Abstract
Matrix metalloproteinases (MMPs) are a family of 23 extracellular proteases that are best known for their collective ability to degrade all components of the extracellular matrix. We previously demonstrated that genetic ablation of MMP-7 reduced tumour multiplicity in multiple intestinal neoplasia (Min) mice possessing a genetic alteration in the adenomatous polyposis coli gene (APC). These mice, commonly referred to as APC-Min mice, are a frequently used model of early intestinal tumourigenesis. To examine further the role of MMPs in intestinal tumour development, we generated APC-Min mice genetically deficient in MMP-2, -9, -12 or -19. Genetic ablation of MMP-2, -12 or -19 did not affect multiplicity or size of intestinal tumours when crossed into the APC-Min system. However, MMP-9 deficient animals developed 40% fewer tumours than littermate controls, although tumour size distribution remained unaffected. Intestinal adenomas from MMP-9 deficient mice demonstrated a 50% decrease in proliferating cells compared with control tissues, with no difference in apoptosis. To determine the cellular origin of MMP-9 in these tumours, immunofluorescent co-staining with markers for different leucocyte lineages was used to demonstrate that intratumoural MMP-9 is largely a product of neutrophils. These studies extend the potential targets for chemoprevention of intestinal adenomas to MMP-9 in addition to MMP-7 and exclude MMP-2,-12,-19 as attractive targets for intervention.
Keywords: APC, cancer, colorectal, Min, MMPs, tumourigenesis
Introduction
MMPs are a family of 23 enzymes best known for their ability to cleave virtually all components of the extracellular matrix (Egeblad & Werb 2002). For reasons of the ability of MMP-2 and MMP-9 to degrade the basement membrane, they are frequently associated with tumour invasion and metastasis (Egeblad & Werb 2002). However, the roles for MMPs have been identified virtually at every stage of tumour development (Chambers & Matrisian 1997) and recent evidence suggests that certain MMPs may function in a protective role at certain stages of tumour development (Jacobs et al. 2007). Furthermore, in recent years, a number of non-matrix substrates have been identified for MMPs including growth factors, chemokines, cytokines, angiogenic factors and pro-apoptotic regulators (Noel et al. 2008). In light of this, rather than simply functioning in pro-metastatic capacity during later stages of tumour progression, MMPs are now known to be involved in both pro- and anti-neoplastic roles throughout all stages of tumour development.
Several MMP family members have been shown to be differentially expressed in both human colorectal tumours and tumours from the APC-Min mouse (multiple intestinal neoplasia), a commonly used model organism for studying intestinal neoplasia (Wagenaar-Miller et al. 2004; Martinez et al. 2005; Moser et al. 1990). Because of a missense mutation in the tumour suppressor gene APC (Su et al. 1992), 100% of mice heterozygous for the APCMin allele develop numerous spontaneous tumours throughout the small and large intestine (Moser et al. 1990). Aetiologically, genetic alterations in the human orthologue of APC have been shown to be a causative agent of both sporadic adenomatous polyposis (Nishisho et al. 1991) and familial adenomatous polyposis (FAP) (Kinzler et al. 1991) human colorectal tumours.
A number of genetic modifiers of tumourigenesis in the APC-Min system have been identified which include both coding (Dietrich et al. 1993) and non-coding (Kwong et al. 2007) regions. Previously, our lab has shown that genetic ablation of MMP-7, a secreted endopeptidase produced by adenomatous epithelial cells, reduces tumour multiplicity in the APC-Min system by >50% suggesting a pro-tumourigenic role for MMP-7 (Wilson et al. 1997). Human colonic tumours commonly express several matrix metalloproteinases (MMPs) not detected in normal colonic tissue that are predictors of both positive and negative clinical response and survival (Wagenaar-Miller et al. 2004).
We chose the APC-Min model of early tumour development to investigate the potential functions of four different MMPs expressed by both stromal and tumour cells in the APC-Min system. MMP-2, MMP-9 and MMP-12 were previously reported to be overexpressed by intestinal polyps of the APC-Min mouse (Martinez et al. 2005). In contrast, MMP-19 is produced by normal intestinal epithelium, but expression is lost upon transformation (Bister et al. 2004).
MMP-2 (common name ‘gelatinase A’) is expressed by various stromal cell populations in both human (Poulsom et al. 1992) and murine (Wilson et al. 1997) intestinal polyps. Normally, in the APC-Min mouse model, only a subset of tumours express MMP-2. However, upon genetic ablation of MMP-7, all tumours that do form express MMP-2 suggesting that in the absence of MMP-7, MMP-2 may function in a compensatory role in promoting tumourigenesis (Wilson et al. 1997). MMP-2 has been shown to contribute to tumour growth in several experimental systems (Itoh et al. 1997; Bergers et al. 2000) making it an attractive target for further study.
