Abstract
The neurotrophin, glial-derived neurotrophic factor (GDNF), is essential for the development of the enteric nervous system (ENS) in both the embryo and neonate and may be important for maintenance and plasticity of ENS. The tapeworm, Hymenolepis diminuta, altered the number of cells containing GNDF in the host’s jejunum and ileum. Numbers and locations of GDNF-containing cells were determined by applying monoclonal anti-GDNF antibody to intestinal segments collected from infected and uninfected age-matched rats during the initial 34 days post-infection (dpi). Most cells staining positive for GDNF were present in the lamina propria of the jejunum and ileum from both infected and uninfected rats. The co-localization of staining by the antibodies, anti-GDNF and anti-ED2 (a nuclear specific antibody for resident macrophages) indicated that at least 74% of the cells staining for GDNF were macrophages. Mast cells did not stain with the anti-GDNF antibody. The increased number of GDNF+ cells in the infected rat intestine suggests that this neurotrophin may play a role in the neural and mucosal responses to lumenal tapeworm infection.
Keywords: ED2, GDNF, macrophages, mast cells, tapeworm
Glial-derived neurotrophic factor (GDNF), a member of the transforming growth factor-β gene superfamily, has trophic effects on both dopaminergic (Lin et al. 1993) and motor neurons (Henderson et al. 1994). During neural development, the distribution of GDNF mRNA indicates that GDNF expression occurs in a number of developing cell types, i.e. skeletal muscle and neural tissues including the spinal cord, peripheral nerves, ventral and dorsal root (Henderson et al. 1994; Choi-Lundberg & Bohn 1995; Trupp et al. 1995). In the adult animal, GDNF aids in maintaining and repairing neurons (Oppenheim et al. 1995).
Analysis of GDNF Family Receptor α1-knockout mice demonstrated that GDNF signalling is involved in the development of enteric nervous system (ENS) (Widenfalk et al. 1997). Mice, deficient in GDNF, the receptor for GDNF (GFRα1) and the receptor-associated signalling subunit, Ret tyrosine kinase receptor for GDNF (RET), do not develop an ENS from the stomach to the rectum (Cacalano et al. 1998; Enomoto et al. 1998).
The GDNF is present in the mucosa and muscle layers of the rat intestine according to study by Peters et al. (1998), which suggested that GDNF may play a role in the maintenance of the adult ENS. Their data suggested that one source of GDNF might be the enteric smooth muscle (Peters et al. 1998). They hypothesized that the increase in GDNF that they observed as resulting from chemically induced smooth muscle hypertrophy may be necessary for the adaptive plasticity of enteric neurons of adult animals.
Enteric infections in animals and humans have a proven link between mucosal inflammation and gastrointestinal motor dysfunction caused by intestinal infections (Castro & Arntzen 1993). Helminth parasite infections (tapeworms and nematodes) can cause significant pathophysiological changes in the intestine including altered smooth muscle contraction, smooth muscle hypertrophy and mastocytosis (Palmer et al. 1984; Palmer & Castro 1986; Castro 1992; Dwinell et al. 1997, 1998).
These parasite infections can affect ENS by altering the distribution of nerves, neuronal functions and the levels of neurochemicals (Mckay & Fairweather 1997). Batchelor et al. (1999, 2000) showed that monocyte-derived cells, such as activated macrophages and microglia, express increased amounts of GDNF mRNA and GDNF after neural injury in the brain. A sprouting response of dopaminergic neurons is observed at the wound edge correlating with the close association of GDNF-containing macrophages and microglia. Within a wound, regenerating fibres from dopaminergic neurons grow towards and intimately surround wound macrophages expressing GDNF.
We hypothesized that chronically parasite infected, rat small intestine would show an alteration in the levels and distribution of GDNF. Using a rat model in which rats were chronically infected with the lumen dwelling tapeworm, Hymenolepis diminuta, we report an increase in macrophages containing GDNF in the small intestinal wall.
Materials and methods
Life cycle maintenance and rat infection
The tapeworm, H. diminuta, was maintained in the laboratory through alternating infection of male rats (150–250 g; Sprague Dawley; Harlan Inc., Madison, WI, USA) and laboratory raised grain beetles, Tenebrio molitor, as described by Dwinell et al. (1997). Cysticercoids were removed from the beetles, suspended in 0.85% NaCl solution, and administered orally (35 cysticercoids/rat) to halothane-anaesthetized rats.
