 1, 56 mice received CCl4 treatment for 4 weeks, after which four were killed and fibrosis of the livers was examined, with that day was designated as day 0. The remaining 52 were divided into group 1 (n= 28) and group 2 (n= 24). Group 1 mice received transplantation of 1 × 105 graft cells (0.1 ml of 1 × 106/ml solution) on day 0, while group 2 mice were injected with 0.1 ml of PBS, instead of graft cells. Mice in both groups were treated with CCl4 twice a week consecutively. Liver sampling was performed after 1, 2, 4, 8 and 12 weeks (W1, W2, W4, W8 and W12 respectively) using four mice from each group, as well as on day 1 using group 1 mice only.
In experiment 2, the remaining four mice in group 1 received a second transplantation of graft cells into the spleen at W12, while those in group 2 received 0.1 ml of PBS, then both groups continued to receive treatment with CCl4 for an additional 4 weeks. Animal grouping and an outline of the experiments are shown in Figure 1.
Figure 1.
Grouping of experimental animals and outline of the experiments. Female C57BL/6 mice (n= 56) received CCl4 treatment for 4 weeks, after which four mice were killed, which was designated as day 0. The remaining 52 mice were divided into group 1 (n= 28) and group 2 (n= 24). Group 1 received transplantation of 1 × 105 graft cells on day 0 and group 2 were injected with 0.1 ml of PBS. Liver sampling was performed at W1, W2, W4, W8 and W12 using four mice from each group, as well as on day 1 in group 1 only. At W12, the remaining four mice in group 1 received a second transplantation of graft cells and those in group 2 received 0.1 ml of PBS into the spleen. All were then subjected to an additional 4 weeks of treatment with CCl4.
Tissue preparation
The livers were thoroughly perfused via the heart with 4% paraformaldehyde to wash out contaminating blood cells. For fixation, the perfused livers were incubated with 4% paraformaldehyde overnight and then soaked in 30% sucrose for 3 days. Tissues were frozen on dry ice and sectioned into 5-mm slices using a cryostat in preparation for staining.
Staining for collagen using picro-Sirius red
Frozen tissue sections were desiccated completely, then washed with distilled water for 5 min and incubated for 40 min in a saturated picric acid aqueous solution (1.1 g of picric acid in 100 ml distilled water). Next, a 0.1% concentration of picro-Sirius red powder was mixed into the solution and tissue sections were additionally soaked for a few minutes. When the sections were determined to be properly stained, they were removed, and rinsed twice in 0.01 N HCl and distilled water. Finally, the sections were briefly dehydrated with 70% ethanol for 30 s and 100% ethanol for 1 min, fixed with xylene and placed under coverslips.
Staining for collagen using Mallory-azan
First, frozen tissue sections were desiccated, then incubated in an azocarmine G aqueous solution (0.1 g of azocarmine G powder and 1 ml of absolute acetic acid in 100 ml of distilled water) at 60 °C for 60 min and then at room temperature for 30 min. Next, after rinsing in distilled water, the tissue sections were soaked in a 5% molybdenum phosphate aqueous solution overnight, after which they were rinsed in distilled water, and soaked in an aniline blue and orange G-mixed aqueous solution (0.5 g of aniline blue, 2 g of orange G and 8 ml of absolute acetic acid in 100 ml of distilled water) at room temperature for 60 min. Finally, the sections were dehydrated, fixed and placed under coverslips.
Immunohistochemistry of GFP
Frozen tissue sections were desiccated completely, then washed with PBS and soaked in PBS containing 5% bovine serum albumin (BSA) for 5 min, after which they were incubated overnight with anti-GFP (1:1000; Nacalai Tesque Inc, Kyoto, Japan). The sections were then washed with PBS and incubated with biotin-conjugated anti-rat IgG secondary antibodies for 1 h at 37 °C (1:200; Vector Laboratories Inc, Burlingame, CA, USA). Next, for the purpose of intrinsic peroxidase elimination, the sections were immersed in 3% hydrogen peroxide (H2O2) for 30 min. The sections were incubated with Vectastain ABC® reagent (Vector Laboratories Inc) for 2 h at 37 °C, then treated with 0.02 M Tris–HCl buffer (pH 7.5) containing 4% diaminobenzidine tetrahydrochloride (Vector Laboratories Inc) in the presence of 0.0004% H2O2 for 2 min at room temperature. Stained sections were rinsed with distilled water and immersed in ethanol and xylene for complete dehydration. Finally, the sections were enclosed with Entellan-new® (Merck, Tokyo, Japan).
