Abstract
As local steroid metabolism controls the bioavailability of active steroidal hormones in the prostate, the aim of this study, was to investigate the effects of absence of 5-alpha reductase (5α-r) and aromatase (Aro) enzymes on prostatic cellular and extracellular components after long-term inhibition. Young, adult and old male Mongolian gerbils were treated orally, once a day, for 30 consecutive days, with Finasteride (10.0 mg/kg) and Letrozole (1.0 mg/kg) (5α-r and Aro enzymes inhibitors respectively) simultaneously or separately. Animals were killed on 1, 7, 14 and 21 days post-treatment. Data obtained after double or single enzymatic inhibition with Finasteride and Letrozole demonstrated marked remodelling of epithelial and stromal compartments. During the post-treatment period, particularly on the first and the last analysed days, prostatic epithelial cells showed decreased cytoplasmic volume and secretory activity. In the stroma, collagen fibres had accumulated in the epithelial base and among smooth muscle cells, which showed reduced diameter and condensed cytoplasm, and some of them had a highly irregular external contour. Also in the sub-epithelial area, some fibroblasts acquired an activated phenotype besides increased deposits of amorphous granular material. In conclusion, the inhibition of 5α-r and Aro enzymes affected, in a persistent manner, the structural and ultrastructural morphology of the prostate, irrespective of the gerbil′s age. Hence these enzymes appear to be crucial in the maintenance of this gland during postnatal development. Also, these data bring more light to the complex issue of the mechanisms of local steroid metabolism and prostatic histology. Thus, the blockade of the steroid-metabolizing enzymes provided an important novel tool to study the relationship between sex steroids and normal physiology and diseases of the prostate.
Keywords: 5-alpha reductase, aromatase, epithelium, gerbil, stroma, ventral prostate
Prostate tissue contains a variety of steroid metabolizing enzymes, such as 5-alpha reductase (5α-r) and Aromatase (Aro), required for the local formation of active androgens and oestrogens from precursor steroids provided by the adrenals (Soronen et al. 2004; Vihko et al. 2005, 2006). The enzymes, 5α-r and Aro, are thought to play important roles as local regulators of androgens and oestrogens within normal and abnormal prostatic tissue (Nakamura et al. 2005) and the products yielded by these enzymatic reactions are factors that influence the behaviour and physiology of the reproductive system and have innumerable implications throughout the life of the individual (Lephart et al. 2001 and Soronen et al. 2004).
Androgens are essential for prostatic growth and development but they also have a significant function in prostate disease pathogenesis (Tindall & Rittmaster 2008). Both normal and pathological growth of the prostate is dependent on dihydrotestosterone (DHT) synthesis, which is catalysed by two types of 5α-r isozymes (type 1 and type 2) (Bruchovsky & Wilson 1999; Ekman 2000; Steers 2001; Thomas et al. 2008). The action of these two types of 5α-r isozymes is responsible for the local conversion of Testosterone (T) into DHT in both the prostate and several other androgen target tissues (Hsing et al. 2002; Yuan-Shan 2005), so that a potential therapeutic benefit could be achieved through the inhibition of these enzymes (Tindall & Rittmaster 2008).
Study of 5α-r in the prostate has resulted in the development of drugs, such as Finasteride, an efficient 5α-r type 2 inhibitor, which has been used clinically for controlling the symptoms of benign prostatic hyperplasia (BPH) (Steers 2001). Azzolina et al. (1997) proposed that in rats, the 5α-r type 1 and type 2 present distinct Finasteride action mechanisms, that is, reversible in the inhibition of type 1, and time-dependent and irreversible in 5α-r type 2 inhibition. The use of Finasteride and the consequent inhibition of this enzyme provoked striking prostatic epithelial and stromal changes in the adult gerbil ventral prostate, probably the result of a homeostatic interaction-imbalance between the epithelium and the underlying stroma (Corradi et al. 2004). Androgen-withdrawal therapy can reverse prostate tumour growth by reducing circulating T. However, 5α-r-catalysed DHT synthesis within the prostate can continue and most tumours eventually develop resistance to androgen-deprivation therapy (Thomas et al. 2008).
Although the prostate is one of the major targets for DHT, this gland is also recognized as a non-classical target for oestrogen, because it expresses both types of oestrogen receptors (ER), especially ERbeta (Oliveira et al. 2007). An alternative pathway for the metabolism of T to oestradiol is the local prostatic oestrogen production, which depends on Aro enzyme, implying that androgen aromatization may be in part responsible for androgen action in non-tumoural and malignant prostate (Härkonen & Mäkelä 2004). Aberrant Aro expression and activity has been reported in prostate tumour tissues and cells, implying that androgen aromatization to oestrogens may play a role in prostate carcinogenesis or tumour progression (Carruba 2007). Oestradiol level in BPH stroma increases with age, which has always been associated with elevated expression of Aro enzymes in prostatic stromal cells, especially through the production of prostaglandin in a paracrine mechanism (Wu et al. 2007). Therapy with the non-steroidal Letrozole provokes inhibition of Aro enzyme activity, thereby blocking the conversion of T into oestrogen. The action of this drug promotes an additional increase of circulating androgen levels and, consequently, an oestradiol decline (Risbridger et al. 2003).
Androgens and oestrogens are each capable of altering the normal growth of the prostate but individually they do not induce prostatic malignancy (Risbridger et al. 2003). Some studies have shown that the combination of these two sex steroid hormones can induce dysplasia, premalignant and malignant changes to the prostatic cells (Wang & Wong 1998; Wang et al. 2000, 2001; Hayward et al. 2001). Rivas et al. (2002) suggest that the balance in action between androgens and oestrogens, rather than their absolute levels, may be of fundamental significance to the normal and abnormal development of some regions of the male reproductive tract, because a decrease of androgenic action sensitizes these tissues to oestrogens.
The rodent Meriones unguiculatus (Criscetidae, Gerbilinae) named Mongolian gerbil has been known as a good model for laboratory use since the 1960s (Corradi et al. 2004). The usefulness of this animal in biomedical research has been recognized in diversified areas besides prostate morphology (Santos et al. 2003; Corradi et al. 2004; Custódio et al. 2004; Scarano et al. 2006; Góes et al. 2007), and more recently, the gerbil has also been suggested as a suitable model for studies on mammalian ageing (Pegorin de Campos et al. 2006). The gerbil prostate has compact lobes, similar to those of the human prostate, but unlike those of rats and mice, which have distinct lobes (Price 1963; Pinheiro et al. 2003; Góes et al. 2007).
Based on the concept that local steroid metabolism is crucial in determining the overall biological impact of hormones in individuals that target normal and abnormal prostate cells (Carruba 2007), we sought to characterize through this study what the absence of the enzymes 5-αr and Aro provoked in prostatic cellular and extracellular compartments after their long-term inhibition and how the prostate responded during the tissue recovering following the drug suspension treatments. The main focus of this study was to investigate how the Finasteride and Letrozole could perturb the prostate microenvironments considering the three distinct phases of postnatal gerbil development.
Materials and methods
Animals
Male gerbils (Meriones unguiculatus, Gerbilinae, Criscetidae) – 100 young (48 days), 100 adults (112 days), and 100 old (78 weeks) – were housed under controlled conditions of temperature (25 °C), relative humidity (40–70%) and lighting (12-h light, 12-h dark cycle), and allowed free access to standard chow and water. Animal handling and experiments were carried out according to the ethical guidelines of the Commission for Ethics in Animal Experimentation (CEEA) at the Campinas State University - UNICAMP, São Paulo, Brazil (Process Nr 1236-1), following the Guide for Care and Use of Laboratory Animals.
