Abstract
Diacetyl, a component of artificial butter flavoring, is a potential etiological agent of obliterative bronchiolitis (OB); however, the toxic dose and mechanisms of toxicity remain controversial. We evaluated the respiratory toxicity of diacetyl in a murine model using several exposure profiles relevant to workplace conditions at microwave popcorn packaging plants. Male C57B1/6 mice were exposed to inhaled diacetyl across several concentrations and duration profiles, or by direct oropharyngeal aspiration. Effects of diacetyl on the respiratory tract were evaluated by histopathology and BALF analyses. Subacute exposure to 200 or 400 ppm diacetyl for 5 days caused deaths, necrotizing rhinitis, necrotizing laryngitis and bronchitis. Reducing the exposure to 1 h/day (100, 200, 400 ppm) for 4 weeks resulted in less nasal and laryngeal toxicity, but led to peribronchial and peribronchiolar lymphocytic inflammation. A similar pattern was observed with intermittent high-dose exposures at 1200 ppm (15 min, twice a day, 4 weeks). Subchronic exposures to 100 ppm (6 h/day, 12 weeks) caused moderate nasal injury, and peribronchial lymphocytic inflammation accompanied by epithelial atrophy, denudation, and regeneration. Treatment with 400 mg/kg by oropharyngeal aspiration to bypass the nose caused foci of fibrohistiocytic proliferation with little or no inflammation at the junction of the terminal bronchiole and alveolar duct. Depending on the route and duration of exposure, diacetyl causes significant epithelial injury, peribronchial lymphocytic inflammation, or fibrohistiocytic lesions in the terminal bronchioles. Collectively these results indicate that clinically relevant diacetyl exposures result in a pattern of injury that replicates features of human OB.
Keywords: diacetyl, inhalation, mice, lymphocytic bronchitis, obliterative bronchiolitis, microwave popcorn, artificial butter flavoring
Diacetyl (2,3-butanedione; CAS #431–03–8) is a common food flavoring used to impart a butter flavor to dairy products, bakery goods, and other confections. The U.S. Food and Drug Agency granted diacetyl GRAS (generally recognized as safe) status as a direct ingredient of food under the Federal Food, Drug and Cosmetic Act, and consumption of the low levels of diacetyl present in food has not been shown to present a human health risk. Inhalation exposure to significant concentrations of diacetyl only occurs in a few occupational settings, and until recently no adverse health effects have been attributed to inhalation of diacetyl vapors. The toxicity of inhaled diacetyl came under scrutiny after an unusually high percentage of young employees at a microwave popcorn packaging plant developed obliterative bronchiolitis (OB), an often fatal lung disease (Kreiss et al., 2002). Although a cause-effect relationship between diacetyl inhalation and obstructive airway disease has not been established, diacetyl was the predominant component isolated from air samples at microwave popcorn plants. The diagnosis of OB in these employees has created considerable concern for popcorn packaging workers and also for thousands of workers who may be exposed to diacetyl in the food and flavoring industries.
Worker exposures to diacetyl vapors are highly variable not only between different industries but also within an industry. In a Midwest microwave popcorn packaging plant, workers in all parts of the production area were exposed to diacetyl; however, the exposure concentrations and durations differed considerably depending upon the specific work assignment. Workers in the production area of the plant were exposed to artificial butter flavoring vapors containing diacetyl at mean concentrations ranging from 0.56 ppm (quality control workers), 1.88 ppm (packaging line) to 32.3 ppm (mixers) (Kreiss et al., 2002). Mixers were exposed to time-weighted average diacetyl concentrations of about 100 ppm, with potential exposure to peak concentrations greater than 1200 ppm when manually adding flavoring to heated oil (Kreiss et al., 2002). These highly variable occupational exposure scenarios make it difficult to establish safe worker exposure limits based upon the epidemiological data.
