Abstract
Several lines of evidence suggest that exploring the neurochemical basis of reward in invertebrate species may provide clues for the fundamental behavioral and neurobiology underpinnings of drug addiction. How the presence of drug-sensitive reward relates to a decrease in drug-seeking behavior and reinstatement of drug seeking behavior in invertebrate systems is not known. The present study of a conditioned place preference (CPP) paradigm in crayfish (Orconectes rusticus) explores morphine-induced reward, extinction and reinstatement. Repeated intra-circulatory infusions of 2.5μg/g, 5.0μg/g and 10.0μg/g doses of morphine over 5 days serve as a reward when paired with a distinct visual or tactile environment. Morphine-induced CPP was extinguished after repeated saline injections for 5 days in the previously morphine-paired compartment. After the previously established CPP had been eliminated during the extinction phase, morphine-experienced crayfish were challenged with 2.5 μg/g, 5.0 μg/g and 10.0 μg/g respectively. The priming injections of morphine reinstated CPP in all training doses, suggesting that morphine-induced CPP is unrelenting, and that with time, it can be reinstated by morphine following extinction in an invertebrate model just like in mammals. Together with other recent studies, this work demonstrates the advantage of using crayfish as an invertebrate animal model to investigate the basic biological processes that underline exposure to mammalian drugs of abuse.
Keywords: Conditioned place preference, Crayfish, Extinction, Morphine, Reward, Reinstatement
1. Introduction
Exposure to mammalian drugs of abuse causes enduring effects on the brain and behavior. One of such effects is that drugs can alter brain functions, and the resulting drug-associated behaviors can, in turn, be activated and maintained when a particular environmental cue is associated with the effect of the drug. In the absence of the drug, the conditioned stimulus can sustain, and even re-establish drug-seeking behavior [1-3]. In fact, the drug-related conditioned stimulus could maintain its efficiency for weeks after the initiation of withdrawal in rats [4]. In humans, the unique stimulus could elicit behavioral sensitization resulting in conditioned response despite abstinence from drugs for years [5]. Studies of the relationship between a behavioral response and a drug-related environmental stimulus in vertebrates led to the inference that the attractiveness or the positive valence of the environmental cues can directly induce behavioral sensitization and promote drug-seeking behavior. Whether such generalization can be extended to an invertebrate model of drug addiction is yet to be explored.
The neurochemical systems in behavioral sensitization of vertebrates and invertebrates exhibit many similarities [6], including the general modes of action [7], process of activation and inactivation [8], and the major receptor components [9] that initiate behavioral sensitization [10]. Behavioral sensitization is thought to indicate an escalation of drug craving behavior in mammals [11]. The expression of sensitization in fruit flies provided an opportunity to study the neurobiological underpinnings of behavioral sensitization in an invertebrate species [12]. Fruit flies display remarkable behavioral sensitization such as stereotypic locomotion and akinesia, when exposed to different doses of cocaine [13]. Such behavior is analogous to a prolonged stimulative effect of drugs of abuse in the mammalian brain [14], indicating that sensitization in fruit flies shares similarities to that of mammals [11]. Interestingly, in both mammals and flies the catecholamine pathway appears to be a neurochemical denominator in behavioral sensitization. For instance, a presynaptic catecholaminergic mechanism modulates the initiation of behavioral sensitization, while post-synaptic elements act in its maintenance, indicating that mammalian drugs of addiction are likely to act on evolutionary conserved brain substrates, and initiate sensitization even in an invertebrate system that is exposed to mammalian drugs of abuse.
Apart from similarities in the neurochemical mechanisms between vertebrates and invertebrates, exploring psychostimulant effects s in drosophila indicate that behavioral sensitization depends on interactions between an analog of dopamine (tyramine) [13], and a member of the circadian gene [15]. Tyramine -a monohydroxyphenol occurs at trace levels in the mammalian brain [16], and its pharmacological profile in vertebrates resembles amphetamine. Tyramine enhances synaptic catecholamines through inhibition of membrane transporter uptake [17], and appears to play a vital role in the enhancement of behavioral sensitization to cocaine such that circadian gene regulation, tyramine biosynthesis and behavioral sensitization are firmly coupled in fruit flies [15]. Previous study in mammals revealed the significance of circadian gene regulation for psychostimulant sensitization and, additionally, identified a population of high-affinity tyramine receptors in the ventral tegmental area [18], a crucial region of dopamine-dominated cell bodies in the mammalian that is considered to be a major part of reward circuit. Despite similarities between invertebrates and vertebrates with respect to the underlying mechanisms of behavioral sensitization during drug addiction, it remains unclear whether a conditioned stimulus can be attractive, salient, and directly induce behavioral sensitization to promote drug-seeking behavior in an invertebrate system. It is also not known whether the elimination of the response-contingent drug could lead to a decrease in drug seeking behavior in an invertebrate model. Although there are extensive studies on fruit flies, because of their small size, most studies have primarily focused on reflexive motor sequences. Despite the unique neuroanatomical substrates of the land edible snail, it has only been used as a model for exploring reward or punishment [19].
Recent reports have shown that amphetamine and cocaine serve as a powerful reward in crayfish when paired with a distinct environment [20]. The Crayfish has a complex behavioral repertoire [20], and its neurochemical systems have been well documented [21, 22, 6]. Even more compelling is the fact that crayfish has proven to be a useful model in the exploration of the proximate neural mechanism of behavioral decisions [23], and neurochemical mechanisms in neuroethological studies [24], suggesting that crayfish may contribute more as an invertebrate model to explore the neurochemical basis of drug addiction apart from showing reward to psychostimulants. Using crayfish in the present study, we explored the behavioral response and drug-related environmental stimuli to know whether attractive, salient or incentive properties of environmental cues can directly induce behavioral sensitization, promote drug-seeking and if the elimination response-contingent drug can attenuate drug-seeking behavior in an invertebrate system.
A conditioned place preference procedure paired morphine, the unconditioned stimulus, with a distinct visual and tactile environment, to test the rewarding properties of morphine in crayfish. The present study first tested for presence of the CPP, and then explored the time course of the expression of the morphine-induced CPP, as well as movement of crayfish between two compartments over the test session. We subsequently designed additional experiments to determine: (1) whether the CPP could be extinguished by the repeated pairing of both environments with saline and (2) whether, following extinction, the CPP could be reinstated by priming injections of different doses of morphine given prior to the test session.
2. Materials and methods
2.1 Animals
Twenty-one male intermolt male crayfish (Orconectes rusticus) with complete and intact appendages were collected from the Portage River near Bowling Green State University OH, USA. In the laboratory, the animals were maintained in a big tank of water that is freshly aerated and flows through holding trays. Once in the laboratory, animals were isolated in individual plastic containers and maintained in flow-through holding trays that received freshly filtered/aerated water at 20±1 °C. Crayfish were fed 1–2 times per week with tuna fish, earth worms or rabbit chow, and housed under a 16:8 h light/dark cycle.
2.2 Apparatus
The place conditioning apparatus consisted of an opaque white Plexiglas aquarium, measuring 220×90×75mm (length, width and height). Water flowed into and out of the arena through tubes to each end of the aquarium. Four strip lamps with 20W florescent bulbs were mounted at the sides of the aquarium. A digital camera (Sony DCR-VX1000-NTSC) was mounted above the tank and its image contained the entire aquarium. Two distinctive cues that comprised of textural and visual cues were used as the environmental stimuli in each aquarium. Each aquarium was divided into two equal compartments such that distinct visual or textured environment was always present in the opposite compartment.