Experimental design
The animals received orally, for 30 consecutive days, simultaneously or separately, Finasteride - 5α-r inhibitor (Sigma Chemical Co., St. Louis, MO, USA; 10 mg/kg per day in 0.1 ml corn oil), based on the results previously detected by Corradi et al. (2004) and Letrozole - Aro inhibitor (Femara, Novartis-Pharma, Basileia, Swiss; 1 mg/kg per day in 0.1 ml corn oil), as described by Tobin and Canny (1998) and Santos et al. (2007). As control groups, some animals were persevered intact, while others received only the vehicle, for the same 30 days. Animals were killed on 1, 7, 14 and 21 days after the end of the drug administration periods, denominated the post-treatment period. In each of these four stages, after being anaesthetized by CO2 inhalation, gerbils of experimental and control groups (n = 5/group) were weighed and decapitated, after which blood samples were collected for serological analysis. The prostatic complex was dissected out, weighed, and fixed according to the different protocols specified below. Only the ventral prostatic lobes were analysed.
Serum steroid hormone assay
Circulating serum testosterone and oestradiol levels were determined by immunochemical assays. Serum was separated by centrifugation and stored at −20 °C for subsequent assays. Measurements were performed in triplicate using automated equipment (Vitros-ECi; Johnson & Johnson, orthoclinical Diagnostics Division, Amershan, UK) for detection by ultrasensitive chemiluminescence. Sensitivity was 0.1–150 ng/ml for testosterone and 0.1–3.814 pg/ml for oestradiol. Intra-assay variations were 1% and 1.1%, and the interassay variations were 2.1% and 1.5% for testosterone and oestradiol respectively.
Structural analysis
In each stage of the post-treatment period, the entire prostate was dissected out, weighed and only the ventral lobe was fixed by immersion in Karnovsky solution (4% parformaldehyde, 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2) for 24 h. After fixation, the tissues were washed with running tap water, dehydrated in an ethanol series and embedded in glycol methacrylate resin (Leica historesin embedding kit, Leica, Nussloch, Germany) and sectioned at 3 μm on a Leica automatic rotatory microtome (Leica RM2155, Nussloch, Germany). Histochemical sections were subjected to Haematoxylin–Eosin (Behmer et al. 1976) staining for general studies. Microscopical analyses were performed on Zeiss-Jenaval (Zeiss-Jenaval, Jena, Germany) or an Olympus BX60 light photomicroscope (Olympus, Hamburg, Germany). Microscopic fields were digitized using the image-pro®plus (Media Cybernetics Inc., Bethesda, MD, USA) version 4.5 for Windows™ software.
Quantitative analysis
Thirty random ventral prostatic areas from the H&E sections of both control and experimental groups were analysed by the image-pro®plus version 4.5 for Windows™ software. The morphometric-stereological analyses were obtained by Weibel's multipurpose graticulate with 120 points and 60 test lines (Weibel 1963) to compare the relative proportion (%) of each prostatic tissue compartment (epithelium, lumen, smooth muscle cells (SMC) and non-muscular stroma).
Ultra-structural analysis
Prostate ventral-lobe fragments were minced into small pieces and fixed by immersion with 3% glutaraldehyde plus 0.25% tannic acid solution in Millonig's buffer, pH 7.3, containing 0.54% glucose for 24 h (Cotta-Pereira et al. 1976). After washing with the same buffer, samples were postfixed with 1% osmium tetroxide for 1 h, washed in buffer, dehydrated in graded acetone series and embedded in Araldite resin. Ultrathin sections (50–75nm) were cut using a diamond knife and stained with 2% alcoholic uranyl acetate for 30 min followed by 2% lead citrate in 1 M solution of sodium hydroxide for 10 min. Samples were evaluated by electron microscopy using a LEO – Zeiss 906 Transmission Electron Microscope operating at 80 Kv.
Statistical analysis
Data were analysed using statistica 6.0 software (StatSoft, Inc., Tulsa, OK, USA). The anova and Tukey honest significant difference (HSD) tests were applied to determine the statistical significance, with the level of significance set at 5% (P ≤ 0.05). Values are presented as mean ± standard deviation (SD).
Results
Body and prostatic complex weights
Table 1 shows the variation in body and prostatic complex weights. Body weights of young and adult gerbils increased significantly after the treatments with Finasteride, Letrozole and Finasteride plus Letrozole. Significant increase in the prostatic complex weight was observed throughout the post-treatment period of adult and old gerbils of all experimental groups. Letrozole treatment did not provoke notable changes in the young prostatic complex weight during the analysed period. The relative weight of young prostatic complex showed increases of 15.7% and 9.7%, 1 and 21 days after Letrozole and Finasteride-plus-Letrozole administration respectively. Besides, after Letrozole treatment, the relative weight of the prostatic complex of old gerbils increased 9.7% and 7.9%, respectively, 1 and 21 days after the end of treatment periods.
Table 1.
Body and prostatic complex weight in control and treated animals of different postnatal developmental ages (mean ± SE)
| Parameter (n = 5) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight (g) | Prostatic complex weight (g)* | Relative weight† | Relative weight variation (%)† | ||||||||||
| Exp. groups | Post-treatment periods | Young | Adult | Old | Young | Adult | Old | Young | Adult | Old | Young | Adult | Old |
| Control | 1 | 51.4 ± 0.3 | 74.4 ± 4.61 | 92.3 ± 3.7 | 0.155 ± 0.08 | 0.588 ± 0.1a,1 | 0.763 ± 0.1a | 100.0 ± 5.2 | 100.0 ± 6.9a | 100.0 ± 4.7a | - | - | - |
| 7 | 52.9 ± 2.8a | 79.5 ± 3.0a,1,2 | 91.6 ± 1.7a | 0.163 ± 0.1 | 0.613 ± 0.1a,1 | 0.823 ± 0.05a | 100.4 ± 12.4 | 97.4 ± 7.2a | 109.1 ± 5.5a | +0.4 | 2.6 | +9.1 | |
| 14 | 52.2 ± 2.7a | 90.9 ± 1.7a,2 | 87.3 ± 1.4 | 0.170 ± 0.02a | 0.765 ± 0.05a,1 | 0.764 ± 0.01a | 106.6 ± 5.3a | 106.9 ± 7.9a,1 | 106.6 ± 1.1a | +6.6 | +6.9 | +6.6 | |
| 21 | 57.1 ± 1.4 | 92.1 ± 3.7a,2 | 95.4 ± 2.5 | 0.198 ± 0.04 | 0.876 ± 0.03a,2 | 0.834 ± 0.04a | 115.3 ± 22.1a | 121.4 ± 6.0a | 106.8 ± 6.2a | +15.3 | +21.4 | +6.8 | |
| Finasteride | 1 | 51.2 ± 2.01 | 67.9 ± 4.6 | 89.8 ± 1.9 | 0.117 ± 0.11 | 0.279 ± 0.05b | 0.481 ± 0.01b | 75.5 ± 8.41 | 50.7 ± 7.5b | 65.3 ± 1.8b,1 | 24.3 | 49.2 | 34.7 |
