Abstract
The liver sinusoidal endothelial cell (LSEC) is damaged by many toxins, including oxidants and bacterial toxins. Any effect on LSECs of the Pseudomonas aeruginosa virulence factor, pyocyanin, may be relevant for systemic pseudomonal infections and liver transplantation. In this study, the effects of pyocyanin on in vivo rat livers and isolated LSECs were assessed using electron microscopy, immunohistochemistry and biochemistry. In particular, the effect on fenestrations, a crucial morphological aspect of LSECs was assessed. Pyocyanin treatment induced a dose-dependent reduction in fenestrations in isolated LSECs. In the intact liver, intraportal injection of pyocyanin (11.9 μM in blood) was associated with a reduction in endothelial porosity from 3.4 ± 0.2% (n = 5) to 1.3 ± 0.1% (n = 7) within 30 min. There were decreases in both diameter and frequency of fenestrations in the intact endothelium. There was also a decrease in endothelial thickness from 175.8 ± 5.8 to 156.5 ± 4.0 nm, an endothelial pathology finding previously unreported. Hepatocyte ultrastructure, liver function tests and immunohistochemical markers of oxidative stress (3-nitrotyrosine and malondialdehyde) were not affected. Pyocyanin induces significant ultrastructural changes in the LSEC in the absence of immunohistochemical evidence of oxidative stress or hepatocyte injury pointing to a novel mechanism for pyocyanin pathogenesis.
Keywords: electron microscopy, immunohistochemistry, liver sinusoidal endothelial cell fenestrations, oxidative stress, Pseudomonas aeruginosa pyocyanin, transplantation
Pseudomonas aeruginosa is an increasingly important cause of sepsis and death in organ transplant recipients, particularly those receiving liver transplants (Wagener & Yu 1992; Singh et al. 2004; Aduen et al. 2005). Pseudomonas aeruginosa has a special affinity for tissue vasculature, typically surrounding blood vessels circumferentially (Soave et al. 1978; Schaber et al. 2007) and congregating in postcapillary venules (Fetzer et al. 1967). Pseudomonas aeruginosa induces apoptosis in the endothelial cell line, ECV304 (Takahashi et al. 1990; Valente et al. 2000) and produces a number of virulence factors including pyocyanin. Pyocyanin is a phenazine dye with broad range of activities including redox activity (Hassan & Fridovich 1980; Britigan et al. 1997), immunomodulation (Muhlradt et al. 1986; Muller et al. 1989), pro-inflammatory effects (Lau et al. 2004), generation of reactive oxidative species (ROS) (Cheluvappa et al. 2007b), succinic dehydrogenase enzyme inactivation (Harman & Macbrinn 1963), cytotoxicity (Britigan et al. 1997; Lau et al. 2004), pro-apoptotic effects (Usher et al. 2002) and induction of senescence (Muller 2006).
There are several reasons to suspect that pyocyanin might have important effects on the liver sinusoidal endothelial cell (LSEC). Pyocyanin has been shown to induce oxidative stress and morphological changes in endothelial cells (Britigan et al. 1992). Within the liver, the LSEC is very sensitive to both oxidative stress (Cogger et al. 2001; Cheluvappa et al. 2007b) and the effects of bacterial lipopolysaccharides (LPS) (Dobbs et al. 1994; Seto et al. 1998). LSECs are perforated with fenestrations, pores with diameters ranging from 30 to 300 nm, that facilitate the transfer of lipoproteins, particularly triglyceride-rich chylomicron remnants, between blood and hepatocytes (Fraser et al. 1995). Previously, we found that pyocyanin reduced porosity in isolated LSECs. This was prevented by treatment with catalase, suggesting that the mechanism involves hydrogen peroxide (H2O2)-induced oxidative stress (Cheluvappa et al. 2007b). Another possible mechanism for these LSEC changes is the alteration of caveolin-1 expression. LPS, which induces defenestration in LSECs (Dobbs et al. 1994), also induces the over-expression of caveolin-1 (Kamoun et al. 2006), a key component of fenestrations (Ogi et al. 2003). Similarly, pyocyanin could influence fenestrations through its interactions with proteins, such as F-actin or caveolin-1, that maintain fenestrations (Braet et al. 2003; Ogi et al. 2003).
The LSEC has a key role in the maintenance of liver function and its viability following transplantation. The rejection of donor livers is associated with demonstrable LSEC antibodies (Sumitran-Holgersson et al. 2004). LSEC apoptosis correlates with preservation–perfusion-related dysfunction of donor rat livers (Zhu et al. 2006) and LSEC apoptosis without concomitant hepatocellular injury occurs in preservation injury during liver transplantation (Gao et al. 1998). For such reasons, changes induced in the LSEC are likely to have significant clinical outcomes in terms of liver transplantation.
Therefore, we postulated that the effects of pyocyanin on the LSEC are an important component of the toxicity of pseudomonal sepsis. Better knowledge of the pathogenesis of the changes in LSECs induced by pyocyanin may partially explain the mechanisms of liver allograft rejection (Sumitran-Holgersson et al. 2004) and hyperlipidaemia of sepsis (Spitzer et al. 1988; Fraser et al. 1995; Harris et al. 2000).