Immunofluorescence analyses
Goat anti-MMP9 (1:100; Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), rabbit anti-F4/80 (1:100; Santa Cruz Biotechnology Inc), and either rabbit or rat anti-GFP (1:500; Molecular Probe Inc, Eugene, OR, USA or Nacalai Tesque Inc, Kyoto, Japan respectively) were used as primary antibodies. For the secondary antibodies, Alexa Fluor® 546 goat anti-rabbit IgG, Alexa Fluor® 488 goat anti-rat IgG or donkey anti-goat IgG, Alexa Fluor 350 goat anti-rabbit IgG and Alexa Fluor® 546 goat anti-rat IgG were purchased from Molecular Prove Inc and used at a final concentration of 1:200. Frozen tissue sections were desiccated completely at room temperature using a dryer, washed with PBS thrice for 5 min each and soaked in PBS containing 5% BSA once for 5 min, then incubated overnight with the primary antibodies. The sections were then washed with PBS thrice for 5 min each and incubated with the Alexa Fluor® conjugated secondary antibodies for 1 h at 37 °C, then washed thrice for 5 min each with PBS and mounted on glass slides from an Anti-fade Kit (Molecular Prove Inc). Positive cells in the liver were observed using a Provis microscope (Olympus, Tokyo, Japan) equipped with a charge coupled device camera. A total of 10 different areas in each liver section were analysed independently.
Quantitative analysis of liver fibrosis
The degree of liver fibrosis was quantified by measuring the relative areas of fibrosis with the aid of computer software, as described previously (Watanabe et al. 2000). Briefly, areas were selected at random and assessed using meta-morph software (Universal Imaging Corporation, Downingtown, PA, USA) at a magnification of ×40. The mean value of six randomly selected areas per sample was used to evaluate the degree of liver fibrosis, with liver fibrosis on day 0 determined to be the standard value of fibrosis index (F.I.) 1.0.
Statistical analysis
Data are presented as the mean ± SD. Differences between groups were analysed by Student’s t-test, with P< 0.05 considered to be significant.
Results
Presence of ES-derived cells in CCl4-treated livers
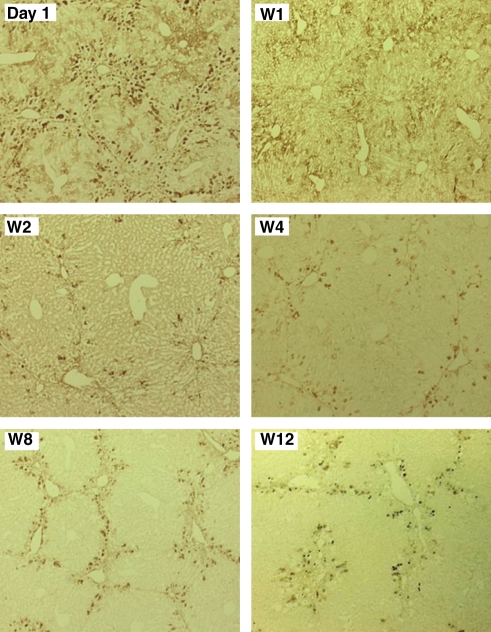
Transplantation was performed after pretreatment with CCl4 for 4 weeks. GFP-immunopositivity in the liver specimens was examined on day 1, as well as at W1, W2, W4, W8 and W12 in the group 1 mice (Figure 2). There were a number of GFP-immunopositive cells in the livers of group 1 mice on day 1. Thereafter, although the numbers of those cells decreased, they remained in the livers throughout the duration of the experiment. The distribution of immunoreactivity against GFP was observed predominantly in the periportal regions of the hepatic lobules.