| 7 | 50.7 ± 1.6a,1 | 74.7 ± 2.1a | 82.5 ± 2.3b | 0.092 ± 0.021 | 0.361 ± 0.03b | 0.501 ± 0.01b | 59.3 ± 12.11 | 62.7 ± 2.5b | 67.3 ± 2.8b,1 | 41.1 | 34.7 | 41.8 | |
| 14 | 38.5 ± 2.1b,2 | 79.8 ± 2.1b | 84.2 ± 5.5 | 0.04 ± 0.01b,2 | 0.47 ± 0.1b | 0.605 ± 0.04b | 30.1 ± 04.7b,2 | 69.1 ± 7.4b | 82.1 ± 2.5b,2 | 76.5 | 37.8 | 24.5 | |
| 21 | 54.6 ± 1.91 | 70.5 ± 3.7b | 82.8 ± 4.0 | 0.075 ± 0.081 | 0.32 ± 0.05b | 0.538 ± 0.05b | 39.3 ± 2.9b,2 | 46.9 ± 5.7b | 72.8 ± 5.7b,1 | 76 | 74.5 | 33.9 | |
| Letrozol | 1 | 48.5 ± 0.91 | 76.5 ± 4.6 | 86.3 ± 2.5 | 0.168 ± 0.2 | 0.562 ± 0,1a,c | 0.77 ± 0.02a,c,1 | 115.7 ± 18.4 | 92.7 ± 7.2a,c | 109.7 ± 1.6a,c,1 | +15.7 | 7.3 | +9.7 |
| 7 | 50.8 ± 1.9a,1 | 78.8 ± 2.1a | 85.4 ± 1.5a | 0.158 ± 0.05 | 0.534 ± 0.04b | 0.654 ± 0.02c,2 | 99.7 ± 28.0 | 93.6 ± 4.4a,c | 85.4 ± 1.9c,2 | 0.7 | 3.7 | 23.7 | |
| 14 | 46.2 ± 1.7a,c,1,2 | 75.8 ± 3.2 | 85.6 ± 3.3 | 0.085 ± 0.03b | 0.48 ± 0.04 | 0.67 ± 0.02a,1,2 | 54.9 ± 15.5b | 73.4 ± 3.9 | 93.4 ± 4.4a,2 | 51.7 | 33.5 | 13.2 | |
| 21 | 55.2 ± 2.41,3 | 76.8 ± 2.9b | 91.9 ± 4.8 | 0.154 ± 0.04 | 0.645 ± 0.1c | 0.92 ± 0.04a,c,3 | 79.9 ± 21.9a | 86.8 ± 5.4c | 114.7 ± 1.4a,c,1 | 35.5 | 34.6 | +7.9 | |
| Fin + Let | 1 | 50.3 ± 0.71 | 80.9 ± 2.31 | 78.1 ± 5.8 | 0.167 ± 0.31 | 0.358 ± 0.04b,1 | 0.45 ± 0.05b | 109.7 ± 20.51 | 55.9 ± 5.4b | 69.5 ± 4.2b,1 | +9.7 | 44.1 | 30.5 |
| 7 | 43.2 ± 1.4b,2 | 69.3 ± 1.9b,2 | 78.9 ± 1.6b | 0.084 ± 0.031 | 0.286 ± 0.04c,1 | 0.41 ± 0.03b | 64.5 ± 21.11 | 60.9 ± 4.7b | 59.2 ± 4.0b,1,2 | 35.9 | 36.5 | 49.9 | |
| 14 | 47.4 ± 0.8a,c,1,2 | 72.4 ± 0.7b,2 | 74.9 ± 6.0 | 0.054 ± 0.1b,2 | 0.373 ± 0.04b,1 | 0.571 ± 0.02b | 35.6 ± 7.8b,2 | 69.6 ± 1.9b | 88.7 ± 5.3b,1,3 | 71 | 37.3 | 17.9 | |
| 21 | 52.0 ± 1.51,3 | 75.4 ± 2.6b,1 | 80.1 ± 6.2 | 0.102 ± 0.031 | 0.517 ± 0.01c,2 | 0.542 ± 0.1b | 56.6 ± 17.4a,2 | 72.3 ± 4.0c | 75.1 ± 9.7b,1 | 58.7 | 49.1 | 31.6 | |
Alphabetic superindices (a, b, c) indicate statistically significant inter-group differences, when comparing treatment kind; numeric superindices (1, 2, 3) indicate statistically differences inter-group differences, when comparing stages of post-treatment period. Significant P ≤ 0.05. Comparisons among different postnatal ages where not executed.
Relative weight corresponds to the ratio between the weight of the prostate and that of the whole body.
Relative weight variation is shown with respect to the control, which was taken as 100%.
Serum steroid hormone assay
Serum steroid hormone levels are shown in Figure 1. As expected, comparing the three different treatment types, serum testosterone levels were elevated after the 30 consecutive days of drug administration. One day after dual enzymatic inhibition, the testosterone concentrations were the highest in the serum of young, adult and old gerbils. Despite oscillating through the post-treatment period, 21 days after the end of enzymatic dual inhibition, serum testosterone levels were similar to those found in the control group of young and old gerbils, except in the adults, where this hormone concentration was lower than in the control group. Finasteride plus Letrozole caused a significant elevation in circulating oestradiol levels of old gerbils, corresponding to more than four times the concentration observed in the old control group on the first post-treatment day.
Figure 1.
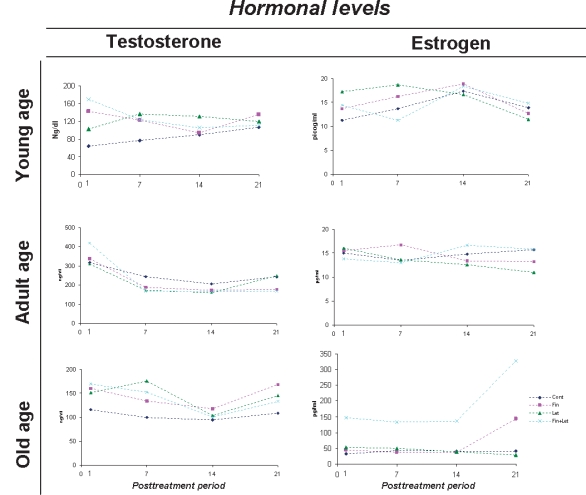
Mean variation of serum Testosterone and Oestradiol concentration following post-treatment periods of young, adult and old animals.
Structural analysis
During the post-treatment periods, young animals of the control group showed an immature prostate gland composed of some acini still in the process of glandular modelling. The epithelium was composed of voluminous cells and showed some signals of secretory activity. SMC and fibroblasts were the main stromal compounds besides collagen fibres. Adult prostate of control group presented a morphological structure that was completely developed and mature. An intense secretory function of the epithelial cells, which now were disposed in a single columnar pattern, probably widened the acinar lumen. In the stromal compartment, fusiform SMC had formed concentric and densely packed layers around acini. Fibroblasts were located at the epithelial base. In the old animals, the prostate was characterized by morphologically heterogeneous areas, some of them with normal histological aspect and others histopathologically compromised. In the normal acinar areas, the secretory epithelium was functionally similar to that found in adult controls, but with small infoldings. In the stromal compartment, the connective tissue adjacent to epithelial layer was denser and SMC slightly less compact.
Many morphological alterations were observed in the prostatic epithelial and stromal compartments of young, adult and old gerbils after the long-term suppression of 5α-r and Aro enzymes. In the Finasteride plus Letrozole groups, including animals of all postnatal developmental ages, the new characteristics assumed by the prostatic compartments were very similar to those caused by the exclusive use of Finasteride. On the first day after the cessation of Finasteride and Finasteride plus Letrozole treatments, the stromal compartment was modified and remodelled, with a remarkable collagen accumulation within the connective tissue adjacent to the epithelial base. In the same area, an apparent increase of phenotypically altered fibroblasts was observed. The cells of smooth muscle had also undergone modifications, as some SMC assumed an irregular wrinkled contour, contributing to the loose rearrangement of this smooth layer, with denser collagen interspersed among them. At the analyses of 21 post-treatment days, these aforementioned aspects of reorganized stromal compartment were still present. Young, adult and old animals treated with Letrozole showed similar morphological modifications in the stromal prostatic compartment; however, the epithelial compartment appeared to be the main target of the action of this drug. In the young prostate, epithelial infoldings into luminal area, besides phenotypically altered epithelial cells, were noted, while in the old animals, the epithelial cells had become shorter and did not return to their normal shape until the late post-treatment phase (Figure 2).
Figure 2.
