Abstract
The nuclear hormone receptor peroxisome proliferator activated receptor gamma (PPARγ) is an important transcription factor regulating adipocyte differentiation, lipid and glucose homeostasis, and insulin sensitivity. Numerous genetic mutations of PPARγ have been identified and these mutations positively or negatively regulate insulin sensitivity. Among these, a relatively common polymorphism of PPARγ, Pro12Ala of PPARγ2, the isoform expressed only in adipose tissue has been shown to be associated with lower body mass index, enhanced insulin sensitivity, and resistance to the risk of type 2 diabetes in human subjects carrying this mutation. Subsequent studies in different ethnic populations, however, have revealed conflicting results, suggesting a complex interaction between the PPARγ2 Pro12Ala polymorphism and environmental factors such as the ratio of dietary unsaturated fatty acids to saturated fatty acids and/or between the PPARγ2 Pro12Ala polymorphism and genetic factors such as polymorphic mutations in other genes. In addition, this polymorphic mutation in PPARγ2 is associated with other aspects of human diseases, including cancers, polycystic ovary syndrome, Alzheimer disease and aging. This review will highlight findings from recent studies.
1. Introduction
Peroxisome proliferator activator receptor gamma (PPARγ) is a member of the nuclear hormone receptor superfamily that transcriptionally regulates genes controlling a variety of biological functions including cell growth, differentiation, and metabolism in response to lipophilic hormones, dietary fatty acids, and their metabolites [1]. Unlike some steroid hormone receptors such as the estrogen receptor, that are bound by heat shock proteins and sequestered in the cytoplasm, PPARγ is constitutively localized in the nucleus [2], heterodimerizes with the retinoid X receptor (RXR) [3], and binds to corepressors [4]. Ligand binding results in a conformational change in the receptor, triggering dissociation of corepressor complex and recruitment of coactivator proteins, leading to activation of gene expression [4].
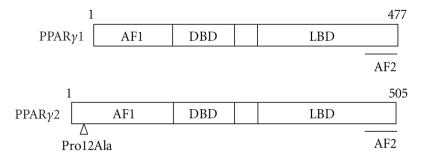
Human PPARγ gene is located in chromosome 3 and spans a genomic segment of >150 kb. It consists of 9 exons (A1, A2, B, and 1–6), from which the two distinct isoforms of PPARγ mRNA and protein, PPARγ1 and PPARγ2, are derived through the use of separate promoters and 5' exons. PPARγ1 mRNA specie is comprised of exons A1, A2, and 1–6, and is translated from P1 promoter while PPARγ2 mRNA is a combination of exons B and 1–6 and is translated from P2 promoter. The two proteins differ by the presence of extra 28 amino acids at the NH2-terminus of PPARγ2 [5, 6]. PPARγ is abundantly expressed in adipose tissue, colon and macrophages while its expression is much lower in skeletal muscle, heart and other tissues [7, 8]. PPARγ1 is ubiquitously expressed whereas PPARγ2 expression is restricted to adipose tissue [9] (Figure 1).
Figure 1.
Domain structure of human PPARγ. AF1, activation function 1; DBD, DNA binding domain; LBD, ligand binding domain; AF2, activation function 2.
PPARγ plays many functional roles in different organs and tissues (Figure 2). In vivo and in vitro studies demonstrate its critical role in regulating adipocyte differentiation and promoting lipid accumulation in adipose tissue [10–13]. It is also important for maintaining the viability and normal function of differentiated adipocytes [14–16]. In macrophages, PPARγ may enhance foam cell formation and atherogenesis upon increased uptake of oxidized low-density lipoprotein (oxLDL) [17, 18] or increases liver X receptor (LXR)-ATP-binding cassette A1 (ABCA1)-dependent cholesterol efflux upon pharmacological activation by its agonist TZDs [19, 20]. PPARγ in macrophages has also been shown to be involved in suppression of inflammatory cytokine production [21, 22] and improvement of insulin sensitivity [23, 24]. PPARγ in Skeletal muscle critically regulates normal glucose metabolism in muscle and lipid homeostasis in fat and the liver [25, 26] while PPARγ in the liver is implicated in controlling systemic glucose and lipid metabolism [27, 28]. PPARγ also plays roles in regulating bone homeostasis [29], heart hypertrophy [30, 31], high fat diet-induced hypertension [32], and urine concentration in the kidney (Cao et al., unpublished data).
Figure 2.
Pleiotropic functions of PPARγ in different organs/tissues.
PPARγ is also intimately implicated in regulation of glucose and lipid homeostasis and insulin sensitivity [33–35]. Not surprisingly, PPARγ has been identified as the target for thiazolidinediones (TZDs) [36], a class of synthetic compounds that improve insulin sensitivity in a variety of insulin resistant animal models and diabetic patients [33–35]. This role of PPARγ in affecting insulin action is consistent with many human genetic studies with various single amino acid mutations, including Pro12Ala, Pro115 Gln, Cys114Arg, Cyc131Tyr, Cyc162Trp, Val290Met, Pro388Leu, Arg425Cyc, His477His, and Pro467Leu that are scattered in activation function domain 1 (AF1), DNA binding domain (DBD), or ligand binding domain (LBD) of the receptor [37–45]. These mutations result in either gain-of-function or loss-of-function of the receptor; human subjects bearing these mutations show decreased or increased lipid accumulation in adipose tissue, enhanced insulin sensitivity or insulin resistance, dyslipidemia, diabetes, and hypertension [46–50]. Among these, Pro12Ala mutation in PPARγ2 (PPARγ2 Pro12Ala ) is the most common. This mutation was first identified by Schuldiner's group in 1997 [37], with different ethnic populations showing various allelic frequencies. Caucasians have the highest frequency (12%), followed by Mexican Americans (10%), West Samoans (8%), African Americans (3%) while Chinese have the lowest (1%) [37]. In the last 10 years, extensive studies have been undertaken to assess the effects of this polymorphism on many aspects of human physiology (Figure 3). This review will summarize the effect of this mutation on human health revealed in these studies.
Figure 3.
Effects of PPARγ2Pro12Ala polymorphism on various aspects of human health. FFAs, free fatty acids, IL-6, interleukin 6.
2. Effect of PPARγ2Pro12Ala on Adiposity
Soon after the identification of Pro12Ala mutation, an independent study demonstrates that Ala12 variant is associated with decreased transactivation function of PPARγ2 and lower body mass index (BMI) [51]. This finding is consistent with reduced adipogenic function of the mutant receptor in 3T3-L1 preadipocytes [52]. However, further studies in various ethnic populations demonstrate that effect of this mutation on body mass is more complex. An association of Ala12 variant with decreased adiposity is confirmed in diabetic, nondiabetic, or healthy subjects [53–58]. In studies involving an African American population and a white American population, this mutation is associated with lower BMI in African Americans and increased BMI in white Americans [59, 60], indicating that same genetic mutation results in different responses in different ethnic groups. PPARγ2 Pro12Ala mutation has been also shown to enhance weight loss brought about by exercise in offspring of type 2 diabetic subject [61] or prevent body weight regain after weight loss [62–64]. However, numerous studies suggests an association of Ala12 variant with increased risk of obesity, including studies in ethnic populations of Mexican Americans [65], male Spanish adults [66] or Spanish children and adolescents [67], French [68], male white Italians [69], French Canadians [70], male Brazilians of European descent [71], native Javanese [72], Uygurs, Kazaks, Hans (Chinese) [73], and Greek young girls [74]. This association can also be found in nondiabetic and nonobese or obese Americans [75], obese Finnish women [76], overweight Korean female subjects [but not in lean female subjects] [77] and in Turkish women with gestational diabetes [78]. In addition, women with the Ala12 allele have also been shown to gain more weight than women with Pro12 allele [79]. Despite these, studies in Germans [80], French [81], Hispanics (Colorado, US) [82], Japanese [83], Koreans [84, 85] and Polish [86] do not show an association between Pro12Ala polymorphism and body fat mass. Meta-analysis of 57 studies on nondiabetic individuals show that Caucasians with the X (Pro or Ala)/12Ala genotype is associated with significantly increased BMI, although no difference can be found in the global population [87]. These results indicate that a mild change in PPARγ2 transcription activity has a significant impact on lipid accumulation in adipose tissue.
It is unclear how a single genetic mutation results in conflict results in different ethnic populations. Given the proadipogenic role of PPARγ, it can be expected that moderate reduction of PPARγ2 transactivation function results in lower BMI in PPARγ2 Pro12Ala carriers. Heterogeneous effects of this polymorphic mutation on adiposity in association studies clearly show that PPARγ regulation of human adipose tissue physiology is a complex process. Several studies suggest roles of genetic or environmental contexts, such as the character of the diet, in shaping the patterns of associations of Pro12Ala polymorphism with body fat composition in different human populations. In at least two studies, ratio of dietary polyunsaturated fatty acid to saturated fatty acid (P : S ratio) have been shown to significantly affects body mass in Ala12 allele carriers. Thus, intake of a diet with higher P : S ratio results in lower BMI while a food with lower P : S ratio is inversely associated with BMI in human subjects carrying Ala12 allele [88]. Similarly, intake of monounsaturated fatty acid also shows such an effect in Ala12 allele carriers [89]. In another study, total fat and saturated fat intake is positively correlated with body mass change in Pro12 homozygotes while Ala12 allele carriers are protected [70]. In addition, changes in genetic context, such as coexistence of other polymorphisms, may have a significant impact on the effect of Pro12Ala polymorphism on body weight composition, resulting opposite findings mentioned above (flip-flop phenomenon) [90]. For example, either Pro12Ala or G174C (promoter region) of interleukin 6 (IL-6) shows an effect on reducing body fat mass or preventing body weight regain after weight loss and the presence of both variants has an additive effect [54, 62]. On the other hand, subjects bearing both Pro12Ala and Trp64Arg of β 3-adrenergic receptor (β 3-AR Trp64Arg ) have increased risk to obesity when compared to those carrying only a single mutation in a case-control study [67] or in a study in dizygotic twins [91], while subjects with the Ala12 allele become more obese only when they also carry the Trp64Arg variant in a Mexican American population [92]. These data suggest complex interactions between genes that both affect lipid metabolism. Yet, there is no study thus far to show that the effect of Pro12Ala polymorphism is negated by mutations in other genes.
3. PPARγ2Pro12Ala Regulating Insulin Sensitivity
PPARγ2 Pro12Ala has also been found to increase insulin sensitivity in middle-aged and elderly Finns [51] and this finding is confirmed by subsequent studies in other populations, assessed by plasma levels of insulin and homeostasis model assessment of insulin resistance (HOMA-IR) [54, 55, 59, 66, 79, 93–95]. In healthy carriers of the Ala12 allele, second-phase insulin secretion in response to free fatty acid infusion or insulin secretion in response to arginine is significantly decreased compared to subjects with Pro12 genotype [96]. Although increased glucose uptake in skeletal muscle is observed only in lean but not in obese subjects in Finns carrying Ala12 allele [97], enhanced insulin sensitivity is observed in obese children [98, 99] as well as in obese adults [100, 101]. Even in diabetic patients, Ala12 allele is associate with lower fasting insulin and increased insulin sensitivity [102], more significant hypoglycemic effect of exercise [103], and increased response to TZD treatment [104]. A population-based study in twins also shows a significant impact of the Ala12 allele on maintaining glucose tolerance and insulin sensitivity [105]. Meta-analysis of such studies confirmed a significantly lower levels of fasting insulin in subjects with the homozygous Ala12Ala genotype compared to the Pro12Pro genotype and significantly greater fasting glucose levels and insulin resistance in obese subjects in the Pro12Pro group [87]. These findings point to a beneficial effect of Ala12 variant on systemic insulin sensitivity.
