Abstract
Objective
To examine the chronic care model (CCM) as a framework for improving provider delivery of 5A tobacco cessation services.
Methods
Cross-sectional surveys were used to obtain data from 497 health care providers in 60 primary care clinics serving low-income patients in New York City. A hierarchical generalized linear modeling approach to ordinal regression was used to estimate the probability of full 5A service delivery, adjusting for provider covariates and clustering effects. We examined associations between provider delivery of 5A services, clinic implementation of CCM elements tailored for treating tobacco use, and the degree of CCM integration in clinics.
Principal Findings
Providers practicing in clinics with enhanced delivery system design, clinical information systems, and self-management support for cessation were 2.04–5.62 times more likely to perform all 5A services (p<.05). CCM integration in clinics was also positively associated with 5As delivery. Compared with none, implementation of one to six CCM elements corresponded with a 3.69–30.9 increased odds of providers delivering the full spectrum of 5As (p<.01).
Conclusions
Findings suggest that the CCM facilitates provider adherence to the Public Health Service 5A clinical guideline. Achieving the full benefits of systems change may require synergistic adoption of all model components.
Keywords: Chronic care model, tobacco cessation, Public Health Service 5A clinical guideline, preventive care, systems change, multilevel analysis
Tobacco use remains the leading cause of preventable death and disability in the United States (McGinnis and Foege 1993; Mokdad et al. 2004). Recent studies rank smoking cessation treatment as the most cost-effective preventive service due to its potential impact on public health and economic savings (Maciosek et al. 2006; Solberg et al. 2006). However, while health services such as tobacco screening and counseling have been thoroughly supported by evidence reviews and are highly recommended by the U.S. Preventive Services Task Force (USPSTF), significant gaps persist between recommended and usual care (Fiore et al. 2000; Hopkins et al. 2001; USPSTF 2005).
Best practices as outlined in the Public Health Service (PHS) Clinical Practice Guideline: Treating Tobacco Use and Dependence (Fiore et al. 2000) recommends the following “5As” approach: (1) ask about tobacco use at every visit, (2) advise smokers to quit, (3) assess smokers’ readiness to quit, (4) assist quit attempts through counseling and pharmacotherapy, and (5) arrange follow-up to prevent relapse. The guideline provides strong evidence that brief advice combined with additional assistance such as counseling and pharmacotherapy can double quit rates (Fiore et al. 2000). Despite this evidence, there is inconsistent adherence to the guideline. Rates for cessation advice range from 30 to 75 percent, with actual treatment ranging from 2 to 38 percent for pharmacotherapy to <10 percent for referral and follow-up (Denny et al. 2003; Quinn et al. 2005; Schroeder 2005; Ferketich, Kahn, and Wewers 2006).
The 5As are targeted to audiences such as primary care providers who are uniquely positioned to interact with smokers. More than 70 percent of smokers are seen by a primary care clinician at least once a year with an average of over three visits annually (Centers for Disease Control and Prevention 1993). Furthermore, many smokers report wanting their physician to discuss cessation with them and report greater satisfaction with the visit when their tobacco use is addressed (Fiore et al. 2000; Quinn et al. 2005). While primary care clinics are strategic venues for delivering cessation services, guideline awareness and dissemination alone are insufficient for routine application in busy care settings (Solberg et al. 2000).
This research examines the potential for the chronic care model (CCM), a systems-level quality improvement framework, to enhance 5As delivery by primary care providers. Studies of the CCM to date have typically focused on improving the care of patients with chronic illnesses including diabetes, hypertension, congestive heart failure, asthma, and depression (Wagner et al. 2001a, b; Bodenheimer, Wagner, and Grumbach 2002a, b; Bonomi et al. 2002; Kilbourne et al. 2004; Sperl-Hillen et al. 2004; Mangione-Smith et al. 2005; Pearson et al. 2005; Stroebel et al. 2005; Parchman et al. 2007). Beyond this, the model has also been preliminarily explored as a template for primary prevention and for delivery of services that address health risk behaviors (Glasgow et al. 2001; Hung et al. 2007). The current research builds on this work in prevention by specifically targeting tobacco use and examining how implementation of the CCM might improve provider adherence to treatment guidelines.
Methodologically, this study also builds on the existing CCM literature by using multilevel modeling techniques to estimate both organizational- and individual-level effects on provider delivery of health services. This approach indicates the extent to which provider delivery of the 5As may be attributed to clinic implementation of the CCM, adjusting for provider covariates and clustering. Thus, this study will: (1) describe the prevalence of CCM features tailored for treating tobacco use in urban primary care clinics; (2) examine relationships between provider 5As delivery and clinic implementation of each CCM element; and (3) examine associations between the degree of CCM integration in clinics and provider delivery of 5A services.
Conceptual Framework
The health systems and organization of health care element of the CCM refers to organizational or system values, structures, and mechanisms that facilitate high-quality care (Improving Chronic Illness Care [ICIC] 2008). Prior research found that a common feature among successful prevention programs was the existence of program directors who reported a strong commitment to preventive care (Glasgow et al. 2001). Practices with organizational cultures valuing quality improvement also offered a greater variety of preventive services for behavioral modification (Hung et al. 2007). Examples of this CCM element in studies of chronic disease management include policies for systems change, support from senior leaders, and incentives or rewards for achieving care delivery goals (Wagner et al. 2001a, b; Bodenheimer, Wagner, and Grumbach 2002a, b; Bonomi et al. 2002; Pearson et al. 2005).
Decision support describes interventions or activities that improve the knowledge and skills of health care providers, and that facilitate care consistent with scientific evidence (ICIC 2008). While treatment decisions based on evidence are important starting points, guidelines may not be as effective unless they are integrated into routine practice (Woolf et al. 1999). Common examples of decision support in prior studies include provider education, integration of clinical guidelines through reminder systems, and distribution of pocket cards to reference clinical information (Solberg et al. 2000; Bonomi et al. 2002; Pearson et al. 2005).
Enhanced delivery system designs assure that the needs of patient populations are met in a proactive and timely manner (Wagner et al. 2001a). This involves regular and well-planned care, not just spontaneous treatment of acute problems (Bodenheimer, Wagner, and Grumbach 2002a). Features of enhanced delivery systems include clearly defined provider roles, appropriate use of specialized health professionals, case management for more complex patients, and planned interactions such as group visits (Wagner 2000; Wagner et al. 2001a; Bodenheimer et al. 2002; Pearson et al. 2005).
Clinical information systems are an essential component of effective care management processes (Wagner et al. 1999; Rundall et al. 2002; Casalino et al. 2003). Such systems provide timely access to both patient and population data, and enable routine documentation of clinical activity and patient care needs. Information systems include disease registries to monitor patient populations, as well as either paper-based or electronic medical records to manage individual patient data (Wagner et al. 2001b; Bonomi et al. 2002; Pearson et al. 2005).
The self-management support element of the CCM supports patient roles in becoming informed, active participants in their own care (Wagner, Austin, and Von Korff 1996). The goal of self-management support is to activate patients by providing them with necessary information and tools to facilitate self-efficacy, i.e., the ability to carry out behaviors in order to reach their health goals (Bodenheimer et al. 2002). A core feature of effective self-management support is the routine application of patient-centered behavior change strategies such as the 5As (Bodenheimer et al. 2002; Glasgow et al. 2004; Wagner et al. 2005).
The final CCM element is that of community resources. This element expands care for patients and may include community programs, local or state health policies, insurance benefits, and advocacy groups (ICIC 2008). An important function of quality care includes leveraging community resources by referring patients to effective programs (Institute of Medicine [IOM] 2001). Thus, the community can play an important role especially in supporting cessation and quit attempts outside of the clinical setting (Barr et al. 2003).