Tissue preparation
Rats were killed with Beuthanasia® solution. Segments of cephalic jejunum (10 cm caudad to the ligament of Treitz) and ileum (20 cm orad to the ileocaecal junction) were removed and rinsed briefly with Krebs’ ringers-tris maleate buffer (pH 7.2) and then cut longitudinally. Tissue was placed serosal side down on #50 filter paper, and both tissue and the filter paper support were immersed in Bouin’s fixative for 3 h at room temperature (RT). After fixation, the tissues were rinsed and stored in 70% ethanol until subsequent alcohol dehydration and paraffin embedment. Sections of the paraffin embedded tissue were cut to 5 μm and placed on slides coated with poly-l-lysine.
Histochemistry
Granulated mast cells were identified according to Duffy et al. (1993) by staining with Astra blue 6GLL (Sigma-Aldrich Co., St Louis, MO, USA), and counter-stained with Mayer’s haematoxylin (Sigma-Aldrich Co.). Separate sections of each tissue were also stained with haematoxylin and eosin for morphological examinations.
Immunohistochemistry
Prior to staining, sections were deparaffinized with xylene and rehydrated through graded ethanol solution, and endogenous peroxide was inhibited by 0.3% solution H2O2 in 0.01 M phosphate-buffered saline (PBS). After each step in this procedure, the sections were rinsed in three changes of PBS. Non-specific protein binding was blocked for 30 min at RT with: (i) 1:50 (v/v) of dilution of normal serum from the same species providing the source of the secondary antibody for blocking secondary antibody nonspecific binding, and (ii) blocking buffer (SuperBlockTM. in Tris buffer Pierce, Rockford, IL, USA) for blocking primary antibody non-specific binding. Tissue sections were incubated with primary antibody at 4 °C for overnight (single staining) or for 72 h (double staining). The two murine-derived primary antibodies, used in these experiments, were: (i) a biotinylated monoclonal anti-human GDNF IgG1 monoclonal antibody (anti-GDNF Ab) (Promega Inc., Madison, WI, USA), and (ii) a monoclonal IgG, anti-rat macrophage antibody (anti-ED2 Ab) (Serotec Ltd, Kidlington, Oxford, UK). The anti-GDNF and anti-ED2 antibodies were used at 1:800–2000 and 1:400 respectively (see protocols below).
When unlabelled primary antibody was used, excess primary antibody was removed by multiple rinses of PBS before a biotinylated secondary antibody (biotin labeled horse anti-mouse IgG; Vector Laboratory, Burlingame, CA, USA) being applied to the tissue (diluted 1:500) for 30 min at RT. Bound primary antibody was localized at RT using a 30-min incubation with avidin–biotin peroxidase complex (ABC; Vectastain ABC-Kit; Vector Laboratory) followed by incubation with diaminobenzidine plus nickel (DAB-nickel). Alternatively, VIP peroxidase substrate (Vector ® VIP substrate kit for peroxidase, Vector Laboratory) was added on each section and incubated for 5–10 min at RT. All steps were carried out in moist chambers maintained at either RT or at 4 °C. After a final rinse in distilled water, the tissues were counterstained with Mayer’s haematoxylin, dehydrated and cover slipped.
Negative control tissues were incubated in PBS alone or PBS plus a 1:500 nonspecific mouse antibody (v/v) dilution (with a starting concentration of 2 μg/ml). This negative control was run following similar procedure described above, exception by the absence of GDNF or ED2. Specificity of GNDF immunostaining was tested by pre-adsorbing anti-GDNF antibody (1.25 μg/ml) with the recombinant protein rhGDNF (0.25 μg/ml; Promega Inc.) for 1 h at RT. Concentrations were chosen to maintain an equal molar ratio between rhGDNF (150 kDa) and the biotin-antiGDNF mAb (30 kDa). The mixture was centrifuged to remove antigen-mAb complexes and the supernatant was applied to sections to examine the specific blocking of GDNF immunostaining. No immunoreactivity was observed under these conditions.