Methods
Synthesis of pyocyanin
Pyocyanin was chemically synthesized by the photolysis of phenazine methosulphate (Knight et al. 1979) and purified (Muller & Sorrell 1992) as described earlier. The purity of pyocyanin was ascertained and the concentration quantified by utilizing its known absorption spectrum and extinction coefficient values as elucidated earlier (Watson et al. 1986).
Animal protocols, LSEC isolation and pyocyanin treatment
Animal studies were approved by the Sydney Southwest Area Health Service Animal Welfare Committee. All rats were specific pathogen-free males obtained from the Animal Research Centre (Perth, Australia). Each rat used was anesthetized with ketamine and xylazine (50 and 5 mg/kg respectively; Troy Laboratories, Smithfield, Australia) by intraperitoneal injection. LSECs were harvested from livers of Sprague-Dawley rats (aged 2–3 months, 250–350 g) according to methodology described previously (Cogger et al. 2004). The resulting cells were plated onto collagen-coated Thermanox cover slips (Nalge Nunc Int., Rochester, NY, USA) at a density of 1.60 × 106 per ml at 37 °C/5% CO2 in RPMI-1640 media with 2% FCS (GIBCO®; Invitrogen Corporation, Carlsbad, CA 92008, USA) and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin) and cultured for at least 16 h. A series of dose–response experiments in triplicate were performed to assess the effects of pyocyanin at the following concentrations: 0, 10, 20, 50 and 100 μM. These concentrations were chosen because pyocyanin has been detected in vivo at concentrations of 1–130 μM (Wilson et al. 1988).
The in vivo experiments were performed in Fisher F344 rats (aged 2–3 months, approximately 200 g). A mid-line laparotomy incision was made in anesthetized animals and saline (n = 5) or pyocyanin (n = 7) was injected into the portal vein. Pyocyanin, when injected portally, was calculated to give a final systemic concentration of 11.9 μM. Total blood volume was calculated for each rat using the following formula (Lee & Blaufox 1985): blood volume (ml) = 0.06 × body weight (g) + 0.77. The laparotomy was closed and respiratory rate monitored. After 30 min, the incision was re-opened. Blood was collected from the inferior vena cava for biochemical analysis, and the liver was removed and processed for scanning electron microscopy, transmission electron microscopy and immunohistochemistry as described previously (Cheluvappa et al. 2007a,b). Two lobes were snap frozen in liquid nitrogen for biochemical analyses.
Electron microscopy
Scanning electron microscopy of LSECs and liver tissue blocks was performed as described previously (Cheluvappa et al. 2007a,b) using a Jeol JSM-6380LV scanning electron microscope (Jeol, Akishima-Shi, Japan). For an analysis of isolated LSECs, three representative micrographs from each of four cellular fields per cover slip were taken at 15,000× magnification. For liver tissue blocks, 12 representative fields from at least three liver blocks per animal were photographed at 25,000× magnification. Micrographs were analysed using ImageJ (http://rsb.info.nih.gov/ij/) to determine endothelial porosity, average fenestration diameter, fenestration density and presence of gaps. Fenestrations are distinctly patent pores in contrast to caveolae which are closed pores. Fenestrations contribute to endothelial porosity, while caveolae do not owing to the mechanical barricade that the latter pose. Hence, open pores (fenestrations) alone were counted from electron micrographs, but not closed or membrane-covered pores (caveolae).
Transmission electron microscopy of liver tissue sections was performed as described previously (Cogger et al. 2004). Briefly, two technically eligible blocks per liver were examined. Sections from each block were chosen at random for ultrastructural measurement. Twenty representative micrographs per animal were taken at 19,000× and 10 at 4600× using a Philips CM10 Transmission Electron Microscope fitted with a Megaview III camera and Analysis® software (Olympus, Tokyo 163-0914, Japan). The 19,000× micrographs were analysed using ImageJ to measure endothelial thickness. The 4600× micrographs were analysed using ImageJ for morphological aberrations in hepatocyte ultrastructure.
Light microscopy and immunohistochemistry
Liver specimens were fixed in 4% paraformaldehyde-buffered saline and embedded in paraffin for light microscopy and immunohistochemistry. Four-micrometre sections were stained with haematoxylin and eosin for light microscopy. Immunohistochemistry was used to detect the differences in staining intensity, distribution and pattern of caveolin-1, which is present on the plasma membrane of LSEC fenestrations (Ogi et al. 2003); 3-nitrotyrosine, which marks tyrosine nitration occurring during oxidative stress; and malondialdehyde, which indicates lipid peroxidation occurring during oxidative stress. Immunohistochemical staining was performed using an indirect polymer immunoperoxidase method. Four-micrometre sections of fixed liver tissue were deparaffinized in xylene (3 × 3 min) and taken to absolute ethanol (3 × 2 min). Endogenous peroxidase was blocked by incubating slides in 3% H2O2 in absolute methanol for 10 min at room temperature. After hydrating the sections, the slides utilized for nitrotyrosine and caveolin-1 immunohistochemistry were heated at 125 °C for 4 min in a Decloaking Chamber (Biocare Medical, Concord, CA 94520, USA) with epitope retrieval buffer and then cooled. This was followed by incubation with goat serum for 20 min. Without washing, the primary antibodies were applied and incubated overnight. The primary antibodies used were rabbit anti-human caveolin-1 antibody (N-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal anti-nitrotyrosine antibody (ab7048; Abcam, Cambridge CB4 OWN, UK) and rabbit polyclonal anti-malondialdehyde antibody (ab6463; Abcam). The epitope retrieval buffers used were citrate buffer (0.01 M, pH 6.0) for caveolin-1 and Tris buffer (0.05 M Tris–EDTA, pH 8.0) for 3-nitrotyrosine immunohistochemistry. No pretreatment was needed for tissues for malondialdehyde immunohistochemistry. During immunostaining, all slides were washed in washing buffer (0.001 M Tris, pH 7.6) containing Tween20. After the primary incubation, the secondary antibody, affinity-purified goat anti-mouse or anti-rabbit immunoglobulin-linked polymeric horseradish peroxidase (AP340P-50ML/AP342P-50ML; Chemicon, Temecula, CA 92590, USA; now Bioscience Research Reagents, Millipore, Billerica, MA 01821, USA) was applied for 30 min. After buffer wash, the sections were treated with diaminobenzidine (DAB) chromogenic substrate solution for 5 min and slides were washed in water. The slides were then immersed in 1% aqueous CuSO4 solution for further intensification of staining and counterstained with haematoxylin, dehydrated and mounted. The slides were graded by three blinded observers and a consensus was reached according to staining distribution (periportal, zone 2, pericentral) and intensity of staining (0, +, ++, +++) and semi-quantitatively assessed.