The soft-texture environment was created by lining the floor of each aquarium compartment with a soft plastic felt material (i.e. soft texture), while the floor of the hard-texture environment was lining with smooth, light colored tiles. The uniform color of the Plexiglas was maintained in the walls of the hard and soft texture compartments (Fig 1A). The striped environment (Fig 1B) was created by lining the opaque inner walls with plastic transparencies that contained a continuous array of 10mm wide alternating black or uniform stripes. The transparencies were positioned vertically to the walls (i.e. striped environment), while the floor was uniform with the color of the plexiglas. For the uniform environment, the white color of the Plexiglas was maintained, and this made the aquarium to be uniformly white. In all, the combinations of the visual or tactile environment are as follows: i) tactile environment comprised of soft texture in one compartment and hard texture in the opposite compartment. ii) visual environment comprised of striped compartment vs. uniformed compartment.
Fig 1.
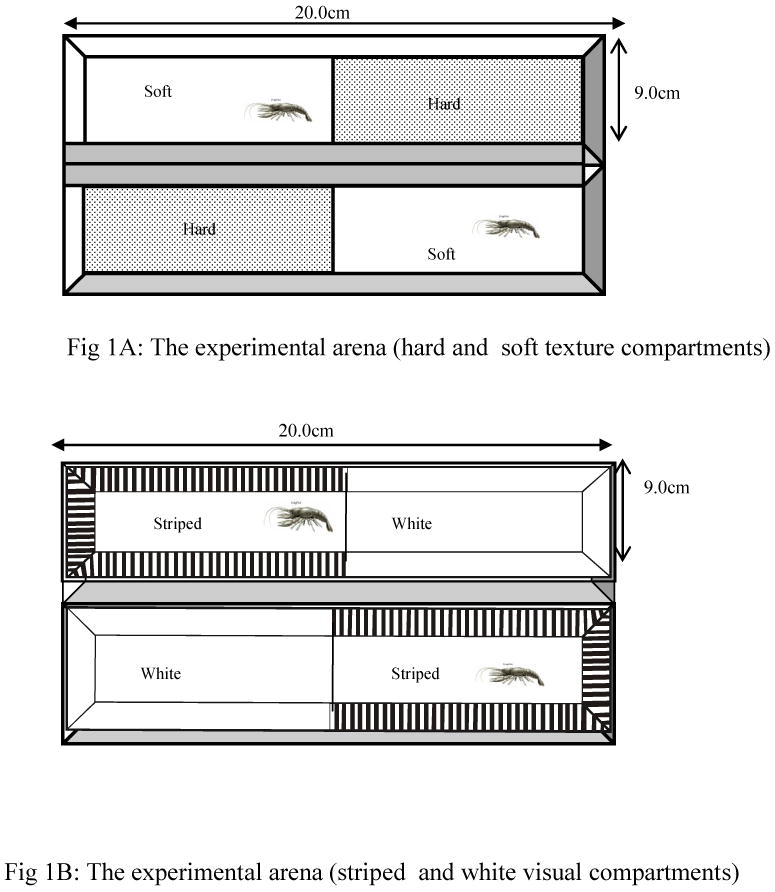
Illustration of the experimental arena for the CPP, Extinction and Reinstatements tests. In Fig 1A, an aquarium was constructed with Plexiglas. Tiles cut to 5cm by 11cm dimensions were placed on the floor. The thick ties represent the hard texture cue. For the soft texture, we used soft woolen materials cut to 5cm by 11cm dimensions and glued to the floor of the compartment. The hard texture and soft texture compartments represent the tactile environment. In Fig 1B, visual environments were created by maintaining the uniform color of the Plexiglas for the white compartment. For the striped compartment, we lined the inner walls of the compartments with plastic transparencies with continuous and alternating arrays of black or white stripes of about 10mm wide. Each compartment was divided into two compartments of equal sizes such that distinct visual or textured environments were always present in the opposite compartments.
2.4 Surgical protocol
During surgery, animals were anesthetized in crushed ice for about 20 minutes. A 26.5 gauge needle was used to gently create an incision in the caudal 1/3 of the dorsal carapace, lateral of the midline to avoid damaging the underlying heart. A 15mm section of deactivated, fine-bore, fused silica (Agilent, i.d. =250μm) was implanted into the pericardial sinus (allowing 3mm to enter the sinus), and reinforced with superglue and bonding material. Following successful surgery, animals were allowed to recover overnight.
2.5 Injection protocols
During injection, 0.5m of deactivated, fine-bore, fused silica needle (Agilent, i.d. =100μm) was coupled to a crayfish with implanted cannula in the pericardial system. The coupling was done using Tygon microbore tubing (Fisher Scientific, i.d. = 250μm). In turn, the tubing was connected to a microdialysis swivel (Intech, 375/25p) that was placed on a small box at the side of the aquarium. Prior to drug administration, we primed the cannula to fill its void volume, to make sure that the drug infusion commences immediately when the pump was turned on. Different doses of morphine (2.5μg, 5.0μg, and 10.0μg/g of the animal body weight) were dissolved in 125mM NaCl and administered. The morphine sulphate used in this study was obtained from Sigma (St. Louis USA). All the doses were expressed as free base concentration of the salt. We administered drugs into the pericardial sinus using a microdialysis pump (CMA/102). For the control condition, 125mM saline was used. The choice of injecting directly into the pericardial system is because the pericardial organs of crustaceans are primary sites of monoamine release, and any manipulations of amines at that site are delivered to the nerve cord [21].
2.6 Behavioral analysis
To provide a detailed analysis of the spatial activities of crayfish, a video tracking system was used. The system was set to extract the spatial coordinates of crayfish from a single video frame at a temporal resolution of 1/3 Hz. The digital camera (Sony DCR-VX1000) that was mounted on the ceiling recorded the behavioral activities of crayfish. The signal from the camcorder was relayed to a video digitizer on a power Macintosh (81001/100AV) computer. Video tracking was was performed using a freeware Java framework for the analysis of behavioral data (available on the Internet at http://caspar.bgsu.edu/∼software/Java/).
2.7 Statistical analysis
Pre-conditioning and CPP test outcomes examined the amount of time spent in each compartment. A direct comparison of time spent between the soft or hard texture, and striped or uniform visual compartments were analyzed using the Student's T-test. We considered the repeated measures ANOVA with a between-subjects factor to determine the existence of significant differences between the doses of morphine (2.5μg/g, 5.0μg/g and 10.0μg/g) to analyze the CPP-induced rewarding effect of morphine. ANOVAs (one for each priming dose of morphine) with a between-subjects factor “Groups of CPP, Extinction and Reinstatement” were evaluated to determine the existence of significant differences between groups of the originally established CPP, subsequent induction of extinction and the reinstatement effects of each dose of morphine. Statistically significant effects were followed by Bonferroni post-hoc comparisons. In using repeated measures ANOVA, we considered the independence of the groups being compared. We used Mauchly's test to test for sphericity to meet the assumption that the relationships between pairs are equal in parametric test. The normal distribution of all data was tested with the exploratory data analysis (EDA) before use of parametric test. Analyses specific to each experiment are outlined in the appropriate result section.
2.8 Experimental design/procedure
2.8.1 Spatial characteristics of crayfish
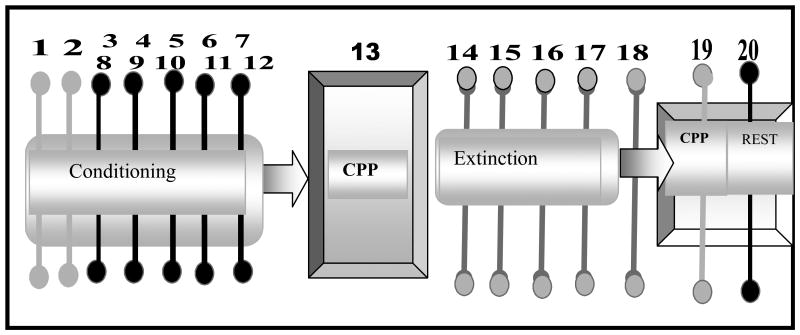
In the preliminary experiments, we explored the spatial activities of crayfish (body weights between 13.5-30.3g) inside the test aquarium. We placed individual crayfish (n=7 per group) in the aquarium for 2 consecutive days and their spatial characteristics were monitored for 60 minutes. The amount of time spent in each compartment was monitored and used to assess the spatial activities and the initial unconditioned preferences. In the next set of experiments, the CPP was used to test the rewarding properties of morphine on crayfish. Furthermore, following extinction training, we determined whether an extinguished morphine-induced CPP could be reinstated by priming injections of three different doses of morphine. The experiments consisted of four phases (see Fig 2): spatial characteristics of crayfish activity in the two compartments, acquisition of CPP, extinction of CPP and reinstatement of CPP.