Experimental groups
Experiment 1: To compare the size of anti-GDNF and anti-ED2 positive (macrophage) populations in normal and tapeworm infected small intestinal wall
Six experimental groups of male rats (n= 5 rats/group) were used: Four groups of rats infected with H. diminuta were killed sequentially at 6, 15, 22 and 34 days post-infection (dpi). Two groups of uninfected control rats, age matched to infected groups, were killed at 0 and 34 dpi to provide baseline cell counts for the beginning and ending points in the experimental period.
Serial single sections of both the jejunum and ileum, as defined in the Immunohistochemistry section above, were used to quantify the individual populations of GDNF containing cells (anti-GDNF+) and macrophages (anti-ED2+; Dijkstra et al. 1985a). Positively staining cells were microscopically identified and counted in the lamina propria of the villi and the crypts, submucosa, muscularis externa and serosa of the intestinal wall. A total of 60 villus-crypt units (VCU) were counted for each rat (n= 5 animals/group at each time point). For cell counting, the observer was unaware of the experimental conditions to avoid operator bias.
Experiment 2: To determine if anti-GDNF staining co-localizes within macrophages and/or mucosal mast cells
For this experiment, we used tissue sections of small intestine of eight animals (four uninfected and four infected rats at 34 dpi) which were stained with Astra blue and either one primary antibody per section (single serial staining) or with both antibodies per section (sequentially double staining) as explained below. A total of 40 villus-crypt units (VCU) for each experiment were counted per animal.
(a) Single antibody staining of adjacent serial sections: To confirm observations from double-stained sections indicating that GDNF co-localized with ED2 and not Astra blue, two adjacent serial sections were mounted on a single slide. Both sections were histochemically stained with Astra blue before the immunostaining procedures. One section on each slide was stained with anti-GDNF antibody while the other section was stained with anti-ED2 antibody. The substrate used on both sections was VIP. Cells staining positive were counted in each layer of both ileum and jejunum. Double staining of mast cells (Astra blue+) or macrophages (ED2+) by anti-GDNF was assessed by comparing the staining of a single cell as it appeared in adjacent but separately stained serial sections.
(b) Two antibody staining of a single section: Two adjacent serial sections were mounted on each slide and both were stained first with Astra blue, then one was stained with anti-GDNF only and another one was double-stained with anti-GDNF and anti-ED2 antibodies. Both serial sections were initially incubated with biotinylated anti-GDNF at 4 °C for 3 days at a dilution of 1:2000. After rinsing with PBS, ABC complex was added for 30 min at RT, rinsed and developed with the VIP chromogen (purple colour). After appropriate rinsing and blocking of non-specific binding, one section was then incubated overnight with the anti-ED2 antibody (1:400) at 4 °C. Following the anti-ED2 antibody incubation, the sections were rinsed and incubated with biotinylated secondary antibody for 30 min at RT. After the secondary antibody incubation, ABC solution and DAB + nickel chromogen (black colour) were added, as described in the Immunohistochemistry section. Cells stained with anti-GDNF and VIP developed a red colour in the cytoplasm, while cells stained with ED2 and VIP plus nickel developed a black colour at the membrane and the nucleus envelope (ED2). Populations of cells displaying GDNF positivity only, cells staining positive for both GDNF + ED2 and cells positive for GDNF and Astra blue were counted.
Statistical analysis
Numbers of GDNF+ and ED2+ stained cells were analysed using one-way of variance (anova). The data were transformed by log (x + 1). The Tukey test was used for comparison of means. Values with a P < 0.05 were considered statistically significant.
Results
Within the intestinal wall of both tapeworm infected (Figure 1d) and uninfected rats (not shown), anti-GDNF and ED2 antibodies bound almost exclusively within cells located in the lamina proprial of the mucosa layer. In addition, we confirmed the earlier works that mast cells (Astra blue stained cells) were primarily found in the same region (Dwinell et al. 1998; Starke & Oaks 1999).