Blood biochemistry
Protein quantification, blood biochemistry and liver function tests were carried out by the Biochemistry Department, Diagnostic Pathology Unit, Concord RG Hospital, using the automated Roche Diagnostics Modular Analytics Serum Work Area (F. Hoffmann-La Roche Ltd). Briefly, the principles utilized in these assays are enclosed in brackets as follows: total protein (biuret/endpoint with blank), albumin (BCG-Citrate buffer), alanine transaminase (ALT) and aspartate transaminase (AST) (IFCC modified) and alkaline phosphatase (ALP) (AMP buffer-IFCC).
Western blot analysis for pyocyanin interaction with caveolin-1
To determine whether pyocyanin irreversibly binds to caveolin-1 and alters protein size, caveolin-1 electromobility was examined using Western blot. Twenty micrograms of cell lysate from SK-HEP-1 cells, an endothelial cell line that expresses caveolin-1 (Heffelfinger et al. 1992), were treated with pyocyanin; and controls were separated by SDS-PAGE. Lanes were run with 100 μM pyocyanin or 2 μM pyocyanin in the presence of lysate or with 100 μM pyocyanin alone. Lysate, when loaded, was always 20 μg per well. After transfer onto nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ 08855-1327, USA; Now part of GE Healthcare, Chalfont St Giles, BUCKS, HP8 4SP, UK), the blot was blocked, incubated with primary antibodies to caveolin-1 (Santa Cruz Biotechnology), washed and incubated with a rabbit anti-goat IgG secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences). Proteins were visualized using chemiluminescence.
Statistical analysis
Statistical analysis was performed using SigmaStat Statistics Software (spss Inc, Chicago, IL, USA). Data are presented as the mean ± standard error of the mean. The Mann–Whitney test was used to compare groups and considered significant when P < 0.05. The Mann–Whitney test was performed only when the Kolmogorov–Smirnov normality test failed on the data sets tested.
Results
Effect of pyocyanin on isolated LSECs
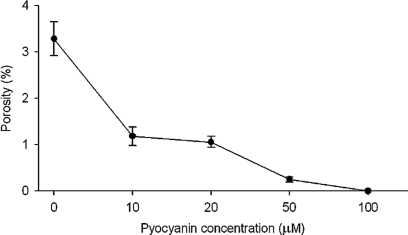
Treatment of LSECs with pyocyanin concentrations ranging from 0 to 100 μM induced a dose-dependent decrease in LSEC porosity (Figure 1) as measured by scanning electron micrograph morphometry.
Figure 1.
Pyocyanin dose and porosity of LSECs. Porosity (%) of isolated LSECs decreased as concentration of pyocyanin increased from 0 to 100 μM.
Scanning electron microscopy of liver sinusoids
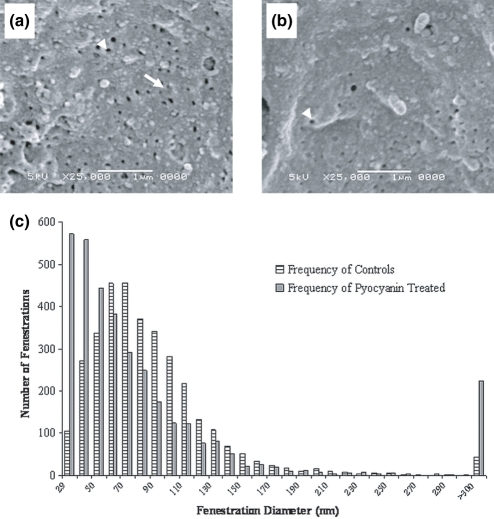
Scanning electron micrograph analysis of liver sinusoids (Figure 2a,b) revealed a decrease in porosity of liver sinusoids with pyocyanin treatment, with significant contributions from both fenestration diameter and fenestration frequency (number of fenestrations per μm2) (Table 1). Pyocyanin treatment was associated with smaller fenestration diameters than those seen in control liver sinusoids (Figure 2c). The number of gaps (diameter >300 nm) was slightly raised with pyocyanin treatment, although statistically insignificant (Figure 2c).
Figure 2.