Figure 2.
Presence of ES-derived cells in CCl4-treated livers. GFP-immunopositivity was examined in liver specimens from group 1 mice on day 1, then at W1, W2, W4, W8 and W12.
Decreased fibrosis following ES transplantation
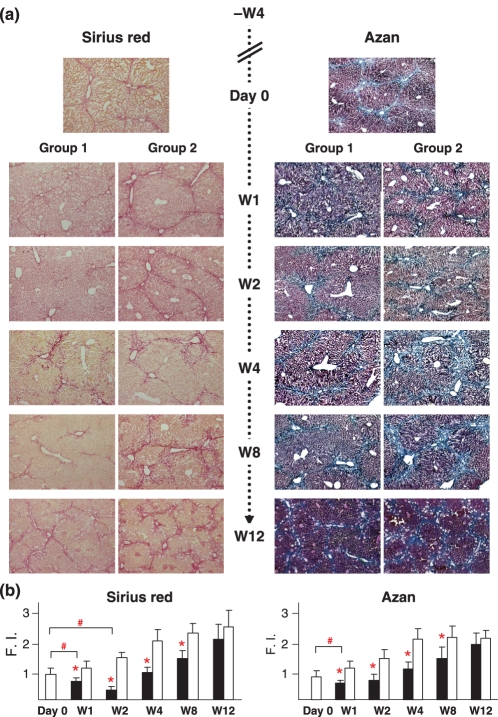
Liver fibrosis was evaluated histologically using picro-Sirius red and Mallory-azan staining. Pretreatment with CCl4 for 4 weeks caused considerable liver fibrosis (Figure 3a, top). In group 2 mice, which received PBS instead of ES cells, liver fibrosis continuously progressed following treatment with CCl4 (Figure 3a, group 2). When compared with group 2 mice, reduced liver fibrosis was observed in group 1 (Figure 3a, group 1). The results of quantitative evaluations using an image analyser are shown in Figure 3b. A significant reduction in liver fibrosis was observed in group 1 as compared with group 2 at all observation points except W12.
Figure 3.
Decreased fibrosis by ES transplantation. (a) Liver histology findings on day 0, and at post-transplantation at W1, W2, W4, W8 and W12. Presumable liver fibrosis was induced by 4 weeks of treatment with CCl4. In group 2 mice, which received PBS, liver fibrosis continuously progressed, whereas that was reduced in group 1 mice, which received ES cell grafts. (b) Quantitative evaluation of liver fibrosis using image analyser. Fibrosis reduction was observed in group 1 (closed squares) as compared with group 2 (open squares) (*P< 0.05). In a comparison with liver fibrosis on day 0, reductions were confirmed at W1 and W2 in group 1 using picro-Sirius red staining and at W1 using Mallory-azan staining (#P< 0.05). Liver fibrosis on day 0 was determined to be the standard value of Fibrosis Index (F.I.) 1.0.
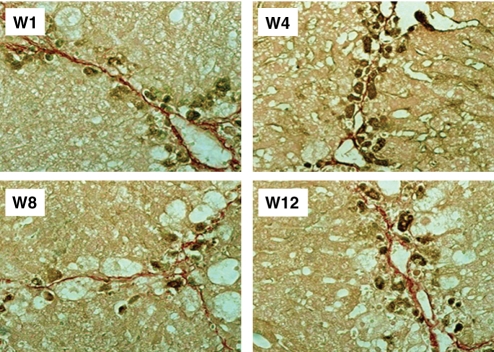
GFP-immunopositive cells in locations neighbouring fibrosis areas
The location of GFP-immunopositive cells was determined using liver specimens from group 1 mice taken at W1, W4, W8 and W12 (Figure 4). GFP-immunopositive cells were found adjacent to or along with fibres, as shown by picro-Sirius red staining.
Figure 4.
GFP-immunopositive cells in locations adjacent to fibrosis areas. GFP-immunopositive cells were found adjacent to or along with fibres in fibrotic areas, as shown by picro-Sirius red staining.