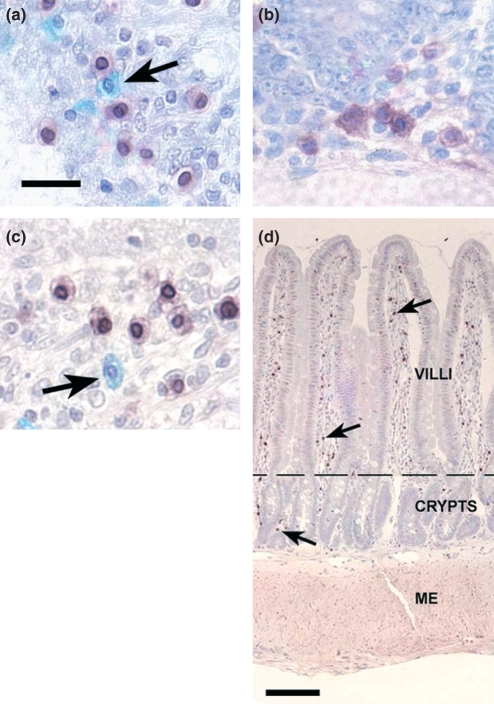
Figure 1.
Distribution of GDNF+ cells, macrophages and mast cells identified in the chronically tapeworm infected small intestine. Images shown are representative of tissue samples taken from both cephalic jejunum and ileum. (a) Anti-ED2 antibody identification of macrophages in the lamina propria. Antibody apparently binds most strongly to the nuclear envelope. Mast cells (arrow) do not bind anti-ED2 antibody. (b) Anti-GDNF antibody binds to cytoplasmic granules in the cytoplasm of cells located in the lamina propria. (c) Colocalization of anti-GDNF (brown cytoplasmic staining) and anti-ED2 (dark nuclear envelope) antibodies indicates that GDNF is present in macrophages and not mast cells (arrow). (d) Photomicrograph of the small intestine wall, taken from the ileal region, show GDNF+ macrophages in the lamina propria in the villi and surrounding the crypts of Lieberkühn. GDNF+ macrophages are indicated by the brown to black spots, a few of which are indicated by the black arrows. The distribution of the GDNF+ macrophages is relatively higher in the lamina propria than in other layers of the intestinal wall: submucosa, muscularis externa (ME) and serosa. The dotted line delimits the lamina propria of the villi from that of the crypts. (a–c) Bar = 20 μm. (d) Bar = 0.11 mm.
Most GDNF antibody binding occurred in the cytoplasm of large round to polygonal cells with oval nuclei (Figure 1b); however, another subpopulation of GDNF+ cells with lobular nuclei, reminiscent of granulocyte nuclear morphology, was also present (not shown). In both these cell populations, GDNF staining was restricted to small particles in the cytoplasm suggesting that GDNF was contained in cytoplasmic granules or vesicles. In contrast to the GDNF antibody, ED2 antibody intensely stained the nuclear envelope (Figure 1a). In addition, cells stained with ED2 showed some barely detectable staining of cytoplasm.
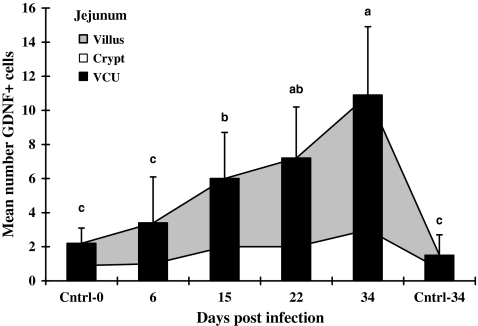
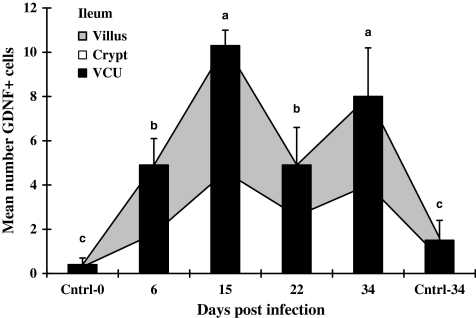
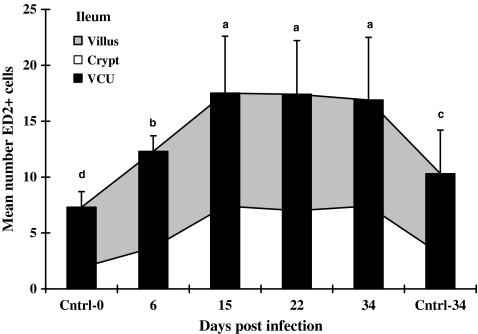
In the infected jejunum (VCU in Figure 2), the means of GDNF+ cells increased progressively through the last sampling day at 34 dpi, while in the ileal mucosa (VCU in Figure 3), the total number of GDNF+ cells rose rapidly, reaching a peak on 15 dpi. Infection caused GDNF+ cells in the jejunum to rise significantly to approximately five- to sevenfold higher levels on 34 dpi than in control tissues (VCU in Figure 2). In contrast, the GDNF+ cell population in the infected ileum did not continue to rise after 15 dpi, 15 dpi values were 7–25 fold above controls collected at 0 or 34 days, and remained significantly above control values from 15 dpi to the end of the experiment (VCU in Figure 3).