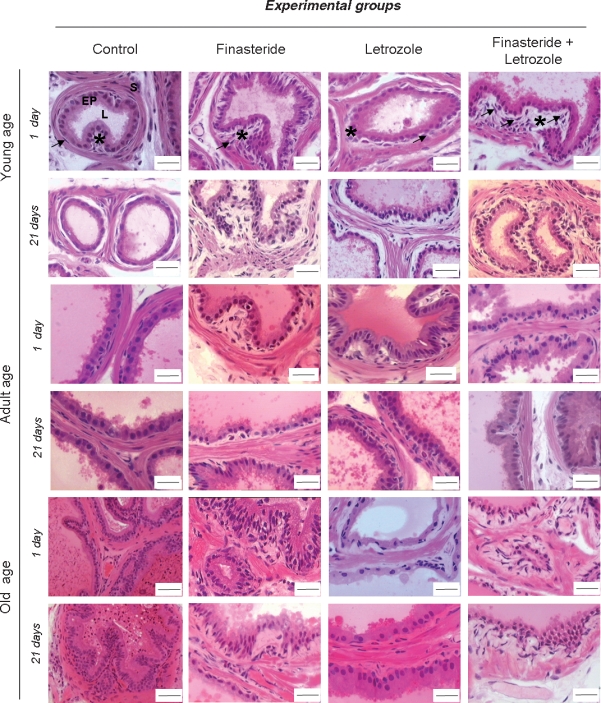
Haematoxylin–Eosin stained sections of Mongolian gerbil ventral prostatic lobe regions. The control prostate tissue of all ages studied presented development characteristic of epithelium (EP) and lumen (L) and stromal compartment (s). Treatments promoted important changes in the epithelial and stromal compartments, mainly in connective tissue in the subepithelial region (*), besides an increase of fibroblasts (arrows) within this area. Morphological modifications occurred in the smooth muscle cells (SMC) during ageing and post-treatment periods. The prostate of animals that received only corn oil did not present either morphological or biometric results significantly different from those of intact animals. Thus, the results obtained in these two groups were aggregated as Control. Barr = 40μm, exception for the Control of Old age where barr = 20 μm.
Quantitative analysis
The stereological data obtained for prostatic compartments of control and experimental groups are shown in Table 2. In the control groups, the percentage of glandular epithelial and stromal compartments remained relatively constant throughout the post-treatment period. In young gerbils, these prostatic compartment percentages were quite similar. On the other hand, the adult prostatic lumen area occupied approximately 50% of the prostatic volume and the stromal compartment was almost equally divided between SMC and non-muscular stroma. In the prostate of the old gerbil, the glandular epithelium presented a higher percentage than the stromal compartment. After the administration periods of Finasteride and Letrozole, an accentuated oscillation behaviour could be noted among prostatic compartments of young, adult and old gerbils. On the first post-treatment day, the percentage of prostatic epithelial volume density of young gerbils treated with Finasteride and Finasteride plus Letrozole decreased and was counterbalanced by a larger lumen area. Letrozole alone was capable of inducing a percentage elevation of epithelium and lumen area. In the young stromal compartment of the prostate gland, SMC and non-muscular stroma diminished after Finasteride, Letrozole and Finasteride plus Letrozole treatment, which remained until the end of the post-treatment period, except for the non-muscular percentage. In the adult gerbil prostate, Finasteride and Finasteride plus Letrozole administration provoked a persistent increase of epithelium and decrease of lumen area, while both SMC and non-muscular stroma were occupying a greater area. In adults, both the prostatic glandular epithelial and stromal compartments having undergone Letrozole treatment presented balanced and expected behaviour. The smooth muscle and non-muscular stromal components were increased during all the Finasteride, Letrozole and Finasteride plus Letrozole old gerbil post-treatment periods. Their prostatic epithelial volume density was slightly diminished, as also occurred in the luminal area.
Table 2.
Percentage of prostatic tissue compartments in control and treated animals of different postnatal developmental ages (mean ± SE)
| Tissues | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epithelium | Lumen | Smooth muscle cell (SMC) | Fibrovascular stroma | ||||||||||
| Exp. groups | Post -treatment periods | Young | Adult | Old | Young | Adult | Old | Young | Adult | Old | Young | Adult | Old |
| Control | 1 | 22.2 ± 0.71 | 18.8 ± 0.9a,1 | 26.1 ± 1.1a,1 | 19.6 ± 1.7a,1 | 49.9 ± 2.3a | 39.4 ± 1.7a | 26.0 ± 0.8a,1 | 12.8 ± 0.7a | 10.4 ± 0.4a,1 | 32.2 ± 1.6a,1 | 19.0 ± 1.5a | 23.6 ± 0.91 |
| 7 | 18.2 ± 0.7a,2 | 16.7 ± 0.7a,1,2 | 24.5 ± 0.91 | 26.8 ± 2.3a,1 | 49.5 ± 2.3a | 42.9 ± 1.2a | 23.6 ± 1.2a,1 | 11.8 ± 0.6a | 13.7 ± 0.5a,2 | 31.2 ± 1.6a | 22.0 ± 1.7a | 18.8 ± 0.9a,2 | |
| 14 | 18.9 ± 0.82 | 20.4 ± 1.3a,1 | 23.3 ± 0.91 | 34.4 ± 2.6a,2 | 50.9 ± 2.9a | 40.8 ± 1.5a | 20.2 ± 1.1a,2 | 12.1 ± 1.0a | 13.9 ± 0.6a,2 | 26.5 ± 1.6 | 16.9 ± 1.4a | 21.9 ± 0.9a,1 | |
| 21 | 21.3 ± 1.0a,1 | 22.0 ± 0.9a,1,3 | 22.1 ± 1.1a,2 | 21.3 ± 2.11 | 48.9 ± 2.0a | 39.1 ± 1.9a | 29.5 ± 1.3a,1,2 | 11.6 ± 0.6a | 14.1 ± 0.7a,2 | 27.8 ± 1.5a | 17.4 ± 1.2a | 24.3 ± 1.01 | |
| Finasteride | 1 | 19.9 ± 0.91 | 19.6 ± 0.8a,1 | 25.5 ± 0.7a | 40.8 ± 1.5b,1 | 44.2 ± 2.1a,1 | 29.3 ± 1.6b,1 | 19.9 ± 0.8b,1 | 14.2 ± 0.9a,c,1 | 16.6 ± 0.7b | 19.4 ± 1.3b,1 | 22.3 ± 1.7a,b | 28.5 ± 1.31 |
| 7 | 20.1 ± 1.1a,b,1 | 26.3 ± 1.2b,2 | 23.6 ± 0.8 | 34.2 ± 2.2a,1 | 36.7 ± 2.4b,1 | 35.0 ± 1.4b,2 | 21.0 ± 1.3a,1 | 12.6 ± 0.6a,b | 16.8 ± 0.4b | 24.6 ± 1.3a,b,1 | 24.4 ± 1.6a | 24.8 ± 1.3b,1 | |