Scanning electron microscopy of liver sinusoids from control and pyocyanin-treated rats. The fenestrations of the control liver sinusoid (a) are larger in diameter and higher in frequency (number of fenestrations per μm2) than those of the pyocyanin-treated liver (b). The arrow-head indicates a single fenestration and the full arrow, a sieve-plate circumscribing many fenestrations. Original magnification 25,000×, scale bar = 1 μm. (c) is a histogram of fenestration diameters measured on scanning electron micrographs, showing a lower proportion of smaller fenestrations in pyocyanin-treated liver endothelium than in control livers. There was a non-significant trend towards an increased number of gaps (>300 nm) with pyocyanin treatment.
Table 1.
Electron micrograph morphometry of the liver endothelium and perisinusoidal hepatocytes from rat livers with and without pyocyanin treatment in vivo
| No treatment (n = 5) | Pyocyanin treatment (n = 7) | P-value | |
|---|---|---|---|
| Scanning electron microscopy | |||
| Porosity % | 3.4 ± 0.2 | 1.3 ± 0.1 | <0.001 |
| No. of fenestrations (μm2) | 5.5 ± 0.3 | 2.4 ± 0.1 | <0.001 |
| Fenestration diameter (nm) | 80.4 ± 0.6 | 71.0 ± 0.8 | <0.001 |
| Transmission electron microscopy | |||
| Endothelial thickness (nm) | 175.8 ± 5.8 | 156.5 ± 4.0 | <0.01 |
With pyocyanin treatment, scanning electron micrograph analysis revealed a decrease in porosity, fenestration diameter and fenestration frequency of liver sinusoids. With pyocyanin treatment, transmission electron micrograph analysis revealed a decrease in endothelial thickness. Data are presented as numerical values ± standard error of the mean.
Transmission electron microscopy of liver sinusoids and the space of Disse
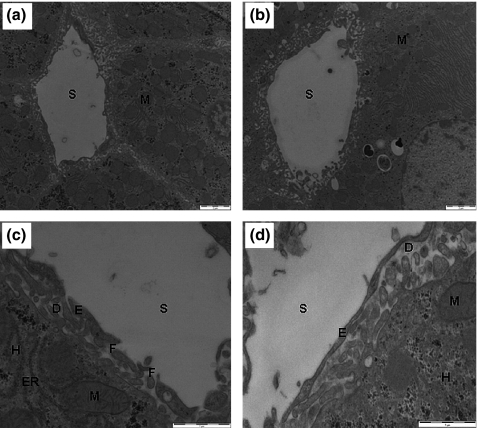
Transmission electron micrograph analysis revealed normal hepatocyte architecture in both control and pyocyanin treatment groups (Figure 3). The hepatocellular nuclei, mitochondria and endoplasmic reticulum were well preserved and had normal morphology. Pyocyanin treatment was associated with a statistically significant decrease in endothelial thickness from 175.8 ± 5.8 to 156.5 ± 4.0 nm (Figure 3c,d, Table 1).
Figure 3.
Transmission electron microscopy of livers from control and pyocyanin-treated rats. Organelle size and number were unaffected by pyocyanin treatment with normal morphology of mitochondria (M) observed in control and pyocyanin-treated rats: (a) control liver sinusoid, (b) pyocyanin-treated liver sinusoid. S; sinusoidal lumen, scale bar = 2 μm, original magnification 4600×. At original magnification 19,000×, a decrease in the thickness of the endothelium (E) was seen in the pyocyanin treatment group. The space of Disse (D), hepatocytes (H) and hepatocellular endoplasmic reticulum (ER) appeared normal in both groups: (c) control liver sinusoid, (d) pyocyanin-treated liver sinusoid. F; fenestrations, scale bar = 1 μm.
Light microscopy and immunohistochemistry of livers
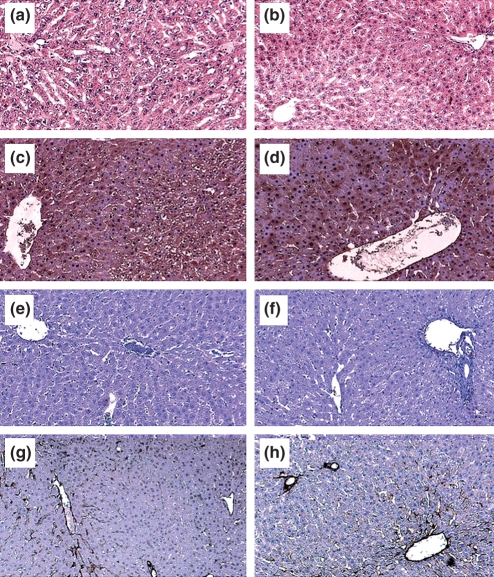
Light microscopy (Figure 4a,b), 3-nitrotyrosine immunohistochemistry (Figure 4c,d) and malondialdehyde immunohistochemistry (Figure 4e,f) of livers revealed no observable changes with pyocyanin treatment.
Figure 4.
Light microscopy and immunohistochemistry of livers from control and pyocyanin groups. Light microscopy of liver sections stained with eosin and haematoxylin staining. (a) Control liver, (b) pyocyanin-treated liver. Malondialdehyde immunohistochemistry [(c) control liver, (d) pyocyanin-treated liver], 3-nitrotyrosine immunohistochemistry [(e) control liver, (f) pyocyanin-treated liver] and caveolin-1 immunohistochemistry [(g) control liver, (h) pyocyanin-treated liver] revealed no changes across the hepatic lobule with pyocyanin treatment. Immunohistochemical stains appear brown, original magnification 100×.