Figure 2.
Mean numbers of GDNF-positive cells in jejunal mucosa of rats. Infected jejunal tissues were collected from rats infected with Hymenolepis diminuta on days 6, 15, 22 and 34 post-infection (dpi) and uninfected age-matched rats on 0 and 34 dpi (n= 60 VCU counted for each of five rats). Bars = standard deviation of mean. Letters above columns indicate groups of statistical significances in relation to control (P< 0.05).
Figure 3.
Mean numbers of GDNF-positive cells in ileal mucosa of rats. Infected ileal tissues were collected from rats infected with Hymenolepis diminuta on days 6, 15, 22 and 34 post-infection (dpi) and uninfected age-matched rats on 0 and 34 dpi (n= 60 VCU counted for each of five rats). Bars = standard deviation of mean. Letters above columns indicate groups of statistical significances in relation to control (P< 0.05).
GDNF+ cells were present in the small intestinal lamina propria of both infected and uninfected rats on all days examined (Figures 2 and 3). In the mucosa of the jejunum and the ileum, the numbers of GDNF+ cells in the infected mucosa were significantly greater than either age-matched controls collected on 0 or 34 day of the experiment.
As some cells associated with the response to intestinal parasites appear to enter and mature in the lamina propria of the crypt region before migrating into the villus (Friend et al. 1996), we examined the ratio of GDNF+ cells in the crypt vs. the villus over the development of infection. When the villus and crypt regions from infected jejunum were compared, the numbers of GDNF+ cells in villi at each time point of infection were 2.0–2.6 fold higher than in the crypt (Figure 2). In the ileum, however, when the numbers of GDNF+ cells in the crypts were compared with those in the villi on any day of infection, the numbers of these cells in these two regions were not significantly different from one another (Figure 3).
To identify potential regional differences in GDNF+ cell populations, the number of GDNF+ cells in each layer (i.e. villus, crypt, VCU and submucosa) of the jejunum was compared with the similar layer in the ileum. No statistical differences were found at all times tested (Figures 2 and 3). Few GDNF+ cells were seen in the submucosa compared to the lamina propria of either section of the intestine. Essentially, no positive cells were observed in the muscularis externa or serosa of either section of infected (Figure 1) or uninfected (not shown) small intestine.
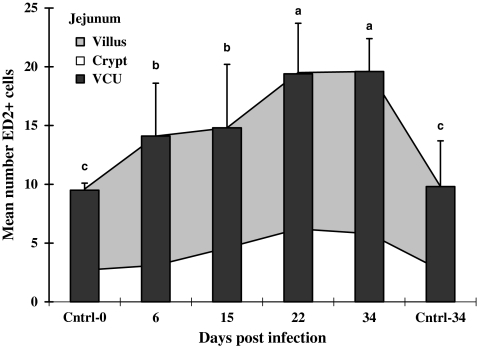
The numbers of ED2+ cells in lamina propria (VCU) of both the jejunum and ileum from infected animals were consistently greater than controls throughout the sampling period (Figures 4 and 5). While in the infected jejunum the total numbers of ED2+ cells in the lamina propria increased progressively through 22 dpi (VCU; Figure 4), the same ED2+ cell population in the ileum peaked earlier, i.e., on 15 dpi (Figure 5). Kinetically similar increases in ED2+ cells occurred individually in both villus and crypt regions for both ileum and jejunum, although the numbers of cells were higher in villus than in crypt regions (Figures 4 and 5).
Figure 4.
Mean numbers of ED2-positive cells in jejunal mucosa of rats. Infected jejunal tissues were collected from rats infected with Hymenolepis diminuta on days 6, 15, 22 and 34 post-infection (dpi) and uninfected age-matched rats on 0 and 34 dpi (n= 60 VCU counted for each of five rats). Bars = standard deviation of mean. Letters above columns indicate groups of statistical significances in relation to control (P< 0.05).