Integration of CCM Elements
The CCM is a multicomponent model outlining six major elements in the organization, health system, and community. Owing to practical limitations, interventions may tend to focus on only one or two components that are viewed as most conducive to change. For this reason, relationships between each CCM component and 5As delivery will be examined. However, the CCM is ideally conceptualized as a holistic combination of all six elements that work together to foster quality improvement (Wagner et al. 2001a). While it is useful to consider individual aspects of the CCM, little is known about the extent to which care processes are affected by the degree of CCM implementation as a whole. To explore this question, associations between clinic integration of the CCM and provider delivery of 5A services will also be examined.
Methods
Data Sources
This study used cross-sectional survey data collected from approximately 500 primary care providers in 60 community clinics located throughout New York City. The vast majority of these sites were located in areas serving low-income, minority patient populations. Sites were affiliated with major teaching hospitals, owned by the NYC public health system, and in some cases were part of a private nonprofit entity or a private medical practice.
Two self-administered surveys were used to collect data at both the organizational level and individual provider level. A clinic survey was distributed to 70 sites, and was completed by a medical director or practice administrator at 60 of the sites (85.7 percent clinic response rate). This survey obtained a description of the organization including practice type, staffing patterns, and clinic structures or processes in place for addressing tobacco use. A provider survey was distributed to all clinical staff members at the 60 sites, and of 632 potential respondents, 497 responded yielding a 78.6 percent provider response rate across clinics. The average within-clinic provider response rate was 76 percent. This survey gathered data on provider demographics, job title or function, and performance of clinical activities including delivery of each of the 5As. Nonrespondents were not significantly different from respondents, except that they were more likely to be internists or obstetricians rather than family practitioners (p<.05). Fifteen clinics (25 percent) obtained survey responses from all clinical team members, and 54 clinics (90 percent) obtained responses from over half of their team members. Additional analyses were conducted that excluded clinics with provider response rates of <50 percent, and yielded no significant difference in study results.
Measures
Dependent Variable: Provider Delivery of 5A Services
The outcome for this study was based on the number of 5A services delivered by health care providers. The first “A” was assessed by the question, “During the past month, for how many of all patients did you ask about tobacco use status, regardless of their tobacco history?” The next two services were evaluated using two additional questions, “For how many of your patients who are tobacco users did you give advice or counsel to quit” and “assess the patient's readiness to quit?”Assistance was captured using the following questions, “For how many tobacco users who were ready to quit did you assist by prescribing nicotine replacement therapy or bupropion,” and “by referring to local cessation programs?” According to the PHS guideline, if providers reported doing either of these, they were considered to have assisted patients. The last 5A service was assessed by the question, “For how many tobacco users who were ready to quit did you arrange follow-up for tobacco use in person or by phone?” Response options for all questions were: none, few, half, many, and all/most patients. For each question, responses of all/most and many were coded “1” and counted to indicate the number of 5A services delivered by each provider. This was treated as a six-level ordinal outcome with categories ranging from 0 to 5 services, allowing for analysis of the extent of service delivery (i.e., how likely providers adhered to the full PHS guideline) without assigning equal weights to each service, particularly as some applied only to smokers.
Clinic-Level Independent Variables: CCM Elements and Integration
We operationalized each CCM element based on conceptual and empirical examples from the literature as previously described. The first element, health systems and organization of care, was characterized by whether or not clinics reported having a written policy, protocol, or guideline regarding tobacco identification and treatment, and whether clinicians were required to deliver each of the 5A services. These questions were surveyed as binary variables, and responses were averaged to create an aggregate measure of this CCM element in each clinic.
Decision support included training of health care providers to deliver brief interventions for cessation, distribution of FDA-approved nicotine replacement therapy lists or formularies, and promotion of evidence-based tobacco cessation guidelines via educational materials and reminders. These binary questions were derived from the clinic survey and averaged to create a score describing levels of clinician decision support for treating tobacco use.
Delivery system design was assessed by whether the clinic conducted group visits or activities for smokers ready to quit; presence of a key person in the clinic to coordinate tobacco cessation activities; and use of tobacco treatment specialists, social workers, case managers, or other dedicated staff to screen or provide brief counseling to tobacco users. All questions were measured as binary variables and averaged to form an aggregate measure of delivery system designs for tobacco cessation in clinics.
Use of clinical information systems was operationalized by whether the practice maintained a patient registry of tobacco users and the extent to which providers documented tobacco-related information in the patient's medical record (i.e., tobacco use status, advice or counsel given, patient's readiness to quit, prescription of tobacco pharmacotherapy). Responses to these provider documentation questions were scaled from 0 (never) to 1 (always) and were aggregated to the clinic level; analysis of variance confirmed greater between- than within-clinic variation (p<.01). All questions were averaged to create a clinic-level score describing the use of clinical information systems.
The 5As represent a patient-centered approach to supporting patient self-management. We therefore operationalized this self-management support element as the use of tools by providers to aid specifically with 5As delivery. We asked whether providers used a formal system (e.g., medical problem list, flow sheet, stamp, or label) to assist in delivering each of the 5As to their patients. While these systems might also be considered as decision support, for this study we define them as indicators of provider activity in helping smokers quit (in contrast, more general features as previously described were used to represent clinician decision support). Patient self-management support items were derived from the provider survey and ranged from 0 to 1 upon aggregation to the clinic level (p<.01).
Last, community resources were measured by the existence of clinic systems to refer patients to community programs or tobacco treatment specialists, perceived adequacy of local community resources, and provider referral to the NY Smokers’ Quitline. Responses were binary coded with the exception of provider referral to the Quitline, which was based on a scale from 0 (never) to 1 (always) and aggregated to the clinic level (p<.05). All clinic-level responses were averaged to create an overall measure of this final CCM element.
The degree of CCM integration in clinics was examined using two different approaches. The first approach examined CCM integration as a single continuous variable that was created by summing the six aggregated CCM scores for each clinic, all of which were measured on scales ranging from 0 to 1. In the second approach, a set of indicator variables was used to characterize the degree of CCM integration in each clinic. These indicator variables were created by first recoding each of the aggregated CCM scores into dichotomous variables (0–0.49=“0” and 0.50–1=“1”), and then summing these scores for each clinic. Each indicator variable was coded “1” if the clinic had implemented the relevant number of CCM element(s), with each indicator ranging from a possible zero to six elements.
Individual-Level Independent Variables: Provider Characteristics
Provider characteristics such as gender, age, and race/ethnicity were included in analyses. Owing to the distribution of health care providers in the sample, provider type was identified as allopathic (M.D.) or osteopathic (D.O.) physicians versus nonphysicians (e.g., nurse practitioners, physician assistants, registered nurses, certified nurse midwives, dentists/dental hygienists, other health professionals). The number of hours of patient care delivered per week was controlled for possible differences between full- or part-time providers and resident physicians in training. Also included was the number of years that providers worked at the clinic in which they were surveyed, which may have affected their familiarity with existing clinic systems and protocols.
Statistical Analysis
Provider and clinic characteristics were described, followed by bivariate analyses of 5As delivery, CCM implementation, and provider covariates across practices. Because provider data were nested within clinic-level data, we used a hierarchical generalized linear modeling (HGLM) approach to ordinal regression. This was a random intercept model with all random coefficients set to zero. The magnitude of variance among clinics in the unconditional model was 0.215 (p<.01), indicating that a sufficiently large portion of outcome variances was accounted for by clinic differences (Snijders and Bosker 1999). We therefore proceeded to model our outcome using HGLM ordinal regression to estimate the probability that providers delivered all 5A services relative to four or fewer services (Raudenbush and Bryk 2002). The equation used to estimate effects of provider covariates within clinics was expressed as the following:
Level 1
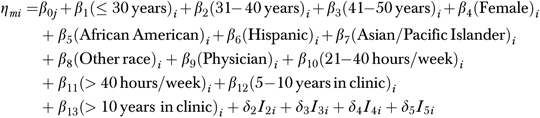 |
where η is the cumulative log-odds of provider i delivering m 5A services, m the number of 5As delivered of M−1 possible ordered categories, δ the threshold difference between categories M−1 and m, and I the indicator for category m.