Diacetyl toxicity has been investigated in several animal species after oral (Colley et al., 1969; Furihata et al., 1985), intraperitoneal injection (Stoner et al., 1973) and dermal (Opdyke, 1979) routes of administration; however, there have been few studies evaluating the toxicity of inhaled diacetyl. In an unpublished study (BASF, 1993), rats were exposed for 4 h to 639, 1477, or 6788 ppm diacetyl vapors. Some deaths occurred at the two highest concentrations and moderate emphysema and focal hyperemia were present in the lungs of survivors at the high dose. More recently Hubbs et al. (2002) exposed rats to artificial butter flavoring vapors containing 203, 285, or 352 ppm diacetyl for 6 h, or a pulsed exposure to butter flavoring vapor containing diacetyl (range 72–940 ppm) for 6 h. Histopathological evaluation revealed suppurative inflammation and necrosis of nasal epithelium and multifocal necrosis of epithelium in the mainstem bronchi. Pulsed exposure also caused epithelial necrosis in the mid-sized bronchioles. Constant exposure to diacetyl for 6 h also caused significant necrosis of the nasal epithelium at ≥ 198 ppm and significant necrosis of tracheal epithelium at 295 ppm.
The acute inhalation studies discussed above were conducted at relatively high diacetyl concentrations that caused animal morbidity and death after exposure for only a few hours. An acute diacetyl exposure incident or a spill could cause obstructive lung disease as found in the microwave popcorn workers. Acute exposures to high concentrations of other reactive gases such as ammonia, chlorine, hydrogen sulfide, nitrogen dioxide, and phosgene can cause obstructive airway lesions (Arora and Aldrich, 1980; King, 1998). However, a distinct episode of diacetyl overexposure preceding the symptoms was not documented by workers (Kreiss et al., 2002), indicating that OB was more likely caused by repeated and/or intermittent exposures to toxic concentrations of diacetyl.
In the presented murine studies we evaluated the toxicity of inhaled diacetyl using well controlled exposure conditions and concentrations of diacetyl that are encountered in the workplace. Because of the different diacetyl exposure scenarios that occur at microwave popcorn packaging plants, we evaluated the respiratory toxicity of diacetyl after subacute, intermittent, and subchronic inhalation exposures at varying concentrations (Table 1). We also evaluated the effects of diacetyl when administered by oropharyngeal aspiration in order to avoid diacetyl reaction in the nose and to assess its direct effects upon the lower respiratory tract.
TABLE 1.
Summary of Diacetyl Exposure Regimens
| Exposure | Route | Diacetyl dose | Duration | End points |
|---|---|---|---|---|
| Subacute inhalation | Whole body | 0, 200, or 400 ppm | 6 h/day, 4 days | Histopathology |
| Intermittent inhalation | Nose only | 0, 100, 200, or 400 ppm | 1 h/day, 5 days/week, 4 weeks | Histopathology |
| Intermittent inhalation | Nose only | 0 or 1200 ppm | 15 min twice a day, 5 days/week, 2 weeks | Histopathology |
| Subchronic inhalation | Whole body | 0, 25, 50, or 100 ppm | 6 h/day, 5 days/week, 12 weeks | Pulmonary function, BAL, histopathology |
| Oropharyngeal aspiration | Oropharyngeal aspiration | 0, 100, 200, or 400 mg/kg body weight, single dose | 4-days postaspiration | Histopathology |
MATERIALS AND METHODS
Animals
This study was conducted under federal guidelines for the care and use of laboratory animals and was approved by the NIEHS Animal Care and Use Committee. Six-week-old, male C57BL/6 mice (Charles River Laboratories, Raleigh, NC) were individually housed in polycarbonate cages for 10 days after arrival. Mice were provided with food (NIH-31) and tap water ad libitum. Mice were acclimated to the inhalation exposure conditions for 3 days prior to exposure. For whole-body exposures mice were acclimated in Hazleton wire exposure batteries for 6 h/day, and for nose-only exposures the mice were acclimated in the holding tubes for 1 h/day. Food was removed during the acclimation periods and during exposures. Water was provided at all times for animals receiving whole-body exposure. Animals were housed in a humidity- and temperature-controlled, high efficiency particulate air (HEPA)-filtered, mass air displacement room in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. Animal rooms were maintained with a light-dark cycle of 12 h (light from 7:00 A.M. to 7:00 P.M.).