| 14 | 21.7 ± 0.81,2 | 18.8 ± 0.7a,1 | 24.4 ± 0.9 | 18.0 ± 1.5b,2 | 45.2 ± 1.9a,1 | 34.7 ± 1.2b,2 | 27.9 ± 0.9b,2 | 9.9 ± 0.7a,b,2 | 16.8 ± 0.4b | 32.2 ± 1.52 | 26.0 ± 1.8b | 23.9 ± 1.3a,b,2 | |
| 21 | 18.2 ± 0.9a,1,3 | 24.7 ± 0.9a,2 | 26.9 ± 1.1b | 20.2 ± 1.92 | 29.2 ± 2.6b,2 | 33.8 ± 1.1a,b,1 | 25.1 ± 1.3b,2 | 21.1 ± 7.1b,3 | 17.7 ± 0.6b | 36.4 ± 1.6a,2 | 23.8 ± 8.1b | 21.6 ± 0.92 | |
| Letrozol | 1 | 23.0 ± 0.91 | 14.5 ± 0.9b,1 | 19.0 ± 1.0b,1 | 27.5 ± 2.3c,1 | 61.9 ± 1.7b,1 | 38.1 ± 2.3a | 22.5 ± 1.1b | 8.8 ± 0.3b,1 | 16.4 ± 0.8b,1 | 26.9 ± 1.5a,1 | 14.8 ± 1.0a,1 | 26.5 ± 1.61 |
| 7 | 22.5 ± 0.9b,1 | 19.0 ± 0.8a,2 | 22.9 ± 1.12 | 28.2 ± 2.6a,1 | 54.3 ± 2.2a,2 | 36.2 ± 1.6b | 19.1 ± 0.9b | 9.6 ± 0.7a,1 | 13.6 ± 0.6a,2 | 30.2 ± 2.0a,1 | 16.7 ± 1.3b,1 | 27.4 ± 1.0b,c,1 | |
| 14 | 19.6 ± 0.92 | 20.9 ± 0.8a,3 | 21.8 ± 1.11 | 32.1 ± 1.8a,1,2 | 47.2 ± 1.7a,3 | 43.1 ± 1.7a | 18.7 ± 1.0a | 11.9 ± 0.7a,2 | 13.6 ± 0.6a,2 | 29.5 ± 1.71 | 19.8 ± 1.1a,2 | 21.6 ± 0.8a,2 | |
| 21 | 20.4 ± 0.8a,1 | 22.9 ± 0.6a,3 | 21.2 ± 0.8a,1 | 22.3 ± 1.91,3 | 44.5 ± 1.7a,3 | 41.8 ± 1.8a,c | 21.9 ± 1.3b | 13.2 ± 0.7a,2 | 15.3 ± 0.8a,1 | 35.3 ± 2.1b,2 | 19.0 ± 1.3a,1 | 21.6 ± 1.02 | |
| Fin + Let | 1 | 21.5 ± 0.81 | 20.8 ± 0.7a,1 | 21.5 ± 0.9b | 25.4 ± 1.1a,c,1 | 38.7 ± 1.6b,1 | 32.7 ± 1.8a,b,1 | 24.7 ± 0.9a,1 | 16.3 ± 1.0c,1 | 18.3 ± 0.8a,1 | 28.3 ± 1.5a,1 | 24.2 ± 1.1b | 28.2 ± 1.41 |
| 7 | 23.5 ± 1.2b,1 | 24.4 ± 0.8b,1 | 22.9 ± 0.8 | 11.9 ± 1.9b,2 | 35.0 ± 1.7b,1 | 29.2 ± 1.3c,1 | 28.3 ± 1.1c,1 | 14.6 ± 0.7b,1,2 | 17.1 ± 0.6a,c,2 | 36.6 ± 2.3a,c,2 | 25.9 ± 1.0a | 30.5 ± 1.1c,1 | |
| 14 | 21.8 ± 0.91 | 28.7 ± 0.7b,2 | 23.4 ± 0.7 | 16.2 ± 1.9b,3 | 31.2 ± 1.2b,2 | 34.3 ± 1.6b,1 | 31.4 ± 1.6b,2 | 14.0 ± 0.6a,c,1,2 | 15.6 ± 0.61 | 30.6 ± 1.41 | 26.1 ± 1.1b | 26.6 ± 1.4b,1 | |
| 21 | 16.3 ± 0.8b,2 | 28.3 ± 0.8b,2 | 22.3 ± 0.6a | 18.0 ± 1.33 | 31.1 ± 1.3b,2 | 40.4 ± 1.2a,c,2 | 24.5 ± 1.1b,1 | 18.3 ± 0.9b,1,3 | 14.1 ± 0.5a,2 | 41.1 ± 1.5b,2 | 22.5 ± 0.7b | 23.2 ± 1.42 | |
Statistical analysis were based on anova and Tukey tests.
Alphabetic superindices (a, b, c) indicate statistically significant inter-group differences, when comparing treatment kind; numeric superindices (1, 2, 3) indicate statistically differences inter-group differences, when comparing stages of post-treatment period. Significant P ≤ 0.05. Comparisons among different postnatal ages where not executed.
Ultrastructural analysis
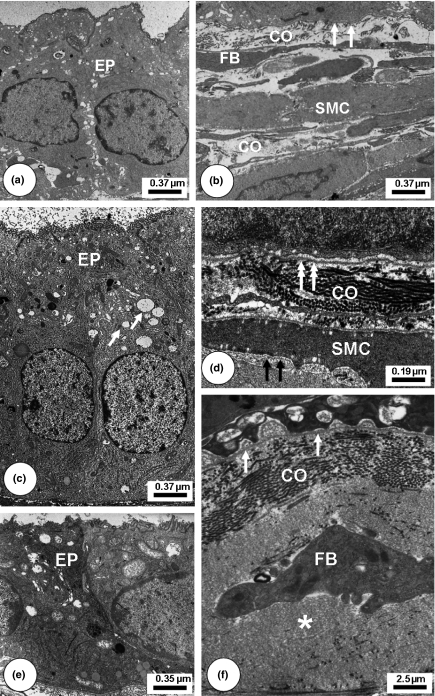
Ultrastructural analysis of the control ventral prostate (Figure 3) confirmed the data obtained by light microscopy. The immature prostate of young control animals showed epithelial cell cytoplasm with scarcity of synthetic organelles. In the stroma, collagen fibrils were intimately associated with the basal lamina and dispersed throughout the stroma. In the adults, the acinar epithelium was composed mostly of secretory cells, in which secretory vesicles were prominently displayed in the cytoplasm indicative of a synthetic phenotype. The collagen fibrils arranged in different directions were observed adjacent to the basal lamina. The SMC were densely packed and regularly organized into periacinar layers with only small intercellular spaces between SMC bundles. In prostate of old animals, the normal secretory epithelial cells presented accumulation of lipid droplets within the cytoplasm. The amount of collagen fibrils was greater at the epithelial base and arranged in a disorganized manner, as also happened among SMC, contributing to the slightly loose aspect of the smooth muscle.
Figure 3.
Transmission Electron Microscopy of control Mongolian gerbil ventral prostatic lobe regions. Young (a–b): Epithelial cells (EP) with few synthetic and secretory organelles. Smooth muscle cells (SMC) interspersed with some collagen fibrils (CO). Scant fusiform fibroblasts (FB) emitting short cytoplasmic projections. Basal lamina (white arrows). Adult (c–d): Cytoplasm of epithelium cells (EP) with numerous synthetic organelles and secretory vesicles (arrows). Collagen fibrils (CO). Intact basement membrane (white double arrows). SMC presenting a contractile pattern with characteristic basement membrane (black arrows). Old (e–f): The secreting epithelium (EP) cells presenting a notable decrease of cytoplasm area and secretory vesicles. Stroma with increased collagen fibrils (CO) and amorphous non-fibrillar material (*). Fibroblast cytoplasm projection (FB) was associated with collagen fibrils. Basal Lamina (white arrow).