The effect of PPARγ2 Pro12Ala polymorphism on insulin sensitivity can be influenced by dietary fatty acids and/or physical activity. Intake of monounsaturated fatty acids is inversely associated with insulin resistance in a Spanish population with Ala12 allele, especially in those with significant obesity [106]. Both dietary P : S ratio and physical activity have been shown to inversely associated with fasting insulin concentration [107]. The effect of dietary P : S ratio on fasting insulin is significant only in physically active, but not in physical inactive subjects carrying Ala12 allele [108]. Ala12 allele also interacts with other genes to influence insulin sensitivity. PPARα Leu162Val allele has been found to be associated with impaired glucose tolerance and this deleterious effect of PPARα mutation is neutralized by the Ala12 variant [109]. Similarly, the Gly > Arg mutation (Gly97Arg) of the insulin receptor substrate 1 (IRS1) is associated with a 15% increased risk of type 2 diabetes, although the difference is not significant [110]. Against this genetic background, insulin sensitivity is almost twice greater in carriers of the 12Ala allele than in subjects with Pro12 allele while no such effect of Ala12 allele can be seen on the Gly97 background [111]. Such a protective effect of Ala12 allele on insulin sensitivity can also be observed in human subjects carrying both the Ala12 allele and the Lys121Gln polymorphism of plasma cell 1 (PC-1) glycoprotein [112]. Subjects bearing PC-1 Lys121Gln variant show higher levels of fasting glucose and decreased insulin sensitivity on Pro12 background, whereas this effect of PC-1 Lys121Gln variant is lost on Ala12 background [113]. These results further support the notion that PPARγ2 Pro12Ala polymorphism interacts with other genetic mutations to affect systemic insulin sensitivity and glucose homeostasis.
4. Association of PPARγ2Pro12Ala with the Risk of Type II Diabetes
A large-scale family-based study shows an association between Pro12Ala mutation and reduced risk of type 2 diabetes (T2D) [110]. A similar result is obtained in twins carrying Ala12 allele [105]. However, further studies clearly show heterogeneous effects of this polymorphism on predicting susceptibility to the risk of diabetes in various populations. Resistance to the risk of diabetes has been found in Ala12 allele carriers compared to Pro12 allele carriers in ethnic populations as diverse as Japanese [114–116], Korean [117], Iranians [118], Scotts [119], Danish [120], Finns [121], French [122], Spanish [106], and American Caucasians [123, 124]. On the other hand, Ala12 allele has also shown to be functional leading to a predisposition to T2D in populations of Germans [125, 126], Finns [127], Italians [128], Dutch [129], US Caucasians [130], French Caucasians [81], British/Irish Caucasians [131], Asian Indians (Sikh) [132], Parkateje Indians [133], and Arabians [134]. Again, no such effect of Ala12 on the risk of type 2 diabetes can be observed in such diverse populations of Italians [135], Tunisians [136], Qatarians [137], Polish [138], and non-Hispanic and Hispanic white women [139]. In spite of such heterogeneity, however, meta-analysis of these studies indicates that Ala12 carriers have an average of 19% reduced risk of T2D compared to Pro12 carriers. BMI seems to be a major factor accountable for the heterogeneous effect of Pro12Ala polymorphism on the risk for T2D since the risk reduction is greater when BMI is lower. Risk reduction is higher in Asians carrying Ala12 allele (35%) than in Northern Americans and Europeans with the Ala12 genotype (18% and 15%, resp.) compared to their own Pro12 allele controls. When adjusted for the BMI of controls, difference between Asians and Europeans is no longer significant. Even among Europeans, Northern Europeans carrying Ala12 allele show significantly reduced risk for T2D (26%) while the risk reduction in Central and Southern Europeans with Ala12 allele is barely significantly (10%) or is not significant at all (0%) [140]. These data suggest a generally beneficial role of Ala12 allele in preventing the pathogenesis of T2D in several populations with lower body fat mass.
While the heterogeneity between Asians and other populations is statistically explained by BMI, this is not the case for the heterogeneity observed in Europeans, indicating that other factors, including different genetic and/or environmental background might cause the heterogeneous Pro12Ala-related T2D risk in Europeans. Indeed, the protective role of Ala12 allele against T2D is considerably affected by dietary lipid levels. In a study in human subjects from Ethiopia, Benin, Ecuador, Italy, and world populations, protection against T2D can be observed mainly in populations where energy from lipids exceeds 30% of total energy intake [141]. However, lipid composition in the diet is a significant determination factor since chronic intake of trans fatty acids and saturated fatty acids predispose to increased risk of T2D and impaired fasting glucose in Ala12 carriers than Pro12 carriers [142]. In addition, intrauterine condition may also determine the risk of T2D in later life. A study in Dutch population suggests that subjects bearing Ala12 allele are associated with a higher prevalence of impaired glucose tolerance and T2D when they are prenatally exposed to famine during midgestation [129]. On the other hand, Finns carrying Ala12 allele who have smaller body weight at birth seem to be protected against insulin resistance and T2D [143]. Again, Pro12Ala polymorphism interacts with other genetic mutations to affect the risk of developing diabetes. Subjects with the Ala12Ala allele and Gly972Gly variant of IRS-1 have significantly higher plasma adiponectin levels compared to those with the Pro12Pro and Gly972Gly genotype [144]. In Mexican Americans, subjects with the Ala12 allele become more obese only when they also carry the Trp64Arg of Beta-3 adrenergic receptor (β-3AR Trp64Arg ) polymorphism [92]. In a study in dizygotic twin pairs, those with both β-3AR Trp64Arg and PPARγ2 Pro12Ala polymorphisms show greater BMI, waist to hip ratio, percent of body fat, and blood glucose [91]. Such interaction between the two polymorphisms also increases the risk of obesity in children and adolescents [67]. In a family-based study in Chinese and Japanese, subjects with both Ala12 allele and the adiponectin T allele are more insulin sensitive than subjects bearing other combinations of genotypes [145]. Recently, an interaction between Ala12 variant and a single nucleotide polymorphism of PPARδ (rs6902123) has been found to contribute to conversion from impaired glucose tolerance to T2D [121]. These studies again emphasize the importance of taking into account of other gene mutations when determining an effect of Pro12Ala polymorphism on the risk of T2D.
5. Effect on Other Components of Metabolic Syndrome
The Ala12 allele has been shown to be associated with reduced prevalence of essential hypertension in Chinese nonagenarians and centenarian [146]. Ala12 allele carriers also show lower blood pressure than subjects carrying Pro12 allele [120, 147] and the Ala12 allele is associated with lower diastolic blood pressure in male, but not in female subjects with T2D [148]. Furthermore, hypertensive subjects with lower birth weight or shorter length at birth and Pro12Pro variant have raised blood systolic blood pressure [149]. However, others have suggested either a potential contribution of Ala12 variant to hypertension [115] or an association of Ala12 allele with higher diastolic blood pressure in obese patients with T2D [150] while couple of studies fails to show an association between the PPARγ2 variant and hypertension [151, 152].
Triglyceride (TAG) and cholesterol metabolism may be regulated by Pro12Ala mutation. Ala12 allele is inversely associated with blood TAG concentrations in one report [54] while it has also been found to be associated with a trend of an increase in TAG and hyperlipidemia in another [152]. This variant has also been shown to be associated with lower levels of serum total and nonhigh-density lipoprotein (non-HDL)-cholesterol in a general population [153], lower low-density lipoprotein (LDL)-cholesterol in T2D patients [154], or higher levels of serum HDL-cholesterol in family-based or population-based studies [155, 156]. however, several studies also show an association of Ala12 allele with higher concentration of low-density lipoprotein (LDL)-cholesterol [68, 157] and lower HDL-cholesterol [70]. Interestingly, Pro12Ala mutation interacts with body size at birth to modulate cholesterol metabolism since an association between increased concentration of serum total, LDL- and non-HDL-cholesterol and Ala12 allele can be found only in those who had birth weights below 3 kilograms [158]. In addition, cholesterol metabolism is also affected by genotype-alcohol interaction since Ala12 allele carriers consuming alcohol have higher serum total and HDL cholesterol while the nondrinkers carrying Ala12 allele show lower serum total and HDL cholesterol compared with Pro12 homozygotes [155].
Due to its role in regulating lipid metabolism, Pro12Ala polymorphism may influence risk of cardiovascular complications such as atherosclerosis and coronary artery diseases. Ala12 allele does not seem to affect the risk of acute myocardial infarction, coronary artery disease, and ischemic stroke in healthy subjects [159, 160]. In a population with an increased risk of T2D and cardiovascular disease, however, improvement in flow-mediated vasodilation and reduction of serum C-reactive protein (CRP), a risk factor for cardiovascular disease, are prominent only in Ala12 allele carriers, but not in Pro12 homozygotes [161]. Consistently, Ala12 allele carriers have been found to have lower carotid intima-media thickness [162, 163] and decreased risk of myocardial infarction [164] in T2D patients. Yet again, studies do show that Ala12 allele either is associated with increased risk of myocardial infarction [165, 166], or attenuates the protective effect of polyunsaturated fatty acids on myocardial infarction [167], or confers excess hazard of developing cardiovascular diseases in patients with diabetic nephropathy [168].
As a result of affecting lipid homeostasis and risk of diabetes, Pro12Ala mutation can be expected to influence diabetic complications. Notably, Ala12 allele is associated with decreased risk of developing diabetic nephropathy compared to Pro12 allele in a case-control study [169]. Ala12 allele carriers also have significantly reduced urinary albumin excretion than noncarriers and the reduction becomes even more dramatic along with increased duration of diabetes [154, 170]. Ala12 variant has also been shown to be associated with decreased risk of diabetic retinopathy in T2D patients [171]. These data suggest a protective effect of the Ala12 allele in relation to complications associated with T2D.
6. Effect on Polycystic Ovary Syndrome
Central obesity, insulin resistance, and hyperinsulinemia are typical feature of polycystic ovary syndrome (PCOS) and significant number of PCOS patients show impaired glucose tolerance and are in increased risk of developing T2D [172]. Studies show that frequency of Ala12 allele is significantly reduced in the PCOS group compared with the control group [173, 174]. Moreover, PCOS subjects carrying Ala12 allele show lower levels of free sex hormones (testosterone, androstenedione, and dehydroepiandrosterone sulfate) and reduced luteinizing hormone/follicle-stimulating hormone ratio compared to PCOS subjects carrying Pro12 allele [174]. Insulin sensitivity, evidenced by fasting insulin and HOMA-IR, is also significantly improved in Ala12 allele carriers than in Pro12 allele carriers [174–177]. Even in first-degree relatives of PCOS subjects, distribution of Ala12 Allele is significantly reduced compared to Pro12 allele [178] and fasting insulin and HOMA-IR are lower in first-degree relatives of PCOS subjects with Ala12 variant compared to first-degree relatives of PCOS subjects with Pro12 allele [178].
