Abstract
The main transmission route of Trypanosoma cruzi is by triatomine bugs. However, T. cruzi is also transmitted through blood transfusion, organ transplantation, ingestion of contaminated food or fluids, or is congenital. Sexual transmission, although suggested since the discovery of Chagas’ disease, has remained unproven. Sexual transmission would require T. cruzi to be located at the testes and ovaries. Here we investigated whether T. cruzi is present in the gonads of mice infected with 104 T. cruzi trypomastigotes from the CL strain. Fourteen days after experimental infection, histopathological examination showed alterations in the extracellular matrix of the lamina propria of the seminiferous tubules. Furthermore, amastigotes were present in seminiferous tubules, within myoid cells, and in the adjacencies of the basal compartment. These results indicate that T. cruzi is able to reach seminiferous tubule lumen, thus suggesting that Chagas’ disease could potentially be transmitted through sexual intercourse. Complementary studies are required to demonstrate that Chagas’ disease can be transmitted by coitus.
Keywords: collagenous, experimental Chagas’ disease, extracellular matrix, myoid cells, seminiferous tubules
Trypanosoma cruzi, a flagellated protozoan, is the aetiological agent of Chagas’ disease, the most important parasitic disease of the Americas. Traditionally confined to Latin America, Chagas’ disease is becoming an important health issue in the United States and Europe (Tarleton et al., 2007a, 2007b). Trypanosoma cruzi is mainly transmitted through blood-feeding triatomine bugs, but can also occur congenitally (Dorn et al., 2007; Gürtler et al., 2003), through blood transfusion (Young et al., 2007) or organ transplantation (Centers for Disease Control and Prevention, 2006), and through the ingestion of contaminated food or fluids (Benchimol Barbosa, 2006). It is estimated that 18 million people are chronically infected with the parasite and about 100 million are at risk of infection in 21 countries in Latin America – about 25% of the region's population (No authors listed 2006 apud Tarleton et al., 2007a, 2007b). Trypanosoma cruzi is a very polymorphic parasite and differences in infectivity and biological characteristics among the strains have been described (Andrade and Magalhães, 1997; Devera et al., 2002; Teixeira et al., 2006). This parasite can invade several organs and tissues whose involvement may contribute to the establishment of the disease (Calabrese et al., 1994; Lenzi et al., 1996; Teixeira et al., 2006); however, little is known about the effect of T. cruzi infection in the reproductive tract (Teixeira et al., 2006).
Since the first report of testis infection by T. cruzi (Vianna, 1911), the colonization of the urogenital system by the parasite has been observed several times in different experimental models. However, more studies are needed to elucidate this issue. Gonçalves da Costa et al. (1986) described the colonization of several structures of the urogenital system in susceptible newborn and 15 days old OF1 mice, both infected with either Y or CL T. cruzi strains. A high density of parasites was observed in the ovaries and testis. In the male reproductive system, interstitial cells and tunica albuginea of seminiferous tubules were largely colonized by the parasite. Lenzi et al. (1996 1998), using inbred BALB/c mice, described the presence of T. cruzi CL strain in the vagina, uterus, oviduct, in the theca layer of the ovary, mesovary, clitoris and mammary glands, epididymis, testicular Leydig cells, preputial glands and skin, penis, testicular albuginea, epididymis, vas deferens, seminal vesicles, prostate, coagulative, bulbi uretral and uretral glands. Carvalho et al. (1991) using the Bolivia strain of T. cruzi found parasites in the lumen of seminiferous tubules and mixed with spermatozoa in the lumen of the epididymal duct of infected mice.
The seminiferous epithelium is encircled by tunica propria (basement membrane and wall formed by type I collagenous fibres, contractile myoid cells, lymph, and lymphatic endothelium) (Siu and Cheng, 2004). Myoid cells are responsible for the rhythmic contractile activity that propels non-motile sperm to rete testis. The space among the seminiferous tubules is occupied by blood vessels, lymphatic sinusoids, macrophages, fibroblasts, aggregates of Leydig cells and extracellular matrix components (Skinner et al., 1985).
The extracellular matrix (ECM) consists of fibrous proteins (collagens and elastin) and amorphous components. Several isotypes of collagens, genetically distinct, have been described, and occur in different connectives tissues (Von der Mark et al., 1976). The Picrosirius-polarization method is a specific histochemical procedure for collagen detection in tissue sections (Montes, 1996). This procedure is also useful for studying differential distribution of distinct collagen types in routinely fixed, paraffin-embedded organs.