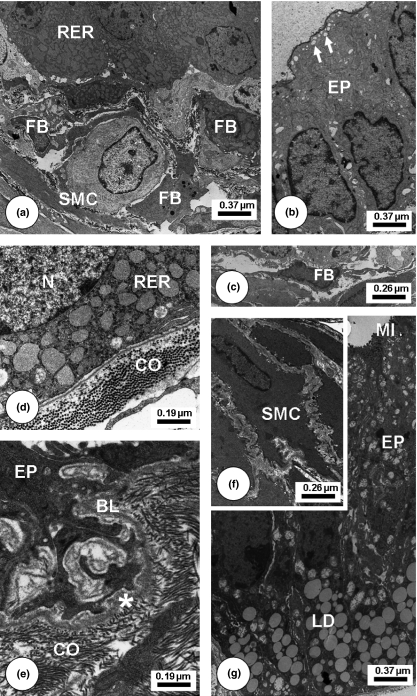
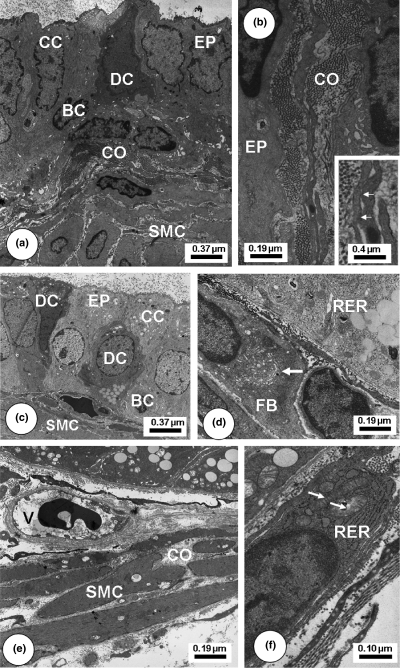
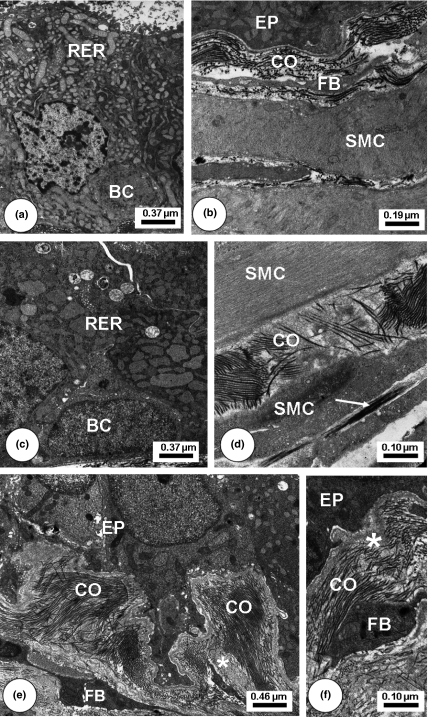
After Finasteride (Figure 4) and Finasteride plus Letrozole (Figure 5) treatments, ventral prostate of young, adult and old animals presented decreased cytoplasmic volume and fewer secretory blebs of epithelial cells, but still showed some secretory vesicles. In the stromal compartment, the connective tissue underlying the epithelium exhibited striking rises in the number and thickness of collagen fibrils accompanied by an increase in deposits of amorphous granular material adjacent to the basal lamina and in the clefts among SMCs. Fibroblast cytoplasm became denser and more voluminous with an enlargement of the perinuclear space and also a notable increase of the Golgi stacks. Some fibroblasts exhibited a more synthetic and activated aspect. Collagen fibrils had accumulated in the epithelial base and among SMC in a disorganized manner. SMC of the periacinar muscle layer had undergone morphological alterations, with reduced diameter and condensed cytoplasm. Other SMC had a highly irregular external contour with numerous spine-like cytoplasmic projections, which were in intimate association with collagen fibrils. After Letrozole treatment (Figure 6), the epithelial and stromal compartments also assumed different ultrastructural patterns, with shrinkage of epithelial layer, diminished epithelial cell cytoplasm and irregular contour of nuclei. As occurred in the stromal compartment of the other treatment types, dilated SMC displayed a loss of synthetic organelles in the perinuclear area and also fibroblasts had assumed an active aspect, besides longer projections, which had established close contact with other fibroblasts.
Figure 4.
Transmission Electron Microscopy of Finasteride-treated Mongolian gerbil ventral prostatic lobe regions. Young (a–c): Epithelial secretory cells (EP) with enlargement of rough endoplasmic reticulum (RER) and secretory vesicles in cellular apices (white arrows). Cellularity increased including fibroblasts (FB) at the subepithelial region. Adult (d): Enlarged cistern of the RER in the basal perinuclear (N) region. Collagen fibrils (CO) increase at epithelial base. Old (e–g) secretory cells with decrease of synthetic organelles in the basal cytoplasm and numerous infoldings were merged with basal lamina compounds (BL). Increased collagen fibrils (CO) adjacent to the basal lamina (BL). Great amount of lipid droplets (LD). Smooth muscle cells assumed a spinous aspect in its contour (SMC) and became interspersed by collagen fibrils. Microvilli (MI) in the apical region of epithelial cells (EP) in intimated contact with lumen area.
Figure 5.
Transmission Electron Microscopy of Letrozole-treated Mongolian gerbil ventral prostatic lobe regions. Young (a–b): Epithelial layer (EP) presented a variation of cellular phenotypes including clear (CC), dark (DC) and basal (BC) cells. Numerous smooth muscle cells (SMC) and collagen fibrils (CO). B-Inset: Detailed view of an activated fibroblast forming cytoplasm projections with focal adhesions (arrows). Adult. (c–d): Cellular heterogeneity as clear (CC), dark (DC) and basal (BC) cells. Rough endoplasmic reticulum (RER) enlarged cisterns in the basal perinuclear region. Fibroblast (FB) showing a large Golgi complex (arrow). Old (e–f): Smooth muscle cells (SMC) loosely packed and interspersed with collagen fibrils (CO). Blood vessel (V). Activated fibroblast adjacent to epithelial base with enlarged RER and mitochondria (arrows).
Figure 6.
Transmission Electron Microscopy of Finasteride + Letrozol-treated Mongolian gerbil ventral prostatic lobe regions. Young (a–b): Enlarged rough endoplasmic reticulum (RER) and secretory vesicles in cell apices. In the subepithelial area, collagen fibrils (CO) increased. Adult. (c–d): RER enlarged cistern occupied the most area among epithelial cytoplasm cells. Increased collagen fibrils (CO) at the epithelial base. Elastic fibre (white arrow). Old (e–f): Basal region of epithelial cells (EP) exhibiting infoldings. Increased amorphous non-fibrillar material (*), collagen fibrils (CO) and fibroblasts (FB) at the subepithelial base. Stroma with increased collagen fibrils (CO) and amorphous non-fibrillar material (*).Basal cell (BC), Smooth muscle cells (SMC).
Discussion
The present study demonstrates that the long-term administration of Finasteride and Letrozole, simultaneously or separately, to gerbils in different phases of postnatal development, causes important and persistent alterations in the prostate gland. Long-term use of Finasteride and Letrozole was capable of eliciting many persistent modifications within the gerbil ventral prostate morphology including changes in the tissue architecture, ultrastructure and extracellular matrix arrangement, besides altering the serum levels of steroidal hormones. The long experimental protocol employed herein resulted in circulating levels of testosterone and oestradiol higher than those measured in control animals.
A complete understanding of steroidal hormone actions requires a more specific mechanism for determination of how stromal and epithelial prostatic compartments normally communicate and how this cross-talk can be modified by hormones (Cunha et al. 2004). Androgen and oestrogen levels had been changed and began to oscillate after the enzymatic blockades by Finasteride and Letrozole, together or separately. However, this new morphological feature assumed by the prostate gland did not easily return to a normal histological pattern, either initially or in the final post-treatment stage at all ages evaluated herein. On the other hand, the adult gerbil prostate presented a more gradual recovery of the normal morphological environment, despite this gland still not showing any normal aspects such as those known and observed in the control gerbils at this age (Pegorin de Campos et al. 2006). With regard to the old animals of experimental groups, the term ‘recovery’ is relative, because in this rodent species, the prostate gland spontaneously develops pathological disorders during ageing as described by Pegorin de Campos et al. (2006). Furthermore, after Finasteride administration, they presented glandular regression, but only with some improvement of the histopathological conditions. At the three postnatal developmental ages of the gerbil experimental groups, the morphological results appeared to be parallel to those obtained with serum steroidal hormone assay, suggesting possible independent events. Based on these data, the steroid metabolizing enzymes 5α-r and Aro seemed to have similar and essential key roles to the maintenance of prostatic morphology during all postnatal gerbil development.
According to Corradi et al. (2004), the alterations provoked by Finasteride are probably the result of an imbalance of the homeostatic interaction between the epithelium and the underlying stroma. The use of Finasteride to inhibit the 5α-r action had provoked remarkable reduction of the prostatic complex weight, which confirms the main therapeutical effect of this drug in reducing the symptoms caused by BPH (Steers 2001). However, data obtained from analyses of the Finasteride post-treatment period reveal that when Finasteride administration was interrupted, the prostatic tissues responds in a different manner to the new hormonal situation established by the long-term absence of enzymatic action. The prostatic epithelial and stromal compartments and serum steroidal hormone levels had also undergone important modifications after Finasteride treatment. Adjacent to the epithelium, a denser layer of collagen surrounding each acinus was evident, which had not been observed during surgical castration periods (Vilamaior et al. 2000 and 2005).