7. Cellular Mechanism of PPARγ2Pro12Ala Polymorphism
Since PPARγ2 is expressed only in adipose tissue, how moderate reduction of PPARγ2 activity in adipose tissue influences insulin sensitivity, diabetes, and other metabolic parameters have been studied but not fully elucidated. Given the role of adipose tissue free fatty acids and adipokines in regulating insulin sensitivity, the effect of Pro12Ala polymorphism can be anticipated to be mediated by changes in these factors. Indeed, subjects with Ala12 allele show lower lipoprotein lipase (LPL) activity [179], which may result in decreased breakdown of lipoproteins and hence, reduced plasma FFAs, which is deleterious to insulin action in skeletal muscle [180]. Consistent with this, Ala12 allele carriers have lower plasma FFAs, higher adipose tissue and skeletal muscle blood flow, and greater insulin-mediated postprandial hormone-sensitive lipase suppression along with greater insulin sensitivity [181]. Besides, insulin suppression of lipolysis in adipose tissue is also increased in lean subjects or in T2D patients carrying Ala12 allele than in subjects with Pro12Pro allele [182, 183]. However, long-term inhibition of lipolysis will, in theory, result in increased adiposity (body mass) rather than lean phenotype in Ala12 allele carriers. Indeed, one study suggests there is an association between Ala12 allele and increased body mass [182]. Obviously, this may not be the true mechanism or may not be the only mechanism underlying the effect of Pro12Ala. Adipose-derived cytokines leptin and adiponectin levels have been shown to increase insulin action [184, 185]. Indeed, Ala12 allele is associated with higher plasma levels of leptin in Spanish diabetic women [186]. In comparison, two Japanese population studies show that Ala12 allele carriers have significantly lower plasma levels of adiponectin than Pro12 allele carriers [187, 188] and another two case-control studies in either diabetic patients or women with PCOS fail to find significant change in serum adiponectin levels [189, 190]. Adiponectin does not seem to play a role in increasing insulin sensitivity in Ala12 allele carriers. Finally, recent studies suggest that increased oxidative stress in adipose tissue is a contributing factor to insulin resistance in obesity [191] and that insulin sensitization by PPARγ agonists is mediated, at least in part, by suppressing oxidative stress in adipose tissue [192]. In adipose tissue-restricted PPARγ heterozygous mice that show reduction of PPARγ in adipose tissue and similarly increased insulin sensitivity as in human subjects carrying Ala12 allele, antioxidant genes are significantly increased; this may be associated with increased resistance to chemical-induced oxidative stress in these animals [193]. Yet, it has not been investigated whether Pro12Ala polymorphism of PPARγ2 is associated with changes in oxidative stress in adipose tissue thus far.
8. PPARγ2Pro12Ala Polymorphism and Risk of Cancers
PPARγ ligands have been shown to inhibit proliferation of many tumor cells in vitro and PPARγ may also be implicated in tumorigenesis in vivo [194]. Although PPARγ2 is exclusively expressed in adipose tissue, genetic variation of PPARγ2 seems to indirectly affect the risk of several forms of tumors. The most studied thus far is the association between Ala12 allele with the risk of colorectal cancer. The Ala12 variant is inversely associated with incident sporadic colorectal adenoma, and the effect of this mutation is especially pronounced in women and those who do not take nonsteroidal anti-inflammatory drugs [195]. In a case-control study, Ala12 allele, together with high lutein intake, low refinery grain intake and a high prudent diet score, is associated with reduced risk of colon cancer [196]. Interestingly, the same study shows an increased rectal cancer risk in Ala12 carriers [196]. In another case-control study, Pro12Pro genotype is associated with increased risk of colorectal cancer while no such association is observed among Ala12 carriers [197]. In comparison, there is no evidence to show a significant association of Ala12 allele with colorectal cancer in an Indian (Asia) population [198]. In 3 studies related to gastric cancer, Ala12 allele has been found to be associated with increased risk of gastric cancer [199–201] and this effect of PPARγ is probably related to gastric mucosa atrophy and Helipobacteria pylori infection since the presence of Ala12 allele does not increase the risk of gastric cancer in H. pylori-negative subjects [199]. In two studies on prostate cancer, one study finds a 2-fold greater risk of prostate cancer in Ala12 allele carriers with BMI above 27.2 kg/m2 compared to those with the Pro12 allele [202] while the other study fails to notice such an association [203]. In addition, a marginally significant increase in the risk of breast cancer is observed in women carrying Ala12 variant [204], but Ala12 allele may decrease the risk of breast cancer associated with alcohol consumption [205]. Finally, Ala12 variant is associated with reduced risk of bladder cancer [206] and renal cell carcinoma [207]. The reason underlying some of the inconsistent findings is unclear, but again may reflect a possibility of gene-gene interaction. In at least one study, Pro12Ala allele interacts with vitamin D receptor (VDR)/bsm/polyA to increase risk of rectal cancer [208].
9. Effect on Aging and Alzheimer Disease
The potential role of genetic variability at Pro/Ala loci of PPARγ2 gene on longevity is studied in a group of centenarians and long-lived men show an increased frequency of Pro/Ala genotype [209]. PPARγ may also be associated with Alzheimer disease (AD) since activation of PPARγ decreases the release of amyloid-β (Aβ), main component of the amyloid plaques associated with AD [210–212]. In line with these observations, a study shows significant overrepresentation of Ala12 allele in octogenarian AD patients, compared to Pro12 allele [213]. However, this result is in contrast with a reported role of Ala12 variant in protecting pathogenesis of AD in female, but not in male subjects in a case-control study [214], while two studies fail to show an association between the Ala12 variant with the genetic risk of AD [215, 216]. Nevertheless, Ala12 allele carriers show an earlier onset of dementia [215], suggesting that Ala12 allele may modify the age of onset in late-onset AD. Ala12 allele carriers also show increased risk of dementia or cognitive impairment without dementia than noncarriers in diabetic patients [217, 218]. It is unclear how PPARγ2 Pro12Ala polymorphism confers such effects on human lifespan or age-related diseases since a change in PPARγ activity by this mutation is supposed to happen only in adipose tissue. Indeed, preliminary studies suggest that the effect of Ala12 allele on human aging may be attributable to decreased IL-6 levels, although there are also reports that healthy elderly have higher levels of IL-6 [219, 220]. In addition, PPARγ2 Pro12Ala polymorphism may affect pathogenesis of AD by modulating cholesterol metabolism since cholesterol levels influence AD pathology [221, 222]. Studies in larger population are required to further elaborate the role of PPARγ2 Pro12Ala polymorphism on blood cholesterol metabolism and AD.
10. Conclusion
Much has been done to evaluate the association between PPARγ2 Pro12Ala polymorphism and body mass, insulin sensitivity, risk of T2D, cancer, and other aspects of human health. However, it is not fully understood how reduction of PPARγ activity in adipose tissue can have such diverse effects on human health. While alteration of fatty acid and cytokine release from adipose tissue may underlie the effect of this mutation on insulin sensitivity and the risk of T2D, it is hard to believe that these factors also account for the effect of Pro12Ala polymorphism on cancer and age-related disease. It is likely that some factors that are overlooked or some unknown factors from adipose tissue may also play a role. Besides, the conflicting results often observed in association studies clearly show the presence of gene-gene interaction. Future association studies should employ a more comprehensive approach, such as linkage disequilibrium or haplotype analyses [223, 224], to examine influence of variants at other genetic loci that may compromise or enhance allelic effect of a genetic polymorphism. PPARγ2 Pro12Ala polymorphism will be a good model to elucidate how alteration of adipose PPARγ activity affects metabolic program and other aspects of human physiology.
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nature Reviews Immunology. 2006;6(1):44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 4.Perissi V, Rosenfeld MG. Controlling nuclear receptors: the circular logic of cofactor cycles. Nature Reviews Molecular Cell Biology. 2005;6(7):542–554. doi: 10.1038/nrm1680. [DOI] [PubMed] [Google Scholar]
- 5.Fajas L, Auboeuf D, Raspé E, et al. The organization, promoter analysis, and expression of the human PPARγ gene. The Journal of Biological Chemistry. 1997;272(30):18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 6.Greene ME, Blumberg B, McBride OW, et al. Isolation of the human peroxisome proliferator activated receptor gamma cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expression. 1995;4(4-5):281–299. [PMC free article] [PubMed] [Google Scholar]
- 7.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137(1):354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 8.Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegelman BM. PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47(4):507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 10.Barak Y, Nelson MC, Ong ES, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 11.Rosen ED, Sarraf P, Troy AE, et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Molecular Cell. 1999;4(4):611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 12.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 13.Kubota N, Terauchi Y, Miki H, et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Molecular Cell. 1999;4(4):597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 14.He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai T, Takakuwa R, Marchand S, et al. Peroxisome proliferator-activated receptor γ is required in mature white and brown adipocytes for their survival in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4543–4547. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JR, Barrick C, Kim K-A, et al. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(17):6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ . Cell. 1998;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 18.Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 19.Chawla A, Boisvert WA, Lee C-H, et al. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Molecular Cell. 2001;7(1):161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 20.Moore KJ, Rosen ED, Fitzgerald ML, et al. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nature Medicine. 2001;7(1):41–47. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 21.