Macrophage metalloelastase, MMP-12, is largely expressed by macrophages, although it can be expressed by tumour epithelial cells (Lavigne & Eppihimer 2005). An elevated level of macrophage derived MMP-12 is commonly associated with an improved prognosis in intestinal neoplasia (Zucker & Vacirca 2004; Asano et al. 2007), although the mechanism behind this protective effect is obscure.
MMP-19, also known as RASI-1, was first identified as an autoantigen associated with rheumatoid arthritis (Sedlacek et al. 1998) and is expressed by a wide spectrum of tissues (Pendas et al. 1997). In the context of intestine, it has been detected on the surface of activated peripheral blood mononuclear cells, TH1 lymphocytes and is normally expressed throughout the intestine in enterocytes, stromal fibroblasts and macrophages (Mueller et al. 2000). Unlike most MMPs, expression of MMP-19 is decreased upon transformation of intestinal epithelial cells (Bister et al. 2004).
Finally, MMP-9 (commonly known as gelatinase B) is produced by several stromal cell populations including neutrophils, mast cells, macrophages, fibroblasts, as well as by the tumour epithelial cells directly (Noel et al. 2008). Previous studies using MMP-9-null mice in a model of squamous cell carcinoma have shown that MMP-9 deficient animals develop fewer tumours than do littermate controls (Coussens et al. 2000). Additionally, in a correlative study examining human colorectal specimens, an increasing abundance of MMP-9 was found to correlate with the progression of normal intestinal mucosa to dysplastic adenomas and eventual invasive carcinomas (Herszenyi et al. 2008). Taken together, these observations suggest that MMP-9 contributes to adenoma progression in a pro-tumourigenic fashion.
We pursued a genetic approach to determining the contribution of MMP-2, -9, -12, and -19 to the formation and growth of intestinal neoplasias in the APC-Min model system.
Materials and methods
Animal housing, diet and genotyping
APC-Min mice (C57Bl/6/J-APCMin/+) and wild type littermates (C57Bl/6J) were bred in our laboratory from breeders obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and genotyped for the APC-Min mutation by PCR as recommended by the supplier. Mice were housed with littermates in microisolator cages lined with CareFresh bedding (Absorption Corp., Ferndale, WA, USA).
Mice lacking MMP-2 (Itoh et al. 1997) were the generous gift of Dr T. Itoh. MMP-12 deficient mice (Shipley et al. 1996) were purchased from The Jackson Laboratory. MMP-9 null mice (Vu et al. 1998) were the generous gift of Dr Z. Werb. MMP-19 knockout mice (Pendas et al. 2004) were obtained from Dr J. Caterina. All experimental mice were bred at Vanderbilt University and genotyped by PCR using previously described protocols.
Male APC-Min mice were bred to female MMP-2−/− mice and ensuing heterozygotes were crossed to generate APC-Min-MMP2−/− mice (C57Bl/6/J-APCMin/+; MMP2−/−) and heterozygous and homozygous control littermates, with the Min allele carried along the paternal lineage. A similar breeding strategy was used to produce Min-MMP9−/− (C57Bl/6/J-APCMin/+; MMP9−/−), Min-MMP12−/− (C57Bl/6/J-APCMin/+; MMP12−/−) and Min-MMP19−/− (C57Bl/6/J-APCMin/+; MMP19−/−) mice. All mouse experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee.
Assessment of tumour multiplicity and size
Mice were maintained on a Purina 5015 chow, an 11% fat diet that has been shown to enhance tumour formation in the APC-Min model system (Wasan et al. 1997). At 17 weeks of age, mice were asphyxiated with CO2 and their intestines harvested, fixed overnight in 4% w/w formaldehyde diluted in PBS (Fisher Scientific, Pittsburgh, PA, USA) and stored in 70% ethanol. Tumour number and size were measured using a binocular dissecting microscope by two independent investigators blinded to the genotype of the sample. Tumour multiplicity was compared using the Mann–Whitney U-test. Tumour size was determined by measuring along the longest diameter using digital calipers to the nearest 100 μm. Tumour diameter measurements were log-transformed and analysed by a mixed models analysis of variance with an autoregressive correlation structure. For these studies, only tumours macroscopically visible (at minimum 0.2 mm in diameter) were included in these analyses.