Fig 2.
Schematic representation of the experimental design used in the present study. Testing for initial preference was carried out in days 1 and 2. The conditioning consists of 10 alternate days (3-12) of drug and saline injections using the unbiased balanced protocol. During conditioning, the compartment in which morphine was administered was assigned randomly. Crayfish were treated for 10 consecutive days with alternate injections of morphine 2.5μg/g, 5.0μg/g and 10.0μg/g). After conditioning, animals were immediately confined to the conditioning compartment for 25 mins. On day 13, the partition separating the compartment was removed, and crayfish were placed at the center and allowed to move freely for 60 mins in a drug-free state to test for the expression of CPP. Following conditioning and the initial CPP test, each crayfish was given pairing of saline with each compartment, one per day, for 5 days (days14-18). Crayfish did not receive morphine during this period. Thereafter, crayfish were given a test for CPP on day 19. The next day (day 20), all crayfish received priming injections of morphine (2.50μg/g, 5.00μg/g, and 10.0μg/g) before the final test for CPP, also on day 20.
2.8.2 Experiment I: Morphine induced CPP
The place conditioning consisted of three phases: pre-exposures that explore the spatial characteristics of crayfish, the conditioning and the CPP test. The place conditioning used in the experiments has been described previously [19]. Briefly, crayfish were randomly assigned into five groups (n=7 per group): control, hard texture/morphine, soft textured/morphine, striped/morphine and uniform/morphine. We tested possible pairwise combinations. For instance, in the hard texture/morphine group, crayfish received morphine injection in the hard-texture compartment, while in the soft-texture compartment the animal received isometric 125mM saline. A similar procedure was adopted for all other environmental/drug combinations. For the control group, crayfish received saline injections at both the hard and soft-texture compartments. The conditioning session commenced when a crayfish was connected to the infusion cannula and placed in the separated visual or texture compartment. The separation was done using a removable Plexiglas enclosure with walls corresponding to the visual or textured compartments. As soon as a crayfish was placed in the aquarium, morphine was infused for the first 5 minutes of the 30 minute session. For 5 consecutive days, each crayfish received two conditioning sessions (1 drug and 1 vehicle injection) per day, separated by 9 hours and in randomized order. Control crayfish received 2 vehicle injections per day. The test condition was carried out on day 6, during which we removed the Plexiglas divider and placed each crayfish at the center of the aquarium. The crayfish was allowed free access to the entire aquarium for 60 minutes. The amount of time spent in each compartment was recorded to assess individual unconditioned preferences. No injections were given on the day of the preference test, thus, maintaining the same procedure as that used during the preliminary baseline test of exploring the spatial activities of crayfish.
2.8.3 Experiment II: Extinction by saline pairing
Twenty-four hours after the conditioning and the initial CPP test, crayfish were given extinction training in which saline was paired 5 times with each of the visual or textured compartments, once per day, for 5 days. Morphine was never administered during this period. After extinction training, crayfish were given a CPP test to determine if the extinction procedure abolished the CPP.
2.8.4 Experiment III: Priming with morphine and restatement of CPP
The day following the last extinction and CPP test, all the crayfish received priming injections of morphine at all training doses (2.5μg/g, and 5.0μg/g and 10.0μg/g body weight). The same dose was used during the conditioning test immediately before the final test for CPP, during which the animals were placed in the center of the aquarium to have access to the entire aquarium for 60mins.
3. Results
3.1 Crayfish without drug treatment display spatial activity in the conditioning aquarium
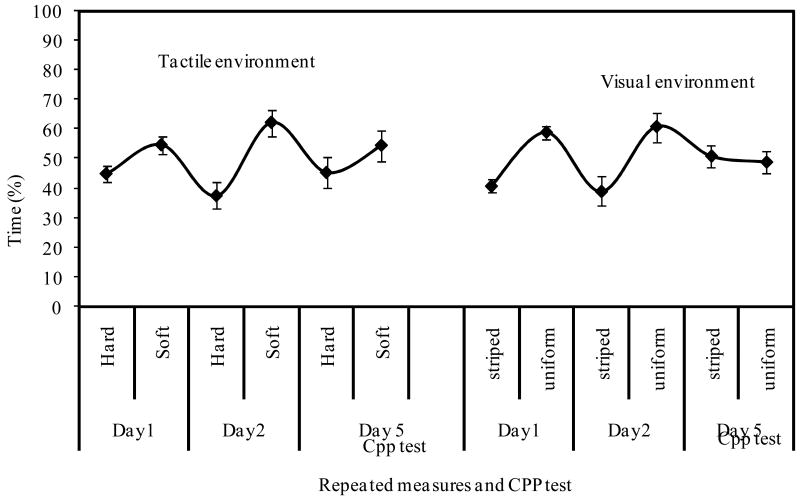
The spatial activity of crayfish was characterized to determine the natural preference of crayfish before drug treatment in the striped or uniform compartments, and soft or hard compartments. Repeated measures of the spatial activity for 1 hour during the first day of test revealed that crayfish significantly spent more time in the uniform environment than the striped environment (T-test (μ =50.0%); t [6] = 3.76, P= <0.05). The preference for the uniform environment was significantly maintained (T-test (μ =50.0%); t [6] = 2.48, P= 0.05) during the second day. In the tactile compartments, crayfish seem to prefer the soft compartment during first day of test (T-test (μ =50.0%); t [6] = 0.93, P= 0.38). Also, during the second day, crayfish showed a significant (T-test (μ =50.0%); t [6] = 2.66, P= 0.04) and stronger preference for the soft compartmen. Taken together, these results indicate a crayfish's natural, unconditioned preference for the uniform and soft compartment. Interestingly, the preferences for the soft and uniform compartments were maintained even during the CPP test (Fig 3). However, in both cases, the preferences were not statistically significant (texture; T-test (μ =50.0%); t [6] = 0.84, P= 0.043), (visual cues; T-test (μ =50.0%); t [6] = 0.20, P= 0.84). These findings represent an important step in the experimental procedure that avoids any preference bias before conditioning.
Fig 3.
The spatial activities of crayfish in three different experiments, following our hypothesis that crayfish will spend equal amount of time in each of the tactile and visual environments. However, it turns out that crayfish showed preference for the uniform compartment following repeated measures of the spatial activities for 1 hour each day. During the first day, the mean time preference for the uniform compartment was 59.02% ±2.34 (SEM) and 40.91±2.33% (SEM) for the striped compartment. The preference was significant (T-test (μ =50.0%); t [6] = 3.76, P= <0.05). In the second day, crayfish maintained their preference for the uniform compartment (62.91%±5.2) while 37.07% ± 5.28 (SEM) of its time was spent in the striped compartment and 62.91% ±5.2 (SEM) inside the uniform compartment. The preference for the uniform compartment was significant (T-test (μ =50.0%); t [6] = 2.48, P= 0.05). During the CPP test, the preference shifted to the striped environment, though such preference was not significant (T-test (μ =50.0%); t [6] = 0.20, P= 0.84). In the tactile environment, crayfish seem to prefer the soft compartment following two days of repeated measures. The mean time spent in the soft compartment was 52.9% ±2.71 (SEM) while crayfish spent an average of 47.71±2.85% of its time in the hard textured compartment. The preference for the soft texture compartment was not significant in the first day (T-test (μ =50.0%); t[6]= 0.93, P=0.38. However, the preference for the soft texture compartment (meantime; 62.29%±4.5 SEM) in the second day when compared with the hard compartment (mean;=37.69±4.53% SEM) was significant (T-test (μ =50.0%); t[6]= 2.66, P= 0.04). During the CPP test, the preference for the soft compartment was maintained, but not statistically significant (T-test (μ =50.0%); t[6]= 0.84, P= 0.043).