Expression of MMP9 on hepatoblast-like cells in the livers
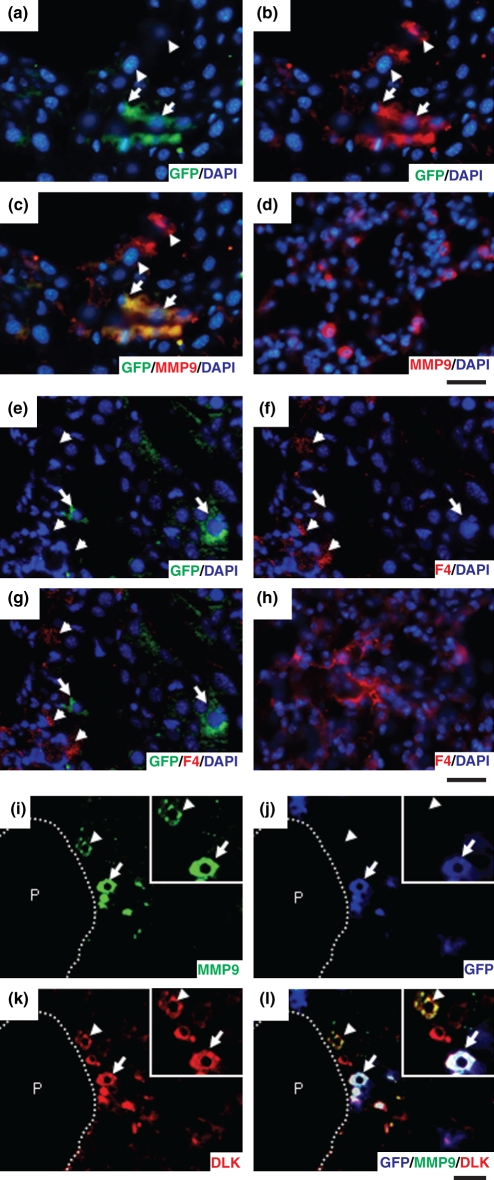
To explore the mechanism of fibrolysis, we examined the expression of MMP9 immunohistochemically using liver specimens from group 1 mice taken at W12 (Figure 5a–c). As expected, the transplanted cells expressed MMP9 (Figure 5a–c, arrows). Further, there were some cells immunopositive for MMP9 that were not immunopositive for GFP (Figure 5a–c, arrowheads), indicating that they had originated from the recipient mice. Our results suggest that not only transplanted ES-derived cells, but also endogenous fibrolysis activities contributed to improved liver fibrosis. Although MMP9 is known to be expressed in alveolar macrophages in the lung (Figure 5d,h), no expression of F4/80, a marker of macrophages, was found in GFP-immunopositive cells in the liver (Figure 5e–g arrows). There were some cells immunopositive for F4/80 in the liver, although they were not immunopositive for GFP (Figure 5e–g arrowheads).
Figure 5.
Immunofluorescence analyses. (a–d) GFP and MMP9-immunopositive findings were examined using liver specimens from group 1 mice taken at W12 (a–c) and specimens from normal C57BL/6 mice at 6 weeks of age (d). GFP-immunopositive cells were also MMP9-immunopositive (arrows), while some MMP9-immunopositive cells were not positive for GFP (arrowheads). Abundant immunopositivity for MMP9 was observed in the lung. Bar = 40 μm. (e–h) Immunopositivity for GFP and F4/80, a marker of macrophages, was examined using liver specimens from group 1 mice taken at W12 (e–g) and from normal C57BL/6 mice at 6 weeks of age (h). GFP-immunopositive cells were negative for F4/80 (arrows), while F4/80-immunopositive cells were not positive for GFP (arrowheads). Abundant immunopositivity for F4/80 was observed in the lung. Bar = 40 μm. (i–l) Immunopositivity for MMP, GFP and DlK-1 was examined using liver specimens from group 1 mice taken at W12. MMP-immunopositive cells (arrows and arrowheads, magnified in insets), derived from ES cells (arrows) and resident liver cells (arrowheads), were positive for DlK-1. P, portal vein; bar = 40 μm.
