Abstract
Raman microspectroscopy was first used to determine the composition of a calcified plaque located at the pterygium-excision site of a 51-year-old female patient's left nasal sclera after surgery. It was unexpectedly found that the Raman spectrum of the calcified sample at 1149, 1108, 1049, 756, 517, 376 and 352/cm was similar to the Raman spectrum of monoclinic form of calcium pyrophosphate dihydrate (CPPD) crystal, but differed from the Raman spectrum of triclinic form of CPPD. An additional peak at 958/cm was also observed in the Raman spectrum of the calcified plaque, which was identical to the characteristic peak at 958/cm of hydroxyapatite (HA). This is the first study to report the spectral biodiagnosis of both monoclinic CPPD and HA co-deposited in the calcified plaque of a patient with sclera dystrophic calcification using Raman microspectroscopy.
Keywords: calcium pyrophosphate dihydrate (CPPD), dystrophic calcification, hydroxyapatite (HA), Raman microspectroscopy, sclera
Pathological calcification of soft tissues may be generally classified as dystrophic, metastatic or idiopathic, in which the prevalence of dystrophic calcification is reported to be about 95–98% (Hussmann et al. 1995; Richardson 2001). The dystrophic calcification usually occurs in areas of diseased or previously damaged tissue in patients with normal serum calcium and phosphate levels. It is distinguished from metastatic calcification, which occurs as a result of biochemical abnormalities of calcium and phosphate, with calcium salt deposition in normal tissue (Shields & Shields 2002; Chan et al. 2002). Ocular dystrophic calcification may be often observed in chronic inflammation, infection, tissue hypoxia, and trauma (Caldemeyer et al. 1995). Dystrophic calcification of the human sclera has been frequently reported in the elderly population (Daicker 1996; Moseley 2000).
Several diagnostic techniques such as histopathological examination, ultrasound biomicroscopy, computed tomography, high-frequency ultrasonography, magnetic resonance imaging, X-ray diffraction analysis and electron microscopy, have been used to observe and detect calcium deposition in ocular tissues (Chang et al. 1996; Raina et al. 2000; Ghadially 2001; Besada et al. 2005; Bernauer et al. 2006; Goldenberg-Cohen et al. 2007). Recently, the application of vibrational microspectroscopy has a great potential over other diagnostic techniques to identify and investigate the chemical compositions of many micronized biological samples (Kalasinsky & Kalasinsky 2005; Dumas et al. 2007). This technique not only has high specificity and sensitivity but also provides a non-destructive analysis with relatively sample preparation. Ocular calcification in senile cataractous lens, cornea or vitreous asteroid bodies had been successfully investigated by using Raman microspectroscopy (Chen et al. 2005, 2006; Lin et al. 2007). The aim of this study was to quickly identify the chemical component of the calcified plaque deposited in the human sclera by using Raman microspectroscopy. This report describes the first documented case of a patient with dystrophic calcification in the sclera by co-deposition of both calcium pyrophosphate dihydrate (CPPD) and hydroxyapatite (HA).
Materials and methods
Material and human tissue specimens
Three crystalline references were selected. The monoclinic and triclinic crystal forms of calcium pyrophosphate dihydrate (CPPD) were obtained from Dr Balic-Zunic and identified by X-ray diffraction before use (Christoffersen et al. 2002). Hydroxyapatite calcium (>100 mesh) was also purchased from Nacalai Tesque, Inc. (Tokyo, Japan).
A 51-year-old female patient visited our department for constant stinging pain and foreign body sensation of her left eye. In tracing her medical history, it was found that she had uneventful pterygium excision surgery in her left eye 5 years previously. About half a year before, she began suffering persistent pain in her left eye, and a small white plaque located at the pterygium-excision site of the left nasal sclera was found by examining the area with a slit-lamp microscope (Figure 1). She was treated with topical lubricants and mild corticosteroid eye drops, but this was in vain. The pain worsened gradually, and this white plaque became bigger. Under an agreed surgical consent, she received an operation of excision for the plaque and amniotic membrane transplantation over the excision site. The lesion was totally excised and was cut into two equal parts to send one for histopathological examination and the other for vibrational microspectroscopic study. During the post-operational follow-up for 3 months, the pain of her left eye was relieved and the wound healed without recurrence of calcification. The excised plaque was simply dried without tissue fixation, and then directly identified by Raman spectral analysis, which was previously approved by the Institutional Review Board at the Taipei Veterans General Hospital according to the Declaration of Helsinki.