Histology and Immunohistochemistry
Immunohistochemistry and quantification
Paraffin embedded, formalin-fixed sections were dewaxed and rehydrated through a series of graded alcohols. Sections were treated for 30 min with 0.6% hydrogen peroxide in methanol to destroy endogenous peroxidase prior to antigen retrieval. Antigen was retrieved by microwaving sections for 10 min in 10 mM sodium citrate buffer. Non-specific binding was inhibited by incubation in a blocking solution (10 mM Tris-HCl pH7.4, 0.1M MgCl2, 0.5% Tween20, 1% BSA, 5% Serum) for 1hr at room temperature. Rabbit polyclonal anti-mouse MMP-9 (Abcam, Cambridge, MA, USA) was diluted 1:100 in blocking solution and applied at 4 °C overnight. Appropriate IgG controls were used on adjacent sections to evaluate background staining. Sections were washed with TBS (150 mM NaCl, 10 mM Tris) and incubated with appropriate biotinylated secondary antibody for 1hr at room temperature. Positive cells were visualized with an avidin-biotin peroxidase complex (Vectastain Avidin-Biotin Complex kit, Vector Laboratories, Burlingame, CA, USA) and 3,3′-diaminobenzidine tetrahydrochloride substrate (Sigma). Nuclei were counterstained with Mayer’s haematoxylin (Sigma), washed in TBS, dehydrated through alcohols, cleared in xylenes and mounted.
Proliferation and apoptosis were assessed by immunohistochemistry for phospho-Histone-H3(Ser10) (1:250 dilution; Millipore, Billerica, MA, USA) and cleaved caspase-3 (1:500 dilution; Cell Signalling Technology, Danvers, MA, USA) respectively. Phospho-Histone H3 positive cells were counted from two adjacent sections from multiple tumours of 4 each Min-MMP-9−/− and Min-MMP-9+/+ littermates. A proliferative index was calculated by determining the ratio of positive cells per constant arbitrary unit of area as determined by Metamorph software (Molecular Devices, Sunnyvale, CA, USA). Apoptotic index was similarly calculated by quantifying cells positive for cleaved caspase-3 immunohistochemistry. Results are reported as mean ± standard deviation (SD).
Immunofluorescence
Five micron, paraffin embedded, formalin fixed sections were dewaxed and rehydrated through alcohols. Sections were microwaved in a 10 mM sodium citrate solution to retrieve antigen for 10 min and allowed to cool. Non-specific staining was prevented by treating sections with a blocking solution for 1 h at room temperature. Sections were simultaneously treated with a rabbit polyclonal antibody to detect MMP-9 and an antibody to detect leucocytes for 6 h at room temperature. Neutrophils were stained using a monoclonal rat anti-neutrophil antibody (AbD Serotec, Oxford, UK) diluted at 1:100 in blocking solution. B cells were visualized using a 1:100 dilution of a rat monoclonal antibody recognizing CD45R/B220 (AbD Serotec). Macrophages were demonstrated by staining for F4/80 antigen using a rat monoclonal antibody (AbD Serotec) diluted at 1:100 in blocking solution. Sections were washed in PBS and incubated with AlexaFluor 594 goat anti-rat (A-11007) or AlexaFluor 488 goat anti-rabbit (A-11008) fluorescently labelled secondary antibodies (Molecular Probes, Carlsbad, CA, USA) and DAPI to visualize nuclei then mounted in aqueous mounting media (Biømeda, Foster City, CA, USA).
Results
MMP-9 ablation reduces intestinal adenoma multiplicity in the APC-Min system
To examine the role of various stromal MMPs in intestinal tumour development, we generated Min-MMP-2−/−, Min-MMP-9−/−, Min-MMP-12−/− and Min-MMP-19−/− mice and corresponding littermate controls. Mice were raised on an 11% fat diet to enhance tumourigenesis (Wasan et al. 1997). Genetic ablation of MMP-2 is known to retard the growth rate of young mice (Mosig et al. 2007), and mice lacking MMP-19 have been shown to be susceptible to diet induced obesity (Pendas et al. 2004). However, at the 17-week time point chosen for this study, animals of both lineages were of normal size. Additionally, all lineages of knockout animals examined herein had no obvious morphological, behavioural or pathological differences when compared with wild-type Min littermates. Mice lacking MMP-9, but not those lacking MMP-2, -12 or -19 developed significantly fewer intestinal adenomas than littermate controls (Figure 1a–d; Table 1). On average, Min-MMP-9−/− developed 24.5 ± 16.1 tumours while wild type littermates developed 41.9 ± 27.6 tumours, indicative of a 40% decrease in tumour multiplicity when MMP-9 is absent (P < 0.05). Min-MMP-9+/− and Min-MMP-9+/+ mice developed a similar number of tumours and comparison of Min-MMP-9−/− (n = 21) and Min-MMP-9+/+ and+/− mice (n = 26) resulted in a similar reduction in tumour multiplicity (P < 0.005).