3.2 Systemic injection of morphine is rewarding to crayfish after 5 days of CPP conditioning and test
The spatial activities of crayfish in the conditioned textured compartment for 5 days indicate a significant environmental preference following treatment with morphine (Fig 4A; (F [2, 12] = 20.88; P < 0.01). By considering the conditioning doses, crayfish prefer to spend 53.56% (2.5, 0μg/g), 62.55% (5.0 0μg/g) and 69.13% (10.0μg/g) of their time in exhibiting spatial activities in the hard textured compartment when compared to saline conditioning, such that a conditioned place preference was established. Eta squared, as a measure of effect size, is large (0.78), indicating that the morphine-induced place preference for the hard texture compartment accounted for about 78% of the overall (effect+error) variance. The ANOVA factor demonstrating the morphine conditioning effect on crayfish was high (statistical power; 1-β=1.00), indicating that morphine-induced CPP can be replicated with a high degree of reliability.
Fig 4.
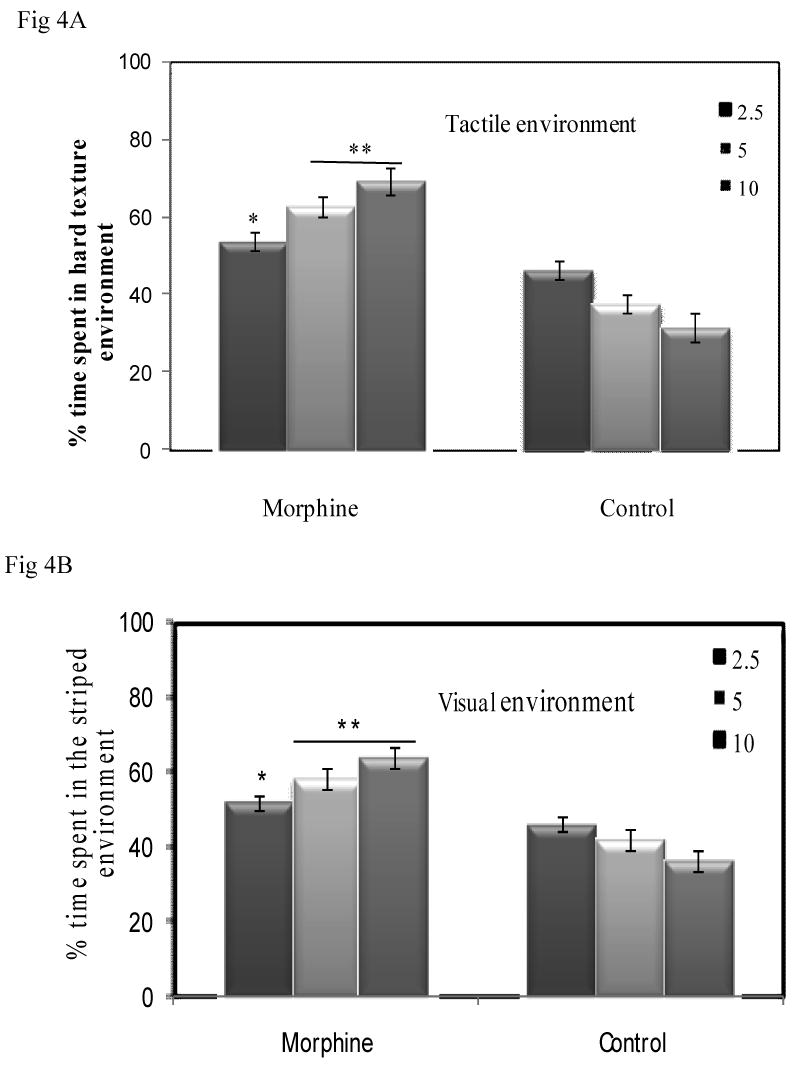
Fig 4A: Repeated infusions of morphine induced CPP in crayfish in the textured environment. Five days of 2.5 μg/g produces 53.56% ± 2.3(SEM), while 5.0 μg/g and 10.0μg/g promote spatial activity of 62.55 % ± 2.6(SEM) and 69.13% ±3.6 respectively, of time preference for the hard textured compartment (F [2, 12] = 20.88; P < 0.01). Post hoc test comparison indicates that crayfish treated with 5.0 μg/g and 10.0μg/g (**P<0.05) were higher and different from crayfish treated with 2.5 μg/g of morphine (*, P<0.05) and saline-paired crayfish.
Fig 4B: Infusions of morphine produced CPP in the visual environment, such that repeated infusions of morphine for 5 days of 2.5 μg/g produces 53.79% ± 1.9 (SEM), while 5.0 μg/g and 10.0μg/g promote spatial activity of 58.20 % ± 2.8(SEM) and 63.59% ±2.8(SEM) respectively of time preference for the striped compartment (F [2, 10] = 13.71; P = <0.01). Bonferroni post hoc test comparison indicates that morphine-conditioned crayfish (*, **P<0.05) were different from saline-paired crayfish. Crayfish treated with 5.0μg/g and 10.0μg/g were significantly different (**P<0.05) from 2.5μg/g (*P<0.05) treated crayfish.
Morphine-induced CPP was apparent in the visual environment such that following 5 days of morphine treatments, 2.5 μg/g of morphine produces 53.79% ± 1.9 (SEM), while 5.0 μg/g and 10.0μg/g promote spatial activity of 58.20 % ± 2.8(SEM), and 63.59% ±2.8(SEM), respectively of time preference for the striped compartment. Morphine conditioning effects were evident when crayfish that were treated with morphine were paired with the naturally un-preferred striped environment. The effect was significant (ANOVA (F [2, 10] = 13.71; P = 0.002), reflecting a greater amount of time being spent in the morphine-paired striped compartment in all doses than the vehicle-paired compartment. The effect of size was large. Eta squared was again large at 0.70, which means that morphine-induced CPP preference for the striped compartment accounted for about 70% of the overall variance. The ANOVA factor demonstrating the morphine-induced CPP in crayfish was very strong (statistical power; 1-β=0.97), indicating that such effect can be reproduced with a high degree of consistency.
Vehicle treated-crayfish exhibited a natural preference for the soft texture compartment. For instance, vehicle treated crayfish for the 2.5 μg/g, 5.0 μg/g and 10.0μg/g morphine treatment groups, showed a preference of 56.00% ± 5.3(SEM), 55.82% ± 2.4(SEM) and 56.78% ± 2.7(SEM) of total time. In the visual environment, at 2.5 μg/g, 5.0 μg/g and 10.0μg/g of morphine treatments, vehicle treated crayfish spent 51.82% ± 2.1(SEM), 59.75% ± 3.4(SEM) and 59.48% ± 2.4(SEM) of their total time in the uniform compartment.