Figure 5.
Mean numbers of ED2-positive cells in ileal mucosa of rats. Infected ileal tissues were collected from rats infected with Hymenolepis diminuta on days 6, 15, 22 and 34 post-infection (dpi) and uninfected age-matched rats on 0 and 34 dpi (n= 60 VCU counted for each of five rats). Bars = standard deviation of mean. Letters above columns indicate groups of statistical significances in relation to control (P< 0.05).
Within either region of the small intestine, the mean number of GNDF+ cells, macrophages (ED2+) and mast cells present in the 0 dpi control intestine were not different from one another (Experiment 2a). When the numbers of cells at 34 dpi in the ileum individually displaying these three markers were compared with those at 34 dpi in the jejunum, no significant differences were found between stained cell numbers, even though the means of all of these cell populations tended increase between 0 and 34 days of infection. Therefore, populations of mast cells or macrophages could not be associated with GDNF content by similar-sized populations. In addition to similarity of population numbers, the distribution within the intestinal wall and the morphology of all three cell types were similar.
To clarify the relationship of these stained cell populations, all three stains were applied to single sections to observe if any of the stains co-localized in single cells (Experiment 2b; Table 1). Single sections from both jejunum and ileum from uninfected (0 dpi) and infected (34 dpi) rats were stained with Astra blue and then double-stained with antibody probes (Figure 1c). Mean counts of co-localized staining by GDNF and ED2 antibodies ranged from 73–74% in the jejunum to 72–78% in the ileum. When counts from all infected and uninfected intestinal segments were pooled, a mean of 74% of all GDNF+ cells were also stained with ED2 (Table 1). These data of the co-localization of these two antibodies indicate that a majority of the cells containing GDNF are macrophages. The morphology of these GDNF+/ED2− cells is similar to that of macrophages, i.e. round to polygonal cell boarders and round to oval nuclei. All cells observed as containing both antigens displayed round to oval nuclei and did not include the few GDNF positive cells that presented lobulated nuclei. Neither GDNF nor ED2 antigen were found localized in Astra blue stained mast cells (Figure 1c at arrow), indicating that this second population of GDNF+ cells does not include mast cells.
Table 1.
Comparisons of single staining and double staining in VCU from jejunum or ileum of control uninfected or tapeworm infected rats
| Mean numbers of cells in VCU (mean ± SD) and percentage | |||||||
|---|---|---|---|---|---|---|---|
| Days post-infection (dpi) | Small intestinal region | Double-stained positive cells for both GDNF & ED2 | % | Double-stained positive cells for GDNF, but not ED2 | % | Total | Single stained positive cells for GDNF, but not ED2 (i.e., in the adjacent section) |
| 0 (uninfected) | Jejunum | 6.7 ± 3.1 | 72.8 | 2.5 ± 1.4 | 27.2 | 9.2 | 8.7 ± 1.7 |
| 34 | Jejunum | 11.0 ± 3.7 | 73.8 | 3.9 ± 2.1 | 26.2 | 14.9 | 13.6 ± 3.9 |
| 0 (uninfected) | Ileum | 6.6 ± 2.5 | 77.6 | 1.9 ± 1.1 | 22.4 | 8.5 | 7.0 ± 2.5 |
| 34 | Ileum | 8.1 ± 1.4 | 71.6 | 3.2 ± 1.4 | 28.3 | 11.3 | 10.1 ± 2.8 |
| Mean and Percentage | 8.1 | 74.3 | 2.9 | 26.6 | 10.9 | 9.85 | |
Results are from single slides with two adjacent serial sections stained simultaneously with anti GDNF antibody and then one section subsequently stained with anti ED2 antibody. See Materials and methods section: Experiment 2b for detail.
SD, standard deviation; VCU = Villus-Crypt Unit = total lamina propria; number of villus-crypt units counted = 40/animal.
Discussion
Unlike many parasites, Hymenolepis is a strictly lumenal parasite of the rat’s intestine. This parasite slows the propulsion of lumenal substances and causes smooth muscle hyperplasia, as well as a mastocytosis and eosinophilic enteritis of the lamina propria without penetrating the mucosal epithelium (Dwinell et al. 1998; Starke & Oaks 1999). In addition, the chronic presence of this tapeworm causes alterations in the ENS (Mckay & Fairweather 1997).