Specific interactions between parasites and extracellular matrix components are an important mechanism in the dissemination of Chagas’ disease. These recent observations of matrix abnormalities in experimental Chagas’ disease and the possibility that parasites may have enzymatic activities that could affect interstitial connective tissue directly (Factor et al., 1993), led us to study morphologic alterations of extracellular matrix in acute murine T. cruzi infection.
In the present work, we used outbred Swiss mice acutely infected with the CL strain of T. cruzi in order to investigate the interaction between parasite and myoid cells, as well as the alterations produced by the infection on the fibrous components of the extracellular matrix of the testis. Our results suggest a role for infected myoid cells in the possible sexual transmission of T. cruzi.
Therefore, 18 outbred Swiss Webster (SW) mice, all males and aging from 4 to 6 weeks old, were obtained from CECAL-FIOCRUZ and divided in two groups: group 1 (G1) comprised six mice which were kept non-infected as a negative control and group 2 (G2) comprised 12 mice that were all infected. During the experiments, all mice were maintained in Pavilhão Carlos Chagas facilities under controlled temperature, receiving food and water ad libitum. All procedures were in accordance with local ethic committee under the number P252/05.
For mice infection, the CL strain of T. cruzi was used. This strain was first isolated from a naturally infected Triatoma infestans, which was collected in a house in Rio Grande do Sul, Brazil (Brener and Chiari, 1963). The parasites were then maintained by serial passages in Swiss Webster mice every 14 days at the Laboratory of Immunomodulation and Protozoology, Oswaldo Cruz Institute. In the present work, blood trypomastigotes were collected from the blood of infected mice and purified chromatography in DEAE–cellulose (Sousa, 1983). Purified parasites were diluted in PBS to a concentration of 104 trypomastigotes in 0.2 ml and injected intraperitoneally (i.p.) in each. The experimental G2 was divided in two subgroups of six mice each: subgroup 1 to verify the parasitaemia and kinetics of mortality and subgroup 2 for histopathological studies.
Parasitaemia was determined microscopically by inspection of blood smears. Five microlitres of blood was collected from the tail vein of each of the six mice and placed under a 22 × 22 mm coverslip. Parasites were counted on 50 fields under a light microscope and this figure was then converted to the number of parasites per ml of blood. The number of parasites/ml was estimated daily as described by Pizzi (1957) until 50% of mice died. The same six mice were checked daily until the 20th day after infection. Mortality rate was recorded by cumulative percentage of six animals.
For histopathological study, six mice from each group (infected and non-infected) were killed 14 days post-infection (d.p.i.). The reproductive systems were removed and fixed in paraformaldehyde 4% in PBS 0.01 M, pH 7.45, 4 °C for 72 h. Tissue samples were taken for identification of genital anatomic structures and routinely processed for paraffin embedding in an Automatic Tissue Processor (Leica TP1020, Germany) and Paraffin Embedding Station (Micron AP280, Germany). Five micrometres thick semi-serial sections was obtained in Rotary Microtome (Micron HM360, Germany). Sections were stained using different methods as indicated: haematoxylin–eosin (H&E), Shorr modified by Carvalho (Carvalho, personal communication 2003), Lennert's Giemsa, Picro-Sirius red (Direct Red 80, Aldrich Milwaukee, WI, USA) and Gordon and Sweet's method for reticular fibres. For elastic fibres we stained tissue with Weigert's resorcin–fuchsin method. Cell types were scored based on their morphologic features analysed in Zeiss Axioplan 2 light microscope. Fragments of different organs were also routinely analysed for analysis tissue invasion pattern of T. cruzi CL strain.
All results presented in this paper are representative of three independent experiments that gave essentially the same results.
Parasitaemia revealed circulating parasites as early as 8 d.p.i. in mice inoculated i.p. with 104 bloodstream trypomastigotes of T. cruzi CL strain. The evolution of parasitaemia was characterized by an exponential increase in the number of trypomastigotes. The highest level of parasitaemia (more than 107 parasites/mm3 of blood) was observed 19 d.p.i.
Clinical signs of infections were detected in the second week after inoculum and mice started to die within 16 d.p.i. The surviving mice were severely sick and presented cachexia. Mean survival time of infected animals was 18.33 days (0/6) ± 0.7 (number of survivors/number of total mice) ± SEM.