The equation modeling random level-1 intercepts β0j as a function of clinic differences in CCM implementation:
Level 2
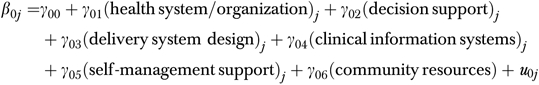 |
The degree of CCM integration in clinics was also analyzed using the level-1 equation and two separate level-2 equations. In the first approach to measuring integration, the summed CCM score for each clinic was entered into the level-2 equation as a single predictor. In the second approach, the set of indicator variables was entered as six predictors. All multilevel analyses were conducted using HLM 6.06 (Raudenbush, Bryk, and Congdon 2000).
Results
Table 1 presents a summary of provider characteristics and delivery of 5A services. Demographic profiles suggest a sample that was diverse in age and racial/ethnic composition. Almost three-quarters of respondents were physicians. The approximate rate of asking all patients about tobacco use regardless of tobacco history was 57.7 percent. Among patients who were identified as tobacco users, providers advised 83.7 percent to quit and assessed 68.8 percent of patients’ readiness to quit. For patients who were ready to quit, providers assisted 55.3 percent by prescribing tobacco pharmacotherapy and/or referring to cessation programs. Providers reported arranging follow-up in person or by phone with approximately 26 percent of patients. The median number of 5A services delivered by providers within and across clinics was three services.
Table 1.
Individual-Level Variables: Provider Characteristics and Delivery of 5A Services
| N | % | |
|---|---|---|
| Provider age (years) | ||
| ≤30 | 73 | 15.9 |
| 31–40 | 150 | 32.8 |
| 41–50 | 123 | 26.9 |
| >50 | 112 | 24.5 |
| Gender | ||
| Male | 190 | 38.2 |
| Female | 307 | 61.8 |
| Race/ethnicity | ||
| Non-Hispanic white | 223 | 45.5 |
| African American | 70 | 14.3 |
| Hispanic | 91 | 18.6 |
| Asian/Pacific Islander | 78 | 15.9 |
| Other | 28 | 5.71 |
| Provider type | ||
| Doctor of medicine | 362 | 72.8 |
| Doctor of osteopathy | 10 | 2.02 |
| Nurse practitioner | 37 | 7.46 |
| Physician assistant | 18 | 3.63 |
| Certified nurse midwife | 11 | 2.21 |
| Registered nurse | 37 | 7.46 |
| Dentist/dental hygienist | 10 | 2.02 |
| Other | 12 | 2.42 |
| Hours of patient care (per week) | ||
| ≤20 | 132 | 26.6 |
| 21–40 | 278 | 56.1 |
| >40 | 86 | 17.3 |
| Years practicing in clinic | ||
| <5 | 226 | 48.0 |
| 5–10 | 143 | 30.4 |
| >10 | 102 | 21.6 |
| Delivery of 5A services | ||
| Ask about tobacco use status of all patients, regardless of tobacco history | 287 | 57.7 |
| Advise patients who are tobacco users to quit | 416 | 83.7 |
| Assess patients’ readiness to quit | 342 | 68.8 |
| Assist patients who are ready to quit | 275 | 55.3 |
| Prescribe tobacco pharmacotherapy | 193 | 38.8 |
| Refer to local cessation programs | 146 | 29.4 |
| Arrange follow-up to address tobacco use | 129 | 26.0 |
| Number of 5A services delivered | ||
| 0 | 41 | 8.3 |
| 1 | 46 | 9.3 |
| 2 | 78 | 15.7 |
| 3 | 141 | 28.4 |
| 4 | 126 | 25.4 |
| 5 | 64 | 12.9 |
Table 2 describes clinic features consistent with the CCM and tailored for treating tobacco use. Survey items used to operationalize each of these CCM components and their corresponding scores are also shown. Across the 60 clinics, mean scores for each of the six CCM elements ranged from 0.31 (health system/organization) to 0.64 (clinical information systems) as measured on a scale from 0 to 1, indicating zero to full implementation. Summing these scores for each clinic, the average degree of CCM integration across all clinics was 3.06 on a scale ranging from a possible 0 to 6. As characterized by the set of indicator variables, the majority of clinics had implemented between two to four CCM elements. These results suggest moderate implementation of CCM features tailored for treating tobacco use.
Table 2.
Clinic-Level Variables: Implementation of Chronic Care Model (CCM) Components Tailored for Treating Tobacco Use
| N | Mean Scores | SD | Range | |
|---|---|---|---|---|
| Health systems/organization of health care | 60 | 0.31 | 0.86 | (0–1) |
| Written policy, protocol, or guideline for identifying and treating tobacco use | 25 | 0.42 | — | — |
| Clinicians required to screen at every visit | 20 | 0.33 | — | — |
| Clinicians required to advise or counsel patients to quit | 13 | 0.22 | — | — |
| Clinicians required to assess readiness to quit | 12 | 0.20 | — | — |
| Clinicians required to prescribe pharmacotherapy for those ready to quit | 8 | 0.13 | — | — |
| Clinicians required to refer to community programs or tobacco specialists | 10 | 0.17 | — | — |
| Clinicians required to arrange follow-up in person or by phone | 9 | 0.15 | — | — |
| Decision support | 60 | 0.55 | 0.35 | (0–1) |
| Provider training to deliver brief interventions | 32 | 0.53 | — | — |
| Distribution of lists or formularies of tobacco pharmacotherapy | 28 | 0.47 | — | — |
| Promotion of evidence-based tobacco guidelines via educational materials, reminders, provider training, etc. | 38 | 0.63 | — | — |
| Delivery system design | 60 | 0.43 | 0.32 | (0–1) |
| Key person to coordinate tobacco cessation activities | 29 | 0.48 | — | — |
| Group visits or activities for patients who want to quit | 18 | 0.30 | — | — |
| Tobacco treatment specialist, social worker/case manager, or other dedicated staff to screen patients for tobacco use | 41 | 0.68 | — | — |
| Tobacco treatment specialist, social worker/case manager, or other dedicated staff to provide brief counseling | 15 | 0.25 | — | — |
| Clinical information systems | 60 | 0.64 | 0.15 | (0.23–0.95) |
| Patient registry of tobacco users | 11 | 0.18 | — | — |
| Documentation of tobacco use status in medical record | 60 | 0.82 | 0.12 | (0.50–1) |
| Documentation of advice or counsel given to tobacco users | 60 | 0.82 | 0.18 | (0.25–1) |
| Documentation of patient's readiness to quit | 60 | 0.66 | 0.26 | (0–1) |
| Documentation of pharmacotherapy prescriptions | 58 | 0.72 | 0.35 | (0–1) |
| Self-management support | 60 | 0.63 | 0.25 | (0–1) |
| Formal system to aid routine screening for tobacco use | 59 | 0.83 | 0.24 | (0–1) |
| Formal system to advise or counsel smokers to quit | 59 | 0.69 | 0.31 | (0–1) |
| Formal system to assess patient's readiness to quit | 59 | 0.61 | 0.32 | (0–1) |
| Formal system to prescribe tobacco pharmacotherapy | 58 | 0.60 | 0.34 | (0–1) |
| Formal system to refer to community programs or tobacco specialists | 60 | 0.55 | 0.30 | (0–1) |
| Formal system to arrange follow-up | 59 | 0.44 | 0.30 | (0–1) |
| Community resources | 60 | 0.50 | 0.22 | (0–1) |
| Clinic-based system to link patients to community programs | 41 | 0.68 | — | — |
| Perceived adequacy of local referral resources | 42 | 0.70 | — | — |
| Referral to NY Smokers’ Quitline | 60 | 0.12 | 0.24 | (0–1) |
| Degree of CCM integration* | ||||
| Sum of CCM scores | 60 | 3.06 | 1.08 | (1.13–5.35) |
| # of elements implemented | ||||
| 0 CCM elements | 2 | 0.03 | — | — |
| 1 CCM element | 7 | 0.12 | — | — |
| 2 CCM elements | 14 | 0.23 | — | — |
| 3 CCM elements | 14 | 0.23 | — | — |
| 4 CCM elements | 12 | 0.20 | — | — |
| 5 CCM elements | 7 | 0.12 | — | — |
| 6 CCM elements | 4 | 0.07 | — | — |
All CCM components were surveyed as binary variables (0=no, 1=yes), except for clinical information system “documentation” items, all self-management support items, and community resources “NY Smokers’ Quitline” item (these variables ranged from 0 to 1 and were aggregated to the clinic level).