3.3 Extinction by saline paring abolishes CPP while priming with morphine and reinstates CPP
For the extinction training, we repeatedly presented crayfish with conditioned stimulus, that is, the visual or tactile environment in the absence of the unconditioned stimulus by paring saline with each of the two different conditioning compartments. In the tactile environment, times spent by crayfish during the test for CPP, extinction and priming by injections of morphine for reinstatement are shown in Figs 5. The main effects of doses were significant (e.g. 2.5 μg/g; F [2, 13] = 7.21; P = 0.008), (5. 0μg/g; (F [2, 14] = 7.39; P = 0.006.) and 10.0 0μg/g; (F [1, 9] = 18.08; P =0.001) for CPP given before, after extinction and during reinstatement trainings in the hard texture compartment. These results indicate that during the CPP test, before extinction training, crayfish in all groups of dose paring, spent a greater amount of their time in the morphine-paired compartment than in saline-paired compartment (2.5 μg/g; *, P < 0.01). Following extinction training, the paring of saline with each of the two different conditioning compartments in the textured compartments leads to a decline in behavioral significance of the drug-related stimuli, due to less time being spent in the previously morphine rewarding compartment. Interestingly, none of the groups did time spent in morphine and saline paired compartments differ (P<0.05). The CPP test for reinstatement reveals a significant effect (*,**(P<0.05), resulting in a greater amount of time spent in the morphine paired compartment after priming injections of 2.5 μg/g, 5.0 μg/g and 10.0μg/g doses when compared to time spent during CPP after extinction training.
Fig 5.
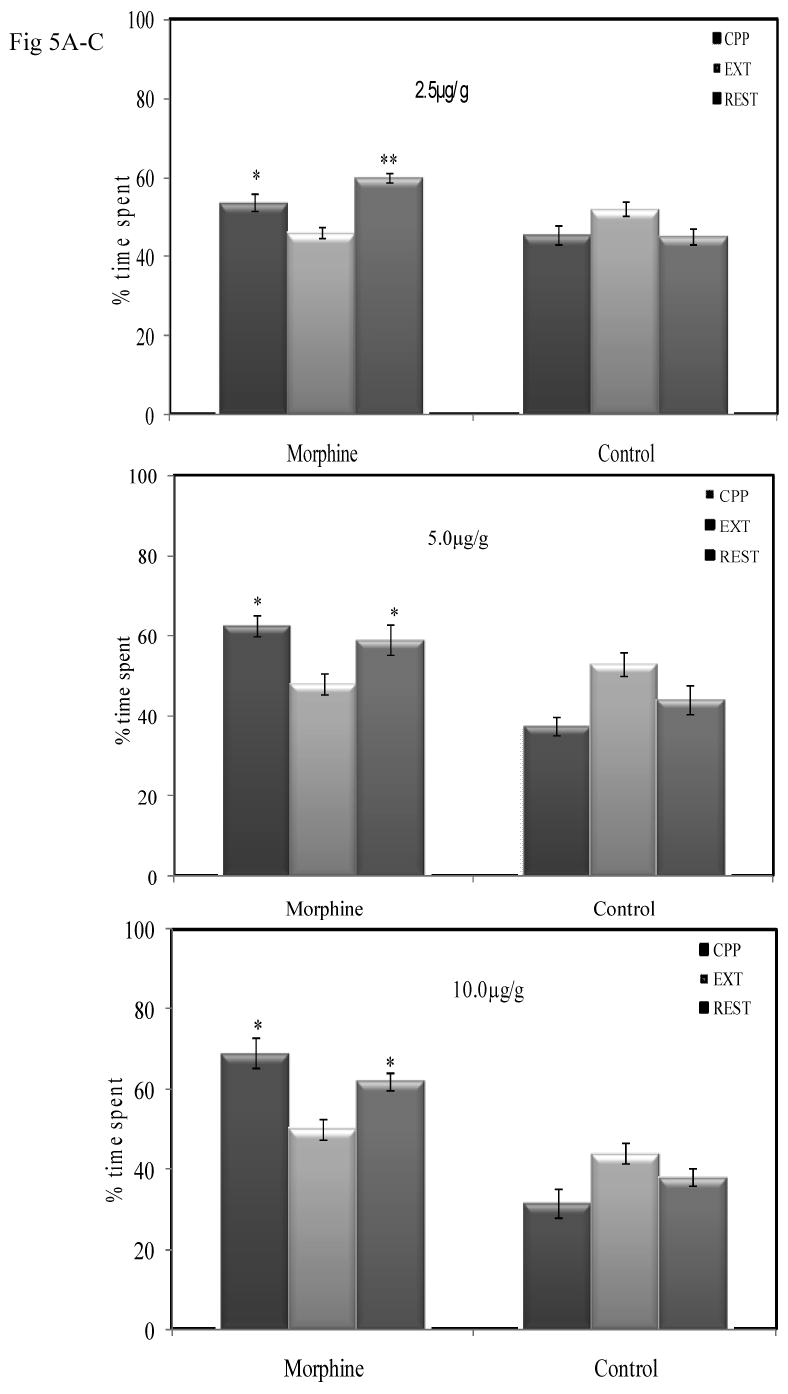
Extinction by saline pairing and reinstatement in the tactile compartments. Mean (± SEM) percentage of time spent in the morphine-paired and saline-paired compartments in pre- and post extinction of 60mins test for CPP. The 60mins test for reinstatement of CPP following a priming injection of 2.5μg/g, 5.0μg/g and 10.0μg/g) is shown on the far right of each panel.
Morphine-induced CPP was rendered in crayfish and then extinguished after repeated saline injections for 5 days, in the previously morphine-paired striped compartment (Fig.6). ANOVA reveals significant differences at all doses tested (2.5 μg/g; F [2, 10] = 11.81; P =0.003), 5. 0μg/g (F [2, 12] = 10.58; P =0.002.) and 10.0μg/g (F [2, 14] = 11.90; P = 0.001). Prior to extinction, crayfish spent a greater amount of time in the morphine -paired compartment than saline-paired compartment (2.5 μg/g; *, P < 0.01), and the preference decreased following extinction training (P<0.05). Following the extinction phase, morphine-experienced crayfish were challenged with all doses of morphine. Priming injections of morphine result in crayfish spending a greater amount of time in the morphine paired compartment at 2.5 μg/g (**P<0.05), (5.0 μg/g(**P<0.05) and 10.0 μg/g (*P<0.05. The amount of time spent during reinstatements were significantly higher (P<0.05), when compared with the amount of time spent during extinction training.
Fig 6.
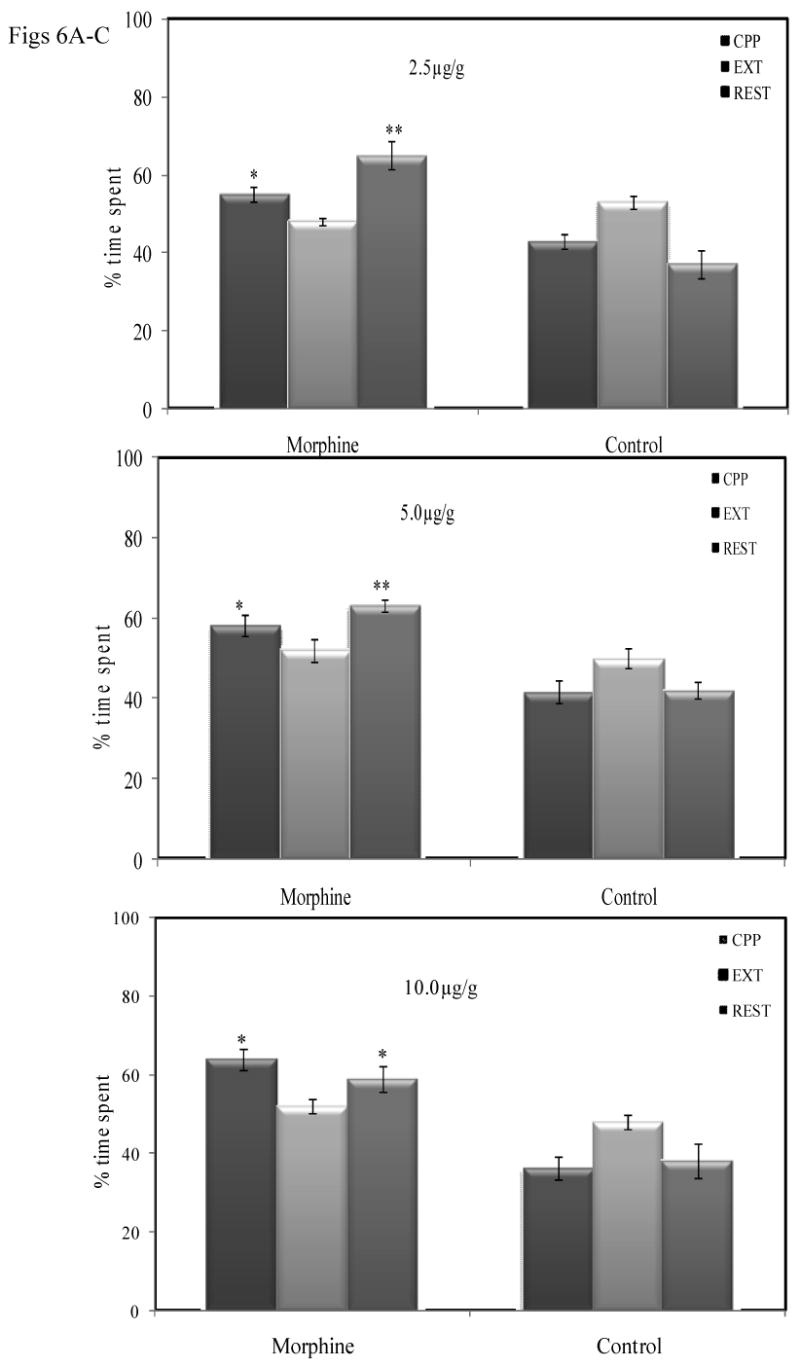
Fig 6A: Extinction by saline pairing and reinstatement in the visual compartments. Mean (± SEM) percentage of time spent in the morphine-paired striped compartment, and saline-paired compartments in pre and post extinction 60mins test for CPP. The 60mins test for reinstatement of CPP following a priming injection of 2.5μg/g, 5.0μg/g and 10.0μg/g) is shown on the far right of each panel.