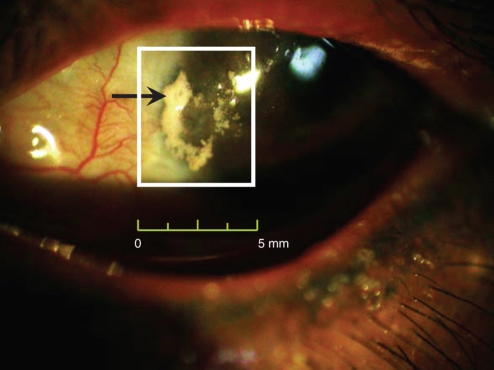
Figure 1.
A photograph of white calcium deposition in the sclera of patient with dystrophic calcification. The gross photography of this patient's left eye was taken by the digital camera equipped on the slit lamp microscope in the outpatient section of Ophthalmology Department. The size of calcified plaque was about 4.5 mm × 4.0 mm.
Histopathological analysis
The microtome sections were cut and examined by optical microscopy after staining with haematoxylin and eosin (H&E staining).
Raman microspectroscopic study
The calcified sample was dried for 1 day at 25 °C, 50% relative humidity (RH) condition, and then used for spectral biodiagnosis to identify the chemical component using a micro-Raman spectrophotometer (Ventuno, Jasco Co., Tokyo, Japan) equipped with a 30 mW green (532 nm) solid-state laser for non-destructive analysis (Chen et al. 2005, 2006; Lin et al. 2007) under 25 °C and 75% RH condition.
Results
The gross picture of the left eye in Figure 1 shows a 4.5 × 4.0 mm calcified plaque located on the pterygium-excised site, on the nasal bare sclera near the corneal limbus. Neovascularization and conjunctival congestion around the calcification were noted. This plaque caused severe irritation, pain and tearing; these symptoms were relieved after it was excised and an amniotic membrane patching was transplanted.
Histopathological examination of the sliced sample revealed a deep blue-purple on the H&E stain, implying the existence of a calcified deposition (Sundaram et al. 2004).
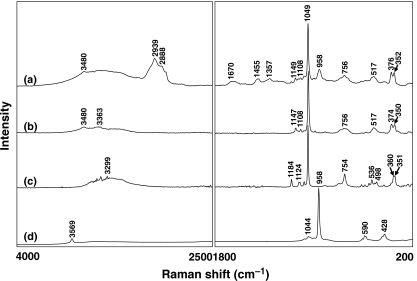
By comparing the characteristic Raman spectral peaks of various calcified samples from the literature, we found that the Raman spectra of our scleral deposits closely resembled the standard Raman spectra of CPPD and HA; thus, three crystals were selected as references (Cornilsen 1984; Hamada et al. 2001; Maugars et al. 1994; Wopenka & Pasteris 2005). Figure 2 shows the Raman spectra of the calcified plaque (a), and three crystalline references [(b) monoclinic CPPD, (c) triclinic CPPD and (d) HA]. Obviously, the peaks at 2939, 2888 and 1670/cm indicate the presence of scleral collagen. A unique peak at 1049/cm assigned to the symmetric P–O stretching mode is a characteristic frequency of CPPD (Maugars et al. 1994; Wopenka & Pasteris 2005). The Raman spectrum of the calcified sample at 1149, 1108, 1049, 756, 517, 376 and 352/cm was essentially identical to the Raman spectrum of monoclinic CPPD, but was different from the Raman spectrum of triclinic CPPD. The peak at 958/cm was also observed in the Raman spectrum of the calcified plaque, which matched to the characteristic peak at 958/cm of the HA (Wopenka & Pasteris 2005; Chen et al. 2005, 2006; Lin et al. 2007). Thus, Raman spectra confirmed the co-existence of monoclinic CPPD and HA deposited in the calcified plaque.
Figure 2.
The Raman spectra of the (a) calcified plaque, (b) monoclinic calcium pyrophosphate dihydrate (CPPD), (c) triclinic CPPD and (d) hydroxyapatite. Spectral acquisition parameter was dispersive micro-Raman, 30 mW, 532 nm diode laser, Andor CCD detector, 1.3 cm/pixel resolution.
Discussion
The present result clearly indicates that, except for the peaks for scleral collagen, the Raman spectrum of the calcified scleral sample was almost identical to that of the standard spectra of HA and monoclinic CPPD, rather than to triclinic CPPD. The co-existence of CPPD and HA in human tissues has also been reported where HA crystals were distributed in the central part of nodules consisting of CPPD crystals (Derfus et al. 1998; Yamagami et al.2000). It has been proposed that HA deposition occurs by a transformation from CPPD (Kawano et al. 1988).