Two approaches were used to characterize the degree of CCM integration: (1) sum of the six CCM element scores and (2) indicator variables describing the number of CCM elements implemented in each clinic.
According to unadjusted bivariate results shown in Table 3, all CCM elements were positively and significantly related to provider 5As delivery across practices (p<.05). CCM integration as measured by summed CCM scores was positively related to 5As delivery (p<.001), as were indicators characterizing the number of CCM elements implemented in each clinic (p<.001). Provider type and hours of patient care were also significantly associated with delivery of 5A services (p<.05).
Table 3.
Bivariate Relationships between Provider 5As Delivery, Chronic Care Model (CCM) Implementation, and Provider Characteristics across Clinics
| Number of 5A Services Delivered | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | Spearman's ρ or χ2 | |
| CCM elements† | |||||||
| Health systems/organization | 0.16 (0.25) | 0.20 (0.32) | 0.17 (0.31) | 0.21 (0.31) | 0.33 (0.38) | 0.31 (0.36) | 0.16*** |
| Decision support | 0.29 (0.35) | 0.50 (0.33) | 0.47 (0.35) | 0.57 (0.32) | 0.54 (0.33) | 0.64 (0.35) | 0.18*** |
| Delivery system design | 0.29 (0.28) | 0.36 (0.25) | 0.39 (0.30) | 0.42 (0.29) | 0.46 (0.30) | 0.40 (0.29) | 0.16*** |
| Clinical information systems | 0.51 (0.17) | 0.59 (0.15) | 0.61 (0.12) | 0.65 (0.12) | 0.66 (0.15) | 0.67 (0.13) | 0.24*** |
| Self-management support | 0.49 (0.17) | 0.53 (0.17) | 0.53 (0.18) | 0.53 (0.20) | 0.57 (0.22) | 0.60 (0.21) | 0.13** |
| Community resources | 0.37 (0.22) | 0.44 (0.26) | 0.46 (0.21) | 0.47 (0.21) | 0.48 (0.23) | 0.49 (0.21) | 0.11* |
| Degree of CCM integration | |||||||
| Sum of CCM scores† | 2.13 (0.80) | 2.65 (0.98) | 2.65 (0.91) | 2.87 (0.94) | 3.06 (1.10) | 3.21 (1.02) | 0.24*** |
| # of elements implemented | |||||||
| 0 CCM elements | 8 (19.5%) | 2 (4.3%) | 2 (2.6%) | 1 (0.7%) | 3 (2.4%) | 0 (0.0%) | 74.45*** |
| 1 CCM element | 10 (24.4%) | 12 (26.1%) | 15 (19.2%) | 24 (17.0%) | 17 (13.5%) | 5 (7.8%) | |
| 2 CCM elements | 10 (24.4%) | 11 (23.9%) | 20 (25.6%) | 37 (26.2%) | 27 (21.4%) | 16 (25.0%) | |
| 3 CCM elements | 7 (17.1%) | 11 (23.9%) | 14 (17.9%) | 26 (18.4%) | 25 (19.8%) | 17 (26.6%) | |
| 4 CCM elements | 6 (14.6%) | 7 (15.2%) | 22 (28.2%) | 38 (27.0%) | 34 (27.0%) | 12 (18.0%) | |
| 5 CCM elements | 0 (0.0%) | 0 (0.0%) | 3 (3.8%) | 9 (6.4%) | 10 (7.9%) | 9 (14.1%) | |
| 6 CCM elements | 0 (0.0%) | 3 (6.5%) | 2 (2.6%) | 6 (4.3%) | 10 (7.9%) | 5 (7.8%) | |
| Provider age (years) | |||||||
| ≤30 | 3 (8.1%) | 7 (20.0%) | 18 (24.7%) | 21 (15.7%) | 16 (13.6%) | 7 (11.7%) | 20.45 |
| 40–54 | 8 (21.6%) | 7 (20.0%) | 22 (30.1%) | 45 (33.6%) | 45 (38.1%) | 23 (38.3%) | |
| 55–64 | 11 (29.7%) | 9 (25.7%) | 20 (27.4%) | 36 (26.9%) | 34 (28.8%) | 13 (21.7%) | |
| ≥65 | 15 (40.5%) | 12 (34.3%) | 13 (17.8%) | 32 (23.9%) | 23 (19.5%) | 17 (28.3%) | |
| Gender | |||||||
| Male | 10 (24.4%) | 23 (50.0%) | 30 (38.5%) | 56 (39.7%) | 41 (32.5%) | 29 (45.3%) | 9.25 |
| Female | 31 (75.6%) | 23 (50.0%) | 48 (61.5%) | 85 (60.3%) | 85 (67.5%) | 35 (54.7%) | |
| Race/ethnicity | |||||||
| Non-Hispanic white | 11 (27.5%) | 23 (51.1%) | 32 (42.1%) | 71 (51.4%) | 52 (41.3%) | 34 (53.1%) | 30.21 |
| Non-Hispanic black | 7 (17.5%) | 7 (15.6%) | 7 (9.2%) | 14 (10.1%) | 22 (17.5%) | 13 (20.3%) | |
| Hispanic | 13 (32.5%) | 3 (6.7%) | 16 (21.1%) | 23 (16.7%) | 28 (22.2%) | 8 (12.5%) | |
| Asian/Pacific Islander | 7 (17.5%) | 6 (13.3%) | 16 (21.1%) | 23 (16.7%) | 16 (12.7%) | 6 (9.4%) | |
| Other | 2 (5.0%) | 6 (13.3%) | 5 (6.6%) | 7 (5.1%) | 8 (6.3%) | 3 (4.7%) | |
| Provider type | |||||||
| Physician | 16 (39.0%) | 32 (69.6%) | 59 (75.6%) | 113 (80.1%) | 99 (78.6%) | 53 (82.8%) | 33.97*** |
| Nonphysician | 25 (61.0%) | 14 (30.4%) | 19 (24.4%) | 28 (19.9%) | 27 (21.4%) | 11 (17.2%) | |
| Hours of patient care (per week) | |||||||
| ≤20 | 8 (19.5%) | 13 (28.3%) | 20 (25.6%) | 37 (26.2%) | 36 (28.6%) | 18 (28.6%) | 20.44* |
| 21–40 | 29 (70.7%) | 25 (54.3%) | 36 (46.2%) | 76 (53.9%) | 68 (54.0%) | 43 (68.3%) | |
| >40 | 4 (9.8%) | 7 (17.4%) | 22 (28.2%) | 28 (19.9%) | 22 (17.5%) | 2 (3.2%) | |
| Years practicing in clinic | |||||||
| <5 | 18 (47.4%) | 12 (30.0%) | 40 (54.1%) | 66 (48.5%) | 59 (49.2%) | 30 (48.4%) | 8.20 |
| 5–10 | 10 (26.3%) | 16 (40.0%) | 20 (27.0%) | 45 (33.1%) | 34 (28.3%) | 18 (29.0%) | |
| >10 | 10 (26.3%) | 12 (30.0%) | 14 (18.9%) | 25 (18.4%) | 27 (22.5%) | 14 (22.6%) | |
p<0.05.
p<0.01.
p<0.001.