3.4 Morphine enhances locomotion at lower dose and decreases locomotion at higher dose in crayfish
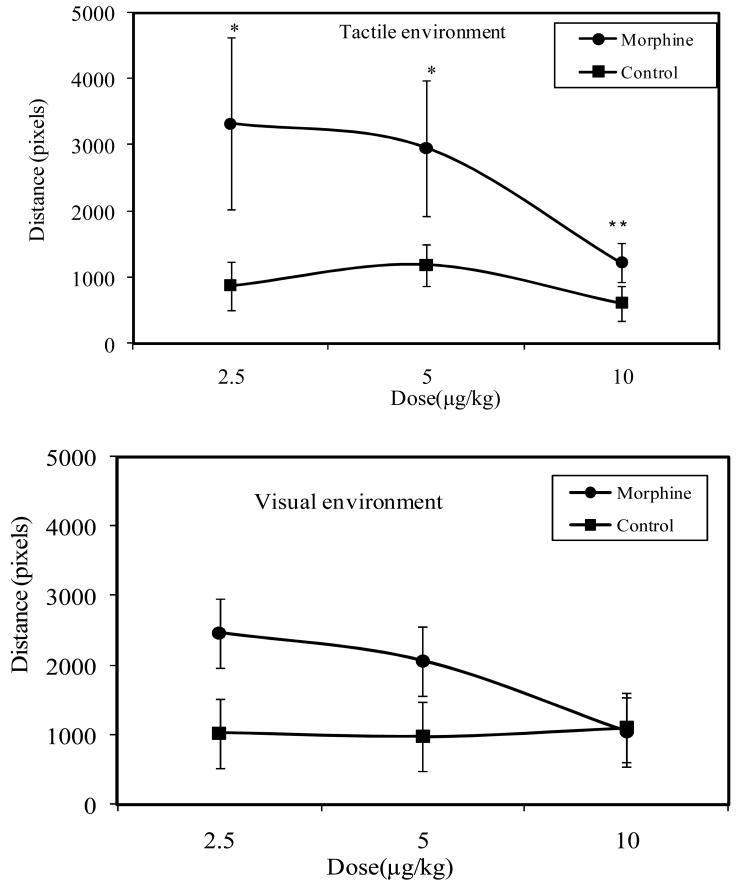
In order to determine the effect of morphine on locomotion, we evaluated locomotion behavior during the CPP morphine-induced reward in all doses. In the texture compartment, injections of morphine significantly increased locomotion (F [5, 41] = 2.5; P = 0.05; Fig 7a) when compared with saline injections. Posthoc comparison confirmed that locomotion behavior of crayfish was highest at 2.5μg, and reduces with increase in doses of morphine 5.0μg/g (*P<0.05), 10.0 μg (**P<0.05). Fig 7b indicates that although locomotion was highest at 2.5 μg/g and 5.0μg/g doses, when compared with 10.0μg/g dose in the visual environment. However, such effect was not significant (F[5, 41] =0.93; P = 0.47).
Fig 7.
Locomotion responses of crayfish pretreated with 2.5μg/g, 5.0μg/g and 10.0μg/g doses of morphine (n=7) or saline (n= 7) during CPP test in the textured and visual compartments. Data are expressed as mean traveled distances (pixels) within the test textured or visual compartments. In the textured compartment, ANOVA reveals that injection of morphine significantly (F [5, 41] = 2.5; P = 0.05) increased locomotion when compared with saline injection. Locomotion was high at 2.5μg/g and 5.0μg/g doses; *P<0.05, and low 10.0μg/g dose of morphine (**P<0.05). Whereas in the visual compartment, although locomotion was higher at lower doses of 2.5 μg and 5.0μg/g when compared with high dose of 10.0μg/g, however, such effect was not statistically significant (F [5, 41] =0.93; P = 0.47).
4. Discussion
Four major findings arise from the experiments in this study. First, we found that morphine-induced CPP was established in crayfish in two contrasting environmental cues (texture and visual stimuli). Second, the established CPP was extinguished by explicit pairing of two environments in both the visual and textured cues with injections of saline alone. Third, we found that after extinction, a priming of injections of morphine at all doses reinstated the morphine-induced CPP in crayfish. Finally, and very interesting, during morphine-induced CPP, locomotion decreased with an increase in the dose of morphine that was repeatedly administered in both the textured and visual cues. Taken together, these results suggest that crayfish may represent an efficient model for studying the primary sites of morphine -an opioid to explore the proximate mechanisms and fundamental neurobiological alterations that underlie drug addiction in an invertebrate model.
Regardless of dose, morphine was perceived as rewarding by the crayfish when paired with a distinct visual or tactile environment. A morphine-induced CPP was established at 2.5μg/g and significantly lower when compared with 5.0μg/g and 10.0μg/g doses of morphine. Although, there was no statistical significant difference between CPP induced at 5.0μg/g and 10.0μg/g in the visual and tactile environments, however, a morphine-induced CPP was established at all training doses because there was tendency for a greater preference of the morphine-paired compartment at higher doses (see figs 4A and 4B). It has been shown that psychostimulants are capable of interfering with the monoamine chemistry and induce reward in crayfish when exposed to a distinct visual environment [20]. Similarly, in the present study, morphine, a mammalian drug of abuse, is rewarding to crayfish when exposed to a distinct visual and tactile environment. Taking results from a previous study [20] and those of the present study together, crayfish as a model of drug addiction illustrate the conceptual validity of the sensitivity and rewarding properties of mammalian drugs of abuse to an invertebrate system, which unlike mammals exhibits simple neuronal organization.