Investigation of relationship of caveolin-1 to pyocyanin-induced endothelial changes
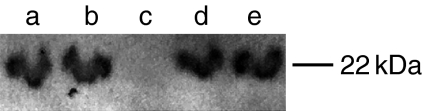
Caveolin-1 immunohistochemistry (Figure 4g,h) of livers revealed no observable changes with pyocyanin treatment. Western blot analysis (Figure 5) showed that pyocyanin did not alter caveolin-1 mobility.
Figure 5.
Western blot analysis for caveolin-1. SDS-PAGE gel run with 20 μl of SK-HEP-1 cell lysate with 2 μM pyocyanin (lane a), 100 μM pyocyanin (lane b) or no pyocyanin (lanes d,e); or with 100 μM (lane c) pyocyanin alone and stained for caveolin-1. Lysate, when loaded, was always 20 μg per well (lanes a,b,d,e). Band migration was unaltered for SK-HEP-1 cell lysate with and without pyocyanin treatment.
Blood biochemistry
Liver function tests (total protein, albumin, ALT, AST and ALP) showed no significant change with pyocyanin treatment (Table 2).
Table 2.
Liver function tests with and without pyocyanin treatment in vivo
| No treatment (n = 5) | Pyocyanin treatment (n = 7) | |
|---|---|---|
| ALP (U/l) | 186.6 ± 16.7 | 174.2 ± 12.2 |
| ALT (U/l) | 69.0 ± 13.7 | 71.5 ± 8.4 |
| AST (U/l) | 90.6 ± 14.3 | 120.2 ± 33.4 |
| Protein (g/l) | 52.0 ± 1.2 | 54.4 ± 1.0 |
| Albumin (g/l) | 33.2 ± 0.8 | 34.4 ± 0.8 |
Liver function tests showed no statistically significant changes with pyocyanin treatment. Data are presented as numerical values ± standard error of the mean.
Discussion
Pyocyanin over a wide range of concentrations is associated with a substantial loss of LSEC porosity. Pyocyanin also induces significant acute changes in the in vivo liver sinusoidal endothelium without evidence that these changes are mediated by reactive oxygen and nitrogen species. These LSEC changes were not accompanied by evidence of structural or biochemical hepatocellular changes. The data indicate that the ultrastructure of LSECs is highly sensitive to pyocyanin.
Many toxins have been shown to induce LSEC injury. In particular, oxidative stress has been shown to have dramatic effects on the morphology of the LSEC. We found that H2O2 (0.7 mM) delivered via the portal vein in the perfused liver had effects that were largely confined to the perisinusoidal areas (Cogger et al. 2001) and that tert-butyl hydroperoxide injected into the portal vein in vivo and in isolated LSECs caused disruption of the liver sieve plates (Cogger et al. 2004). As regards pyocyanin, Britigan et al. showed that pyocyanin induced oxidative injury in pulmonary artery endothelial cells (Britigan et al. 1992; Miller et al. 1996). Pyocyanin induced H2O2 production by human umbilical vein endothelial cells with marked depletion of intracellular glutathione, and these changes were preventable by catalase (Muller 2002). In our previous study exploring the effects of pyocyanin on isolated LSECs (Cheluvappa et al. 2007b), catalase which inactivates hydrogen peroxide to water, prevented pyocyanin-induced morphological changes in the LSECs, specifically defenestration. In the current study, pyocyanin treatment induced a dose-dependent loss of porosity in isolated LSECs. Furthermore, portally injected pyocyanin in vivo led to a significant reduction in porosity of the endothelium showing that this effect is seen both in vivo and in vitro. In addition, we noted a decrease in endothelial thickness with pyocyanin, a change which has not been reported with any other toxic injury to the LSEC.
It is important to note that the ultramicroscopic LSEC changes were present without any morphological hepatocellular alterations including mitochondrial morphology and frequency or any other signs of hepatocyte injury or oxidative stress. This indicates that the LSEC is an initial site of injury induced by pyocyanin, and indeed may even have a role in protecting hepatocytes from endo- and xenobiotics. There were no changes in malondialdehyde and 3-nitrotyrosine immunohistochemistry. This is in contradistinction to our studies in isolated LSECs where an increase in markers of oxidative stress was found. It is plausible that in vivo, hepatocyte-derived antioxidants prevented any overall changes in markers of oxidative stress but not sufficient to prevent defenestration of the LSEC. Alternatively, it is possible that pyocyanin induces defenestration in vivo through mechanisms independent of oxidative stress such as influencing caveolin-1.