We also investigated the expression of DLK to elucidate the characteristics of the cells that were MMP9-immunopositive and F4/80-negative. DLK is a molecule identified as a possible hepatoblast marker and known to be strongly expressed on hepatoblasts in mouse embryonic livers until embryonic day 16.5, after which it becomes downregulated and disappears by birth (Tanimizu et al. 2003). Interestingly, MMP9-immunopositive cells, irrespective of their origin from graft cells or the recipient, were immunopositive for DlK-1 (Figure 5i–l arrows or arrowheads respectively), indicating that they were hepatoblast-like cells.
Liver fibrosis improved by secondary ES cell transplantation
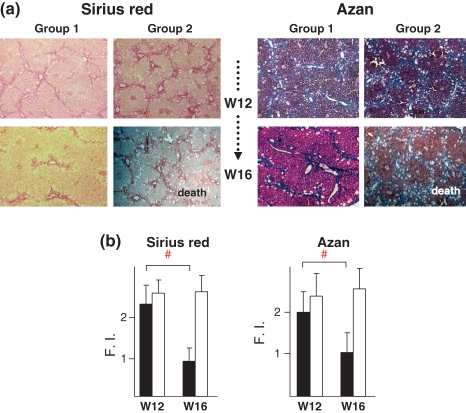
At W12, the remaining four mice in group 1 received a second transplantation of ES cells into the spleen, while those in group 2 received 0.1 ml of PBS, after which mice in both groups were subjected to an additional 4 weeks of treatment with CCl4. However, all mice in group 2 died no later than W15 and exhibited ascites before death, leading us to conclude that the cause of death was liver failure. The livers were isolated within 8 h after death and histology findings for group 1 mice at 4 weeks after the second transplantation (W16) showed reduced fibrosis as compared with W12 (Figure 6a), which was determined quantitatively by image analysis (Figure 6b). In group 2, histological findings of the liver showed advanced fibrosis with lipid accumulation, although there was no significant difference between W12 and W16.
Figure 6.
Liver fibrosis improved by second ES cell transplantation. (a) Liver histology results for group 1 mice at W16 and group 2 at death. At W12, the remaining four mice in each group received a second transplantation of ES cells (group 1) or PBS (group 2) into the spleen. The mice in group 1 survived until W16, whereas all in group 2 died by W15. (b) Quantitative evaluation of liver fibrosis using image analyser. Liver fibrosis was improved at W16 in group 1. Closed and open squares show group 1 and group 2 respectively. Liver fibrosis on day 0 was determined to be the standard value of Fibrosis Index (F.I.) 1.0.
Tumour formation
All of the livers in the experimental animals were removed and the lobes sliced into 2-mm widths, with specimens from the middle lobes used for microscopic analysis. No tumour formation was observed macroscopically or microscopically in any of the livers and spleens. Organs other than the liver and lungs were found to be macroscopically intact.
Discussion
Recently, transplantation of bone marrow cells (Sakaida et al. 2004), bone marrow stem cells (Wu et al. 2005), bone marrow mesenchymal stem cells (Fang et al. 2004; Zhao et al. 2005; Oyagi et al. 2006) and foetal liver progenitor cells (Zheng et al. 2006) has been reported to reduce liver fibrosis. However, the effect of ES cells on liver fibrosis has not been reported. We previously reported that ES cells transplanted into the spleens of CCl4-treated mice migrated to the liver and differentiated into hepatocyte-like cells (Moriya et al. 2007). In that study, we also noted the possibility that ES cells might reduce fibrosis of the liver, although the total experimental period was only 5 weeks, which consisted of a 1-week pretreatment with CCl4 before intrasplenic transplantation of ES cells and then an additional 4 weeks of treatment. In this study, we conducted a long-term experiment to examine the effects of ES cell transplantation on liver fibrosis. Mice were pretreated with CCl4 for 4 weeks to produce considerable liver fibrosis prior to transplantation, then fibrosis was observed histochemically for up to 16 weeks after the initial transplantation.