Mean (SD), Spearman's ρ used as test statistic.
Adjusting for provider covariates and all other CCM elements, the first HGLM analysis in Table 4 shows that delivery system design, clinical information systems, and self-management support for cessation were most significantly related to 5As delivery. Providers practicing in clinics with these CCM features were 2.04–5.62 times more likely to offer all 5A services (p<.05). Results from the second HGLM analysis in Table 4 show that CCM integration in clinics was also positively associated with 5As delivery. Each unit increase corresponded with a 1.81 odds that providers adhered more closely to 5A clinical guidelines (p<.001). This trend was observed again in the third HGLM analysis when varying degrees of integration were analyzed as indicator variables. Compared with providers in clinics that implemented none of the CCM, providers in clinics that implemented one and two elements were 3.69 and 5.33 times more likely to deliver all 5As (p<.01), while those in clinics implementing three and four elements had similar 8.32 and 7.68 odds of full 5As delivery (p<.001). Providers in clinics with the highest degree of CCM integration, featuring five and six elements tailored for treating tobacco use, were 20.4–30.9 times more likely to deliver the full spectrum of 5A services (p<.001).
Table 4.
Ordinal HGLM: Effects of CCM Elements and Degree of CCM Integration on Provider Delivery of 5A Services
| HGLM 1: CCM Elements | HGLM 2: Degree of CCM Integration (Sum) | HGLM 3: Degree of CCM Integration (Indicators) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient (SE) | t (p-value) | OR (CI) | Coefficient (SE) | t (p-value) | OR (CI) | Coefficient (SE) | t (p-value) | OR (CI) | |
| CCM elements | |||||||||
| Health systems/organization | 0.33 | 1.03 | 1.39 | — | — | — | — | — | — |
| (0.32) | (0.308) | (0.73, 2.61) | |||||||
| Decision support | 0.37 | 1.59 | 1.44 | — | — | — | — | — | — |
| (0.23) | (0.117) | (0.90, 2.31) | |||||||
| Delivery system design | 0.71*** | 3.78 | 2.04 | — | — | — | — | — | — |
| (0.18) | (0.001) | (1.40, 2.98) | |||||||
| Clinical information systems | 1.73** | 2.80 | 5.62 | — | — | — | — | — | — |
| (0.62) | (0.008) | (1.63, 19.36) | |||||||
| Self-management support | 1.09* | 2.22 | 2.98 | — | — | — | — | — | — |
| (0.49) | (0.031) | (1.11, 7.98) | |||||||
| Community resources | 0.12 | 0.48 | 1.12 | — | — | — | — | — | — |
| (0.25) | (0.636) | (0.68, 1.84) | |||||||
| Degree of CCM integration | |||||||||
| Sum of CCM scores | — | — | — | 0.60*** | 6.77 | 1.81 | — | — | — |
| (0.09) | (<0.001) | (1.52,2.16) | |||||||
| # of elements implemented (ref: 0) | |||||||||
| 1 CCM element | — | — | — | — | — | — | 1.31** | 3.31 | 3.69 |
| (0.40) | (0.002) | (1.67, 8.15) | |||||||
| 2 CCM elements | — | — | — | — | — | — | 1.67** | 3.77 | 5.33 |
| (0.44) | (0.001) | (2.19, 12.9) | |||||||
| 3 CCM elements | — | — | — | — | — | — | 2.12*** | 5.28 | 8.32 |
| (0.40) | (<0.001) | (3.72, 18.5) | |||||||
| 4 CCM elements | — | — | — | — | — | — | 2.04*** | 5.01 | 7.68 |
| (0.41) | (<0.001) | (3.40,17.3) | |||||||
| 5 CCM elements | — | — | — | — | — | — | 3.43*** | 7.18 | 30.9 |
| (0.47) | (<0.001) | (11.8,80.7) | |||||||
| 6 CCM elements | — | — | — | — | — | — | 3.01*** | 5.40 | 20.4 |
| (0.55) | (<0.001) | (6.67,62.2) | |||||||
| Provider characteristics | |||||||||
| Age (years) (ref: >50) | |||||||||
| ≤30 | 0.24 | 0.62 | 1.28 | 0.26 | 0.62 | 1.30 | 0.26 | 0.65 | 1.30 |
| (0.39) | (0.531) | (0.58,2.78) | (0.42) | (0.533) | (0.56,2.99) | (0.40) | (0.511) | (0.59,2.87) | |
| 31–40 | 0.53 | 1.70 | 1.71 | 0.51 | 1.64 | 1.66 | 0.51 | 1.71 | 1.67 |
| (0.31) | (0.090) | (0.92,3.16) | (0.31) | (0.100) | (0.90,3.07) | (0.30) | (0.087) | (0.92,3.01) | |
| 41–50 | 0.20 | 0.78 | 1.22 | 0.21 | 0.86 | 1.23 | 0.20 | 0.82 | 1.22 |
| (0.25) | (0.433) | (0.73,2.03) | (0.24) | (0.390) | (0.76,2.02) | (0.24) | (0.411) | (0.75,1.97) | |
| Gender (ref: male) | |||||||||
| Female | 0.08 | 0.43 | 1.08 | 0.10 | 0.55 | 1.10 | 0.09 | 0.50 | 1.09 |
| Race (ref: white) | (0.18) | (0.661) | (0.74,1.57) | (0.18) | (0.578) | (0.77,1.58) | (0.18) | (0.614) | (0.76,1.57) |
| African American | −0.02 | −0.07 | 0.97 | −0.03 | −0.14 | 0.96 | −0.01 | −0.02 | 0.99 |
| (0.28) | (0.940) | (0.56,1.70) | (0.27) | (0.885) | (0.56,1.63) | (0.27) | (0.988) | (0.57,1.71) | |
| Hispanic | −0.17 | −0.74 | 0.83 | −0.18 | −0.79 | 0.82 | −0.12 | −0.48 | 0.88 |
| (0.24) | (0.457) | (0.52,1.34) | (0.23) | (0.429) | (0.52,1.32) | (0.25) | (0.631) | (0.53,1.46) | |
| Asian/Pacific Islander | −0.61* | −2.27 | 0.54 | −0.60* | −2.17 | 0.54 | −0.58* | −2.12 | 0.55 |
| (0.26) | (0.024) | (0.31,0.92) | (0.27) | (0.030) | (0.31,0.94) | (0.27) | (0.034) | (0.32,0.95) | |
| Other | 0.06 | 0.15 | 1.06 | 0.03 | 0.08 | 1.03 | 0.04 | 0.11 | 1.04 |
| (0.40) | (0.880) | (0.48,2.33) | (0.37) | (0.930) | (0.49,2.17) | (0.38) | (0.906) | (0.48,2.24) | |
| Provider type (ref: nonphysician) | |||||||||
| Physician | 0.79* | 2.54 | 2.21 | 0.82** | 2.78 | 2.29 | 0.77* | 2.47 | 2.17 |
| (0.31) | (0.012) | (1.20,4.08) | (0.29) | (0.006) | (1.27,4.11) | (0.31) | (0.014) | (1.17,4.01) | |
| Hours of patient care (per week) (ref: ≤20) | |||||||||
| 21–40 | −0.06 | −0.29 | 0.93 | −0.04 | −0.19 | 0.95 | −0.02 | −0.13 | 0.97 |
| (0.21) | (0.766) | (0.61,1.42) | (0.21) | (0.845) | (0.63,1.45) | (0.21) | (0.897) | (0.63,1.49) | |
| >40 | −0.29 | −1.46 | 0.74 | −0.31 | −1.56 | 0.73 | −0.39 | −1.73 | 0.67 |
| (0.20) | (0.143) | (0.49,1.10) | (0.19) | (0.118) | (0.49,1.08) | (0.22) | (0.084) | (0.43,1.05) | |
| Years in clinic (ref: <5) | |||||||||
| 5–10 | −0.25 | −1.33 | 0.77 | −0.25 | −1.33 | 0.77 | −0.22 | −1.14 | 0.79 |
| (0.18) | (0.184) | (0.53,1.12) | (0.19) | (0.182) | (0.53,1.12) | (0.19) | (0.252) | (0.54,1.17) | |
| >10 | −0.21 | −0.70 | 0.80 | −0.17 | −0.62 | 0.83 | −0.16 | −0.56 | 0.84 |
| (0.30) | (0.481) | (0.44,1.46) | (0.28) | (0.53) | (0.48,1.46) | (0.29) | (0.571) | (0.47,1.50) | |
| Intercept | −5.01*** | −10.3 | 0.01 | −4.41*** | −8.79 | 0.01 | −4.61*** | −9.10 | 0.01 |
| (0.48) | (<0.001) | (0.01,0.02) | (0.50) | (<0.001) | (0.01,0.03) | (0.50) | (<0.001) | (0.01,0.03) | |
| Threshold differences (ref: 0 5A services) | |||||||||
| δ2 (m=4 services) | 1.56*** | 11.5 | 4.77 | 1.55*** | 11.6 | 4.73 | 1.55*** | 11.9 | 4.73 |
| (0.13) | (<0.001) | (3.65,6.23) | (0.13) | (<0.001) | (3.64,6.14) | (0.12) | (<0.001) | (3.67,6.11) | |
| δ3 (m=3 services) | 2.92*** | 18.5 | 18.6 | 2.90*** | 18.8 | 18.2 | 2.90*** | 20.1 | 18.2 |