It is interesting to note that in our studies, morphine-induced CPP was established only in the hard-texture compartment and not in the soft-texture compartment. In the visual environment, crayfish only formed reward when morphine treatment was paired with the striped environment. These findings indicate that the hard texture environment was novel when compared to the soft texture environment, or that the striped environment was novel to crayfish when compared with the uniform environment. The preference for the striped environment could be attributed to the inherent perceptual saliency of the striped environment [20]. This is because the visual system of crayfish is particularly proficient in extracting vertical oriented contrast information [25, 26]. In their natural environment, crayfish hide under hard structures, such as a rock for protection[27]. It is possible that crayfish might have explored and perceived the hard texture to be relatively attractive when compared with the soft and uniform environments, suggesting that stimulus salience when paired with drugs indicates the significance or noticeability of the hard-texture or striped visual environment as novel by crayfish.
Extinction training by repeated pairings of the two environments in the tactile and visual environments with saline alone for 5 days led to reduction in already established CPP, indicating that paring with saline without the drug provides measures of the incentive or motivational properties of morphine in crayfish. That being the case, the reduction in drug-seeking behavior in the absence of response-contingent drug availability probably led to the observed decline in the significance of the drug-paired stimuli in crayfish.
The present study also demonstrated that, following a priming of injection, CPP was again re-established at all training doses, such that crayfish again approached and remained in the presence of a hard texture-drug and striped-drug stimuli. This finding indicates the renewal of incentive saliency and attractiveness of the visual and tactile stimuli by the presence of morphine. In mammals for example, morphine increases craving for drugs in drug-free addicts associated with drug stimuli, and reinstate drug-seeking after prolonged periods of extinction in morphine-experienced conditions [28, 29]. Similar to mammals, the behavior of crayfish in re-approaching and showing preference for the hard texture and striped stimuli following the priming injections of morphine seems to be determined by drug-related stimuli, and the priming of injections probably increase the salience, attractiveness, or even the positive valence of the stripped or hard texture cue. It is possible that the reinstating properties of morphine in crayfish could be as a result of the hedonic properties of drugs of abuse, which in mammals, produce an incentive motivational state in addicts, and promote drug craving that leads an addict to search for those environmental stimuli that are associated with the presence of the drug. The fact that crayfish was sensitive to incentive properties of the conditioned stimuli when re-instated with morphine and paired to a distinct visual or tactile environment supports our idea, and suggests that potential and even unanticipated hypotheses in drug addiction can emerge from studies of addiction in an invertebrate system such as crayfish.
Interpretations of crayfish morphine-induced reward or reinstatement of CPP after extinction maybe has evolutionary explanations. In mammals, all neurochemical processes of drug-induced reward process is generally hinged on the dopaminergic system [14, 30-32, 34,35]. The association of motivation salience to morphine rewarding is evidence in recent hypotheses of dopamine functions in regulating motivational-affective responses [33-34], reward-related stimuli [37], in learning process [38, 39], or even in the Pavlovian mechanism of a cue's attractiveness. The dopamine is a neurochemical signal that is conserved and shared across all mammals and non mammalian species [40, 41]. For instance, in invertebrate models of drug addiction including Bees [41], arthropods [42] and specifically in crayfish [21], which is the focus of the present study. The fact that morphine is rewarding to an invertebrate system with simple neuronal organization, indicates that mammalian drugs of addiction are likely to act on evolutionary conserved brain substrates to initiate reward beyond those peculiar to humans.
The different doses of morphine when paired with distinct environmental cues seem to have strengthened the expression of behavioral sensitization in crayfish. This is evidence in more time being spent in previously drug-paired compartments in animals tested for extinction, and this as well supports the idea that the reinstating effect led to the development of sensitization. Behavioral sensitization to opiates is a consequence of repeated drug administration that results in augmentation of the behavioral effects of opiate upon re-administration [43,44]. For this reason, behavioral response of crayfish to morphine could be attributed not only to a direct pharmacological effect of the drug but also to learned associations of the distinct visual and tactile stimuli with the drug rewarding experience. The inhibition of such situational associations between drug-taking and environmental cues of the tactile and visual compartments during the 5 days of extinction training with saline seem to attenuate behavioral sensitization in crayfish. Taken together, our data suggest that the link between environmental cues and behavioral sensitization in relapse to opiate dependence following extinction of morphine conditioned place preference that has been established in different studies in mammals [37, 45-48], is also present in crayfish.
The repeated administration of morphine at a lower dose increased locomotion while repeated treatments of crayfish at a higher dose suppressed locomotion. In fact, opioids in mammals, depending on dose and time protocols, may induce both reduction and enhancement of locomotor activity [49, 50]. In crayfish, multiple administration of morphine seemed to develop behavioral sensitization, manifested in enhancing locomotion behavior at 2.5μg/g. Instead of behavioral sensitization, the multiple effects of higher doses of 5.0μg/g and 10.0μg/g seem to suppress locomotion activity in crayfish, and its effects on locomotion develop tolerance after repeated administration. Therefore, instead of behavioral sensitization, morphine seems to dose-dependently block the development, interrupt the transfer and suppress the expression of behavioral sensitization to morphine following repeated administration of higher doses. This may be a distinct characteristic of morphine on locomotion in crayfish that could be explored further.
In conclusion, the nervous system of crayfish contains less than 1000 large and accessible monoamine-containing neurons [21]. Crayfish has a dopaminergic system of 30-35 somata located in the brain, 25-30 of the dopamine neurons are located in the suboesophageal ganglion [21]. In exploiting the simplicity of the neuronal organization in crayfish, we have demonstrated that crayfish with simple neural and neuromodulatory organization may thus represent an efficient model for studying the fundamental behavioral and neurobiology underpinnings of drug addiction, relating to decrease in drug-seeking behavior with extinction of drug-associated stimuli, and reinstatement of drug seeking behavior.
Acknowledgments
We are grateful to Celeste Greer and Antonio Alcaro who help in sampling and in laboratory setting up of the behavioral experiments. We thank Emily Ruckel and Mary Rita DeNucci for their help in behavioral analysis. Chris Hess, Deb Mclean and Steve Queen provided the technical support. This study was funded by the NIH grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis W, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlov J Biol Sci. 1976;11(4):222–36. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- 2.Burbassi S, Cervo L. Stimulation of serotonin(2C) receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 2008;(1):15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- 3.Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28(6):1150–9. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Grimm J, Hope B, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47 1:214–26. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien C, Childress AR, McLellan AT. Classical conditioning in drug dependent humans. Ann N Y Acad Sci. 1992;654:400–15. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 6.Rhanor G. Evolution and Function in Serotonergic Systems. Integrative and Comparative Biology. 2006;46:838–846. doi: 10.1093/icb/icl024. [DOI] [PubMed] [Google Scholar]
- 7.Vernier P, Cardinaud B, Valdenair O, Philippe H, Vincent JD. An evolutionary view of drug receptor interaction: the bioamine receptor family. Trends Pharm Sci. 1995;16:375–381. doi: 10.1016/s0165-6147(00)89078-1. [DOI] [PubMed] [Google Scholar]