As expected, obvious fibrosis was induced in the livers by pretreatment with CCl4. First, we determined whether the transplanted ES cells migrated to and were retained in the liver for a sustained period, because we noted only few grafted cells at 30 days after transplantation in mouse livers in a previous experiment (Moriya et al. 2007). In this study, we found a considerable number of transplanted cells in the liver even at W12. These contrasting results were probably because of the length of the CCl4 pretreatment protocol, which consisted of two intraperitoneal injections over 1 week in the previous study, whereas we gave eight injections of CCl4 over a 4-week period in this study. We considered that a more favourable microenvironment might be induced in the liver by the present repeated CCl4 administration, under which ES cells were able to migrate to and reside in the liver. Recently, the CXCR4/SDF1 system has been suggested to be involved in the migration of bone marrow cells to inflamed organs (Dalakas et al. 2005), including CCl4-treated livers (Kollet et al. 2003; Jung et al. 2006; Son et al. 2006). Although the involvement of the CXCR4/SDF1 axis in hepatic migration of ES cells transplanted into the spleen is unknown, we noted a much higher level of SDF1 expression immunohistochemically in the livers of mice pretreated with CCl4 for 4 weeks as compared with those pretreated for 1 week (data not shown).
These results demonstrated that transplantation of ES cells improves liver fibrosis, while immunohistochemistry findings also revealed GFP-immunopositive cells in locations neighbouring areas of fibrosis at all observation time-points. Bone marrow cells and bone marrow-derived mesenchymal stem cells have been reported to migrate to fibrosis areas of the liver and show activities of matrix metalloproteinases after transplantation (Kollet et al. 2003; Sakaida et al. 2004; Higashiyama et al. 2007). It is conceivable that ES cells elicit fibrolysis activity similar to that of bone marrow cells. As expected, ES-derived cells in the present livers were immunopositive for MMP9, some of which were negative for GFP. These findings suggest that the improvement in liver fibrosis after transplantation of ES cells was a result of fibrolysis activities by both ES-derived and recipient-derived cells. Next, we attempted to identify the nature of the MMP9-immunopositive cells. As the GFP-immunopositive cells were negative for F4/80, we considered that the ES-derived MMP9-immunopositive cells were also negative for F4/80. Interestingly, MMP9-immunopositive cells, irrespective of their origin from ES cells or the recipient, were immunopositive for DlK-1, a hepatoblast marker (Tanimizu et al. 2003), suggesting that hepatoblast-like cells play an important role in the regression of liver fibrosis. Investigation of the pathophysiology of the generation of MMP9-expressing hepatoblast-like cells from ES cells and liver resident cells in CCl4-injured livers may lead to discovery of a new treatment approach for liver fibrosis.
We performed a second transplantation of ES cells at W12, which led to an additive effect for improved fibrosis seen at W16. All four mice in group 1 that received ES cell transplantation twice, day 0 and W12, survived to the final observation point at W16. However, none of the mice that received PBS instead of ES cells at the same time-points survived to W16, but rather showed accumulated ascites, leading us to conclude that the cause of death was liver failure. Thus, these findings suggest the potential usefulness of ES cell transplantation to treat liver cirrhosis.
Recently, a novel trial of intravenous transplantion of autologous bone marrow-derived stromal cells (BMSCs) in patients with liver cirrhosis reported improved serum albumin and total protein levels (Terai et al. 2006). In contrast to that method, the present ES cells used as grafts and host mice were an allogeneic combination, as the cells were derived from 129SvJ mice and transplanted into C57BL/6 mice. In the future, it is considered that human ES cells used in therapeutic strategies will likely be allogeneic to the host, except in cases where ES cells are produced with a nuclear transfer technique using nuclei from the host cells (Lavoir et al. 2005 or by introducing four recently identified genes into host somatic cells (Takahashi et al. 2007; Yu et al. 2007). Although a high incidence of teratomas in livers was reported following syngeneic transplantation of undifferentiated ES cells and differentiating EBs into C57BL/6 mice receiving CCl4 treatment (Chinzei et al. 2002; Yamamoto et al. 2003), we found no tumours in the livers of mice that received undifferentiated allogeneic ES cells during the 16-week experimental period.