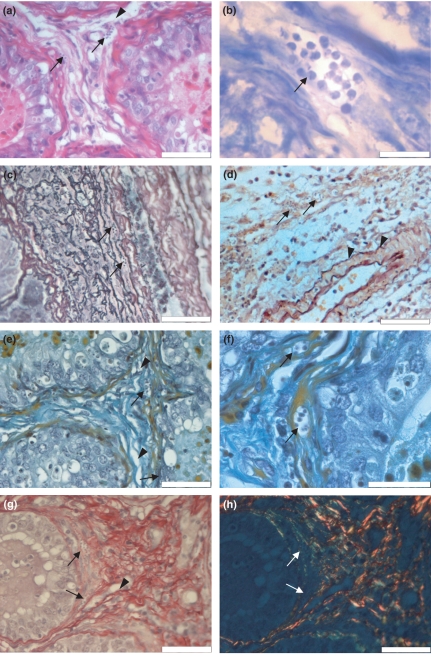
Histopathological findings revealed the presence of T. cruzi nests in the lamina propria (Figure 1a) and in myoid cells (Figure 1b) at 14 d.p.i. Myoid cells are stained orange by the modified Shorr method, providing a better contrast between myoid cells and surrounding tissue. Trypanosoma cruzi amastigotes are observed in the cytoplasm of myoid cells (Figure 1e, f) and surrounding basal compartment of a Sertoli cell (Figure 1f). We observed dissociation of collagen fibres near seminiferous tubules (Figure 1a, e–h) and vasculitis in the layer of the testis albuginea (data not shown). Histochemical analysis for fibres with silver affinity shows an intimate relationship between type III collagen and parasite nests (Figure 1c). Traditionally used for demonstration of fibres from the elastic system, the Weigert stain with prior oxidation by oxone, showed no alterations in the intertubular conjunctive tissue (Figure 1d). Picrosirius red staining showed a reduction of type I collagen, which might be due to enzymatic activity produced by the parasite, and enhancement of type III collagen, especially around parasite nests. In addition, appearance of neoformed type I collagen was observed, suggesting tissue to repair activity after type I collagen degradation by the parasite (Figure 1g, transmitted light and Figure 1h, polarized light). Furthermore, scrotum skin showed dissociation of collagen fibres and the presence of T. cruzi amastigotes, mainly near sebaceous glands and within hair erector muscles (data not shown).
Figure 1.
Histopathological analysis of Swiss mice testis, 14 days after infection with Trypanosoma cruzi, CL strain. (a) Nests of T. cruzi amastigotes (arrows) are observed in the intertubular connective tissue near the blood–testis barrier. Dissociation of collagen fibres near seminiferous tubers are also seen (arrow head), (haematoxylin–eosin); (b) nests of T. cruzi are observed within myoid cells (arrow) (Lennert's Giemsa); (c) histochemical analysis for fibres with silver affinity puts in evidence an intimate relationship between type III collagen and parasite nests (arrow) (Gordon and Sweet's methods contrasted with eosin); (d) intertubular connective tissue in which are seen a T. cruzi nest (arrow) and non-modified elastic fibres (arrow head) (Weigert's resorcin–fucsin after oxone, oxidation counterstained with orange G); (e) intertubular connective tissue presenting collagen dissociation (arrow head) and parasitized myoid cell (arrow) (modified Shorr); (f) amastigote nest in a myoid cell cytoplasm (arrow) (modified Shorr); (g) intertubular connective tissue presenting parasite nest (arrow) and type I collagen dissociation (arrow head) (Picrosirius Red, non-polarized light), (h) intertubular connective tissue showing type III collagen supporting the parasite nest (green) (arrow), type I typical collagen (orange and red) and neoformed type I collagen (yellow) (Picrosirius Red, polarized light). All scale bars = 50 μm, except for B and F in which scale bars = 20 μm.
Trypanosoma cruzi can invade and proliferate in almost all vertebrate host cell types. In experimental models, several authors have observed that different T. cruzi strains present various tissue tropisms (Bice and Zeledon, 1970; Melo and Brener, 1978). The CL strain is described as a myotropic strain, which preferentially infects heart and skeletal muscle (Melo and Brener, 1978). However, Gonçalves da Costa et al. (1986) and Lenzi et al. (1996) described the T. cruzi CL strain is ubiquitous in its infectivity spreading over diverse tissues and anatomical structures. In the present work, we demonstrated that T. cruzi CL strain may also infect myoid cells in testis, which, in light of our knowledge, has not yet been described in the literature.