Calcium pyrophosphate dihydrate deposition disease is the accumulation of CPPD in human tissues such as cartilage, synovium, tendons or ligaments (Rosenthal 2001; Bencardino & Hassankhani 2003). The interaction of the CPPD crystals with inflammatory cells is considered an important factor in crystal-induced inflammation (Prudhommeaux et al. 1996). It generally occurs in elderly patients and probably increases with age (Daicker 1996; Moseley 2000). The location of CPPD deposition has been divided into articular and extra-articular calcification (Genant 1976). Compared with intra-articular CPPD deposition disease, the extra-articular type is relatively rare (Steinbach & Resnick 2000). The eye is another extra-articular site for CPPD deposition, but it is an infrequent finding. The present study is the first documented case of a Taiwanese patient with scleral calcification associated with CPPD and HA depositions.
Although the pathogenesis of CPPD crystal deposition is still unclear, two hypotheses had been proposed: (i) overproduction or reduced removal of CPPD crystals from cartilage or abnormality of collagen in cartilage and (ii) deposition of intracellular pyrophosphate (PPi) released from cells in the extracellular space by trauma or surgery (Chen et al. 2003; Costello & Ryan 2004). Moreover, it has been reported that lower concentrations of PPi showed an apatitic mineral formation while high concentrations formed an immature CPPD (Garimella et al. 2006). In the present case, the calcified plaque was located on the bare sclera site after pterygium excision. We assume that it might be related to the dystrophic scleral calcification.
We propose that two possible factors might result in this dystrophic scleral calcification. The first factor might be an over manipulation of the sclera during the pterygium excision such as drug treatment (mitomycin C treatment), mechanical irritation and inflammation (Dunn et al.1991; Rubinfeld et al. 1992). The second reason might be due to a vascular zone of the sclera after excision, local inflammation, local tear insufficiency or poor tear turnover. This was consistent with the dystrophic calcifications of scleral scars after trauma, surgery or scleritis (Leitch et al. 1992; Daicker 1996). Moreover, the abnormalities of tear film and eye surface, similar to what is seen in eyes with band keratopathy, might also be responsible for this calcium deposition.
Although the sclera is one of the sites of calcification and has been reported in a few studies (Daicker 1996; Moseley 2000), dystrophic scleral calcification has been found less often (Dunn et al. 1991). In particular, the co-deposition of both monoclinic CPPD and HA crystals in the human sclera is a very rare case. The physiological mineralization in a tissue has been reported to be the result of the dynamic interaction between local phospho-substrate concentration, enzyme kinetics and transporter functions (Garimella et al. 2006). As HA is assumed to be the final biochemical product of CPPD (Kawano et al. 1988; Yamagami et al. 2000), this suggests that CPPD played a predominant role in the process of calcification. A study has pointed out that the nature of the crystal product formed was also highly dependent on the ambient Mg2+ concentration, although it was well known that crystal formation varied with Ca2+ and PPi (Cheng & Pritzker 1981). Cheng and Pritzker found that lower concentrations of Mg2+ and PPi favoured the formation of triclinic crystal form of CPPD, whereas higher concentrations of both easily caused a monoclinic crystal form of CPPD formation. In this study, the co-deposition of both HA and monoclinic CPPD in the scleral deposits may be due to the ionic balance in the ocular microenvironment. Furthermore, Raman microspectroscopy was first used to quickly and accurately identify the calcium deposition of dystrophic scleral calcification, suggesting that the sensitivity and specificity of Raman spectroscopy should be suitable for clinical diagnosis of CPPD disease.