Caveolin-1 is a membrane protein involved in the maintenance of fenestrations (Braet et al. 2003; Ogi et al. 2003). Normal liver caveolin-1 immunohistochemical staining is usually evident in LSECs which line the sinusoids, bile canaliculi, as well as portal and hepatic arterioles and venules (Yokomori et al. 2001). It has been demonstrated that LSEC defenestration, which occurs in models of pathological liver states, such as cirrhosis (Nopanitaya et al. 1976) and type 1 diabetic liver (Jamieson et al. 2007), is accompanied by caveolin-1 over-expression. Similarly, LPS which induces defenestration in LSECs (Dobbs et al. 1994) also induces over-expression of caveolin-1 (Kamoun et al. 2006). However, we were unable to demonstrate any caveolin-1 immunohistochemical changes with the liver endothelial defenestration induced by pyocyanin. We also investigated the possibility of pyocyanin binding to caveolin-1 or altering the properties of caveolin-1. We were unable to demonstrate any alteration in caveolin-1 using an immunoblot method. These results exclude the possibility that pyocyanin induces defenestration via any major effects on caveolin-1. Therefore, it is possible that pyocyanin induces defenestration through mechanisms independent of oxidative stress or interaction with caveolin-1. As pyocyanin has been shown to influence the expression and secretion of numerous cytokines (Muhlradt et al. 1986; Leidal et al. 2001), further investigations into the expression and activity of these cytokines may serve to reveal the mechanism.
The novelties of the present study are manifold. First, we have shown that the in vitro findings elucidated in our previous study (Cheluvappa et al. 2007b) were reproducible in vivo, which is more pathophysiologically relevant. Secondly, the contributing parameters leading to the loss of endothelial porosity were different in the in vitro model (Cheluvappa et al. 2007b) in contrast to the in vivo model that we utilized here. In the in vitro model, fenestration frequency was the only determining factor. In this in vivo model, the fenestration diameter was as significant a contributing factor as fenestration frequency. The ultrastructural changes recorded in this in vivo study are more likely to be pathophysiologically representative. Thirdly, using transmission electron microscopy, we have described endothelial thinning, a novel ultrastructural change induced by pyocyanin. The significance of endothelial thinning, a pathological change unreported in extant literature, is yet to be elucidated. Fourthly, the mechanism of pyocyanin pathogenesis has always been attributed to oxidative stress injury. In this study, we demonstrated with clarity that pyocyanin induces in vivo sinusoidal changes in the absence of clear-cut oxidative stress indicators. These findings point to a novel mechanism for pyocyanin pathogenesis.
The observation that pyocyanin influences endothelial morphology may have significant clinical implications. LSECs have been shown to be important in tolerance induction in liver transplantation and rejection of donor livers correlates closely with the presence of LSEC antibodies (Sumitran-Holgersson et al. 2004). Thus, there is a possibility that pyocyanin-induced LSEC perturbations with or without the induction of free radicals may impact graft outcome and prognosis following pseudomonal sepsis. It has also been reported that hyperlipidaemia is an important response to sepsis. The mechanism for sepsis-associated hyperlipidaemia is multifactorial, but impaired catabolism of lipoproteins is a contributory factor (Spitzer et al. 1988; Harris et al. 2000). LSECs, which are perforated with fenestrations that facilitate transfer of lipoproteins between blood and hepatocytes, have an increasingly recognized role in hyperlipidaemia (Fraser et al. 1995). We have shown that conditions associated with reduced numbers of fenestrations, such as aging (Hilmer et al. 2005) and treatment with the surfactant poloxamer 407 (Cogger et al. 2006), are associated with impaired lipoprotein uptake by the liver and hypertriglyceridaemia. The results with pyocyanin support the concept that hyperlipidaemia associated with sepsis might in part be a result of LSEC defenestration. In the current study, the 30 min duration of pyocyanin exposure would not have been sufficient to cause profound changes in blood lipoprotein levels.
In conclusion, the P. aeruginosa toxin, pyocyanin, caused loss of fenestrations over a range of concentrations in isolated LSECs as well as the in vivo liver endothelium. The ultrastructural LSEC changes in the absence of hepatocellular injury indicate that the LSEC is a prime target for pyocyanin. Pyocyanin-induced LSEC changes seen in vivo in the absence of free radical or oxidative stress injury points to a novel mechanism for the pathogenesis of P. aeruginosa pyocyanin.
Acknowledgments
We would like to thank Mr Andrew Fitzhardinge and Dr Margaret Janu, Biochemistry Department, Diagnostic Pathology Unit, Concord RG Hospital, for carrying out the blood biochemistry and Prof. Robin Fraser, Department of Pathology, Christchurch School of Medicine, University of Otago, Christchurch, New Zealand, for his continued guidance and inspiration.
Sources of support
This work was supported by the Australian National Health and Medical Research Council (Research Grant no. 352342) and the Ageing and Alzheimer’s Research Foundation.
Potential conflicts of interest
None.