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 22.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metabolism. 2006;4(1):13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hevener AL, Olefsky JM, Reichart D, et al. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. The Journal of Clinical Investigation. 2007;117(6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hevener AL, He W, Barak Y, et al. Muscle-specific Pparg deletion causes insulin resistance. Nature Medicine. 2003;9(12):1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 26.Norris AW, Chen L, Fisher SJ, et al. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. The Journal of Clinical Investigation. 2003;112(4):608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavrilova O, Haluzik M, Matsusue K, et al. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. The Journal of Biological Chemistry. 2003;278(36):34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 28.Matsusue K, Haluzik M, Lambert G, et al. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. The Journal of Clinical Investigation. 2003;111(5):737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Y, Chong L-W, Evans RM. PPAR-γ regulates osteoclastogenesis in mice. Nature Medicine. 2007;13(12):1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 30.Ding G, Fu M, Qin Q, et al. Cardiac peroxisome proliferator-activated receptor γ is essential in protecting cardiomyocytes from oxidative damage. Cardiovascular Research. 2007;76(2):269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specffic knockout and agonist of peroxisome proliferator-activated receptor-γ both induce cardiac hypertrophy in mice. Circulation Research. 2005;97(4):372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 32.Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARγ in endothelial cells influences high fat diet-induced hypertension. American Journal of Hypertension. 2005;18(4):549–556. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Evans RM, Barish GD, Wang Y-X. PPARs and the complex journey to obesity. Nature Medicine. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 34.Kahn CR, Chen L, Cohen SE. Unraveling the mechanism of action of thiazolidinediones. The Journal of Clinical Investigation. 2000;106(11):1305–1307. doi: 10.1172/JCI11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. The Journal of Clinical Investigation. 2000;106(4):467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) The Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 37.Chung-Jen Y, Beamer BA, Negri C, et al. Molecular scanning of the human peroxisome proliferator activated receptor γ (hPPARγ) gene in diabetic Caucasians: identification of a Pro12Ala PPARγ2 missense mutation. Biochemical and Biophysical Research Communications. 1997;241(2):270–274. doi: 10.1006/bbrc.1997.7798. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-γ gene in a patient with familial partial lipodystrophy. The Journal of Clinical Endocrinology & Metabolism. 2002;87(1):408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- 39.Agostini M, Gurnell M, Savage DB, et al. Tyrosine agonists reverse the molecular defects associated with dominant-negative mutations in human peroxisome proliferator-activated receptor γ . Endocrinology. 2004;145(4):1527–1538. doi: 10.1210/en.2003-1271. [DOI] [PubMed] [Google Scholar]
- 40.Agostini M, Schoenmakers E, Mitchell C, et al. Non-DNA binding, dominant-negative, human PPARγ mutations cause lipodystrophic insulin resistance. Cell Metabolism. 2006;4(4):303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barroso I, Gurnell M, Crowley VEF, et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 42.Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51(12):3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- 43.Ristow M, Müller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. The New England Journal of Medicine. 1998;339(14):953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 44.Savage DB, Agostini M, Barroso I, et al. Digenic inheritance of severe insulin resistance in a human pedigree. Nature Genetics. 2002;31(4):379–384. doi: 10.1038/ng926. [DOI] [PubMed] [Google Scholar]
- 45.Savage DB, Tan GD, Acerini CL, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-γ . Diabetes. 2003;52(4):910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 46.Berg JP. Pluripotent PPARγ polymorphisms. European Journal of Endocrinology. 1999;140(4):293–295. doi: 10.1530/eje.0.1400293. [DOI] [PubMed] [Google Scholar]
- 47.Gurnell M. PPARγ and metabolism: insights from the study of human genetic variants. Clinical Endocrinology. 2003;59(3):267–277. doi: 10.1046/j.1365-2265.2003.01767.x. [DOI] [PubMed] [Google Scholar]
- 48.Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochimica et Biophysica Acta. 2007;1771(8):999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kagawa Y, Yanagisawa Y, Hasegawa K, et al. Single nucleotide polymorphisms of thrifty genes for energy metabolism: evolutionary origins and prospects for intervention to prevent obesity-related diseases. Biochemical and Biophysical Research Communications. 2002;295(2):207–222. doi: 10.1016/s0006-291x(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 50.Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARγ in humans. Molecular Genetics and Metabolism. 2004;83(1-2):93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARγ2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nature Genetics. 1998;20(3):284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 52.Masugi J, Tamori Y, Mori H, Koike T, Kasuga M. Inhibitory effect of a proline-to-alanine substitution at codon 12 of peroxisome proliferator-activated receptor-γ 2 on thiazolidinedione-induced adipogenesis. Biochemical and Biophysical Research Communications. 2000;268(1):178–182. doi: 10.1006/bbrc.2000.2096. [DOI] [PubMed] [Google Scholar]
- 53.Doney A, Fischer B, Frew D, et al. Haplotype analysis of the PPARγ Prol 12Ala and CI431T variants reveals opposing associations with body weight. BMC Genetics. 2002;3, article 21:1–8. doi: 10.1186/1471-2156-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbieri M, Rizzo MR, Papa M, et al. Role of interaction between variants in the PPARG and interleukin-6 genes on obesity related metabolic risk factors. Experimental Gerontology. 2005;40(7):599–604. doi: 10.1016/j.exger.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Damcott CM, Moffett SP, Feingold E, et al. Genetic variation in fatty acid-binding protein-4 and peroxisome proliferator-activated receptor γ interactively influence insulin sensitivity and body composition in males. Metabolism: Clinical and Experimental. 2004;53(3):303–309. doi: 10.1016/j.metabol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Rosado EL, Bressan J, Martins MF, Cecon PR, Martínez JA. Polymorphism in the PPARgamma2 and beta2-adrenergic genes and diet lipid effects on body composition, energy expenditure and eating behavior of obese women. Appetite. 2007;49(3):635–643. doi: 10.1016/j.appet.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Tankó LB, Siddiq A, Lecoeur C, et al. ACDC/adiponectin and PPAR-γ gene polymorphisms: implications for features of obesity. Obesity Research. 2005;13(12):2113–2121. doi: 10.1038/oby.2005.262. [DOI] [PubMed] [Google Scholar]
- 58.Bouhaha R, Meyre D, Kamoun HA, et al. Effect of ENPP1/PC-1-K121Q and PPARγ-Pro12Ala polymorphisms on the genetic susceptibility to T2D in the Tunisian population. Diabetes Research and Clinical Practice. 2008;81(3):278–283. doi: 10.1016/j.diabres.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Fornage M, Jacobs DR, Jr., Steffes MW, Gross MD, Bray MS, Schreiner PJ. Inverse effects of the PPARγ2 Pro12Ala polymorphism on measures of adiposity over 15 years in African Americans and whites: the CARDIA study. Metabolism: Clinical and Experimental. 2005;54(7):910–917. doi: 10.1016/j.metabol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Wei Q, Jacobs DR, Jr., Schreiner PJ, Siscovick DS, Steffes MW, Fornage M. Patterns of association between PPARγ genetic variation and indices of adiposity and insulin action in African-Americans and whites: the CARDIA Study. Journal of Molecular Medicine. 2006;84(11):955–965. doi: 10.1007/s00109-006-0088-7. [DOI] [PubMed] [Google Scholar]
- 61.Østergård T, Ek J, Hamid Y, et al. Influence of the PPAR-γ2 Pro12Ala and ACE I/D polymorphisms on insulin sensitivity and training effects in healthy offspring of type 2 diabetic subjects. Hormone and Metabolic Research. 2005;37(2):99–105. doi: 10.1055/s-2005-861174. [DOI] [PubMed] [Google Scholar]
- 62.Goyenechea E, Parra MD, Martínez JA. Weight regain after slimming induced by an energy-restricted diet depends on interleukin-6 and peroxisome-proliferator-activated-receptor-γ2 gene polymorphisms. British Journal of Nutrition. 2006;96(5):965–972. doi: 10.1017/bjn20061901. [DOI] [PubMed] [Google Scholar]
- 63.Vogels N, Mariman EC, Bouwman FG, Kester AD, Diepvens K, Westerterp-Plantenga MS. Relation of weight maintenance and dietary restraint to peroxisome proliferator-activated receptor γ2, glucocorticoid receptor, and ciliary neurotrophic factor polymorphisms. The American Journal of Clinical Nutrition. 2005;82(4):740–746. doi: 10.1093/ajcn/82.4.740. [DOI] [PubMed] [Google Scholar]
- 64.Adamo KB, Dent R, Langefeld CD, et al. Peroxisome proliferator-activated receptor γ 2 and acyl-CoA synthetase 5 polymorphisms influence diet response. Obesity. 2007;15(5):1068–1075. doi: 10.1038/oby.2007.630. [DOI] [PubMed] [Google Scholar]
- 65.Cole SA, Mitchell BD, Hsueh W-C, et al. The Pro12Ala variant of peroxisome proliferator-activated receptor-γ2 (PPAR-γ2) is associated with measures of obesity in Mexican Americans. International Journal of Obesity and Related Metabolic Disorders. 2000;24(4):522–524. doi: 10.1038/sj.ijo.0801210. [DOI] [PubMed] [Google Scholar]
- 66.González Sánchez JL, Serrano Ríos M, Fernández Pérez C, Laakso M, Martínez Larrad MT. Effect of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor γ-2 gene on adiposity, insulin sensitivity and lipid profile in the Spanish population. European Journal of Endocrinology. 2002;147(4):495–501. doi: 10.1530/eje.0.1470495. [DOI] [PubMed] [Google Scholar]
- 67.Ochoa MC, Marti A, Azcona C, et al. Gene-gene interaction between PPARγ2 and ADRβ3 increases obesity risk in children and adolescents. International Journal of Obesity. 2004;28(supplement 3):S37–S41. doi: 10.1038/sj.ijo.0802803. [DOI] [PubMed] [Google Scholar]
- 68.Meirhaeghe A, Tanck MWT, Fajas L, et al. Study of a new PPARγ2 promoter polymorphism and haplotype analysis in a French population. Molecular Genetics and Metabolism. 2005;85(2):140–148. doi: 10.1016/j.ymgme.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 69.Morini E, Tassi V, Capponi D, et al. Interaction between PPARγ2 variants and gender on the modulation of body weight. Obesity. 2008;16(6):1467–1470. doi: 10.1038/oby.2008.225. [DOI] [PubMed] [Google Scholar]