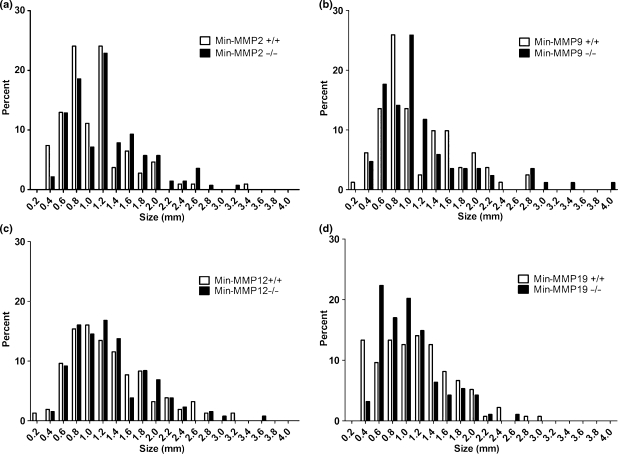
Figure 1.
Min mice lacking MMP-9 develop fewer macroscopically visible intestinal tumours than littermates, but MMP-2, -12 or -19 ablation does not affect tumour multiplicity. (a) Min mice deficient for MMP-2 developed a similar number of tumours as wild type littermates. A total of 20 Min mice, 7 Min-MMP-2 (APCMin/+; MMP-2−/− open diamonds) and 13 Min (APCMin/+;MMP-2+/+ closed diamonds) were analysed for tumour multiplicity; bar, mean. Difference is not statistically significant. (b) MMP-9 deficient Min mice develop significantly fewer tumours than wild-type or heterozygous littermates. A total of 32 mice, 21 deficient for MMP-9 (APCMin/+;MMP-9−/− open diamonds) and 11 littermate controls (APCMin/+;MMP-9+/+ closed diamonds) were counted for tumours; bar, mean. *, P < 0.05, difference is statistically significant, Mann–Whitney U-test. (c) Mice deficient for MMP-12 develop a similar number of tumours as do wild type littermates. A total of 26 mice, 16 Min-MMP-12 (APCMin/+; MMP-12−/− open diamonds) and 10 littermate controls (APCMin/+;MMP-12+/+ closed diamonds) were counted for tumour multiplicity; bar, mean. Difference is not statistically significant. (d) Mice deficient for MMP-19 develop a similar number of tumours as do wild type littermates. A total of 18 mice, nine deficient for MMP-19 (APCMin/+; MMP-19−/− open diamonds) and nine littermate controls (APCMin/+; MMP-19+/+ closed diamonds) were counted for tumours; bar, mean. Difference is not statistically significant.
Table 1.
Tabular format of tumour multiplicity and size data for various MMP knockout mouse lines
| Group | Multiplicity average tumours ± St. Dev. (n) | Mean | Size (mm) 95% CI low | 95% CI high | |
|---|---|---|---|---|---|
| MMP-2 | +/+ | 36.3 ± 10.6 (15) | 0.94 | 0.87 | 1.03 |
| −/− | 35.7 ± 14.8 (7) | 1.08 | 0.77 | 1.49 | |
| MMP-9 | +/+ | 41.9 ± 27.6 (10) | 0.76 | 0.76 | 1.54 |
| −/− | 24.5 ± 16.1 (21) | 0.88 | 0.88 | 1.20 | |
| MMP-12 | +/+ | 47.6 ± 35.5 (7) | 0.69 | 0.69 | 1.07 |
| −/− | 53.8 ± 34.5 (15) | 0.86 | 0.86 | 1.63 | |
| MMP-19 | +/+ | 49.7 ± 28.9 (9) | 0.60 | 0.60 | 1.54 |
| −/− | 36.6 ± 26.8 (9) | 0.76 | 0.76 | 1.35 |
MMP ablation does not affect intestinal adenoma size
To determine if ablation of selected MMPs affected tumour size, we measured tumours throughout the intestinal track in five each of MMP single deficient animals and littermate controls and compared the size distribution using a mixed model analysis (Figure 2a–d). Mean tumour diameter and 95% confidence intervals presented in a tabular format are listed in Table 1. The mean diameter for all groups examined was 1.2 mm and diameter ranged from 0.2 mm to 4.7 mm. On the basis of these results, ablation of any of the tested MMPs did not significantly affect the tumour size. Because no affect on tumour multiplicity or size was observed upon genetic ablation of MMP-2, -12 or -19, only Min-MMP-9 mice were further examined.
Figure 2.