References
- Aduen JF, Hellinger WC, Kramer DJ, et al. Spectrum of pneumonia in the current era of liver transplantation and its effect on survival. Mayo Clin. Proc. 2005;80:1303–1306. doi: 10.4065/80.10.1303. [DOI] [PubMed] [Google Scholar]
- Braet F, Muller M, Vekemans K, Wisse E, Le Couteur DG. Antimycin A-induced defenestration in rat hepatic sinusoidal endothelial cells. Hepatology. 2003;38:394–402. doi: 10.1053/jhep.2003.50347. [DOI] [PubMed] [Google Scholar]
- Britigan BE, Roeder TL, Rasmussen GT, Shasby DM, McCormick ML, Cox CD. Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells. Implications for Pseudomonas-associated tissue injury. J. Clin. Invest. 1992;90:2187–2196. doi: 10.1172/JCI116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan BE, Rasmussen GT, Cox CD. Augmentation of oxidant injury to human pulmonary epithelial cells by the Pseudomonas aeruginosa siderophore pyochelin. Infect. Immun. 1997;65:1071–1076. doi: 10.1128/iai.65.3.1071-1076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheluvappa R, Hilmer SN, Kwun SY, et al. The effect of old age on liver oxygenation and the hepatic expression of VEGF and VEGFR2. Exp. Gerontol. 2007a;42:1012–1019. doi: 10.1016/j.exger.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Cheluvappa R, Jamieson HA, Hilmer SN, Muller M, Le Couteur DG. The effect of Pseudomonas aeruginosa virulence factor, pyocyanin, on the liver sinusoidal endothelial cell. J. Gastroenterol. Hepatol. 2007b;22:1350–1351. doi: 10.1111/j.1440-1746.2007.05016.x. [DOI] [PubMed] [Google Scholar]
- Cogger VC, Mross PE, Hosie MJ, Ansselin AD, McLean AJ, Le Couteur DG. The effect of acute oxidative stress on the ultrastructure of the perfused rat liver. Pharmacol. Toxicol. 2001;89:306–311. doi: 10.1034/j.1600-0773.2001.d01-165.x. [DOI] [PubMed] [Google Scholar]
- Cogger VC, Muller M, Fraser R, McLean AJ, Khan J, Le Couteur DG. The effects of oxidative stress on the liver sieve. J. Hepatol. 2004;41:370–376. doi: 10.1016/j.jhep.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Cogger VC, Hilmer SN, Sullivan D, Muller M, Fraser R, Le Couteur DG. Hyperlipidemia and surfactants: the liver sieve is a link. Atherosclerosis. 2006;189:273–281. doi: 10.1016/j.atherosclerosis.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Dobbs BR, Rogers GW, Xing HY, Fraser R. Endotoxin-induced defenestration of the hepatic sinusoidal endothelium: a factor in the pathogenesis of cirrhosis? Liver. 1994;14:230–233. doi: 10.1111/j.1600-0676.1994.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Fetzer AE, Werner AS, Hagstrom JW. Pathologic features of pseudomonal pneumonia. Am. Rev. Respir. Dis. 1967;96:1121–1130. doi: 10.1164/arrd.1967.96.6.1121. [DOI] [PubMed] [Google Scholar]
- Fraser R, Dobbs BR, Rogers GW. Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21:863–874. [PubMed] [Google Scholar]
- Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652–1660. doi: 10.1002/hep.510270626. [DOI] [PubMed] [Google Scholar]
- Harman JW, Macbrinn MC. The effect of phenazine methosulphate, pyocyanine and EDTA on mitochondrial succinic dehydrogenase. Biochem. Pharmacol. 1963;12:1265–1278. doi: 10.1016/0006-2952(63)90194-1. [DOI] [PubMed] [Google Scholar]
- Harris HW, Gosnell JE, Kumwenda ZL. The lipemia of sepsis: triglyceride-rich lipoproteins as agents of innate immunity. J. Endotoxin Res. 2000;6:421–430. [PubMed] [Google Scholar]
- Hassan HM, Fridovich I. Mechanism of the antibiotic action of pyocyanine. J. Bacteriol. 1980;141:156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffelfinger SC, Hawkins HH, Barrish J, Taylor L, Darlington GJ. SK HEP-1: a human cell line of endothelial origin. In Vitro Cell. Dev. Biol. 1992;28A:136–142. doi: 10.1007/BF02631017. [DOI] [PubMed] [Google Scholar]
- Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42:1349–1354. doi: 10.1002/hep.20937. [DOI] [PubMed] [Google Scholar]
- Jamieson HA, Cogger VC, Twigg SM, et al. Alterations in liver sinusoidal endothelium in a baboon model of type 1 diabetes. Diabetologia. 2007;50:1969–1976. doi: 10.1007/s00125-007-0739-4. [DOI] [PubMed] [Google Scholar]
- Kamoun WS, Karaa A, Kresge N, Merkel SM, Korneszczuk K, Clemens MG. LPS inhibits endothelin-1-induced endothelial NOS activation in hepatic sinusoidal cells through a negative feedback involving caveolin-1. Hepatology. 2006;43:182–190. doi: 10.1002/hep.20940. [DOI] [PubMed] [Google Scholar]
- Knight M, Hartman PE, Hartman Z, Young VM. A new method of preparation of pyocyanin and demonstration of an unusual bacterial sensitivity. Anal. Biochem. 1979;95:19–23. doi: 10.1016/0003-2697(79)90179-9. [DOI] [PubMed] [Google Scholar]
- Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 2004;72:4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, Blaufox MD. Blood volume in the rat. J. Nucl. Med. 1985;26:72–76. [PubMed] [Google Scholar]
- Leidal KG, Munson KL, Denning GM. Small molecular weight secretory factors from Pseudomonas aeruginosa have opposite effects on IL-8 and RANTES expression by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001;25:186–195. doi: 10.1165/ajrcmb.25.2.4273. [DOI] [PubMed] [Google Scholar]