- 70.Robitaille J, Després J-P, Pérusse L, Vohl M-C. The PPAR-gamma P12A polymorphism modulates the relationship between dietary fat intake and components of the metabolic syndrome: results from the Québec Family Study. Clinical Genetics. 2003;63(2):109–116. doi: 10.1034/j.1399-0004.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 71.Mattevi VS, Zembrzuski VM, Hutz MH. Effects of a PPARG gene variant on obesity characteristics in Brazil. Brazilian Journal of Medical and Biological Research. 2007;40(7):927–932. doi: 10.1590/s0100-879x2006005000114. [DOI] [PubMed] [Google Scholar]
- 72.Danawati CW, Nagata M, Moriyama H, et al. A possible association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor γ2 gene with obesity in native Javanese in Indonesia. Diabetes/Metabolism Research and Reviews. 2005;21(5):465–469. doi: 10.1002/dmrr.543. [DOI] [PubMed] [Google Scholar]
- 73.Li L-L, Ma X-L, Ran J-X, et al. Genetic polymorphism of peroxisome proliferator-activated receptor-γ2 Pro12Ala on ethnic susceptibility to diabetes in Uygur, Kazak and Han subjects. Clinical and Experimental Pharmacology and Physiology. 2008;35(2):187–191. doi: 10.1111/j.1440-1681.2007.04796.x. [DOI] [PubMed] [Google Scholar]
- 74.Lagou V, Scott RA, Manios Y, et al. Impact of peroxisome proliferator-activated receptors γ and δ on adiposity in toddlers and preschoolers in the GENESIS study. Obesity. 2008;16(4):913–918. doi: 10.1038/oby.2008.1. [DOI] [PubMed] [Google Scholar]
- 75.Beamer BA, Yen C-J, Andersen RE, et al. Association of the Pro12Ala variant in the peroxisome proliferator- activated receptor-γ2 gene with obesity in two Caucasian populations. Diabetes. 1998;47(11):1806–1808. doi: 10.2337/diabetes.47.11.1806. [DOI] [PubMed] [Google Scholar]
- 76.Valve R, Sivenius K, Miettinen R, et al. Two polymorphisms in the peroxisome proliferator-activated receptor-γ gene are associated with severe overweight among obese women. The Journal of Clinical Endocrinology & Metabolism. 1999;84(10):3708–3712. doi: 10.1210/jcem.84.10.6061. [DOI] [PubMed] [Google Scholar]
- 77.Kim KS, Choi SM, Shin SU, Yang HS, Yoon Y. Effects of peroxisome proliferator-activated receptor-γ2 Pro12Ala polymorphism on body fat distribution in female Korean subjects. Metabolism: Clinical and Experimental. 2004;53(12):1538–1543. doi: 10.1016/j.metabol.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 78.Tok EC, Ertunc D, Bilgin O, Erdal EM, Kaplanoglu M, Dilek S. PPAR-γ2 Pro12Ala polymorphism is associated with weight gain in women with gestational diabetes mellitus. European Journal of Obstetrics Gynecology and Reproductive Biology. 2006;129(1):25–30. doi: 10.1016/j.ejogrb.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 79.Nicklas BJ, van Rossum EFC, Berman DM, Ryan AS, Dennis KE, Shuldiner AR. Genetic variation in the peroxisome proliferator-activated receptor-γ2 gene (Pro12Ala) affects metabolic responses to weight loss and subsequent weight regain. Diabetes. 2001;50(9):2172–2176. doi: 10.2337/diabetes.50.9.2172. [DOI] [PubMed] [Google Scholar]
- 80.Evans D, Mann WA, de Heer J, et al. Variation in the gene for human peroxisome proliferator activated receptor γ (PPARγ) does not play a major role in the development of morbid obesity. International Journal of Obesity. 2000;24(5):647–651. doi: 10.1038/sj.ijo.0801214. [DOI] [PubMed] [Google Scholar]
- 81.Ghoussaini M, Meyre D, Lobbens S, et al. Implication of the Pro12Ala polymorphism of the PPAR-gamma 2 gene in type 2 diabetes and obesity in the French population. BMC Medical Genetics. 2005;6, article 11:1–8. doi: 10.1186/1471-2350-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nelson TL, Fingerlin TE, Moss L, Barmada MM, Ferrell RE, Norris JM. The PPARγ Pro12Ala polymorphism is not associated with body mass index or waist circumference among hispanics from Colorado. Annals of Nutrition and Metabolism. 2007;51(3):252–257. doi: 10.1159/000104145. [DOI] [PubMed] [Google Scholar]
- 83.Mori Y, Kim-Motoyama H, Katakura T, et al. Effect of the Pro12Ala variant of the human peroxisome proliferator-activated receptor γ2 gene on adiposity, fat distribution, and insulin sensitivity in Japanese men. Biochemical and Biophysical Research Communications. 1998;251(1):195–198. doi: 10.1006/bbrc.1998.9421. [DOI] [PubMed] [Google Scholar]
- 84.Oh EY, Min KM, Chung JH, et al. Significance of Pro12Ala mutation in peroxisome proliferator-activated receptor-γ2 in Korean diabetic and obese subjects. The Journal of Clinical Endocrinology & Metabolism. 2000;85(5):1801–1804. doi: 10.1210/jcem.85.5.6499. [DOI] [PubMed] [Google Scholar]
- 85.Kim K, Lee S, Valentine RJ. Association of Pro12Ala polymorphism in the peroxisome proliferative-activated receptor γ2 gene with obesity and hypertension in Korean women. Journal of Nutritional Science and Vitaminology. 2007;53(3):239–246. doi: 10.3177/jnsv.53.239. [DOI] [PubMed] [Google Scholar]
- 86.Stefanski A, Majkowska L, Ciechanowicz A, et al. Lack of association between the Pro12Ala polymorphism in PPAR-γ2 gene and body weight changes, insulin resistance and chronic diabetic complications in obese patients with type 2 diabetes. Archives of Medical Research. 2006;37(6):736–743. doi: 10.1016/j.arcmed.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 87.Tönjes A, Scholz M, Loeffler M, Stumvoll M. Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor γ with pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care. 2006;29(11):2489–2497. doi: 10.2337/dc06-0513. [DOI] [PubMed] [Google Scholar]
- 88.Luan J, Browne PO, Harding A-H, et al. Evidence for gene-nutrient interaction at the PPARγ locus. Diabetes. 2001;50(3):686–689. doi: 10.2337/diabetes.50.3.686. [DOI] [PubMed] [Google Scholar]
- 89.Memisoglu A, Hu FB, Hankinson SE, et al. Interaction between a peroxisome proliferator-activated receptor γ gene polymorphism and dietary fat intake in relation to body mass. Human Molecular Genetics. 2003;12(22):2923–2929. doi: 10.1093/hmg/ddg318. [DOI] [PubMed] [Google Scholar]
- 90.Lin P-I, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. American Journal of Human Genetics. 2007;80(3):531–538. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen T-J, Ji C-Y, Zheng X-Y, Hu Y-H. Association of β3 adrenergic receptor and peroxisome proliferator-activated receptor gamma 2 polymorphisms with insulin sensitivity: a twin study. Biomedical and Environmental Sciences. 2007;20(2):99–105. [PubMed] [Google Scholar]
- 92.Hsueh W-C, Cole SA, Shuldiner AR, et al. Interactions between variants in the β3-adrenergic receptor and peroxisome proliferator-activated receptor-γ2 genes and obesity. Diabetes Care. 2001;24(4):672–677. doi: 10.2337/diacare.24.4.672. [DOI] [PubMed] [Google Scholar]
- 93.Fritsche A, Madaus A, Tschritter O, et al. Pro12Ala polymorphism in peroxisome proliferator-activated receptorγ 2 (PPARγ 2): beta-cell function and insulin sensitivity. Deutsche Medizinische Wochenschrift. 2001;126(20):580–584. doi: 10.1055/s-2001-14103. [DOI] [PubMed] [Google Scholar]
- 94.Helwig U, Rubin D, Kiosz J, et al. The minor allele of the PPARγ2 Pro12Ala polymorphism is associated with lower postprandial TAG and insulin levels in non-obese healthy men. British Journal of Nutrition. 2007;97(5):847–854. doi: 10.1017/S0007114507665179. [DOI] [PubMed] [Google Scholar]
- 95.Kahara T, Takamura T, Hayakawa T, et al. PPARγ gene polymorphism is associated with exercise-mediated changes of insulin resistance in healthy men. Metabolism: Clinical and Experimental. 2003;52(2):209–212. doi: 10.1053/meta.2003.50038. [DOI] [PubMed] [Google Scholar]
- 96.Stefan N, Fritsche A, Häring H, Stumvoll M. Effect of experimental elevation of free fatty acids on insulin secretion and insulin sensitivity in healthy carriers of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-γ2 gene. Diabetes. 2001;50(5):1143–1148. doi: 10.2337/diabetes.50.5.1143. [DOI] [PubMed] [Google Scholar]
- 97.Vänttinen M, Nuutila P, Pihlajamäki J, et al. The effect of the Ala12 allele of the peroxisome proliferator-activated receptor-γ2 gene on skeletal muscle glucose uptake depends on obesity: a positron emission tomography study. The Journal of Clinical Endocrinology & Metabolism. 2005;90(7):4249–4254. doi: 10.1210/jc.2005-0101. [DOI] [PubMed] [Google Scholar]
- 98.Buzzetti R, Petrone A, Caiazzo AM, et al. PPAR-γ2 Pro12Ala variant is associated with greater insulin sensitivity in childhood obesity. Pediatric Research. 2005;57(1):138–140. doi: 10.1203/01.PDR.0000147728.62185.21. [DOI] [PubMed] [Google Scholar]
- 99.Scaglioni S, Verduci E, Salvioni M, et al. PPAR-γ2 Pro12Ala variant, insulin resistance and plasma long-chain polyunsaturated fatty acids in childhood obesity. Pediatric Research. 2006;60(4):485–489. doi: 10.1203/01.pdr.0000238259.41560.00. [DOI] [PubMed] [Google Scholar]
- 100.Buzzetti R, Petrone A, Ribaudo MC, et al. The common PPAR-γ2 Pro12Ala variant is associated with greater insulin sensitivity. European Journal of Human Genetics. 2004;12(12):1050–1054. doi: 10.1038/sj.ejhg.5201283. [DOI] [PubMed] [Google Scholar]
- 101.Koch M, Rett K, Maerker E, et al. The PPARγ2 amino acid polymorphism Pro 12 Ala is prevalent in offspring of type II diabetic patients and is associated to increased insulin sensitivity in a subgroup of obese subjects. Diabetologia. 1999;42(6):758–762. doi: 10.1007/s001250051225. [DOI] [PubMed] [Google Scholar]
- 102.Tavares V, Hirata RDC, Rodrigues AC, et al. Association between Pro12Ala polymorphism of the PPAR-γ2 gene and insulin sensitivity in Brazilian patients with type-2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2005;7(5):605–611. doi: 10.1111/j.1463-1326.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 103.Adamo KB, Sigal RJ, Williams K, Kenny G, Prud'homme D, Tesson F. Influence of Pro12Ala peroxisome proliferator-activated receptor γ2 polymorphism on glucose response to exercise training in type 2 diabetes. Diabetologia. 2005;48(8):1503–1509. doi: 10.1007/s00125-005-1827-y. [DOI] [PubMed] [Google Scholar]
- 104.Kang ES, Park SY, Kim HJ, et al. Effects of Pro12Ala polymorphism of peroxisome proliferator-activated receptor γ2 gene on rosiglitazone response in type 2 diabetes. Clinical Pharmacology & Therapeutics. 2005;78(2):202–208. doi: 10.1016/j.clpt.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 105.Poulsen P, Andersen G, Fenger M, et al. Impact of two common polymorphisms in the PPARγ gene on glucose tolerance and plasma insulin profiles in monozygotic and dizygotic twins: thrifty genotype, thrifty phenotype, or both? Diabetes. 2003;52(1):194–198. doi: 10.2337/diabetes.52.1.194. [DOI] [PubMed] [Google Scholar]
- 106.Soriguer F, Morcillo S, Cardona F, et al. Pro12Ala polymorphism of the PPARG2 gene is associated with type 2 diabetes mellitus and peripheral insulin sensitivity in a population with a high intake of oleic acid. The Journal of Nutrition. 2006;136(9):2325–2330. doi: 10.1093/jn/136.9.2325. [DOI] [PubMed] [Google Scholar]
- 107.Harding A-H, Williams DEM, Hennings SHJ, Mitchell J, Wareham NJ. Is the association between dietary fat intake and insulin resistance modified by physical activity? Metabolism: Clinical and Experimental. 2001;50(10):1186–1192. doi: 10.1053/meta.2001.26702. [DOI] [PubMed] [Google Scholar]