MMP ablation does not affect tumour size. (a–d): Size distribution of Min adenomas from MMP-2 (Panel A), MMP-9 (Panel B), MMP-12 (Panel C) and MMP-19 (Panel D) deficient Min mice (solid bars) and wild type littermates (open bars). Tumours were measured with digital calipers from single deficient Min-MMP-x−/− mice and their respective Min littermate controls (n = 5 each group). Differences are not statistically significant.
MMP-9 ablation reduces tumour cell proliferation; apoptosis remains unaffected
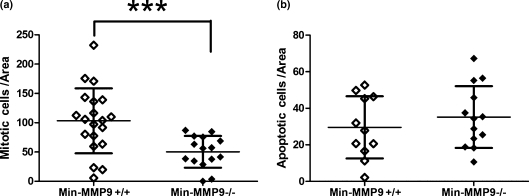
To examine if MMP-9 ablation affected the rate of tumour cell proliferation or apoptosis, we performed immunohistochemical staining and calculated an index of positive cells per unit area using Metamorph image analysis software. Using a monoclonal antibody to phospho-HistoneH3(Ser10), a marker specific for late anaphase and mid-metaphase mitosis (Hendzel et al. 1997), tumours from Min-MMP-9−/− mice had fewer than half as many actively proliferating cells as did Min-MMP-9+/+ littermates (50.3 ± 27.1 vs. 103.2 ± 55.4 cells/unit area) (Figure 3a). However, tumours from both Min-MMP-9−/− and Min-MMP-9+/+ mice had a rate of apoptotic cells similar to that measured by immunohistochemical detection of cleaved caspase-3 (35.2 ± 16.9 vs. 29.6 ± 17.0 cells/unit area, Figure 3b), suggesting that MMP-9 functions as a pro-tumourigenic capacity by stimulating tumour cell mitosis.
Figure 3.
Reduced rate of proliferation in tumours from Min-MMP-9 −/− mice compared with Min-MMP9+/+ littermates. (a) Intestinal adenomas were assayed for proliferative cells by phospho-Histone H3 immunohistochemistry. Positive cells were counted per unit area using Metamorph software. Tumours from Min-MMP9−/− mice (50.3 ± 27.1 cells/area; n = 15) have half as many actively dividing cells compared with tumours isolated from littermate controls (103.2 ± 55.4 cells/area; SD=55.4; n = 20); bar, mean, whiskers, SD. ***P = 0.0015, difference is statistically significant, t-test. (b) Adenomas isolated from Min-MMP9−/− mice and littermate controls were stained for the cleaved caspase-3, a marker of apoptosis. Min-MMP9+/+ (29.6 ± 17.0 cells/unit area; n = 13) and Min-MMP9−/− (35.2 ± 16.9 cells/unit area; n = 11) adenomas have a similar ratio of apoptotic cells; bar, mean, whiskers, SD. Difference is not statistically significant.
Neutrophils are the major source of intratumoural MMP-9
Several different cellular lineages produce MMP-9 including tumour cells, endothelium, macrophages, neutrophils, mast cells and fibroblasts (Noel et al. 2008). To determine the cellular source of MMP-9 in APC-Min tumours, we performed immunohistochemical staining. No MMP-9 positive epithelial or endothelial cells were observed. Positive staining was restricted to stromal populations that morphologically appeared to be leucocytes within the vasculature or lymphatic vessels (Figure 4a). Immunofluorescent co-staining for MMP-9 and leucocyte markers revealed that neutrophils, but not macrophages or B cells, express MMP-9 in the context of our tumours (Figure 4b–d). However, neutrophil abundance was similar in both Min-MMP-9−/− and littermate controls (data not shown). Taken together, these data suggest that neutrophil derived MMP-9 promotes the development of APC-Min adenomas by enhancing epithelial cell proliferation.
Figure 4.
Neutrophils are a major source of intratumoural MMP-9 in Min adenomas. (a) Low (left), medium (centre), and high (right) power photomicrographs of MMP-9 immunohistochemistry. Bounding boxes superimposed onto the low power photomicrograph indicate the region depicted in medium and high power pictures. No MMP-9 positive epithelial cells were observed, all MMP-9 expression was restricted to intratumoural stromal cells (centre) and leucocytes within the vasculature (right). Brown represents positive staining, low power scale bar indicates 1 mm (left), high power scale bar indicates 50 μm (centre) or 100 μm (right). (b–d): High power (63×) photomicrographs of tumours stained to co-localize MMP-9 with neutrophils (b), macrophages (c) and B cell (d) populations. Leucocyte populations were detected with a red-labelled secondary antibody, MMP-9 expression was stained with a green labelled secondary antibody and nuclei were visualized with DAPI (blue). Arrows indicate double positive cells.