Chemicals
Diacetyl (2,3-butanedione), CAS#431–03–8, was purchased from Fluka Chemical Company (Milwaukee, WI) as a single lot number 1131562. The purity was ≥ 99.0%. Bradford reagent (CN 23208) was purchased from Sigma-Aldrich (St Louis, MO). LDH Reagent kit (CN-L7572) was purchased from Pointe Scientific (Canton, MI).
Inhalation Exposures
Diacetyl Vapor Generation and Monitoring
For all exposure regimens the desired diacetyl concentrations were achieved by metering liquid diacetyl (room temperature) through an atomization nozzle. The atomized diacetyl was injected into the warmed (40°C) process air stream, countercurrent to the process air flow to enhance vaporization of aerosols. Any remaining aerosols were removed by vaporization and impaction in the delivery lines in transit to the inhalation chambers. Verification of aerosol vaporization was performed by analyzing chamber air samples using a handheld aerosol monitor (model 1055, PPM, Inc., Knoxville, TN). Aerosol levels at all diacetyl concentrations were not significantly different from conditioned air without diacetyl, confirming that diacetyl aerosols were not present in the chamber air.
The diacetyl vapor concentration in each chamber was monitored continuously throughout the exposures using dedicated Fourier-transform infrared (FTIR) spectrometers (Hamilton Sundstrand, Pomona, CA). The output from the FTIRs provided feedback for control of the metering pumps. The actual chamber concentrations were held well within 5% of the target concentrations. Diacetyl concentrations were verified by gas chromatography with flame ionization detection analyses of chamber samples.
Whole-body exposures
Subacute and subchronic inhalation exposures were conducted by whole-body exposure in Hazleton 1000 exposure chambers for 6 h/day. Conditioned air (HEPA and charcoal filtered, warmed, and humidified) with or without diacetyl was supplied to the chambers at approximately 400 l/min. The chamber concentrations were monitored continuously throughout the exposures. Actual chamber concentrations were held within 5% of target concentrations for all exposures.
In subacute studies, mice received whole-body exposures to 0 (conditioned air), 200 or 400 ppm diacetyl, 6 h/day for up to 5 days. These concentrations were selected based upon results of an acute inhalation study in rats reported by Hubbs et al. (2002). This experiment was conducted to determine if diacetyl inhalation caused lesions in mice similar to those reported for rats.
Because popcorn packaging workers in some areas of the plant may have received a constant, repeated exposure to lower concentrations of diacetyl a subchronic study also was conducted. Mice (25/concentration) were exposed to 0, 25, 50, or 100 ppm diacetyl for 6 h/day, 5 days/week for either 6 or 12 weeks by whole-body exposure. A subgroup of mice (5/concentration) designated for pulmonary function testing was held without further exposure for an additional 6 weeks after the 12-week exposure (week 18). All mice were weighed weekly and clinical signs of toxicity were recorded at the time of weighing.
Nose-only exposures
Intermittent exposures were conducted by nose-only inhalation exposure using Cannon 52-port nose-only exposure systems (Lab Products, Inc., Seaford, DE). Intermittent exposures were conducted by nose only because animals can be immediately removed from the exposure atmosphere after short, precise exposure durations. Rapid removal from the exposure is not possible with the whole-body exposure system where the mice would continue to be exposed to a decreasing diacetyl concentration for 12–15 min until the chamber could be safely opened. Diacetyl was generated as described above and supplied to the nose-only exposure systems.
Intermittent exposures to low concentrations
In an effort to reduce the nasal toxicity observed after subacute diacetyl exposure and to simulate exposure conditions for some workers, the daily exposure duration was decreased and a lower diacetyl concentration was included. In this intermittent/low concentration exposure profile, 10 mice/concentration were exposed to 0, 100, 200, or 400 ppm diacetyl for 1 h/day, 5 days/week for either 2 or 4 weeks by nose only. Histopathology of the respiratory tract was the only end point in this study. After 2 or 4 weeks of exposure 5 mice/concentration were euthanized and tissues collected, processed, and evaluated as described below.