- 108.Franks PW, Luan J, Browne PO, et al. Does peroxisome proliferator-activated receptor γ genotype (Pro12ala) modify the association of physical activity and dietary fat with fasting insulin level? Metabolism: Clinical and Experimental. 2004;53(1):11–16. doi: 10.1016/j.metabol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 109.Bossé Y, Weisnagel SJ, Bouchard C, Després J-P, Pérusse L, Vohl M-C. Combined effects of PPARγ 2 P12A and PPARα L162V polymorphisms on glucose and insulin homeostasis: the Québec Family Study. Journal of Human Genetics. 2003;48(12):614–621. doi: 10.1007/s10038-003-0087-2. [DOI] [PubMed] [Google Scholar]
- 110.Altshuler D, Hirschhorn JN, Klannemark M, et al. The common PPARγ Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nature Genetics. 2000;26(1):76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 111.Stumvoll M, Stefan N, Fritsche A, et al. Interaction effect between common polymorphisms in PPARγ 2 (Pro12Ala) and insulin receptor substrate 1 (Gly972Arg) on insulin sensitivity. Journal of Molecular Medicine. 2002;80(1):33–38. doi: 10.1007/s001090100282. [DOI] [PubMed] [Google Scholar]
- 112.Baratta R, Di Paola R, Spampinato D, et al. Evidence for genetic epistasis in human insulin resistance: the combined effect of PC-1 (K121Q) and PPARγ2 (P12A) polymorphisms. Journal of Molecular Medicine. 2003;81(11):718–723. doi: 10.1007/s00109-003-0466-3. [DOI] [PubMed] [Google Scholar]
- 113.Pizzuti A, Frittitta L, Argiolas A, et al. A polymorphism (K121Q) of the human glycoprotein PC-1 gene coding region is strongly associated with insulin resistance. Diabetes. 1999;48(9):1881–1884. doi: 10.2337/diabetes.48.9.1881. [DOI] [PubMed] [Google Scholar]
- 114.Hara K, Okada T, Tobe K, et al. The Pro12Ala polymorphism in PPAR γ2 may confer resistance to type 2 diabetes. Biochemical and Biophysical Research Communications. 2000;271(1):212–216. doi: 10.1006/bbrc.2000.2605. [DOI] [PubMed] [Google Scholar]
- 115.Horiki M, Ikegami H, Fujisawa T, et al. Association of Pro12Ala polymorphism of PPARγ gene with insulin resistance and related diseases. Diabetes Research and Clinical Practice. 2004;66(supplement 1):S63–S67. doi: 10.1016/j.diabres.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 116.Mori H, Ikegami H, Kawaguchi Y, et al. The Pro12 → Ala substitution in PPAR-γ is associated with resistance to development of diabetes in the general population: possible involvement in impairment of insulin secretion in individuals with type 2 diabetes. Diabetes. 2001;50(4):891–894. doi: 10.2337/diabetes.50.4.891. [DOI] [PubMed] [Google Scholar]
- 117.Moon MK, Cho YM, Jung HS, et al. Genetic polymorphisms in peroxisome proliferator-activated receptor γ are associated with type 2 diabetes mellitus and obesity in the Korean population. Diabetic Medicine. 2005;22(9):1161–1166. doi: 10.1111/j.1464-5491.2005.01599.x. [DOI] [PubMed] [Google Scholar]
- 118.Meshkani R, Taghikhani M, Larijani B, et al. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-γ2 (PPARγ-2) gene is associated with greater insulin sensitivity and decreased risk of type 2 diabetes in an Iranian population. Clinical Chemistry and Laboratory Medicine. 2007;45(4):477–482. doi: 10.1515/CCLM.2007.095. [DOI] [PubMed] [Google Scholar]
- 119.Doney ASF, Fischer B, Cecil JE, et al. Association of the Pro12Ala and C1431T variants of PPARG and their haplotypes with susceptibility to type 2 diabetes. Diabetologia. 2004;47(3):555–558. doi: 10.1007/s00125-003-1323-1. [DOI] [PubMed] [Google Scholar]
- 120.Frederiksen L, Brødbæk K, Fenger M, et al. Studies of the Pro12Ala polymorphism of the PPAR-γ gene in the Danish MONICA cohort: homozygosity of the Ala allele confers a decreased risk of the insulin resistance syndrome. The Journal of Clinical Endocrinology & Metabolism. 2002;87(8):3989–3992. doi: 10.1210/jcem.87.8.8732. [DOI] [PubMed] [Google Scholar]
- 121.Andrulionytè L, Zacharova J, Chiasson J-L, Laakso M. Common polymorphisms of the PPAR-γ2 (Pro12Ala) and PGC-1α (Gly482Ser) genes are associated with the conversion from impaired glucose tolerance to type 2 diabetes in the STOP-NIDDM trial. Diabetologia. 2004;47(12):2176–2184. doi: 10.1007/s00125-004-1577-2. [DOI] [PubMed] [Google Scholar]
- 122.Jaziri R, Lobbens S, Aubert R, et al. The PPARG Pro12Ala polymorphism is associated with a decreased risk of developing hyperglycemia over 6 years and combines with the effect of the APM1 G-11391A single nucleotide polymorphism: the data from an epidemiological study on the insulin resistance syndrome (DESIR) study. Diabetes. 2006;55(4):1157–1162. doi: 10.2337/diabetes.55.04.06.db05-0676. [DOI] [PubMed] [Google Scholar]
- 123.Memisoglu A, Hu FB, Hankinson SE, et al. Prospective study of the association between the proline to alanine codon 12 polymorphism in the PPARγ gene and type 2 diabetes. Diabetes Care. 2003;26(10):2915–2917. doi: 10.2337/diacare.26.10.2915. [DOI] [PubMed] [Google Scholar]
- 124.Radha V, Vimaleswaran KS, Babu HNS, et al. Role of genetic polymorphism peroxisome proliferator-activated receptor-γ2 Pro12Ala on ethnic susceptibility to diabetes in South-Asian and Caucasian subjects: evidence for heterogeneity. Diabetes Care. 2006;29(5):1046–1051. doi: 10.2337/diacare.2951046. [DOI] [PubMed] [Google Scholar]
- 125.Evans D, de Heer J, Hagemann C, et al. Association between the P12A and c1431t polymorphisms in the peroxisome proliferator activated receptor γ (PPARγ) gene and type 2 diabetes. Experimental and Clinical Endocrinology and Diabetes. 2001;109(3):151–154. doi: 10.1055/s-2001-14838. [DOI] [PubMed] [Google Scholar]
- 126.Herder C, Rathmann W, Strassburger K, et al. Variants of the PPARG, IGF2BP2, CDKAL1, HHEX, and TCF7L2 genes confer risk of type 2 diabetes independently of BMI in the German KORA studies. Hormone and Metabolic Research. 2008;40(10):722–726. doi: 10.1055/s-2008-1078730. [DOI] [PubMed] [Google Scholar]
- 127.Kilpeläinen TO, Lakka TA, Laaksonen DE, et al. SNPs in PPARG associate with type 2 diabetes and interact with physical activity. Medicine and Science in Sports and Exercise. 2008;40(1):25–33. doi: 10.1249/mss.0b013e318159d1cd. [DOI] [PubMed] [Google Scholar]
- 128.Fiorito M, Torrente I, De Cosmo S, et al. Interaction of DIO2 T92A and PPARγ2 P12A polymorphisms in the modulation of metabolic syndrome. Obesity. 2007;15(12):2889–2895. doi: 10.1038/oby.2007.343. [DOI] [PubMed] [Google Scholar]
- 129.de Rooij SR, Painter RC, Phillips DIW, et al. The effects of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-γ2 gene on glucose/insulin metabolism interact with prenatal exposure to famine. Diabetes Care. 2006;29(5):1052–1057. doi: 10.2337/diacare.2951052. [DOI] [PubMed] [Google Scholar]
- 130.Florez JC, Jablonski KA, Sun MW, et al. Effects of the type 2 diabetes-associated PPARG P12A polymorphism on progression to diabetes and response to troglitazone. The Journal of Clinical Endocrinology & Metabolism. 2007;92(4):1502–1509. doi: 10.1210/jc.2006-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeggini E, Parkinson JRC, Halford S, et al. Examining the relationships between the Pro12Ala variant in PPARG and type 2 diabetes-related traits in UK samples. Diabetic Medicine. 2005;22(12):1696–1700. doi: 10.1111/j.1464-5491.2005.01717.x. [DOI] [PubMed] [Google Scholar]
- 132.Sanghera DK, Ortega L, Han S, et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Medical Genetics. 2008;9, article 59:1–9. doi: 10.1186/1471-2350-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vieira-Filho JPB, Reis AF, Kasamatsu TS, et al. Influence of the polymorphisms Tpr64Arg in the β3-adrenergic receptor gene and Pro12Ala in the PPARγ2 gene on metabolic syndrome-related phenotypes in an indigenous population of the Brazilian Amazon. Diabetes Care. 2004;27(2):621–622. doi: 10.2337/diacare.27.2.621. [DOI] [PubMed] [Google Scholar]
- 134.Wakil SM, Al-Rubeaan K, Alsmadi O, et al. The peroxisome proliferator-activated receptor-γ2 P12A polymorphism and type 2 diabetes in an Arab population. Diabetes Care. 2006;29(1):171–172. doi: 10.2337/diacare.29.1.171. [DOI] [PubMed] [Google Scholar]
- 135.Mancini FP, Vaccaro O, Sabatino L, et al. Pro12Ala substitution in the peroxisome proliferator-activated receptor- γ2 is not associated with type 2 diabetes. Diabetes. 1999;48(7):1466–1468. doi: 10.2337/diabetes.48.7.1466. [DOI] [PubMed] [Google Scholar]
- 136.Bouassida KZ, Chouchane L, Jellouli K, et al. The peroxisome proliterator activated receptorγ2 (PPARγ2) Pro12Ala variant: lack of association with type 2 diabetes in obese and non obese Tunisian patients. Diabetes and Metabolism. 2005;31(2):119–123. doi: 10.1016/s1262-3636(07)70177-5. [DOI] [PubMed] [Google Scholar]
- 137.Badii R, Bener A, Zirie M, et al. Lack of association between the Pro12Ala polymorphism of the PPAR-γ2 gene and type 2 diabetes mellitus in the Qatari consanguineous population. Acta Diabetologica. 2008;45(1):15–21. doi: 10.1007/s00592-007-0013-8. [DOI] [PubMed] [Google Scholar]
- 138.Malecki MT, Frey J, Klupa T, et al. The Pro12Ala polymorphism of PPARγ2 gene and susceptibility to type 2 diabetes mellitus in a Polish population. Diabetes Research and Clinical Practice. 2003;62(2):105–111. doi: 10.1016/s0168-8227(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 139.Moffett SP, Feingold E, Barmada MM, et al. The C161 → T polymorphism in peroxisome proliferator-activated receptor gamma, but not P12A, is associated with insulin resistance in Hispanic and non-Hispanic white women: evidence for another functional variant in peroxisome proliferator-activated receptor gamma. Metabolism: Clinical and Experimental. 2005;54(11):1552–1556. doi: 10.1016/j.metabol.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 140.Ludovico O, Pellegrini F, Di Paola R, et al. Heterogeneous effect of peroxisome proliferator-activated receptor γ2 Ala12 variant on type 2 diabetes risk. Obesity. 2007;15(5):1076–1081. doi: 10.1038/oby.2007.617. [DOI] [PubMed] [Google Scholar]
- 141.Scacchi R, Pinto A, Rickards O, et al. An analysis of peroxisome proliferator-activated receptor gamma (PPAR-γ2) Pro12Ala polymorphism distribution and prevalence of type 2 diabetes mellitus (T2DM) in world populations in relation to dietary habits. Nutrition, Metabolism and Cardiovascular Diseases. 2007;17(9):632–641. doi: 10.1016/j.numecd.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 142.Pisabarro RE, Sanguinetti C, Stoll M, Prendez D. High incidence of type 2 diabetes in peroxisome proliferator-activated receptor γ2 Pro12Ala carriers exposed to a high chronic intake of trans fatty acids and saturated fatty acids. Diabetes Care. 2004;27(9):2251–2252. doi: 10.2337/diacare.27.9.2251. [DOI] [PubMed] [Google Scholar]
- 143.Eriksson JG. Gene polymorphisms, size at birth, and the development of hypertension and type 2 diabetes. The Journal of Nutrition. 2007;137(4):1063–1065. doi: 10.1093/jn/137.4.1063. [DOI] [PubMed] [Google Scholar]
- 144.Mousavinasab F, Tähtinen T, Jokelainen J, et al. Effect of the Pro12Ala polymorphism of the PPARγ2 gene on serum adiponectin changes. Endocrine. 2005;27(3):307–309. doi: 10.1385/endo:27:3:307. [DOI] [PubMed] [Google Scholar]