Myoid cells are responsible for the rhythmic contractile activity that propels non-motile sperm to rete testis (Skinner et al., 1985). According to Vianna (1911), in the guinea pig model, Gonçalves da Costa et al. (1986) and Calabrese et al. (1994), in a murine model, T. cruzi can reach the sperm through the seminiferous tubules, epididymis and genital annexa. Teixeira et al. (1970, 2006) described the presence of amastigote nests in seminiferous tubes of the human testis. Unlike us, who observed a marked parasitism in testis, Carvalho et al. (1991) observed that parasitism was less marked in testis itself than in adjoining ducts and structures. There was no further analysis of the cell types infected by T. cruzi in these tissues. Our study suggests that myoid cells are the main targets of T. cruzi infection in the testis. The general structure of the testis was preserved in infected animals, confirming the results of Carvalho et al. (1991).
Lenzi et al. (1998) observed that the CL strain of T. cruzi has a preference for cells expressing contractile proteins, e.g. the muscular layer of reproductive organs. Our data show infection of intertubular myoid cells that also contain contractile proteins and therefore, are in agreement with Lenzi et al. (1998).
In the present study, we suggest that parasite migration to the seminal fluid can occur by myoid cell contraction and rupture, which leads to liberation of amastigotes into the seminiferous ducts.
Specific interactions between parasites and extracellular matrix components are an important mechanism in Chagas’ disease dissemination. Observations of ECM abnormalities in experimental Chagas’ disease, and the possibility that the parasite may have enzymatic activities that could directly affect the interstitial connective tissue (Factor et al., 1993), led us to study the morphologic alterations in the ECM of acute T. cruzi infection. Indeed, binding between extracellular matrix proteins and T. cruzi receptors has been described as a significant step in this phenomenon (Santana et al., 1997). It is important to report that Magalhães-Santos et al. (2004), working with Calomys callosus, did not observe an increase of collagen deposits in damaged tissues.
Taken together, our findings demonstrates that T. cruzi CL strain can colonize different cells, including myoid cells, which could have a pivotal role in the sexual transmission of this protozoan. Nattan Larrier (1921) hypothesized that Chagas’ disease could be transmitted by sexual route due to the liberation of amastigotes in the seminal fluid; our findings support this idea. Furthermore, we also have showed that fibrous extracellular matrix components play an active role during infection, since type III collagen sustains parasite nests.
Acknowledgments
The authors would like to thank Dr. Fabio Santoro for English revision, Dr Gregorio Santiago Montes (in memoriam) for providing Direct Red 80, to Mr. Arlindo Caldeira da Rocha (in memoriam) for histological sections. Equally we thank the instrumental support given by Call Zeiss of Brazil.
This work was supported by grants from Instituto Oswaldo Cruz, FIOCRUZ/Ministry of Health, Brazil.
References
- Andrade SG, Magalhães JB. Biodemes and zimodemes of Trypanosoma cruzi strains: correlations with clinical data and experimental pathology. Rev. Soc. Bras. Med. Trop. 1997;30:27–35. doi: 10.1590/s0037-86821997000100006. [DOI] [PubMed] [Google Scholar]
- Benchimol Barbosa PR. The oral transmission of Chagas’ disease: an acute form of infection responsible for regional outbreaks. Int. J. Cardiol. 2006;112:132–133. doi: 10.1016/j.ijcard.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Bice DE, Zeledon R. Comparation of the infectivity of strains of Trypanosoma cruzi (Chagas, 1909) J. Parasitol. 1970;56:663. [PubMed] [Google Scholar]
- Brener Z, Chiari E. Variações morfológicas observadas em diferentes amostras do Trypanosoma cruzi. Rev. Inst. Med. Trop. 1963;5:220–224. [PubMed] [Google Scholar]
- Calabrese KS, Lagrange PH, Gonçalves da Costa SC. Trypanosoma cruzi: histopathology of endocrine system in immunocompromised mice. Int. J. Exp. Pathol. 1994;75:453–462. [PMC free article] [PubMed] [Google Scholar]
- Carvalho TL, Ribeiro RD, Lopes RA. The male reproductive organs in experimental Chagas’ disease. I. Morphometric study of the vas deferens in the acute phase of the disease. Exp. Pathol. 1991;41:203–214. doi: 10.1016/s0232-1513(11)80092-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Chagas disease after organ transplantation – Los Angeles, California, 2006. MMWR Morb. Mortal. Wkly Rep. 2006;55:798–800. [PubMed] [Google Scholar]