Intermittent exposures to high concentrations
An intermittent/high concentration exposure profile was designed to replicate the intermittent exposure to high concentrations that occur in the workplace when mixing butter flavoring in a large tank of heated oil. In this experiment, mice were exposed by nose only to 1200 ppm (n = 5) or conditioned air (n = 5) for 15 min twice a day, 5 days/week for 2 weeks. Histopathology of the respiratory tract was the only end point in this study. After exposure for 2 weeks animals were euthanized and tissues collected, processed, and evaluated as described below.
Oropharyngeal Aspiration
Mice were treated with diacetyl by oropharyngeal aspiration in order to bypass the nose. Animals were lightly anesthetized (isofluorane), and administered 100, 200, or 400 mg diacetyl/kg body weight in a total volume of 0.05 ml of sterile water by oropharyngeal aspiration (Foster et al., 2001). Controls received the same volume of sterile water vehicle. Mice received a single aspiration and were euthanized 4 days after treatment.
Histopathology
In all studies, animals were euthanized (Nembutal/thoracotomy) and the lungs with ½ trachea were collected. In the inhalation exposure studies, the nasal cavity tissues, and frequently the larynx, were also collected. Lungs were inflated via the trachea with 10% neutral-buffered formalin and then the trachea was ligated. Lungs and larynx were placed in cassettes in 10% formalin. The nasal cavities were fixed in 10% formalin and then decalcified for 5 h in Rapid Bone Decalcifier (Apex Engineering Products Corporation, Plainfield, IL). Fixed tissues were trimmed, processed, embedded in paraffin, sectioned at 5 µm and stained with hematoxylin and eosin. Some lung sections were also stained with Masson’s trichrome stain.
Histopathologic assessment by light microscopy was quantified by recording the number of animals with each lesion and the total number of animals evaluated in each exposure profile. Average severity grades were assigned for animals with nasal or lung lesions and the severity was graded as: minimal = 1, mild = 2, moderate = 3, marked = 4. Total group scores were calculated for different exposure profiles by multiplying the number of animals with each lesion times the average severity grade for that lesion, and then adding the products obtained for each of these lesions. Some lesions could not be evaluated in all animals because the pertinent histological structure, such as the posterior region of the olfactory epithelium or the trachea, was not represented in the section. For lesions in which less than five animals were available for evaluation, scores were weighted by multiplying 5× the number of animals with the lesion/number of animals evaluated × the average severity grade.
For the purposes of these studies, the term bronchiole was considered to include (1) terminal bronchioles, (2) preterminal bronchioles (airway divisions just proximal to the terminal bronchioles), and (3) cross-sectional airway profiles < 250 µm in diameter (a size range encompassing terminal and preterminal bronchioles). All other intrapulmonary airways proximal to the preterminal bronchiole, or ≥ 250 µm in cross-sectional diameter, were designated as bronchi.
Bronchoalveolar Lavage
After subchronic exposure for 6 and 12 weeks, 5 mice/concentration were euthanized (Nembutal/thoracotomy) and the lungs were lavaged three times with 1.0 ml of saline in situ. The bronchoalveolar lavage (BAL) samples were centrifuged (500 × g) to pellet cells. The cell pellets from the three BAL samples were combined and the total numbers of cells were determined electronically (Coulter ZB, Coulter Electronics, Inc., Marietta, GA). Cell differentials were determined from manual cell counts of stained cytospin preparations. The acellular BAL fluids from the first lavages were analyzed for total protein and lactate dehydrogenase (LDH) activity. Total protein was measured in the BAL fluid as an indication of pulmonary vascular leakage caused by diacetyl exposure. Total protein in the BAL fluid samples was measured using a microplate adaptation of the Sigma-Aldrich Bradford reagent method (Sigma-Aldrich, CN 23208). Absorbance of the protein-dye complex was measured at 592 nm (Bradford, 1976). Because LDH is a cytosolic enzyme, detection of extracellular LDH activity was used as a marker of cell membrane damage. LDH activity was measured in BAL fluid samples using a microplate adaptation of a commercially available liquid LDH Reagent kit (CN-L7572, Pointe Scientific, Canton, MI). The rate of reduction of nucleotide adenine dinucleotide was measured as an increase in absorbance at 340 nm and was directly related to LDH activity.