Although androgens are the main steroids controlling prostate growth, increasing evidence demonstrates that oestrogens also regulate prostate development and growth including epithelial proliferation (McPherson et al. 2001; Härkonen & Mäkelä 2004). An increase of serum oestradiol concentration occurred after the Letrozole post-treatment period mainly in young and old gerbils. The elevated serum T level confirms the action of this drug in blocking its aromatization, but simultaneously raises another question regarding the activation and function of parallel pathways of intracrine steroid metabolism (Labrie et al. 2000). The rise of oestradiol levels within the experimental groups where the Aro enzyme was blocked is supported by the fact that this inhibition occurred in the direct local conversion of oestradiol, while those indirect pathways of oestrogen metabolism were still carried out by other specific enzymes (Soronen et al. 2004). Moreover, it has been noticed that DHT may also act indirectly through its oestrogenic metabolite 5-alpha-androstane-3-beta, 17-beta-diol, besides binding strongly to the oestrogen receptors (Soronen et al. 2004; Oliveira et al. 2007). Histological and biometric evaluations demonstrated enlargement of gerbil prostate even 21 days after the end of Letrozole administration. McPherson et al. (2001) obtained similar results in Aromatase knockout mice, concluding that, despite a long-term elevation of androgen and prolactin, the absence of oestrogen in these animals does not result in induction of prostate malignancy.
Finasteride plus Letrozole treatment may be used as an efficient tool in the attempt to understand that balance between androgens and oestrogens is essential for maintenance of the functional status of the prostate. The individual inhibition of 5α-r and Aro and consequently the withdrawal of DHT or oestradiol may mask the effect provoked by the hormone that has its concentration elevated. The simultaneous inhibition of these two enzymes was capable of inducing significant alterations in prostatic morphology at all studied postnatal ages. Although Finasteride and Letrozole individually induce their specific actions, when together, the effects of this simultaneous administration resemble those provoked by Finasteride only. In adult and old gerbils, the known function of Finasteride in reducing prostate size (Steers 2001) and weight remained active when used together with Letrozole, thus diminishing prostate weight. According to Huynh et al. (2001), blocking of oestrogen activity can also disrupt paracrine production of growth factors that act on the epithelial cells. To these authors, the loss of autocrine stimulatory activity on the stromal cells or paracrine activity on the epithelium by co-administration of Finasteride and anti-oestrogen inhibits epithelial cell activity, because prostate size was reduced, as was the cytoplasmic volume of luminal cells. A synergic action between Finasteride and Letrozole may be used to indicate a new mechanism by which oestrogens influence the normal growth and maintenance of the prostate. Thus, the withdrawal of DHT and oestrogen, provoked by Finasteride plus Letrozole treatment, modified the prostatic hormonal and morphological microenvironment of the young, adult and old gerbils, as Suzuki et al. (1998); Ito et al. (2000) and Huynh et al. (2001) had observed previously.
Ishikawa et al. (2006), using an in vitro model to assess the production of oestrogenic steroids, synthesized them via an Aromatase-independent pathway in non-breast cancer cells (HeLa) and in breast cancer cells, and noted that when oestrogens are no longer produced because of the absence of Aro action, the oestrogenicity of other less oestrogenic steroids synthesized from testosterone is readily detected. However, this oestrogenicity is reduced by additional treatment of cells with Finasteride. These effects may be occurring because of the long-term suppression of 5α-r and Aro enzymes by Letrozole and Finasteride plus Letrozole, and probably become persistent despite the cessation of treatments, which supports the morphological results found.
In conclusion, evidence from morphological findings demonstrates that the activity of 5α-r and Aro steroid-metabolizing enzymes appears crucial to the normal maintenance of prostatic biology during all the postnatal development. The blockade of these enzymes imposed a totally new condition on the prostate gland, which probably disrupted the epithelium-–stroma interaction and created a different microenvironment. All data obtained in this study, in association with the easy handling of gerbils plus the anatomical characteristics of their prostatic complex (Pegorin de Campos et al. 2006), indicate this rodent as an excellent model for studying the relationship between prostatic architecture and sex steroid hormones. Although serum hormone levels are important in prostate cancer, the role of local synthesis of steroids has assumed increasing significance in some diseases, particularly of glandular tissues, such as breast and prostate, wherein abnormal levels of oestradiol promote development and proliferation in early stages of malignant transformation of epithelial cells (Risbridger et al. 2003). Our study brings more light to the complex issue of mechanisms of local steroid metabolism in prostate cancer and how the histology of this gland behaves in a new hormonal microenvironment. However, further studies are needed, especially with regard to the intraprostatic hormones. As evidence is accumulating that the prostate gland is primarily influenced by local hormone steroid synthesis during the postnatal development period, it may be important to determine whether and/or how these enzymes act in the process of initiation, promotion and progression of prostate cancer.
Acknowledgments
This paper is part of the thesis presented by LSC to the Institute of Biology, UNICAMP, in partial fulfilment of the requirement for a PhD degree, and was supported by grants from the Brazilian agencies FAPESP – São Paulo Research Foundation (Proc. Nr. 03/9570-2 and 04/01603-1) and CNPq - Brazilian National Research and Development Council (Proc. Nr. 301111/05-7 research fellowship to SRT). The authors wish to thank Mr. Luiz Roberto Falleiros Júnior and MSc Rosana Silistino de Souza for their technical assistance as well as all other researchers at the Microscopy and Microanalysis Laboratory. Thanks are also due to James Welsh for English-language revision of this paper.
References
- Azzolina B, Ellsworth K, Andersson S, Geissler W, Bull HG, Harris GS. Inhibition of rat 5α-reductase by finasteride: evidence for isozyme differences in the mechanism of inhibition. J. Steroid Biochem. Mol. Biol. 1997;61:55–64. doi: 10.1016/s0960-0760(97)00002-2. [DOI] [PubMed] [Google Scholar]
- Behmer AO, Tolosa EMC, Neto AGF. São Paulo, Brazil: Edart-Edusp; 1976. Manual de práticas para histologia normal e patológica. [Google Scholar]
- Bruchovsky N, Wilson JD. Discovery of the role of dihydrotestosterone in androgen action. Steroids. 1999;64:753–759. doi: 10.1016/s0039-128x(99)00054-9. [DOI] [PubMed] [Google Scholar]
- Carruba G. Estrogen and prostate cancer: An eclipsed truth in an androgen-dominated scenario. J. Cell. Biochem. 2007;102:899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- Corradi LS, Góes RM, Carvalho HF, Taboga SR. Inhibition of 5-alpha-reductase activity as an inductor of stromal remodeling and smooth muscle dedifferentiation in adult gerbil ventral prostate. Differentiation. 2004;72:198–208. doi: 10.1111/j.1432-0436.2004.07205004.x. [DOI] [PubMed] [Google Scholar]
- Cotta-Pereira G, Rodrigo FG, David-Ferreira JF. The use of tannic acid-glutaraldehyde in the study of elastic related fibers. Stain Technol. 1976;51:7–11. doi: 10.3109/10520297609116662. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Ricke W, Thomson A, et al. Hormonal, cellular and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 2004;92:221–236. doi: 10.1016/j.jsbmb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Custódio AM, Góes RM, Taboga SR. Acid phosphatase activity in gerbil prostate: comparative study in male and female during postnatal development. Cell Biol. Int. 2004;28:335–344. doi: 10.1016/j.cellbi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Ekman P. The prostate as an endocrine organ: androgens and estrogens. Prostate Suppl. 2000;10:14–18. [PubMed] [Google Scholar]