- Devera R, Illarramendi X, Montoya-Araújo R, Pirmez C, Fernandes O, Coura JR. Biodemes of Trypanosoma cruzi strains isolated from humans from three endemic areas in Minas Gerais State. Rev. Soc. Bras. Med. Trop. 2002;35:323–330. doi: 10.1590/s0037-86822002000400008. [DOI] [PubMed] [Google Scholar]
- Dorn P, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, Wesson D. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerging Infect. Dis. 2007;13:605–507. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor SM, Tanowitz H, Wittner M, Ventura MC. Interstitial connective tissue matrix alterations in acute murine Chagas’ disease. Clin. Immunol. Immunopathol. 1993;68:147–152. doi: 10.1006/clin.1993.1111. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Segura EL, Cohen JE. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerging Infect. Dis. 2003;9:29–32. doi: 10.3201/eid0901.020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves da Costa SC, Calabrese KS, Alencar AA, Lagrange PH. Trypanosoma cruzi invasion of structure related to development and central nervous system. Rev. Bras. Neur. 1986;22:183–190. [Google Scholar]
- Lenzi HL, Oliveira DN, Lima MT, Gattass CR. Trypanosoma cruzi: paninfectivity of CL strain during murine acute infection. Exp. Parasitol. 1996;84:16–27. doi: 10.1006/expr.1996.0086. [DOI] [PubMed] [Google Scholar]
- Lenzi HL, Castelo-Branco MTL, Pelajo-Machado M, Oliveira DN, Gattass CR. Trypanosoma cruzi: compromise of reproductive system in acute murine infection. Acta Trop. 1998;71:117–129. doi: 10.1016/s0001-706x(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Magalhães-Santos IF, Souza MM, Andrade SG. Infection of Calomys callosus (Rodentia Cricetidae) with strains of different Trypanosoma cruzi biodemes: pathogenicity, histotropism, and fibrosis induction. Mem. Inst. Oswaldo Cruz. 2004;99:407–413. doi: 10.1590/s0074-02762004000400011. [DOI] [PubMed] [Google Scholar]
- Melo RC, Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J. Parasitol. 1978;64:475. [PubMed] [Google Scholar]
- Montes GS. Structural biology of the fibers of the collagenous and elastic systems. Cell Biol. Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- Nattan Larrier LA. La schizotrypanomiase americaine peut elle êttre trasnise par contagion genitale. C. R. Seances Soc. Biol. Fil. 1921;84:773–776. [Google Scholar]
- No authors listed. Chagas’ disease—an epidemic that can no longer be ignored. Lancet. 2006;368 doi: 10.1016/S0140-6736(06)69217-9. apud. [DOI] [PubMed] [Google Scholar]
- Pizzi T. In Immunologia de la enfermidad de Chagas. Prensa Universitario en Chile; 1957. Material y Metodos; pp. 40–42. [Google Scholar]
- Santana JM, Grellier P, Schrével J, Teixeira AR. A Trypanosoma cruzi-secreted 80 kDa proteinase with specificity for human collagen types I and IV. Biochem. J. 1997;325:129–137. doi: 10.1042/bj3250129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MK, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J. Cell Biol. 1985;100:1941–1947. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa MA. A simple method to purify biologically and antigenically preserved bloodstream trypomastigotes of Trypanosoma cruzi using DEAE-cellulose columns. Mem. Inst. Oswaldo Cruz. 1983;78:317–333. doi: 10.1590/s0074-02761983000300009. [DOI] [PubMed] [Google Scholar]
- Tarleton RL, Reithinger R, Urbina JA, Kitron U, Gürtler RE. The challenges of Chagas Disease-grim outlook or glimmer of hope. PLoS Med. 2007a;4:e332. doi: 10.1371/journal.pmed.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira AR, Nascimento RJ, Mott KE. Acute Chagas’ disease. Gaz. Méd. Bahia. 1970;70:176–186. [Google Scholar]
- Teixeira AR, Roters F, Sturm NR. Evolution and pathology in Chagas disease – a review. Mem. Inst. Oswaldo Cruz. 2006;101:463–491. doi: 10.1590/s0074-02762006000500001. [DOI] [PubMed] [Google Scholar]
- Vianna G. Contribuição para o estudo da anatomia patolojica da “Molestia de Carlos Chagas”. Mem. Inst. Oswaldo Cruz. 1911;3:276–292. [Google Scholar]
- Von der Mark H, von der Mark K, Gray S. Study of differential collagen synthesis during development of the chick embryo by immunofluorescence. Dev. Biol. 1976;48:237–249. doi: 10.1016/0012-1606(76)90088-9. [DOI] [PubMed] [Google Scholar]
- Young C, Losikoff P, Chawla A, Glasser L, Forman E. Transfusion-acquired Trypanosoma cruzi infection. Transfusion. 2007;47:540–547. doi: 10.1111/j.1537-2995.2006.01147.x. [DOI] [PubMed] [Google Scholar]