| (0.15) | (<0.001) | (13.6,25.2) | (0.15) | (<0.001) | (13.5,24.7) | (0.14) | (<0.001) | (13.7,24.2) | |
| δ4 (m=2 services) | 3.90*** | 18.6 | 49.7 | 3.88*** | 18.8 | 48.7 | 3.88*** | 19.7 | 48.7 |
| (0.20) | (<0.001) | (32.9,74.9) | (0.20) | (<0.001) | (32.5,72.9) | (0.19) | (<0.001) | (33.1,71.8) | |
| δ5 (m=1 service) | 4.71*** | 20.7 | 111.6 | 4.68*** | 20.9 | 108.8 | 4.70*** | 21.4 | 110.1 |
| (0.22) | (<0.001) | (71.5,174.4) | (0.22) | (<0.001) | (70,168.9) | (0.21) | (<0.001) | (71.6,169.3) | |
p<0.05.
p<0.01.
p<0.001.
HGLM, hierarchical generalized linear model; CCM, chronic care model; SE, standard error; OR, odds ratio; CI, confidence interval.
Discussion
This study finds that providers practicing in clinics with greater CCM implementation adhered more closely to the PHS 5A clinical guideline for treating tobacco use. The CCM has been recognized for its role in improving the quality of chronic care, and we find new evidence for its potential role in addressing a major behavioral health issue as well. Primary care providers in urban clinics with enhanced delivery system designs, clinical information systems, and patient self-management support for tobacco cessation were more likely to offer 5A services. Also, there was an upward trend of improved 5As delivery with increasing CCM integration in clinics, suggesting that the model is most effective when more fully implemented in health care settings. While positive effects were observed with as few as one or two CCM elements, a much larger effect on guideline adherence came with fuller integration of the CCM. Findings suggest that achieving maximum benefits of systems change strategies may require synergistic adoption of all model components.
We used a novel multilevel modeling approach to examine provider practice patterns that has not to our knowledge been previously employed in studies of the CCM. Prior research has typically aggregated outcomes across patients or providers in an organization, with the exception of two studies that disaggregated patient outcomes (Mangione-Smith et al. 2005; Parchman et al. 2007). While aggregation of provider data may be appropriate to indicate clinic-level care processes, it does not offer more detailed information about care as delivered by providers themselves. This study models individual-level care processes while highlighting the influence of organizational structures on provider behavior. We note the relatively large effect sizes associated with the CCM and particularly with the trend of increasing CCM integration.
Adjusting for all other CCM elements, use of clinical information systems was the strongest correlate of 5As delivery. This finding reinforces the value of population-based approaches such as using patient registries to monitor and integrate tobacco treatment into routine clinical care (Casalino et al. 2003; Keller et al. 2005). This finding is also consistent with results from a study of participatory approaches to systems change, which found that requiring documentation of smoking status and readiness to quit, as well as instituting an electronic medical record, significantly increased clinical intervention (Fisher et al. 2005).
In light of the goal of full CCM integration, a practical consideration remains in terms of the relative ease of implementing each of the CCM components. For example, it may be easier to require documentation as part of the clinical information system or to designate specialized roles as part of the delivery system design, compared with changing health system and organizational values or the external environment of community resources. One study that assessed implementation of the CCM in quality improvement collaboratives found that while substantial changes were made to integrated systems of chronic care, not all CCM elements were implemented to the same degree (Pearson et al. 2005). Of all six elements, the greatest number of changes and depth of improvement were made to clinical information systems. This taken together with our findings suggests that tailoring information systems for tobacco treatment may be one of the most feasible and effective places to start in facilitating provider adherence to 5A guidelines.
Our results are also consistent with prior studies, which find that implementation of at least one CCM element is associated with better outcomes (Sperl-Hillen et al. 2004; Tsai et al. 2005). However, this study goes further to demonstrate an upward trend of improvement in service delivery with implementation of each additional CCM element. A Cochrane review in diabetes care similarly found that the most successful approaches were often multifaceted and that the more comprehensive the intervention, the more likely it was to achieve desired results (Renders et al. 2001). A meta-analysis of smoking cessation interventions also concluded that the greatest difference was made by multiple interventions rather than a single modality (Kottke et al. 1988).
Some limitations of this study include the limited sample size for evaluating other organizational characteristics. With 60 units of analysis at the clinic level, we examined only the six CCM elements to avoid overfitting the models. Secondly, we did not use the Assessment for Chronic Illness Care (ACIC) tool to evaluate CCM activities (Bonomi et al. 2002), as our survey instrument was originally developed to evaluate a statewide tobacco control effort. However, we adapted and cited extensive examples from the ACIC and other empirical work in chronic care to operationalize the CCM specifically for tobacco use treatment. Thirdly, our results are based on self-reported data. At the organizational level, we relied on accurate reporting by medical directors and practice administrators regarding tobacco-related systems and protocols in their clinics. At the individual level, we relied on provider self-reports, which may overestimate activities such as health behavior counseling (Thorndike et al. 1998). Alternative methods of assessing provider practice patterns such as patient report, direct observation, and medical record review each have their unique limitations (Stange et al. 1998; Pbert et al. 1999; Nicholson et al. 2000). However, a study comparing methods specifically to assess provider delivery of the 5As found that patient reports, and to some extent medical record reviews, did not significantly differ from provider self-reported data (Conroy et al. 2005).