- 145.Yang W-S, Hsiung CA, Ho L-T, et al. Genetic epistasis of adiponectin and PPARγ2 genotypes in modulation of insulin sensitivity: a family-based association study. Diabetologia. 2003;46(7):977–983. doi: 10.1007/s00125-003-1136-2. [DOI] [PubMed] [Google Scholar]
- 146.Lu Z, Dong B, Mo X, et al. Pro12Ala polymorphism in PPAR γ 2 associated with essential hypertension in Chinese nonagenarians/centenarians. Experimental Gerontology. 2008;43(12):1108–1113. doi: 10.1016/j.exger.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 147.Rodríguez-Esparragón FJ, Rodríguez-Pérez JC, Macías-Reyes A, Alamo-Santana F. Peroxisome proliferator-activated receptor-gamma2-Pro12Ala and endothelial nitric oxide synthase-4a/b gene polymorphisms are associated with essential hypertension. Journal of Hypertension. 2003;21(9):1649–1655. doi: 10.1097/01.hjh.0000084719.53355.20. [DOI] [PubMed] [Google Scholar]
- 148.Östgren CJ, Lindblad U, Melander O, Melander A, Groop L, Råstam L. Peroxisome proliferator-activated receptor-γPro12Ala polymorphism and the association with blood pressure in type 2 diabetes: Skaraborg Hypertension and Diabetes Project. Journal of Hypertension. 2003;21(9):1657–1662. doi: 10.1097/01.hjh.0000084734.53355.0d. [DOI] [PubMed] [Google Scholar]
- 149.Ylihärsilä H, Eriksson JG, Forsén T, et al. Interactions between peroxisome proliferator-activated receptor-γ2 polymorphisms and size at birth on blood pressure and the use of antihypertensive medication. Journal of Hypertension. 2004;22(7):1283–1287. doi: 10.1097/01.hjh.0000125438.28861.a4. [DOI] [PubMed] [Google Scholar]
- 150.Stefański A, Majkowska L, Ciechanowicz A, et al. Association between the Pro12Ala variant of the peroxisome proliferator-activated receptor-gamma2 gene and increased 24-h diastolic blood pressure in obese patients with type II diabetes. Journal of Human Hypertension. 2006;20(9):684–692. doi: 10.1038/sj.jhh.1002040. [DOI] [PubMed] [Google Scholar]
- 151.Gouni-Berthold I, Giannakidou E, Müller-Wieland D, et al. Peroxisome proliferator-activated receptor-γ2 Pro12Ala and endothelial nitric oxide synthase-4a/b gene polymorphisms are not associated with hypertension in diabetes mellitus type 2. Journal of Hypertension. 2005;23(2):301–308. doi: 10.1097/00004872-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 152.Swarbrick MM, Chapman CM, McQuillan BM, Hung J, Thompson PL, Beilby JP. A Pro12Ala polymorphism in the human peroxisome proliferator-activated receptor-γ2 is associated with combined hyperlipidaemia in obesity. European Journal of Endocrinology. 2001;144(3):277–282. doi: 10.1530/eje.0.1440277. [DOI] [PubMed] [Google Scholar]
- 153.Iwata E, Matsuda H, Fukuda T, et al. Mutations of the peroxisome proliferator-activated receptor γ (PPARγ) gene in a Japanese population: the Pro12Ala mutation in PPARγ2 is associated with lower concentrations of serum total and non-HDL cholesterol. Diabetologia. 2001;44(10):1354–1355. doi: 10.1007/s001250100647. [DOI] [PubMed] [Google Scholar]
- 154.Pollex RL, Mamakeesick M, Zinman B, Harris SB, Hegele RA, Hanley AJG. Peroxisome proliferator-activated receptor γ polymorphism Pro12Ala is associated with nephropathy in type 2 diabetes. Journal of Diabetes and Its Complications. 2007;21(3):166–171. doi: 10.1016/j.jdiacomp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 155.Brand-Herrmann S-M, Kuznetsova T, Wiechert A, et al. Alcohol intake modulates the genetic association between HDL cholesterol and the PPARγ2 Pro12Ala polymorphism. Journal of Lipid Research. 2005;46(5):913–919. doi: 10.1194/jlr.M400405-JLR200. [DOI] [PubMed] [Google Scholar]
- 156.Tai ES, Corella D, Deurenberg-Yap M, et al. Differential effects of the C1431T and Pro12Ala PPARγ gene variants on plasma lipids and diabetes risk in an Asian population. Journal of Lipid Research. 2004;45(4):674–685. doi: 10.1194/jlr.M300363-JLR200. [DOI] [PubMed] [Google Scholar]
- 157.Hamada T, Kotani K, Tsuzaki K, et al. Association of Pro12Ala polymorphism in the peroxisome proliferator-activated receptor γ2 gene with small dense low-density lipoprotein in the general population. Metabolism: Clinical and Experimental. 2007;56(10):1345–1349. doi: 10.1016/j.metabol.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 158.Eriksson J, Lindi V, Uusitupa M, et al. The effects of the Pro12Ala polymorphism of the PPARγ-2 gene on lipid metabolism interact with body size at birth. Clinical Genetics. 2003;64(4):366–370. doi: 10.1034/j.1399-0004.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 159.Rhee EJ, Kwon CH, Lee WY, et al. No association of Pro12Ala polymorphism of PPAR-γ gene with coronary artery disease in Korean subjects. Circulation Journal. 2007;71(3):338–342. doi: 10.1253/circj.71.338. [DOI] [PubMed] [Google Scholar]
- 160.Zafarmand MH, van der Schouw YT, Grobbee DE, de Leeuw PW, Bots ML. Peroxisome proliferator-activated receptor gamma-2 P12A polymorphism and risk of acute myocardial infarction, coronary heart disease and ischemic stroke: a case-cohort study and meta-analyses. Vascular Health and Risk Management. 2008;4(2):427–436. doi: 10.2147/vhrm.s2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rittig K, Thamer C, Machicao F, et al. The Pro12Ala polymorphism in PPARG2 increases the effectiveness of primary prevention of cardiovascular disease by a lifestyle intervention. Diabetologia. 2007;50(6):1345–1347. doi: 10.1007/s00125-007-0664-6. [DOI] [PubMed] [Google Scholar]
- 162.Al-Shali KZ, House AA, Hanley AJG, et al. Genetic variation in PPARG encoding peroxisome proliferator-activated receptor γ associated with carotid atherosclerosis. Stroke. 2004;35(9):2036–2040. doi: 10.1161/01.STR.0000138784.68159.a5. [DOI] [PubMed] [Google Scholar]
- 163.Iwata E, Yamamoto I, Motomura T, et al. The association of Pro12Ala polymorphism in PPARγ2 with lower carotid artery IMT in Japanese. Diabetes Research and Clinical Practice. 2003;62(1):55–59. doi: 10.1016/s0168-8227(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 164.Doney ASF, Fischer B, Leese G, Morris AD, Palmer CNA. Cardiovascular risk in type 2 diabetes is associated with variation at the PPARG locus: a Go-DARTS study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(12):2403–2407. doi: 10.1161/01.ATV.0000147897.57527.e4. [DOI] [PubMed] [Google Scholar]
- 165.Li L, Cheng L-X, Nsenga R, He M-A, Wu T-C. Association between Pro12Ala polymorphism of peroxisome proliferator-activated receptor-gamma 2 and myocardial infarction in the Chinese han population. Clinical Cardiology. 2006;29(7):300–304. doi: 10.1002/clc.4960290706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pischon T, Pai JK, Manson JE, et al. Peroxisome proliferator-activated receptor-γ2 P12A polymorphism and risk of coronary heart disease in US men and women. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(8):1654–1658. doi: 10.1161/01.ATV.0000171993.78135.7e. [DOI] [PubMed] [Google Scholar]
- 167.Ruiz-Narváez EA, Kraft P, Campos H. Ala12 variant of the peroxisome proliferator-activated receptor-γ gene (PPARG) is associated with higher polyunsaturated fat in adipose tissue and attenuates the protective effect of polyunsaturated fat intake on the risk of myocardial infarction. The American Journal of Clinical Nutrition. 2007;86(4):1238–1242. doi: 10.1093/ajcn/86.4.1238. [DOI] [PubMed] [Google Scholar]
- 168.Szeto C-C, Chow K-M, Poon PY-K, Kwan BC-H, Li PK-T. Peroxisome proliferator-activated receptor-gamma gene polymorphism and risk of cardiovascular disease in patients with diabetic nephropathy. American Journal of Nephrology. 2008;28(5):715–722. doi: 10.1159/000127452. [DOI] [PubMed] [Google Scholar]
- 169.Caramori ML, Canani LH, Costa LA, Gross JL. The human peroxisome proliferator-activated receptor γ2 (PPARγ2) Pro12Ala polymorphism is associated with decreased risk of diabetic nephropathy in patients with type 2 diabetes. Diabetes. 2003;52(12):3010–3013. doi: 10.2337/diabetes.52.12.3010. [DOI] [PubMed] [Google Scholar]
- 170.Herrmann S-M, Ringel J, Wang J-G, Staessen JA, Brand E. Peroxisome proliferator-activated receptor-γ2 polymorphism Pro12Ala is associated with nephropathy in type 2 diabetes: the Berlin Diabetes Mellitus (BeDiaM) Study. Diabetes. 2002;51(8):2653–2657. doi: 10.2337/diabetes.51.8.2653. [DOI] [PubMed] [Google Scholar]
- 171.Malecki MT, Cyganek K, Mirkiewicz-Sieradzka B, et al. Alanine variant of the Pro12Ala polymorphism of the PPARγ gene might be associated with decreased risk of diabetic retinopathy in type 2 diabetes. Diabetes Research and Clinical Practice. 2008;80(1):139–145. doi: 10.1016/j.diabres.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 172.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. The Journal of Clinical Endocrinology & Metabolism. 1999;84(1):165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 173.Korhonen S, Heinonen S, Hiltunen M, et al. Polymorphism in the peroxisome proliferator-activated receptor-γ gene in women with polycystic ovary syndrome. Human Reproduction. 2003;18(3):540–543. doi: 10.1093/humrep/deg128. [DOI] [PubMed] [Google Scholar]
- 174.Yilmaz M, Ergün MA, Karakoç A, Yurtçu E, Çakir N, Arslan M. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-γ gene in women with polycystic ovary syndrome. Gynecological Endocrinology. 2006;22(6):336–342. doi: 10.1080/09513590600733357. [DOI] [PubMed] [Google Scholar]
- 175.Hahn S, Fingerhut A, Khomtsiv U, et al. The peroxisome proliferator activated receptor gamma Pro12Ala polymorphism is associated with a lower hirsutism score and increased insulin sensitivity in women with polycystic ovary syndrome. Clinical Endocrinology. 2005;62(5):573–579. doi: 10.1111/j.1365-2265.2005.02261.x. [DOI] [PubMed] [Google Scholar]
- 176.Hara M, Alcoser SY, Qaadir A, Beiswenger KK, Cox NJ, Ehrmann DA. Insulin resistance is attenuated in women with polycystic ovary syndrome with the Pro12Ala polymorphism in the PPARγ gene. The Journal of Clinical Endocrinology & Metabolism. 2002;87(2):772–775. doi: 10.1210/jcem.87.2.8255. [DOI] [PubMed] [Google Scholar]
- 177.Tok EC, Aktas A, Ertunc D, Erdal EM, Dilek S. Evaluation of glucose metabolism and reproductive hormones in polycystic ovary syndrome on the basis of peroxisome proliferator-activated receptor (PPAR)-γ2 Pro12Ala genotype. Human Reproduction. 2005;20(6):1590–1595. doi: 10.1093/humrep/deh769. [DOI] [PubMed] [Google Scholar]
- 178.Yilmaz M, Ergün MA, Karakoç A, et al. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-γ gene in first-degree relatives of subjects with polycystic ovary syndrome. Gynecological Endocrinology. 2005;21(4):206–210. doi: 10.1080/09513590500231593. [DOI] [PubMed] [Google Scholar]
- 179.Schneider J, Kreuzer J, Hamann A, Nawroth PP, Dugi KA. The proline 12 alanine substitution in the peroxisome proliferator-activated receptor-γ2 gene is associated with lower lipoprotein lipase activity in vivo. Diabetes. 2002;51(3):867–870. doi: 10.2337/diabetes.51.3.867. [DOI] [PubMed] [Google Scholar]
- 180.Delarue J, Magnan C. Free fatty acids and insulin resistance. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10(2):142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 181.Tan GD, Neville MJ, Liverani E, et al. The in vivo effects of the Pro12Ala PPARγ2 polymorphism on adipose tissue NEFA metabolism: the first use of the Oxford Biobank. Diabetologia. 2006;49(1):158–168. doi: 10.1007/s00125-005-0044-z. [DOI] [PubMed] [Google Scholar]