Discussion
In this study, we chose to examine the potential contribution of four members of the MMP family that have been shown to be differentially expressed in intestinal tumours to early tumour development. Importantly, the model system we chose to employ in this study is best suited for studying effects on early tumourigenesis and would not assess roles for these enzymes during later stage tumour development such as malignant conversion, angiogenesis and metastatic spread. Genetic ablation of three of these MMPs – MMP-2, -12, and -19 – had no discernable effect on adenoma multiplicity or size. We did, however, observe a 40% reduction in tumour multiplicity upon genetic ablation of MMP-9 and a >50% reduction in tumour cell proliferation.
The effect observed here suggests a role for MMP-9 early in neoplastic development, i.e. the stages of tumour initiation and/or promotion. One potential way that MMP-9 could impact tumour initiation, in this case, by the loss of the normal APC allele (Luongo et al. 1994), is through the generation of reactive oxygen species (ROS), which are commonly associated with damage to both DNA and proteins (Kundu & Surh 2008). An ROS-based mechanism of tumour initiation has been previously identified for MMP-3, which incidentally can also act as an activator of pro-MMP-9 (Inuzuka et al. 2000). MMP-3 expression induces an alternatively spliced form of the small GTPase rac1 known as rac1b. This variant induces an increase in intracellular ROS, elevated levels of the transcription factor Snail and ultimately, genomic instability, although the initial cleavage product leading to this change has not yet been identified (Radisky et al. 2005). Importantly, MMP-9 has been shown to be capable of substituting for MMP-3 in this same pathway (Radisky et al. 2005).
MMP-9 is well known for its role as a master regulator of angiogenesis. In an animal model of pancreatic tumourigenesis, genetic ablation of MMP-9 has been shown to inhibit angiogenic switching, as well as reduce tumour multiplicity and growth (Bergers et al. 2000). However, in the APC-Min model, adenomas are, in general, less than 2 mm in size and therefore unlikely to be dependent on angiogenesis for growth (Folkman 1992). As expected, no difference in tumour angiogenesis or total vasculature was detected between MMP-9+/+ and MMP-9−/− adenomas (data not shown) suggesting that MMP-9 influences tumour cell proliferation prior to the angiogenic switch. Despite a reduction in proliferative index, the lack of a difference in tumour size between MMP-9+/+ and MMP-9−/− mice is consistent with the tumours being growth-limited by the lack of an angiogenic event in this tumour model.
MMP-9 co-localized with neutrophils, a finding consistent with the previous findings that MMP-9 is stored in the tertiary granules of neutrophils (Opdenakker et al. 2001). Although angiogenesis does not appear to contribute to the biological effect observed following MMP-9 ablation, neutrophils have been implicated as a key regulator of the initial angiogenic switch (Nozawa et al. 2006) by virtue of their granule contents that uniquely contain TIMP-1 free MMP-9 (Ardi et al. 2007). Defects in neutrophil migration associated with MMP-9 deficiency have been reported in some (Khandoga et al. 2006), but not in all (Felkel et al. 2001) model systems. However, in our samples, there was no difference between the number or distribution of neutrophils in APC-Min tumours of wild-type and MMP-9 null mice, suggesting that MMP-9 is not essential for neutrophil recruitment or migration of tumours in the gastrointestinal tract.
The observed effect may be the result of MMP-9 activity influencing neutrophil degranulation, even though neutrophil numbers are not affected. Neutrophils generate reactive oxygen and nitrogen species capable of damaging adjacent cells (Kuby 1997; Klebanoff 2005), and as discussed above, can result in tumour initiation (Josephy & Coomber 1998). MMP-9 activity could be processing cytokines, a mechanism that has been shown to be a major regulator of neutrophil activity. For example, MMP-9 mediated cleavage of full length IL-8 (1-77) to a truncated form (7-77) enhances IL-8 activity by more than tenfold (Van den Steen et al. 2000). This enhanced activity stimulates neutrophils via a positive feedback loop resulting in increased IL-8 binding, migration, production of MMP-9 (Opdenakker et al. 2001) and ROS (Guichard et al. 2005) and ultimately, degranulation (Van den Steen et al. 2000). Thus, it is possible that MMP-9 mediated differences in cytokine signalling are involved in modulating neutrophil-mediated genetic instability.