References
- Bencardino JT, Hassankhani A. Calcium pyrophosphate dihydrate crystal deposition disease. Semin. Musculoskelet. Radiol. 2003;7:175–185. doi: 10.1055/s-2003-43228. [DOI] [PubMed] [Google Scholar]
- Bernauer W, Thiel MA, Kurrer M, et al. Corneal calcification following intensified treatment with sodium hyaluronate artificial tears. Br. J. Ophthalmol. 2006;90:285–288. doi: 10.1136/bjo.2005.082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besada E, Barr R, Natu A. Bilateral disk edema with unilateral macular serous fluid secondary to neurocysticercosis. Optometry. 2005;76:239–249. doi: 10.1016/s1529-1839(05)70299-2. [DOI] [PubMed] [Google Scholar]
- Caldemeyer KS, Smith RR, Edwards-Brown MK. Familial hypophosphatemic rickets causing ocular calcification and optic canal narrowing. Am. J. Neuroradiol. 1995;16:1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am. J. Respir. Crit. Care Med. 2002;165:1654–1669. doi: 10.1164/rccm.2108054. [DOI] [PubMed] [Google Scholar]
- Chang YC, Huang CC, Liu CC. Frequency of linear hyperechogenicity over the basal ganglia in young infants with congenital rubella syndrome. Clin. Infect. Dis. 1996;22:569–571. doi: 10.1093/clinids/22.3.569. [DOI] [PubMed] [Google Scholar]
- Chen CF, Chang MC, Wang ST, Liu CL, Chen W. Calcium pyrophosphate dihydrate crystal deposition disease in cervical radiculomyelopathy. J. Chin. Med. Assoc. 2003;66:256–259. [PubMed] [Google Scholar]
- Chen KH, Cheng WT, Li MJ, Yang DM, Lin SY. Calcification of senile cataractous lens determined by Fourier transform infrared (FTIR) and Raman microspectroscopies. J. Microsc. 2005;219:36–41. doi: 10.1111/j.1365-2818.2005.01491.x. [DOI] [PubMed] [Google Scholar]
- Chen KH, Cheng WT, Li MJ, Lin SY. Corneal calcification: chemical composition of calcified deposit. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:407–410. doi: 10.1007/s00417-005-1191-0. [DOI] [PubMed] [Google Scholar]
- Cheng PT, Pritzker KPH. The effect of calcium and magnesium ions on calcium pyrophosphate crystal formation in aqueous solutions. J. Rheumatol. 1981;8:772–782. [PubMed] [Google Scholar]
- Christoffersen MR, Balic-Zunic T, Christoffersen J. Kinetics and mechanisms of dissolution and growth of acicular triclinic calcium pyrophosphate dihydrate crystals. Cryst. Growth Des. 2002;2:567–571. [Google Scholar]
- Cornilsen BC. Solid state vibrational spectra of calcium pyrophosphate dihydrate. J. Mol. Struct. 1984;117:1–9. [Google Scholar]
- Costello JC, Ryan LM. Modulation of chondrocyte production of extracellular inorganic pyrophosphate. Curr. Opin. Rheumatol. 2004;16:268–272. doi: 10.1097/00002281-200405000-00017. [DOI] [PubMed] [Google Scholar]
- Daicker B. Tophus-like, conglomerated, crystalline calcification of the sclera. Ophthalmologica. 1996;210:223–228. doi: 10.1159/000310713. [DOI] [PubMed] [Google Scholar]
- Derfus B, Kranendonk S, Camacho N, et al. Human osteoarthritic cartilage matrix vesicles generate both calcium pyrophosphate dihydrate and apatite in vitro. Calcif. Tissue Int. 1998;63:258–262. doi: 10.1007/s002239900523. [DOI] [PubMed] [Google Scholar]
- Dumas P, Sockalingum GD, Sule-Suso J. Adding synchrotron radiation to infrared microspectroscopy: what's new in biomedical applications? Trends Biotechnol. 2007;25:40–44. doi: 10.1016/j.tibtech.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Dunn JP, Seamone CD, Ostler HB, Nickel BL, Beallo A. Development of scleral ulceration and calcification after pterygium excision and mitomycin therapy. Am. J. Ophthalmol. 1991;112:343–344. doi: 10.1016/s0002-9394(14)76738-8. [DOI] [PubMed] [Google Scholar]
- Garimella R, Bi X, Anderson HC, Camacho NP. Nature of phosphate substrate as a major determinant of mineral type formed in matrix vesicle-mediated in vitro mineralization: an FTIR imaging study. Bone. 2006;38:811–817. doi: 10.1016/j.bone.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Genant HK. Roentgenographic aspects of calcium pyrophosphate dihydrate crystal deposition disease (pseudogout) Arthritis Rheum. 1976;19(Suppl. 3):307–328. doi: 10.1002/1529-0131(197605/06)19:3+<307::aid-art1780190705>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ghadially FN. As you like it, Part 3: a critique and historical review of calcification as seen with the electron microscope. Ultrastruct. Pathol. 2001;25:243–267. doi: 10.1080/019131201300343874. [DOI] [PubMed] [Google Scholar]