Conclusion
Delivery of health services for tobacco cessation is a key metric in quality improvement initiatives, pay for performance, hospital accreditation, and provider recertification (IOM 2003). The PHS 5As approach to treating tobacco use is a patient-centered model of behavioral counseling that is also increasingly being used to manage chronic illnesses such as diabetes and hypertension (Glasgow et al. 2005). This broadening application of the 5As necessitates continued efforts to improve systems of care that will facilitate behavior change. The current study suggests that more comprehensive integration of the CCM in primary care settings may be an effective means for accomplishing this goal.
Acknowledgments
Joint Acknowledgement/Disclosure Statement: This study was made possible by the New York State Department of Health Tobacco Control Program and the Agency for Healthcare Research and Quality grant K02-HS017007-01. The authors would like to thank Albert Farias and John Wedeles for their assistance with data collection, and the anonymous reviewers for their constructive comments in improving this work.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Barr V J, Robinson S, Marin-Link B, Underhill L, Dotts A, Ravensdale D, Salivaras S. The Expanded Chronic Care Model: An Integration of Concepts and Strategies from Population Health Promotion and the Chronic Care Model. Hospital Quarterly. 2003;7(1):73–81. doi: 10.12927/hcq.2003.16763. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient Self-Management of Chronic Disease in Primary Care. Journal of the American Medical Association. 2002;288(19):2469–75. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Wagner E H, Grumbach K. Improving Primary Care for Patients with Chronic Illness. Journal of the American Medical Association. 2002a;288(14):1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T. Improving Primary Care for Patients with Chronic Illness: The Chronic Care Model, Part 2. Journal of the American Medical Association. 2002b;288(15):1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- Bonomi A E, Wagner E H, Glasgow R E, VonKorff M. Assessment of Chronic Illness Care (ACIC): A Practical Tool to Measure Quality Improvement. Health Service Research. 2002;37(3):791–820. doi: 10.1111/1475-6773.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino L, Gillies R R, Shortell S M, Schmittdiel J A, Bodenheimer T, Robinson J C, Rundall T, Oswald N, Schauffler H, Wang M C. External Incentives, Information Technology, and Organized Processes to Improve Health Care Quality for Patients with Chronic Diseases. Journal of the American Medical Association. 2003;289(4):434–41. doi: 10.1001/jama.289.4.434. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Physician and Other Healthcare Professional Counseling of Smokers to Quit—United States, 1991. Morbidity and Mortality Weekly Report. 1993;42:854–7. [PubMed] [Google Scholar]
- Conroy M, Majchrzak N, Silverman C, Chang Y, Regan S, Schneider L, Rigotti N. Measuring Provider Adherence to Tobacco Treatment Guidelines: A Comparison of Electronic Medical Record Review, Patient Survey, and Provider Survey. Nicotine and Tobacco Research. 2005;7(suppl 1):S35–43. doi: 10.1080/14622200500078089. [DOI] [PubMed] [Google Scholar]
- Denny C H, Serdula M K, Holtzman D, Nelson D E. Physician Advice about Smoking and Drinking: Are U.S. Adults Being Informed? American Journal of Preventive Medicine. 2003;24(1):71–4. doi: 10.1016/s0749-3797(02)00568-8. [DOI] [PubMed] [Google Scholar]
- Ferketich A, Kahn Y, Wewers M E. Are Physicians Asking about Tobacco Use and Assisting with Cessation? Results from the 2001–2004 National Ambulatory Medical Care Survey (NAMCS) Preventive Medicine. 2006;43(6):472–6. doi: 10.1016/j.ypmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Fiore M, Bailen W, Cohen S, Dorfman S, Goldstein M, Gritz E, Heyman R, Jaen C, Kottke T, Lando H, Mecklenburg R, Mullen P, Nett L, Robinson L, Stitzer M, Tommasello A, Villejo L, Wewers M. Treating Tobacco Use and Dependence: Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000. [Google Scholar]
- Fisher E, Musick J, Scott C, Miller J, Gram R, Richardson V, Clark J, Pachalla V. Improving Clinic- and Neighborhood-Based Smoking Cessation Services within Federally Qualified Health Centers Serving Low-Income, Minority Neighborhoods. Nicotine and Tobacco Research. 2005;7(suppl 1):S45–56. doi: 10.1080/14622200500078105. [DOI] [PubMed] [Google Scholar]
- Glasgow R, Goldstein M, Ockene J, Pronk N. Translating What We Have Learned into Practice. Principles and Hypotheses for Interventions Addressing Multiple Behaviors in Primary Care. American Journal of Preventive Medicine. 2004;2004(27):88–101. doi: 10.1016/j.amepre.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Glasgow R E, Nelson C C, Whitesides H, King D K. Use of the Patient Assessment of Chronic Illness Care with Diabetic Patients. Diabetes Care. 2005;28(11):2655–61. doi: 10.2337/diacare.28.11.2655. [DOI] [PubMed] [Google Scholar]
- Glasgow R E, Orleans C T, Wagner E H, Curry S J, Solberg L I. Does the Chronic Care Model Serve Also as a Template for Improving Prevention? Milbank Quarterly. 2001;79(4):579–612. doi: 10.1111/1468-0009.00222. iv–v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D, Briss P, Ricard C, Husten C, Carande-Kulis V, Fielding J, Alao M, McKenna J, Sharp D, Harris J, Woollery T, Harris K. Reviews of Evidence Regarding Interventions to Reduce Tobacco Use and Exposure to Environmental Tobacco Smoke. American Journal of Preventive Medicine. 2001;20(suppl 2):16–66. doi: 10.1016/s0749-3797(00)00297-x. [DOI] [PubMed] [Google Scholar]
- Hung D Y, Rundall T G, Tallia A F, Cohen D, Halpin H A, Crabtree B F. Rethinking Prevention in Primary Care: Applying the Chronic Care Model to Address Health Risk Behaviors. Milbank Quarterly. 2007;85(1):69–91. doi: 10.1111/j.1468-0009.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Improving Chronic Illness Care (ICIC) “The Chronic Care Model”. [accessed on February 28, 2008.]. Available at http://www.improvingchroniccare.org.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academic Press; 2001. [PubMed] [Google Scholar]
- Institute of Medicine. Priority Areas for National Action: Transforming Health Care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Keller P A, Fiore M C, Curry S J, Orleans C T. Systems Change to Improve Health and Health Care: Lessons from Addressing Tobacco in Managed Care. Nicotine and Tobacco Research. 2005;7(suppl 1):S5–8. doi: 10.1080/14622200500077966. [DOI] [PubMed] [Google Scholar]
- Kilbourne A, Schulberg H, Post E, Rollman B, Belnap B, Pincus H. Translating Evidence-Based Depression Management Services to Community-Based Primary Care Practices. Milbank Quarterly. 2004;82(4):631–59. doi: 10.1111/j.0887-378X.2004.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T E, Battista R N, DeFriese G H, Brekke M L. Attributes of Successful Smoking Cessation Interventions in Medical Practice. A Meta-Analysis of 39 Controlled Trials. Journal of the American Medical Association. 1988;259(19):2883–9. doi: 10.1001/jama.259.19.2883. [DOI] [PubMed] [Google Scholar]