As observed by Lin et al. (1993), Henderson et al. (1994) and Ribchester et al. (1998), the trophic effects of GDNF can be involved in changes in the ENS. Batchelor et al. (1999) found that macrophages expressed increased amounts of GDNF mRNA in relation to dopaminergic sprouting after striatal injury in the brain. Dopaminergic fibres were seen growing towards and intimately surrounding wound macrophages which, together with microglia, were expressing GDNF mRNA (Batchelor et al. 2000). On finding that GDNF levels increased during chemically induced small intestinal smooth muscle hyperplasia, Peters et al. (1998) suggested that GDNF was involved in the modification of the ENS necessary to accommodate new muscle. GDNF’s involvement in neuroprotection and neural restoration (Gash et al. 1996) and neuronal survival and remodelling function in adult mammals (Tomac et al. 1995; Bar et al. 1997) is consistent with the increased numbers of GDNF containing macrophages, and the smooth muscle and ENS plasticity that occurs during the initial 4 weeks of tapeworm infection in the small intestine.
The kinetics of the increasing GDNF+ cells differed between these small intestinal regions. GDNF+ cells peaked higher and later in the jejunum than in the ileum. This is consistent with the observations of Dwinell et al. (1998), who noted that during the establishment of tapeworm infection in the rat, there is an initiation of thickening of the muscularis externa by 15 dpi. This thickening of the muscularis externa is apparently greater in the ileum than in the jejunum. The thickening was caused by a hypertrophy of individual smooth muscle cells in this layer. We speculate that the separate peaks of GDNF+ cells in these individual regions of the small intestine may reflect the individual thresholds required to stabilize the evolving neural function necessary for the hypertrophying muscle.
Our Astra blue histochemistry and double immunostaining studies revealed that GDNF reactivity was primarily associated with the increasing macrophage population, but not the mast cells, of the jejunal and ileal mucosa of both uninfected and tapeworm infected rats. Because of the very low intensity and diffuseness of the cytoplasmic staining by ED2 antibody and the documented recognition by this antibody of cytoplasmic and surface membrane antigens of most tissue macrophages (Dijkstra et al. 1985a,b; Kawashima et al. 2003), the clear perinuclear staining served as our marker of ED2 positive (ED2+) cells for identification, localization and census of macrophages.
However, of all the cells staining with GDNF, only 74% of them also had co-localization of ED2. As 26% were stained with GDNF only, these data and our observation of a GDNF+ cell with a lobulated nucleus indicate that there are at least two other cell types in the wall of the small intestine expressing intracytoplasmatic GDNF antigens, other than macrophages. The identity of these other cells types containing GDNF remains unknown.
Our demonstration that GDNF is present in ED2+ rat macrophages is the first observation of this neurotrophin in macrophages of the intestine. As normal uninfected intestinal tissue contains a small population of GDNF+ macrophages, we speculate that the GDNF upregulation, which we observed during tapeworm infection, may not be a specific response to the presence of the intestinal parasite, but a normal consequence of conditions stimulating inflammation in this organ and other organs, such as observed during interstitial cystitis (Okragly et al. 1999). The normal low level of macrophages containing GDNF may be a result of the enteric bacterial flora and other inflammatory stimulants present in the animal’s diet.
In summary, the observations presented here show that GDNF is involved in the response to parasitic infections of the intestine. The functional role for GDNF in these pathologies is uncertain, but suggests that a segment of the response to enteric parasitism is neurologically based.
Acknowledgments
This work was supported by a grant from the NIH-USA.