MMP-9 alters the proliferation of APC-Min adenoma cells suggesting a likely effect on tumour promotion through the expansion of initiated cells that are lacking APC function. Although, we observed difference in tumour multiplicity rather than size in Min-MMP-9−/− mice, this may be reflective of the fact that we only counted macroscopically visible tumours (>0.2 mm). In addition to cleaving matrix components, MMPs have been demonstrated to act upon a number of non-matrix substrates including cytokines, cell surface makers, growth factors and their inhibitory binding proteins (Sternlicht & Werb 2001; Noel et al. 2008). Specifically, MMP-9 activity has been shown to liberate a number of growth factors from the matrix including vascular endothelial growth factor (VEGF) (Bergers et al. 2000; Belotti et al. 2003), transforming growth factor-β (TGF-β) (Mott & Werb 2004) and basic fibroblast growth factor (bFGF) (Brauer 2006). Further, MMP-9 has the ability to convert pro-TNFα into an active form (Brauer 2006) and has been shown to degrade IGF-BPs, thus allowing for higher levels of circulating insulin-like growth factors (IGFs). In particular, IGF-II (Hassan & Howell 2000), VEGF (Goodlad et al. 2006; Alferez et al. 2008) and EGF (Alferez et al. 2008) have all been shown to affect growth and multiplicity of APC-Min tumours.
The APC-Min model has been particularly useful for identifying targets for chemoprevention of colonic tumours (Corpet & Pierre 2003; Hawk et al. 2005). Here, we have demonstrated that MMP-9, but not MMPs-2, -12 or -19, contributes to the development of intestinal adenomas. This would suggest that a selective inhibitor of MMP-9 in combination with inhibition of MMP-7, as identified in our previous work (Wilson et al. 1997), may be a useful strategy for the prevention of adenoma formation. Conversely, broad-spectrum inhibition of multiple MMPs would not have additional benefit and may instead be associated with adverse effects (Fingleton 2008).
In conclusion, we have identified MMP-9, but not the related MMP, MMP-2 or MMP-12 and -19 as a contributor to early intestinal tumourigenesis. This expands both the number of molecules important for early tumour formation and the potential roles of MMP-9.
References
- Alferez D, Wilkinson RW, Watkins J, et al. Dual inhibition of VEGFR and EGFR signaling reduces the incidence and size of intestinal adenomas in Apc(Min/+) mice. Mol. Cancer Ther. 2008;7:590–598. doi: 10.1158/1535-7163.MCT-07-0433. [DOI] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl Acad. Sci. U.S.A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Tada M, Cheng S, et al. Prognostic values of matrix metalloproteinase family expression in human colorectal carcinoma. J. Surg. Res. 2007;146:32–42. doi: 10.1016/j.jss.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Belotti D, Paganoni P, Manenti L, et al. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister VO, Salmela MT, Karjalainen-Lindsberg ML, et al. Differential expression of three matrix metalloproteinases, MMP-19, MMP-26, and MMP-28, in normal and inflamed intestine and colon cancer. Dig. Dis. Sci. 2004;49:653–661. doi: 10.1023/b:ddas.0000026314.12474.17. [DOI] [PubMed] [Google Scholar]
- Brauer PR. MMPs – role in cardiovascular development and disease. Front. Biosci. 2006;11:447–478. doi: 10.2741/1810. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- Corpet DE, Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol. Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WF, Lander ES, Smith JS, et al. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Felkel C, Scholl U, Mader M, et al. Migration of human granulocytes through reconstituted basement membrane is not dependent on matrix metalloproteinase-9 (MMP-9) J. Neuroimmunol. 2001;116:49–55. doi: 10.1016/s0165-5728(01)00294-6. [DOI] [PubMed] [Google Scholar]
- Fingleton B. MMPs as therapeutic targets – still a viable option? Semin. Cell Dev. Biol. 2008;19:61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. The role of angiogenesis in tumour growth. Semin. Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- Goodlad RA, Ryan AJ, Wedge SR, et al. Inhibiting vascular endothelial growth factor receptor-2 signaling reduces tumour burden in the ApcMin/+ mouse model of early intestinal cancer. Carcinogenesis. 2006;27:2133–2139. doi: 10.1093/carcin/bgl113. [DOI] [PubMed] [Google Scholar]
- Guichard C, Pedruzzi E, Dewas C, et al. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. J. Biol. Chem. 2005;280:37021–37032. doi: 10.1074/jbc.M506594200. [DOI] [PubMed] [Google Scholar]
- Hassan AB, Howell JA. Insulin-like growth factor II supply modifies growth of intestinal adenoma in Apc(Min/+) mice. Cancer Res. 2000;60:1070–1076. [PubMed] [Google Scholar]