- 182.Stumvoll M, Häring H. Reduced lipolysis as possible cause for greater weight gain in subjects with the Pro12Ala polymorphism in PPARγ 2? Diabetologia. 2002;45(1):152–153. doi: 10.1007/s125-002-8257-y. [DOI] [PubMed] [Google Scholar]
- 183.Stumvoll M, Wahl HG, Löblein K, et al. Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-γ 2 gene is associated with increased antilipolytic insulin sensitivity. Diabetes. 2001;50(4):876–881. doi: 10.2337/diabetes.50.4.876. [DOI] [PubMed] [Google Scholar]
- 184.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401(6748):73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 185.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 186.Simón I, Vendrell J, Gutiérrez C, et al. Pro12Ala substitution in the peroxisome proliferator-activated receptor-gamma is associated with increased leptin levels in women with type-2 diabetes mellitus. Hormone Research. 2002;58(3):143–149. doi: 10.1159/000064490. [DOI] [PubMed] [Google Scholar]
- 187.Takata N, Awata T, Inukai K, et al. Pro12Ala substitution in peroxisome proliferator-activated receptorγ2 is associated with low adiponectin concentrations in young Japanese men. Metabolism: Clinical and Experimental. 2004;53(12):1548–1551. doi: 10.1016/j.metabol.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 188.Yamamoto Y, Hirose H, Miyashita K, et al. PPARγ 2 gene Pro12Ala polymorphism may influence serum level of an adipocyte-derived protein, adiponectin, in the Japanese population. Metabolism: Clinical and Experimental. 2002;51(11):1407–1409. doi: 10.1053/meta.2002.35586. [DOI] [PubMed] [Google Scholar]
- 189.Orio F, Jr., Palomba S, Cascella T, et al. Lack of an association between peroxisome proliferator-activated receptor-γ gene Pro12Ala polymorphism and adiponectin levels in the polycystic ovary syndrome. The Journal of Clinical Endocrinology & Metabolism. 2004;89(10):5110–5115. doi: 10.1210/jc.2004-0109. [DOI] [PubMed] [Google Scholar]
- 190.Radha V, Vimaleswaran KS, Babu S, et al. Lack of association between serum adiponectin levels and the Pro12Ala polymorphism in Asian Indians. Diabetic Medicine. 2007;24(4):398–402. doi: 10.1111/j.1464-5491.2006.02069.x. [DOI] [PubMed] [Google Scholar]
- 191.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 193.Luo W, Cao J, Li J, He W. Adipose tissue-specific PPARγ deficiency increases resistance to oxidative stress. Experimental Gerontology. 2008;43(3):154–163. doi: 10.1016/j.exger.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 194.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ . Annual Review of Biochemistry. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 195.Gong Z, Xie D, Deng Z, et al. The PPARγ Pro12Ala polymorphism and risk for incident sporadic colorectal adenomas. Carcinogenesis. 2005;26(3):579–585. doi: 10.1093/carcin/bgh343. [DOI] [PubMed] [Google Scholar]
- 196.Murtaugh MA, Ma K, Caan BJ, et al. Interactions of peroxisome proliferator-activated receptor γ and diet in etiology of colorectal cancer. Cancer Epidemiology Biomarkers & Prevention. 2005;14(5):1224–1229. doi: 10.1158/1055-9965.EPI-04-0681. [DOI] [PubMed] [Google Scholar]
- 197.Theodoropoulos G, Papaconstantinou I, Felekouras E, et al. Relation between common polymorphisms in genes related to inflammatory response and colarectal cancer. World Journal of Gastroenterology. 2006;12(31):5037–5043. doi: 10.3748/wjg.v12.i31.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jiang J, Gajalakshmi V, Wang J, et al. Influence of the C161T but not Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma on colorectal cancer in an Indian population. Cancer Science. 2005;96(8):507–512. doi: 10.1111/j.1349-7006.2005.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liao S-Y, Zeng Z-R, Leung WK, et al. Peroxisome proliferator-activated receptor-gamma Pro12Ala polymorphism, Helicobacter pylori infection and non-cardia gastric carcinoma in Chinese. Alimentary Pharmacology & Therapeutics. 2006;23(2):289–294. doi: 10.1111/j.1365-2036.2006.02739.x. [DOI] [PubMed] [Google Scholar]
- 200.Prasad KN, Saxena A, Ghoshal UC, Bhagat MR, Krishnani N. Analysis of Pro12Ala PPAR gamma polymorphism and Helicobacter pylori infection in gastric adenocarcinoma and peptic ulcer disease. Annals of Oncology. 2008;19(7):1299–1303. doi: 10.1093/annonc/mdn055. [DOI] [PubMed] [Google Scholar]
- 201.Tahara T, Arisawa T, Shibata T, et al. Influence of peroxisome proliferator-activated receptor (PPAR)γ Plo12Ala polymorphism as a shared risk marker for both gastric cancer and impaired fasting glucose (IFG) in Japanese. Digestive Diseases and Sciences. 2008;53(3):614–621. doi: 10.1007/s10620-007-9944-8. [DOI] [PubMed] [Google Scholar]
- 202.Zmuda JM, Modugno F, Weissfeld JL, et al. Peroxisome proliferator-activated receptor-γ polymorphism, body mass and prostate cancer risk: evidence for gene-environment interaction. Oncology. 2006;70(3):185–189. doi: 10.1159/000093805. [DOI] [PubMed] [Google Scholar]
- 203.Paltoo D, Woodson K, Taylor P, Albanes D, Virtamo J, Tangrea J. Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma (PPAR-γ) gene and risk of prostate cancer among men in a large cancer prevention study. Cancer Letters. 2003;191(1):67–74. doi: 10.1016/s0304-3835(02)00617-1. [DOI] [PubMed] [Google Scholar]
- 204.Wang Y, McCullough ML, Stevens VL, et al. Nested case-control study of energy regulation candidate gene single nucleotide polymorphisms and breast cancer. Anticancer Research. 2007;27(1B):589–593. [PubMed] [Google Scholar]
- 205.Vogel U, Christensen J, Nexø BA, Wallin H, Friis S, Tjønneland A. Peroxisome profilerator-activated receptorγ2 Pro12Ala, interaction with alcohol intake and NSAID use, in relation to risk of breast cancer in a prospective study of Danes. Carcinogenesis. 2007;28(2):427–434. doi: 10.1093/carcin/bgl170. [DOI] [PubMed] [Google Scholar]
- 206.Leibovici D, Grossman HB, Dinney CP, et al. Polymorphisms in inflammation genes and bladder cancer: from initiation to recurrence, progression, and survival. Journal of Clinical Oncology. 2005;23(24):5746–5756. doi: 10.1200/JCO.2005.01.598. [DOI] [PubMed] [Google Scholar]
- 207.Smith WM, Zhou X-P, Kurose K, et al. Opposite association of two PPARG variants with cancer: overrepresentation of H449H in endometrial carcinoma cases and underrepresentation of P12A in renal cell carcinoma cases. Human Genetics. 2001;109(2):146–151. doi: 10.1007/s004390100563. [DOI] [PubMed] [Google Scholar]
- 208.Slattery ML, Sweeney C, Murtaugh M, et al. Associations between vitamin D, vitamin D receptor gene and the androgen receptor gene with colon and rectal cancer. International Journal of Cancer. 2006;118(12):3140–3146. doi: 10.1002/ijc.21791. [DOI] [PubMed] [Google Scholar]
- 209.Barbieri M, Bonafè M, Rizzo MR, et al. Gender specific association of genetic variation in peroxisome proliferator-activated receptor (PPAR)γ-2 with longevity. Experimental Gerontology. 2004;39(7):1095–1100. doi: 10.1016/j.exger.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 210.Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B. Peroxisome proliferator-activated receptor γ induces a clearance mechanism for the amyloid-β peptide. The Journal of Neuroscience. 2004;24(48):10908–10917. doi: 10.1523/JNEUROSCI.3987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.d'Abramo C, Massone S, Zingg J-M, et al. Role of peroxisome proliferator-activated receptor γ in amyloid precursor protein processing and amyloid β-mediated cell death. Biochemical Journal. 2005;391(3):693–698. doi: 10.1042/BJ20050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sastre M, Dewachter I, Rossner S, et al. Nonsteroidal anti-inflammatory drugs repress β-secretase gene promoter activity by the activation of PPARγ . Proceedings of the National Academy of Sciences of the United States of America. 2006;103(2):443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Scacchi R, Pinto A, Gambina G, Rosano A, Corbo RM. The peroxisome proliferator-activated receptor gamma (PPAR-γ2) Pro12Ala polymorphism is associated with higher risk for Alzheimer's disease in octogenarians. Brain Research. 2007;1139(1):1–5. doi: 10.1016/j.brainres.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 214.Hamilton G, Proitsi P, Jehu L, et al. Candidate gene association study of insulin signaling genes and Alzheimer's disease: evidence for SOS2, PCK1, and PPARγ as susceptibility loci. American Journal of Medical Genetics Part B. 2007;144(4):508–516. doi: 10.1002/ajmg.b.30503. [DOI] [PubMed] [Google Scholar]
- 215.Koivisto AM, Helisalmi S, Pihlajamäki J, et al. Association analysis of peroxisome proliferator-activated receptor gamma polymorphisms and late onset Alzheimer's disease in the Finnish population. Dementia and Geriatric Cognitive Disorders. 2006;22(5-6):449–453. doi: 10.1159/000095857. [DOI] [PubMed] [Google Scholar]
- 216.Sauder S, Kölsch H, Lütjohann D, et al. Influence of peroxisome proliferator-activated receptor γ gene polymorphism on 24S-hydroxycholesterol levels in Alzheimer's patients. Journal of Neural Transmission. 2005;112(10):1381–1389. doi: 10.1007/s00702-004-0267-z. [DOI] [PubMed] [Google Scholar]
- 217.Johnson W, Harris SE, Starr JM, Whalley LJ, Deary IJ. PPARG Pro12Ala genotype and risk of cognitive decline in elders? Maybe with diabetes. Neuroscience Letters. 2008;434(1):50–55. doi: 10.1016/j.neulet.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 218.West NA, Haan MN, Morgenstern H. The PPAR-gamma Pro12Ala polymorphism and risk of cognitive impairment in a longitudinal study. doi: 10.1016/j.neurobiolaging.2008.06.005. Neurobiology of Aging. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fagiolo U, Cossarizza A, Scala E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. European Journal of Immunology. 1993;23(9):2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 220.Myśliwska J, Bryl E, Foerster J, Myśliwski A. Increase of interleukin 6 and decrease of interleukin 2 production during the ageing process are influenced by the health status. Mechanisms of Ageing and Development. 1998;100(3):313–328. doi: 10.1016/s0047-6374(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 221.Pregelj P. Involvement of cholesterol in the pathogenesis of Alzheimer's disease: role of statins. Psychiatria Danubina. 2008;20(2):162–167. [PubMed] [Google Scholar]
- 222.Reid PC, Urano Y, Kodama T, Hamakubo T. Alzheimer's disease: cholesterol, membrane rafts, isoprenoids and statins. Journal of Cellular and Molecular Medicine. 2007;11(3):383–392. doi: 10.1111/j.1582-4934.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. The American Journal of Human Genetics. 2004;74(1):106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Crawford DC, Carlson CS, Rieder MJ, et al. Haplotype diversity across 100 candidate genes for inflammation, lipid metabolism, and blood pressure regulation in two populations. The American Journal of Human Genetics. 2004;74(4):610–622. doi: 10.1086/382227. [DOI] [PMC free article] [PubMed] [Google Scholar]