References
- Bar KJ, Facer P, Williams NS, Tam PKH, Anand P. Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprungs disease. Gastroenterology. 1997;112:1381–1385. doi: 10.1016/s0016-5085(97)70154-9. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JYF, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell-derived neurotrophic factor. J. Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Porritt MJ, Donnan GA, Howells DW. Inhibition of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression reduces dopaminergic sprouting in the injured striatum. Eur. J. Neurosci. 2000;12:3462–3468. doi: 10.1046/j.1460-9568.2000.00239.x. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang I, et al. GDFRα1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro GA. Intestinal physiology in the parasitized host: integration, disintegration, and reconstruction of systems. Ann. N. Y. Acad. Sci. 1992;664:369–379. doi: 10.1111/j.1749-6632.1992.tb39775.x. [DOI] [PubMed] [Google Scholar]
- Castro GA, Arntzen CJ. Immunophysiology of the gut: a research frontier for integrative studies of the common mucosal immune system. Review. Am. J. Physiol. Gastrointest. Liver Physiol. 1993;265:G599–G610. doi: 10.1152/ajpgi.1993.265.4.G599. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial-cell line-derived neurotrophic factor (GDNF) messenger RNA in rat. Dev. Brain Res. 1995;85:80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. Adv. Exp. Med. Biol. 1985a;186:409–419. doi: 10.1007/978-1-4613-2463-8_50. [DOI] [PubMed] [Google Scholar]
- Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985b;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- Duffy JP, Smith PJ, Crocker J, et al. Combined staining method for demonstration of tissue eosinophils and mast cells. J. Histotechnol. 1993;16:143–144. [Google Scholar]
- Dwinell MB, Bass P, Schaefer DM, Oaks JA. Diminished intestinal transit and decreased aerobic bacterial populations in tapeworm infected rats. Am. J. Physiol. Gastrointest. Liver Physiol. 1997;373:G480–G485. doi: 10.1152/ajpgi.1997.273.2.G480. [DOI] [PubMed] [Google Scholar]
- Dwinell MB, Bass P, Wise RM, Oaks JA. Mucosal mastocytosis and intestinal smooth muscle hypertrophy in tapeworm infected rats. Exp. Parasitol. 1998;89:92–102. doi: 10.1006/expr.1998.4271. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, et al. GFRα1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Friend DS, Ghildyal N, Austen N, Gurish MF, Matsumoto R. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J. Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, et al. Functional recovery in parkinsonian monkey treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, et al. GDNF: a potent survival factor for motor neurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Kawashima M, Ide M, Nakanishi M, Kuwamura M, Kumagai D, Yamate J. Immunophenotypical changes of neoplastic cells and tumor-associated macrophages in a rat dendritic cell sarcoma-derived transplantable tumor line (KB-D8) Virchows Arch. 2003;442:141–150. doi: 10.1007/s00428-002-0716-8. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Becktesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Mckay DM, Fairweather I. A role for the enteric nervous system in the response to helminth infections. Parasitol. Today. 1997;13:63–69. doi: 10.1016/s0169-4758(96)10079-x. [DOI] [PubMed] [Google Scholar]
- Okragly AJ, Niles AL, Saban R, et al. Elevated tryptase, nerve growth factor,neurotropin-3 and glial cell derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J. Urol. 1999;161:438–441. [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Johnson JE, et al. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Palmer JM, Castro GA. Anamnestic stimulus-specific myoelectric responses associated with intestinal immunity in the rat. Am. J. Physiol. 1986;250:G266–G273. doi: 10.1152/ajpgi.1986.250.2.G266. [DOI] [PubMed] [Google Scholar]
- Palmer JM, Weisbrodt NW, Castro GA. Trichinella spiralis: intestinal myoelectric activity during enteric infection in the rat. Exp. Parasitol. 1984;57:132–141. doi: 10.1016/0014-4894(84)90072-9. [DOI] [PubMed] [Google Scholar]
- Peters RJ, Osinski MA, Hongo JA, et al. GDNF is abundant in the adult rat gut. J. Auton. Nerv. Syst. 1998;70:115–122. doi: 10.1016/s0165-1838(98)00044-7. [DOI] [PubMed] [Google Scholar]
- Ribchester RR, Thomson D, Haddow LJ, Ushkariov YA. Enhancement of spontaneous transmitter release at neonatal mouse neuromuscular junctions by the glial cell line-derived neurotrophic factor (GDNF) J. Physiol. 1998;512:635–641. doi: 10.1111/j.1469-7793.1998.635bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke WA, Oaks JA. Hymenolepis diminuta: praziquantel removal of adult tapeworm is followed by apoptotic down-regulation of mucosal mastocytosis. Exp. Parasitol. 1999;92:171–181. doi: 10.1006/expr.1999.4409. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, et al. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J. Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L. Neurturin and glial cell line-derived neurotrophic factor receptor-b (GDNFR-b), novel proteins related to GDNF and GDNFR-a with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J. Neurosci. 1997;17:8506–8519. doi: 10.1523/JNEUROSCI.17-21-08506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]