- 8.Vernier P, Cardinaud B, Philippe H, Vincent JD. The classification of bioamine receptors. How helpful are molecular phylogenies. Ann N Y Acad Sci. 1997;812 doi: 10.1111/j.1749-6632.1997.tb48154.x. [DOI] [PubMed] [Google Scholar]
- 9.Porzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Molec Pharmacol. 2001;59(1):83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Hen R. Structural and functional conservation of serotonin receptors throughout evolution. EXS. 1993;63:266–278. doi: 10.1007/978-3-0348-7265-2_14. EXS 63:266-278. [DOI] [PubMed] [Google Scholar]
- 11.Berridge K, Robinson T. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 12.Wolf F, Heberlein U. Invertebrate models of drug abuse. J Neurobiol. 2003;54(1):161–78. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- 13.Mcclung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr Biol. 1999;9:853–60. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- 14.Ikemoto S, Panksepp J. The role of the nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 15.Haidie SL, Zhang JX, Hirssh J. Trace amines differentially regulate adult locomotor activity, cocaine sensitivity, and female fertility in Drosophila melanogaster. Dev Biology. 2007:1396–405. doi: 10.1002/dneu.20459. [DOI] [PubMed] [Google Scholar]
- 16.Durden DA, Davis BA. Determination of regional distributions of phenylethylamine and meta- and para-tyramine in rat brain regions and presence in human and dog plasma by an ultra sensitive negative chemical ion gas chromatography-mass spectrometric (NCI-GC MS) method. Neurochem Res. 1993;18:995–1002. doi: 10.1007/BF00966759. [DOI] [PubMed] [Google Scholar]
- 17.Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated release, transporter rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem. 1998;71:1289–97. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- 18.Burchett SA, Jicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;6:223. doi: 10.1016/j.pneurobio.2006.07.003. 2-methamphetamine. [DOI] [PubMed] [Google Scholar]; Mol Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- 19.Balaban PM, Chase R. Interrelationships of the emotionally positive and negative regions of the brain of the edible snail. Neurosci Behav Physiol. 1991;21(2):172–80. doi: 10.1007/BF01182895. [DOI] [PubMed] [Google Scholar]
- 20.Panksepp J, Huber R. Ethological analyses of Crayfish behavior: a new invertebrate system for measuring rewarding properties of Psychostimulants. Behavioral Brain Research. 2004;153:171–80. doi: 10.1016/j.bbr.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tierney A, Kim T, Abrams R. Dopamine in crayfish and other crustaceans: distribution in the central nervous system and physiological functions. Microsc Res Tech. 2003;60:325–35. doi: 10.1002/jemt.10271. [DOI] [PubMed] [Google Scholar]
- 22.Avali A, Harris-Warrick RM. Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network. J Neurosci. 1999;19:6712–6722. doi: 10.1523/JNEUROSCI.19-15-06712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullonev B. During fictive locomotion, graded synaptic currents drive bursts of impulses in swimmeret motor neurons. J Neurosci. 2003;23:5953–5962. doi: 10.1523/JNEUROSCI.23-13-05953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panksepp J, Huber R. Long-term changes in serotonin function: dynamic neurochemical properties in agonistic behavior of the crayfish Orconectes rusticus. J Neurobiol. 2002;50(4):276–290. doi: 10.1002/neu.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Falcón J, Serrato J, Ramón F. Evoked potentials elicited by natural stimuli in the brain of unanesthesized crayfish. Physiol Behav. 1999;66(3):397–407. doi: 10.1016/s0031-9384(98)00279-0. [DOI] [PubMed] [Google Scholar]
- 26.Hernández-Falcón J, Serrato J, Ramón F. Evoked potentials elicited by natural stimuli in the brain of unanesthesized crayfish. Physiol Behav. 1999;66(3):397–407. doi: 10.1016/s0031-9384(98)00279-0. [DOI] [PubMed] [Google Scholar]
- 27.McMahon A, Blair WP, Macmillan DW. Exploration in a T-Maze by the Cray fish Cherax destructor Suggests Bilateral Comparison of Antennal Tactile Information Biol. Bull. 2005;208:183–88. doi: 10.2307/3593150. [DOI] [PubMed] [Google Scholar]
- 28.Shaham Y, Rodaros D, Stewart J. Reinstatement of Heroin-reinforced behavior following long term extinction: Implications for the treatment of relapse to drug taking. Behav Phramacology. 1994;5:360–464. doi: 10.1097/00008877-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–21. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 30.Carr KD. The physiology of opiate hedonic effects and the role of opioids in motivated behavior. Adv Alcohol Subst Abuse. 1984;3(3):5–18. doi: 10.1300/J251v03n03_02. [DOI] [PubMed] [Google Scholar]
- 31.Tassin JP. Role of dopamine in drug dependence processes. Bull Acad Natl Med. 2002;186(2):295–304. [PubMed] [Google Scholar]
- 32.Marinelli M, Barrot M, Simon H, Oberlander C, Dekeyne A, Le Moal M, et al. Pharmacological stimuli decreasing nucleus accumbens dopamine can act as positive reinforcers but have a low addictive potential. Eur J Neurosci. 1998;10(10):3269–75. doi: 10.1046/j.1460-9568.1998.00340.x. [DOI] [PubMed] [Google Scholar]
- 33.Panksepp J. Affective neuroscience. The foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- 34.Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, Huber R. The role of emotional systems in addiction: a neuroethological perspective. In: Bevins RA, Bardo MT, editors. Motivational factors in the etiology of drug abuse. University of Nebraska Press; Lincoln: 2004. pp. 85–126. [PubMed] [Google Scholar]
- 35.Crisp KM, Mesce KA. Beyond the central pattern generator: amine modulation of decision-making neural pathways descending from the brain of the medicinal leech. Journal of Experimental Biology. 2006;209:1746–1756. doi: 10.1242/jeb.02204. [DOI] [PubMed] [Google Scholar]
- 36.Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence of the motivational salience of drug cues in opiate addiction. Psychol Med. 2007;37(8):1203–9. doi: 10.1017/S0033291707009932. [DOI] [PubMed] [Google Scholar]
- 37.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 38.Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neurosci Biobehav Rev. 2007:123–34. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–97. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 40.Myers PR, Livengood DR. Dopamine depolarising response in a vertebrate neuronal somatic cell hybrid. Nature. 1975;255:235–237. doi: 10.1038/255235a0. [DOI] [PubMed] [Google Scholar]
- 41.Ilona CK, Paul RE, Barbara SK, Alison RM. Distribution of dopamine receptors and dopamine receptor homologs in the brain of the honey bee, Apis mellifera L. Microscopy Research and Technique. 1999;44(23) doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<179::AID-JEMT9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 42.Murdock LL, Wirtz RA, Köhler G. 3,4-Dihydroxyphenylalanine (dopa) decarboxylase activity in the arthropod nervous system. Biochem J. 1973;132(4):681–88. doi: 10.1042/bj1320681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heidbreder CA, Thompson CA, Shpppenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- 44.Mattingly BA, Ragozzimo ME, Gold PE. Stimulus and response factors affecting the development of behavioral sensitization to apormorphine. Psychopharmacology. 1997;130:109–16. doi: 10.1007/s002130050217. [DOI] [PubMed] [Google Scholar]
- 45.Lu L, Xu NJ, Ge X, Yue W, Su WJ, Pei G, et al. Reactivation of morphine conditioned place preference by drug priming: role of environmental cues and sensitization. Psychopharmacology (Berl) 2002;159(2):125–32. doi: 10.1007/s002130100885. [DOI] [PubMed] [Google Scholar]
- 46.Fattore L, Deiana S, Spano SM, Cossu G, Fadda P, Scherma M, et al. Endocannabinoid system and opioid addiction: behavioral aspects. Pharmacol Biochem Behav. 2005;81(2):349–59. doi: 10.1016/j.pbb.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 47.Bartoletti M, Gaiardi M, Gubellini C, Bacchi A, Babbini M. Previous treatment with morphine and sensitization to the excitatory actions of opiates: dose-effect relationship. Neuropharmacology. 1987;26(23):115–9. doi: 10.1016/0028-3908(87)90197-3. [DOI] [PubMed] [Google Scholar]
- 48.Tao PL, Liang KW, Sung WY, Wu YT, Huang EY. Nalbuphine is effective in decreasing the rewarding effect induced by morphine in rats. Drug Alcohol Depend. 2006;84(2):175–81. doi: 10.1016/j.drugalcdep.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Timára J, Gyarmatia Z, Fürsta Z. The development of tolerance to locomotor effects of morphine and the effect of various opioid receptor antagonists in rats chronically treated with morphine. Brain Research Bulletin. 2005;64(5):417–424. doi: 10.1016/j.brainresbull.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Zhao W, Gong Z, Liang J. A new buprenorphine analogy, thenorphine, inhibits morphine-induced behavioral sensitization in mice1. Acta Pharmacol. 2004;25(11):1413–1418. [PubMed] [Google Scholar]