- Maciosek M, Coffield B, Edwards N, Flottemesch T, Goodman M, Solberg L. Priorities among Effective Clinical Preventive Services: Results of a Systematic Review and Analysis. American Journal of Preventive Medicine. 2006;31(1):52–61. doi: 10.1016/j.amepre.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Mangione-Smith R, Schonlau M, Chan K S, Keesey J, Rosen M, Louis T A, Keeler E. Measuring the Effectiveness of a Collaborative for Quality Improvement in Pediatric Asthma Care: Does Implementing the Chronic Care Model Improve Processes and Outcomes of Care? Ambulatory Pediatrics. 2005;5(2):75–82. doi: 10.1367/A04-106R.1. [DOI] [PubMed] [Google Scholar]
- McGinnis J M, Foege W H. Actual Causes of Death in the United States. Journal of the American Medical Association. 1993;270(18):2207–12. [PubMed] [Google Scholar]
- Mokdad A H, Marks J S, Stroup D F, Gerberding J L. Actual Causes of Death in the United States, 2000. Journal of the American Medical Association. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Nicholson J, Hennrikus D, Lando H, McCarty M, Vessey J. Patient Recall versus Physician Documentation in Report of Smoking Cessation Counseling Performed in an Inpatient Setting. Tobacco Control. 2000;9:382–8. doi: 10.1136/tc.9.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchman M L, Zeber J E, Romero R R, Pugh J A. Risk of Coronary Artery Disease in Type 2 Diabetes and the Delivery of Care Consistent with the Chronic Care Model in Primary Care Settings: A STARNet Study. Medical Care. 2007;45(12):1129–34. doi: 10.1097/MLR.0b013e318148431e. [DOI] [PubMed] [Google Scholar]
- Pbert L, Adams A, Quirk M, Hebert J, Ockene J. Patient Exit Interview as an Assessment of Physician-Delivered Smoking Intervention: A Validation Study. Health Psychology. 1999;18:183–8. doi: 10.1037//0278-6133.18.2.183. [DOI] [PubMed] [Google Scholar]
- Pearson M L, Wu S Y, Schaefer J, Bonomi A, Shortell S M, Mendel P, Marsteller J A, Louis T A, Rosen M, Keeler E B. Assessing the Implementation of the Chronic Care Model in Quality Improvement Collaboratives. Health Services Research. 2005;40(4):978–96. doi: 10.1111/j.1475-6773.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn V, Stevens V, Hollis J, Rigotti N, Solberg L, Gordon N, Ritzwoller D, Smith K, Hu W, Zapka J. Tobacco-Cessation Services and Patient Satisfaction in Nine Nonprofit HMOs. American Journal of Preventive Medicine. 2005;29(2):77–84. doi: 10.1016/j.amepre.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk T. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd Edition. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Raudenbush S, Bryk T, Congdon R. HLM 6: Hierarchical Linear and Nonlinear Modeling. Lincolnwood, IL: Scientific Software International Inc; 2000. [Google Scholar]
- Renders C M, Valk G D, Griffin S, Wagner E H, Eijk J T, Assendelft W J. Interventions to Improve the Management of Diabetes Mellitus in Primary Care, Outpatient and Community Settings. Cochrane Database of Systematic Reviews. 2001;(1):CD001481. doi: 10.1002/14651858.CD001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundall T G, Shortell S M, Wang M C, Casalino L, Bodenheimer T, Gillies R R, Schmittdiel J A, Oswald N, Robinson J C. As Good as It Gets? Chronic Care Management in Nine Leading US Physician Organisations. British Medical Journal. 2002;325(7370):958–61. doi: 10.1136/bmj.325.7370.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder S. What to Do with a Patient Who Smokes. Journal of the American Medical Association. 2005;294(4):482–7. doi: 10.1001/jama.294.4.482. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- Solberg L, Maciosek M, Edwards N, Khanchandani H, Goodman M. Repeated Tobacco-Use Screening and Intervention in Clinical Practice: Health Impact and Cost Effectiveness. American Journal of Preventive Medicine. 2006;31(1):62–71. doi: 10.1016/j.amepre.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Solberg L I, Kottke T E, Brekke M L, Magnan S. Improving Prevention Is Difficult. Effective Clinical Practice. 2000;3(3):153–5. [PubMed] [Google Scholar]
- Sperl-Hillen J M, Solberg L I, Hroscikoski M C, Crain A L, Engebretson K I, O'Connor P J. Do All Components of the Chronic Care Model Contribute Equally to Quality Improvement? Joint Commission Journal on Quality and Safety. 2004;30(6):303–9. doi: 10.1016/s1549-3741(04)30034-1. [DOI] [PubMed] [Google Scholar]
- Stange K, Zyzanski S, Smith T, Kelly R, Langa D, Flocke S, Jaen C. How Valid Are Medical Records and Patient Questionnaires for Physician Profiling and Health Services Research? A Comparison with Direct Observation of Patient Visits. Medical Care. 1998;36:851–67. doi: 10.1097/00005650-199806000-00009. [DOI] [PubMed] [Google Scholar]
- Stroebel R J, Gloor B, Freytag S, Riegert-Johnson D, Smith S A, Huschka T, Nassens J, Kottke T E. Adapting the Chronic Care Model to Treat Chronic Illness at a Free Medical Clinic. Journal of Health Care for the Poor and Underserved. 2005;16:286–96. doi: 10.1353/hpu.2005.0041. [DOI] [PubMed] [Google Scholar]
- Thorndike A, Rigotti N, Stafford R, Singer D. National Patterns in the Treatment of Smokers by Physicians. Journal of the American Medical Association. 1998;279(8):604–8. doi: 10.1001/jama.279.8.604. [DOI] [PubMed] [Google Scholar]
- Tsai A C, Morton S C, Mangione C M, Keeler E B. A Meta-Analysis of Interventions to Improve Care for Chronic Illnesses. American Journal of Managed Care. 2005;11(8):478–88. [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Guide to Clinical Preventive Services 2005. Washington, DC: U.S. Department of Health and Human Services; 2005. [Google Scholar]
- Wagner E H. The Role of Patient Care Teams in Chronic Disease Management. British Medical Journal. 2000;320(7234):569–72. doi: 10.1136/bmj.320.7234.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E H, Austin B T, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving Chronic Illness Care: Translating Evidence into Action. Health Affairs (Millwood) 2001a;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- Wagner E H, Austin B T, Von Korff M. Organizing Care for Patients with Chronic Illness. Milbank Quarterly. 1996;74(4):511–44. [PubMed] [Google Scholar]
- Wagner E H, Bennett S M, Austin B T, Greene S M, Schaefer J, Von Korff M. Finding Common Ground: Patient-Centeredness and Evidence-Based Chronic Illness Care. Journal of Alternative and Complementary Medicine. 2005;11(suppl 1):S7–15. doi: 10.1089/acm.2005.11.s-7. [DOI] [PubMed] [Google Scholar]
- Wagner E H, Davis C, Schaefer J, Von Korff M, Austin B. A Survey of Leading Chronic Disease Management Programs: Are They Consistent with the Literature? Managed Care Quarterly. 1999;7(3):56–66. [PubMed] [Google Scholar]
- Wagner E H, Glasgow R E, Davis C, Bonomi A E, Provost L, McCulloch D, Carver P, Sixta C. Quality Improvement in Chronic Illness Care: A Collaborative Approach. Joint Commission Journal on Quality and Safety. 2001b;27(2):63–80. doi: 10.1016/s1070-3241(01)27007-2. [DOI] [PubMed] [Google Scholar]
- Woolf S H, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical Guidelines: Potential Benefits, Limitations, and Harms of Clinical Guidelines. British Medical Journal. 1999;318(7182):527–30. doi: 10.1136/bmj.318.7182.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