- Góes RM, Zanetoni C, Tomiosso TK, Ribeiro DL, Taboga SR. Histological response on dorsal and ventral gerbil prostatic lobes induced by different testosterone withdrawal procedures. Micron. 2007;38:231–236. doi: 10.1016/j.micron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Härkonen PL, Mäkelä SI. Role of estrogens in development of prostate cancer. J. Steroid Biochem. Mol. Biol. 2004;92:297–305. doi: 10.1016/j.jsbmb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Wang Y, Cao M, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. [PubMed] [Google Scholar]
- Hsing AW, Reichardt JKV, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002;52:213–235. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]
- Huynh H, Alpert L, Alaoui-Jamali MA, Ng CY, Chan TW. Co-administration of finasteride and the pure anti-oestrogen ICI 182,780 act synergistically in modulating the IGF system in rat prostate. J. Endocrinol. 2001;171:109–118. doi: 10.1677/joe.0.1710109. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Glidewell-Kenney C, Jameson JL. Aromatase-independent testosterone conversion into estrogenic steroids is inhibited by a 5α-reductase inhibitor. J. Steroid Biochem. Mol. Biol. 2006;98:133–138. doi: 10.1016/j.jsbmb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Ito K, Fukabori Y, Shibata Y, et al. Effects of a new steroidal aromatase inhibitor, TZA-2237, and/or chlormadinone acetate on hormone-induced spontaneous canine benign prostatic hyperplasia. Eur. J. Endocrinol. 2000;143:543–554. doi: 10.1530/eje.0.1430543. [DOI] [PubMed] [Google Scholar]
- Labrie F, The-Luu V, Lin SX, Simard J, Labrie C. Role of 17β-hydroxysteroid dehydrogenase in sex steroid formation in peripheral intracrine tissue. Trends Endocrinol. Metab. 2000;11:421–427. doi: 10.1016/s1043-2760(00)00342-8. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Lund TD, Horvath LT. Brain androgen and progesserone metabolizing enzymes: biosynthesis, distribution and function. Brain. Res. Rev. 2001;37:25–37. doi: 10.1016/s0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- McPherson SJ, Wang H, Jones ME, et al. Elevated androgens and prolactin in aromatase deficient mice cause enlargment, but not malignancy of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Suzuki T, Nakabayashi M, et al. In situ androgen producing enzymes in human prostate cancer. Endocr. Relat. Cancer. 2005;12:101–107. doi: 10.1677/erc.1.00914. [DOI] [PubMed] [Google Scholar]
- Oliveira A, Coelho PH, Guedes FD, Mahecha GAB, Hess RA, Oliveira C. 5α-Androstane-3β,17β-diol (3β-diol), na estrogenic metabolite of 5α-dihydrotestosterone, is a potent modulator of estrogen receptor ERβ expression in the ventral prostate of adult rats. Steroids. 2007;72:914–922. doi: 10.1016/j.steroids.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Pegorin de Campos SG, Zanetoni C, Góes RM, Taboga SR. Biological behavior of the gerbil ventral prostate in three phases of postnatal development. Anat. Rec. A. Discov. Mol. Cell Evol. Biol. 2006;288:723–733. doi: 10.1002/ar.a.20347. [DOI] [PubMed] [Google Scholar]
- Pinheiro PFF, Almeida CCD, Segatelli TM, Martinez M, Padovani CR, Martinez FE. Structure of the pelvic and penile urethrarelationship with the ducts of the sex accessory glands of the Mongolian gerbil (Meriones unguiculatus) J. Anat. 2003;202:431–444. doi: 10.1046/j.1469-7580.2003.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. Comparative aspects of development and structure in the prostate. Natl. Cancer Inst. Monogr. 1963;12:1–27. [PubMed] [Google Scholar]
- Risbridger GP, Bianco JJ, Ellem SJ, Mc Pherson SJ. Oestrogens and prostate cancer. Endocr. relat. Cancer. 2003;10:187–191. doi: 10.1677/erc.0.0100187. [DOI] [PubMed] [Google Scholar]
- Rivas A, Fisher JS, McKinnell C, Atanassova N, Sharpe RM. Induction of reproductive tract developmental abnormalities in the male rat by lowering androgen production or action in combination with a low dose of diethylstilbestrol: evidence for importance of the androgen-estrogen balance. Endocrinology. 2002;143:4797–4808. doi: 10.1210/en.2002-220531. [DOI] [PubMed] [Google Scholar]
- Santos FCA, Góes RM, Carvalho HF, Taboga SR. Structure, histochemistry and ultrastructure of the epithelium and stroma in the gerbil (Meriones unguiculatus) female prostate. Tissue Cell. 2003;35:447–457. doi: 10.1016/s0040-8166(03)00071-5. [DOI] [PubMed] [Google Scholar]
- Santos FCA, Falleiros-Júnior LR, Corradi LS, Vilamaior PSL, Taboga SR. Experimental endocrine therapies promote epithelial cytodifferentiation and ciliogenesis in the gerbil female prostate. Cell Tissue Res. 2007;328:617–624. doi: 10.1007/s00441-007-0381-y. [DOI] [PubMed] [Google Scholar]
- Scarano WR, Vilamaior PS, Taboga SR. Tissue evidence of the testosterone role on the abnormal growth and aging effects reversion in the gerbil (Meriones unguiculatus) prostate. Anat. Rec. 2006;288:1190–1200. doi: 10.1002/ar.a.20391. [DOI] [PubMed] [Google Scholar]
- Soronen P, Laiti M, Törn S, et al. Sex steroid hormone metabolism and prostate cancer. J. Steroid. Biochem. Mol. Biol. 2004;92:281–286. doi: 10.1016/j.jsbmb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Steers WD. 5α-reductase activity in the prostate. Urology. 2001;58(6 Suppl. 1):17–24. doi: 10.1016/s0090-4295(01)01299-7. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Okazaki H, Kurokawa K, et al. Effects of dual inhibition of 5-alpha-reductase and aromatase on spontaneously developed canine prostatic hypertrophy. Prostate. 1998;37:70–76. doi: 10.1002/(sici)1097-0045(19981001)37:2<70::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Thomas LN, Douglas RC, Lazier CB, Too CK, Rittmaster RS, Tindall DJ. Type 1 and type 2 5aplha-reductase expression in the development and progression of prostate cancer. Eur. Urol. 2008;53:244–252. doi: 10.1016/j.eururo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Tindall DJ, Rittmaster RS. The rationale for inhibition 5alpha-reductase isoenzymes in the prevention and treatment of prostate cancer. J. Urol. 2008;179:1235–1242. doi: 10.1016/j.juro.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Canny BJ. The regulation of gonadotropin-releasing hormone induce calcium signals in male rat gonadotrophs by testosterone is mediated by dihydrotestosterone. Endocrinology. 1998;139:1038–1045. doi: 10.1210/endo.139.3.5796. [DOI] [PubMed] [Google Scholar]
- Vihko P, Herrala A, Härkönen P, Törn S, et al. Enzymes as modulators in malignant transformation. J. Steriod Biochem. Mol. Biol. 2005;93:277–283. doi: 10.1016/j.jsbmb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Vihko P, Herrala A, Härkönen P, et al. Control of cell proliferation by steroids: the role of 17HSDs. Mol. Cell. Endocrinol. 2006;248:141–148. doi: 10.1016/j.mce.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Vilamaior PSL, Felisbino SR, Taboga SR, Carvalho HF. Collagen fiber reorganization in the rat ventral prostate following androgen deprivation: an possible role for the smooth muscle cells. Prostate. 2000;45:253–258. doi: 10.1002/1097-0045(20001101)45:3<253::aid-pros8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Vilamaior PSL, Carvalho HF, Taboga SR. Modulation of smooth muscle cells function: morphological evidence for a contractile to synthetic transition in the rat ventral prostate after castration. Cell Biol. Int. 2005;29:809–816. doi: 10.1016/j.cellbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Wong YC. Sex hormone-induced prostatic carcinogenesis in the noble rat: the role of insulin-like growth factor-I (IGF-I) and vascular endothelial growth factor (VEGF) in the development of prostate cancer. Prostate. 1998;35:165–177. doi: 10.1002/(sici)1097-0045(19980515)35:3<165::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hayward SW, Donjacour AA, et al. Sex hormone-induce carcinogenesis in Rb-deficient prostate tissue. Cancer Res. 2000;60:6008–6017. [PubMed] [Google Scholar]
- Wang Y, Sudilovsky D, Zhang B, et al. A human prostatic epithelial model of hormonal carcinogenesis. Cancer Res. 2001;61:6064–6072. [PubMed] [Google Scholar]
- Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab. Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- Wu Q, Zhou Y, Chen L, et al. Benign prostatic hyperplasia (BPH) epithelial cell line BPH-1 induces aromatase expression in prostatic stromal cells via prostaglandin E2. J. Endocrinol. 2007;195:89–94. doi: 10.1677/JOE-06-0181. [DOI] [PubMed] [Google Scholar]
- Yuan-Shan Z. Molecular basis of steroid action in the prostate. Cellscience. 2005;1:27–55. doi: 10.1901/jaba.2005.1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]