- Miller RA, Rasmussen GT, Cox CD, Britigan BE. Protease cleavage of iron-transferrin augments pyocyanin-mediated endothelial cell injury via promotion of hydroxyl radical formation. Infect. Immun. 1996;64:182–188. doi: 10.1128/iai.64.1.182-188.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlradt PF, Tsai H, Conradt P. Effects of pyocyanine, a blue pigment from Pseudomonas aeruginosa, on separate steps of T cell activation: interleukin 2 (IL 2) production, IL 2 receptor formation, proliferation and induction of cytolytic activity. Eur. J. Immunol. 1986;16:434–440. doi: 10.1002/eji.1830160421. [DOI] [PubMed] [Google Scholar]
- Muller M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Rad. Biol. Med. 2002;33:1527–1533. doi: 10.1016/s0891-5849(02)01087-0. [DOI] [PubMed] [Google Scholar]
- Muller M. Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic. Biol. Med. 2006;41:1670–1677. doi: 10.1016/j.freeradbiomed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Muller M, Sorrell TC. Leukotriene B4 omega-oxidation by human polymorphonuclear leukocytes is inhibited by pyocyanin, a phenazine derivative produced by Pseudomonas aeruginosa. Infect. Immun. 1992;60:2536–2540. doi: 10.1128/iai.60.6.2536-2540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PK, Krohn K, Muhlradt PF. Effects of pyocyanine, a phenazine dye from Pseudomonas aeruginosa, on oxidative burst and bacterial killing in human neutrophils. Infect. Immun. 1989;57:2591–2596. doi: 10.1128/iai.57.9.2591-2596.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopanitaya W, Grisham JW, Carson JL, Dotson MM. Surface features of cirrhotic liver. Virchows Arch. A Pathol. Anat. Histol. 1976;372:97–108. doi: 10.1007/BF00427085. [DOI] [PubMed] [Google Scholar]
- Ogi M, Yokomori H, Oda M, et al. Distribution and localization of caveolin-1 in sinusoidal cells in rat liver. Med. Electron. Microsc. 2003;36:33–40. doi: 10.1007/s007950300004. [DOI] [PubMed] [Google Scholar]
- Schaber JA, Triffo WJ, Suh SJ, et al. Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infect. Immun. 2007;75:3715–3721. doi: 10.1128/IAI.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto S, Kaido T, Yamaoka S, et al. Hepatocyte growth factor prevents lipopolysaccharide-induced hepatic sinusoidal endothelial cell injury and intrasinusoidal fibrin deposition in rats. J. Surg. Res. 1998;80:194–199. doi: 10.1006/jsre.1998.5472. [DOI] [PubMed] [Google Scholar]
- Singh N, Wagener MM, Obman A, Cacciarelli TV, de Vera ME, Gayowski T. Bacteremias in liver transplant recipients: shift toward gram-negative bacteria as predominant pathogens. Liver Transpl. 2004;10:844–849. doi: 10.1002/lt.20214. [DOI] [PubMed] [Google Scholar]
- Soave R, Murray HW, Litrenta MM. Bacterial invasion of pulmonary vessels. Pseudomonas bacteremia mimicking pulmonary thromboembolism with infarction. Am. J. Med. 1978;65:864–867. doi: 10.1016/0002-9343(78)90807-0. [DOI] [PubMed] [Google Scholar]
- Spitzer JJ, Bagby GJ, Meszaros K, Lang CH. Alterations in lipid and carbohydrate metabolism in sepsis. JPEN J. Parenter. Enteral. Nutr. 1988;12:53S–58S. doi: 10.1177/014860718801200604. [DOI] [PubMed] [Google Scholar]
- Sumitran-Holgersson S, Ge X, Karrar A, et al. A novel mechanism of liver allograft rejection facilitated by antibodies to liver sinusoidal endothelial cells. Hepatology. 2004;40:1211–1221. doi: 10.1002/hep.20434. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Sawasaki Y, Hata J, Mukai K, Goto T. Spontaneous transformation and immortalization of human endothelial cells. In Vitro Cell. Dev. Biol. 1990;26:265–274. doi: 10.1007/BF02624456. [DOI] [PubMed] [Google Scholar]
- Usher LR, Lawson RA, Geary I, et al. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J. Immunol. 2002;168:1861–1868. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- Valente E, Assis MC, Alvim IM, Pereira GM, Plotkowski MC. Pseudomonas aeruginosa induces apoptosis in human endothelial cells. Microb. Pathog. 2000;29:345–356. doi: 10.1006/mpat.2000.0400. [DOI] [PubMed] [Google Scholar]
- Wagener MM, Yu VL. Bacteremia in transplant recipients: a prospective study of demographics, etiologic agents, risk factors, and outcomes. Am. J. Infect. Control. 1992;20:239–247. doi: 10.1016/s0196-6553(05)80197-x. [DOI] [PubMed] [Google Scholar]
- Watson D, MacDermot J, Wilson R, Cole PJ, Taylor GW. Purification and structural analysis of pyocyanin and 1-hydroxyphenazine. Eur. J. Biochem. 1986;159:309–313. doi: 10.1111/j.1432-1033.1986.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Wilson R, Sykes DA, Watson D, Rutman A, Taylor GW, Cole PJ. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 1988;56:2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori H, Oda M, Ogi M, Kamegaya Y, Tsukada N, Ishii H. Endothelial nitric oxide synthase and caveolin-1 are co-localized in sinusoidal endothelial fenestrae. Liver. 2001;21:198–206. doi: 10.1034/j.1600-0676.2001.021003198.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Dong JH, Chen P, Yang SZ, Zhang YJ. Relationship between sinusoidal endothelial cell apoptosis and hepatocyte injury after their transplantation into rats. Zhonghua Gan Zang Bing Za Zhi. 2006;14:114–117. [PubMed] [Google Scholar]