- Hawk ET, Umar A, Lubet RA, Kopelovich L, Viner JL. Can animal models help us select specific compounds for cancer prevention trials? Recent Results Cancer Res. 2005;166:71–87. doi: 10.1007/3-540-26980-0_6. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Herszenyi L, Sipos F, Galamb O, et al. Matrix metalloproteinase-9 expression in the normal mucosa-adenoma-dysplasia-adenocarcinoma sequence of the colon. Pathol. Oncol. Res. 2008;14:31–37. doi: 10.1007/s12253-008-9004-5. [DOI] [PubMed] [Google Scholar]
- Inuzuka K, Ogata Y, Nagase H, Shirouzu K. Significance of coexpression of urokinase-type plasminogen activator, and matrix metalloproteinase 3 (stromelysin) and 9 (gelatinase B) in colorectal carcinoma. J. Surg. Res. 2000;93:211–218. doi: 10.1006/jsre.2000.5952. [DOI] [PubMed] [Google Scholar]
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J. Biol. Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- Jacobs ET, Thompson PA, Martinez ME. Diet, gender, and colorectal neoplasia. J. Clin. Gastroenterol. 2007;41:731–746. doi: 10.1097/MCG.0b013e3180338e56. [DOI] [PubMed] [Google Scholar]
- Josephy PD, Coomber BL. The 1996 Veylien Henderson Award of the Society of Toxicology of Canada. Current concepts: neutrophils and the activation of carcinogens in the breast and other organs. Can. J. Physiol. Pharmacol. 1998;76:693–700. doi: 10.1139/cjpp-76-7-8-693. [DOI] [PubMed] [Google Scholar]
- Khandoga A, Kessler JS, Hanschen M, et al. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J. Leukoc. Biol. 2006;79:1295–1305. doi: 10.1189/jlb.0805468. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Kuby J. Immunology. New York, NY: W. H. Freeman; 1997. [Google Scholar]
- Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat. Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Kwong LN, Shedlovsky A, Biehl BS, Clipson L, Pasch CA, Dove WF. Identification of Mom7, a novel modifier of Apc(Min/+) on mouse chromosome 18. Genetics. 2007;176:1237–1244. doi: 10.1534/genetics.107.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne MC, Eppihimer MJ. Cigarette smoke condensate induces MMP-12 gene expression in airway-like epithelia. Biochem. Biophys. Res. Commun. 2005;330:194–203. doi: 10.1016/j.bbrc.2005.02.144. [DOI] [PubMed] [Google Scholar]
- Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54:5947–5952. [PubMed] [Google Scholar]
- Martinez C, Bhattacharya S, Freeman T, Churchman M, Ilyas M. Expression profiling of murine intestinal adenomas reveals early deregulation of multiple matrix metalloproteinase (Mmp) genes. J. Pathol. 2005;206:100–110. doi: 10.1002/path.1755. [DOI] [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Mosig RA, Dowling O, DiFeo A, et al. Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum. Mol. Genet. 2007;16:1113–1123. doi: 10.1093/hmg/ddm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MS, Harnasch M, Kolb C, Kusch J, Sadowski T, Sedlacek R. The murine ortholog of matrix metalloproteinase 19: its cloning, gene organization, and expression. Gene. 2000;256:101–111. doi: 10.1016/s0378-1119(00)00369-3. [DOI] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Noel A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumour-host interface. Semin. Cell Dev. Biol. 2008;19:52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl Acad. Sci. U.S.A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G, Van den Steen PE, Dubois B, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- Pendas AM, Knauper V, Puente XS, et al. Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location, and tissue distribution. J. Biol. Chem. 1997;272:4281–4286. doi: 10.1074/jbc.272.7.4281. [DOI] [PubMed] [Google Scholar]
- Pendas AM, Folgueras AR, Llano E, et al. Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol. Cell. Biol. 2004;24:5304–5313. doi: 10.1128/MCB.24.12.5304-5313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsom R, Pignatelli M, Stetler-Stevenson WG, et al. Stromal expression of 72 kda type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am. J. Pathol. 1992;141:389–396. [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlacek R, Mauch S, Kolb B, et al. Matrix metalloproteinase MMP-19 (RASI-1) is expressed on the surface of activated peripheral blood mononuclear cells and is detected as an autoantigen in rheumatoid arthritis. Immunobiology. 1998;198:408–423. doi: 10.1016/S0171-2985(98)80049-1. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar-Miller RA, Gorden L, Matrisian LM. Matrix metalloproteinases in colorectal cancer: is it worth talking about? Cancer Metastasis Rev. 2004;23:119–135. doi: 10.1023/a:1025819214508. [DOI] [PubMed] [Google Scholar]
- Wasan HS, Novelli M, Bee J, Bodmer WF. Dietary fat influences on polyp phenotype in multiple intestinal neoplasia mice. Proc. Natl Acad. Sci. U.S.A. 1997;94:3308–3313. doi: 10.1073/pnas.94.7.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumourigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc. Natl Acad. Sci. U.S.A. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]