In conclusion, our results showed that undifferentiated ES cells grafted into the spleen migrated to the liver and reduced liver fibrosis in mice chronically treated with CCl4. For the coming era of controlled cell growth, ES cells are a promising cell source for treatment of liver cirrhosis.
References
- Chinzei R, Tanaka Y, Shimizu-Saito K, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22–29. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- Dalakas E, Newsome PN, Harrison DJ, Plevris JN. Hematopoietic stem cell trafficking in liver injury. FASEB J. 2005;19:1225–1231. doi: 10.1096/fj.04-2604rev. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotent cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–88. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- Higashiyama R, Inagaki Y, Hong YY, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- Ishizaka S, Shiroi A, Kanda S, et al. Development of hepatocytes from ES cells after transfection with the HNF-3beta gene. FASEB J. 2002;11:1444–1446. doi: 10.1096/fj.01-0806fje. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Ryu KH, Cho SJ, et al. Syngenic bone marrow cells restore hepatic function in carbon tetrachloride-induced mouse liver injury. Stem Cells Dev. 2006;15:687–695. doi: 10.1089/scd.2006.15.687. [DOI] [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34 + stem cell recruitment to the liver. J. Clin. Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoir MC, Weier J, Conaghan J, Pedersen RA. Poor development of human nuclear transfer embryos using failed fertilized oocytes. Reprod. Biomed. Online. 2005;11:740–744. doi: 10.1016/s1472-6483(10)61693-8. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya K, Yoshikawa M, Saito K, et al. Embryonic stem cells develop into hepatocytes after intrasplenic transplantation in CCl4-treated mice. World J. Gastroenterol. 2007;13:866–873. doi: 10.3748/wjg.v13.i6.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura F, Yoshikawa M, Kanda S, et al. Potential use of embryonic stem cells for the treatment of mouse parkinsonian models: improved behavior by transplantation of in vitro differentiated dopaminergic neurons from embryonic stem cells. Stem Cells. 2003;21:171–180. doi: 10.1634/stemcells.21-2-171. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Oyagi S, Hirose M, Kojima M, et al. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J. Hepatol. 2006;44:742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- Shiroi A, Yoshikawa M, Yokota H, et al. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells. 2002;20:284–292. doi: 10.1634/stemcells.20-4-284. [DOI] [PubMed] [Google Scholar]
- Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- Sugie Y, Yoshikawa M, Ouji Y, et al. Photoreceptor cells from mouse ES cells by co-culture with chick embryonic retina. Biochem. Biophys. Res. Commun. 2005;332:241–247. doi: 10.1016/j.bbrc.2005.04.125. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:1–12. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tanimizu N, Nishikawa M, Saito H, et al. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J. Cell Sci. 2003;116:1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Niioka M, Hozawa S, et al. Gene expression of interstitial collagenase in both progressive and recovery phase of rat liver fibrosis induced by carbon tetrachloride. J. Hepatol. 2000;33:224–235. doi: 10.1016/s0168-8278(00)80363-3. [DOI] [PubMed] [Google Scholar]
- Wu LM, Li LD, Liu H, Ning KY, Li YK. Effects of Guiyuanfang and autologous transplantation of bone marrow stem cells on rats with liver fibrosis. World J. Gastroenterol. 2005;11:1155–1160. doi: 10.3748/wjg.v11.i8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Yoshikawa M, Takaki M, et al. In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells. 2002;20:41–49. doi: 10.1634/stemcells.20-1-41. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Quinn G, Asari A, et al. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37:983–993. doi: 10.1053/jhep.2003.50202. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J. Gastroenterol. 2005;11:3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JF, Liang LJ, Wu CX, Chen JS, Zhang ZS. Transplantation of fetal liver epithelial progenitor cells ameliorates experimental liver fibrosis in mice. World J. Gastroenterol. 2006;12:7292–7298. doi: 10.3748/wjg.v12.i45.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]