- Goldenberg-Cohen N, Bahar I, Barash D, Naphtalaiv E, Segev Y. Sonographic features of senile scleral calcification. Ophthalmic. Surg. Lasers Imaging. 2007;38:115–117. doi: 10.3928/15428877-20070301-05. [DOI] [PubMed] [Google Scholar]
- Hamada J, Ono W, Tamai K, Saotome K, Hoshino T. Analysis of calcium deposits in calcific periarthritis. J. Rheumatol. 2001;28:809–813. [PubMed] [Google Scholar]
- Hussmann J, Russell RC, Kucan JO, Khardori R, Steinau HU. Soft-tissue calcifications: differential diagnosis and therapeutic approaches. Ann. Plast. Surg. 1995;34:138–147. [PubMed] [Google Scholar]
- Kalasinsky KS, Kalasinsky VF. Infrared and Raman microspectroscopy of foreign materials in tissue specimens. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005;61:1707–1713. doi: 10.1016/j.saa.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Kawano N, Matsuno T, Miyazawa S, et al. Calcium pyrophosphate dihydrate crystal deposition disease in the cervical ligamentum flavum. J. Neurosurg. 1988;68:613–620. doi: 10.3171/jns.1988.68.4.0613. [DOI] [PubMed] [Google Scholar]
- Leitch RJ, Bearn MA, Watson PG. Exudative retinal detachment and posterior scleritis associated with massive scleral thickening and calcification treated by scleral decompression. Br. J. Ophthalmol. 1992;76:109–112. doi: 10.1136/bjo.76.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Chen KH, Cheng WT, Ho CT, Wang SL. Preliminary identification of beta-carotene in the vitreous asteroid bodies by micro-Raman spectroscopy and HPLC analysis. Microsc. Microanal. 2007;13:128–132. doi: 10.1017/S143192760707002X. [DOI] [PubMed] [Google Scholar]
- Maugars YM, Peru LF, el Messaoudi B, et al. Pelvic pseudotumoral calcium pyrophosphate dihydrate deposition: an ultrastructural study. J. Rheumatol. 1994;21:573–576. [PubMed] [Google Scholar]
- Moseley I. Spots before the eyes: a prevalence and clinicoradiological study of senile scleral plaques. Clin. Radiol. 2000;55:198–206. doi: 10.1053/crad.1999.0348. [DOI] [PubMed] [Google Scholar]
- Prudhommeaux F, Schiltz C, Liote F, et al. Variation in the inflammatory properties of basic calcium phosphate crystals according to crystal type. Arthritis Rheum. 1996;39:1319–1326. doi: 10.1002/art.1780390809. [DOI] [PubMed] [Google Scholar]
- Raina UK, Tuli D, Arora R, Mehta DK, Taneja M. Tubercular endophthalmitis simulating retinoblastoma. Am. J. Ophthalmol. 2000;130:843–845. doi: 10.1016/s0002-9394(00)00646-2. [DOI] [PubMed] [Google Scholar]
- Richardson ML. Approaches to differential diagnosis in musculoskeletal imaging. http://www.rad.washington.edu/mskbook/softtissueca.html.
- Rosenthal AK. Pathogenesis of calcium pyrophosphate crystal deposition disease. Curr. Rheumatol. Rep. 2001;3:17–23. doi: 10.1007/s11926-001-0046-x. [DOI] [PubMed] [Google Scholar]
- Rubinfeld RS, Pfister RR, Stein RM, et al. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992;99:1647–1654. doi: 10.1016/s0161-6420(92)31749-x. [DOI] [PubMed] [Google Scholar]
- Shields JA, Shields CL. CME review: sclerochoroidal calcification: the 2001 Harold Gifford Lecture. Retina. 2002;22:251–261. doi: 10.1097/00006982-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Steinbach LS, Resnick D. Calcium pyrophosphate dihydrate crystal deposition disease: imaging perspectives. Curr. Probl. Diagn. Radiol. 2000;29:209–229. doi: 10.1016/s0363-0188(00)90014-8. [DOI] [PubMed] [Google Scholar]
- Sundaram S, Kuruvilla S, Thirupuram S. Idiopathic arterial calcification of infancy – a case report. Images Paediatr. Cardiol. 2004;18:6–12. [PMC free article] [PubMed] [Google Scholar]
- Wopenka B, Pasteris JD. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C. 2005;25:131–143. [Google Scholar]
- Yamagami T, Kawano N, Nakano H. Calcification of the cervical ligamentum flavum – case report. Neurol. Med. Chir. (Tokyo) 2000;40:234–238. doi: 10.2176/nmc.40.234. [DOI] [PubMed] [Google Scholar]