4-Hydroxyproline Assay
The lavaged lungs of mice collected after subchronic exposure for 6 and 12 weeks were analyzed for 4-hydroxyproline (4-HP), a marker for collagen. Lungs were digested and 4-HP quantified spectrophotometrically by the method of Woessner (1961).
Pulmonary Function Analysis
During the subchronic study pulmonary function testing was conducted repetitively on five designated mice per exposure group after 3, 6, and 12 weeks of exposure and 6 weeks after the 12-week exposure (week 18). Lung function in spontaneously breathing mice was evaluated by whole-body plethysmography (Buxco Electronics, Wilmington, NC). Baseline measurements were collected the day prior to the first exposure. Testing consisted of 30 min of baseline measurements followed by 20-min measurements of responses to saline aerosol or methacholine (6.25, 12.5, 25, 50, or 100 mg/ml) aerosol challenge. After pulmonary function measurements at week 18, mice were euthanized and tissues collected for histopathology as described above.
Statistical Analyses
Data from pulmonary function testing and BAL end points were evaluated for statistically significant differences among treatment and control groups using one-way analysis of variance and Dunnett’s multiple comparison tests. Differences were considered significant at a level of p < 0.05. Analyses were performed using JMP statistical software (SAS, version 6.02 Cary, NC).
RESULTS
Subacute Inhalation Exposures
Mice initially were exposed to either 0 (n = 7), 200 (n = 10), or 400 ppm (n = 15) diacetyl 6 h/day for up to 5 days. In the 200 ppm concentration group, three animals were euthanized in moribund condition after two exposures and three more after three exposures. Moribund mice were dyspneic, hypoactive, and had lost > 10% body weight (data not shown). In the four remaining mice that survived to the end of the study, five exposures to 200 ppm caused acute necrotizing rhinitis and either erosive or necrotizing laryngitis. In the 400 ppm concentration group, two mice were found dead, and nine were euthanized in moribund condition after three exposures. The four remaining mice were euthanized in moribund condition after four exposures to 400 ppm diacetyl. Morbidity was likely a result of nasal lesions and reduced food consumption. Histopathologic evaluation of the respiratory tract of moribund mice exposed to 400 ppm revealed acute necrotizing rhinitis, laryngitis, and bronchitis (proximal large bronchi).
Histopathology
The respiratory epithelium in nasal cavity Levels 1 and 2 of exposed mice had regions of coagulative necrosis with infiltrates of neutrophilic leukocytes, foci of respiratory epithelial sloughing, and neutrophilic exudates in the adjacent nasal cavity (Figs. 1a and 1b). The nasal epithelial necrosis typically affected the lateral walls of the nasal cavity and the naso- and maxillo-turbinates, although in many animals the nasal septum was also involved. In addition, exposure to 400 ppm often resulted in lesions of the Level 3 olfactory epithelium, characterized by vacuolar degeneration and the presence of numerous apoptotic bodies. Similarly, the laryngeal mucosal epithelium of all 400 ppm mice exhibited extensive coagulative necrosis, accompanied by a more mixed inflammatory infiltrate of neutrophils and lymphocytes. Neutrophilic exudates were often noted on the necrotic mucosal surface of the larynx, and the neutrophilic infiltrate frequently extended beyond the submucosa into the adjacent muscle. In mice exposed to 200 ppm diacetyl, the laryngeal changes were generally less severe. Foci of erosive laryngitis were present in 9/10 mice, with two of these mice also showing focal necrotizing laryngitis. One of the 200 ppm mice exhibited necrotizing laryngitis comparable to that of the 400 ppm mice.
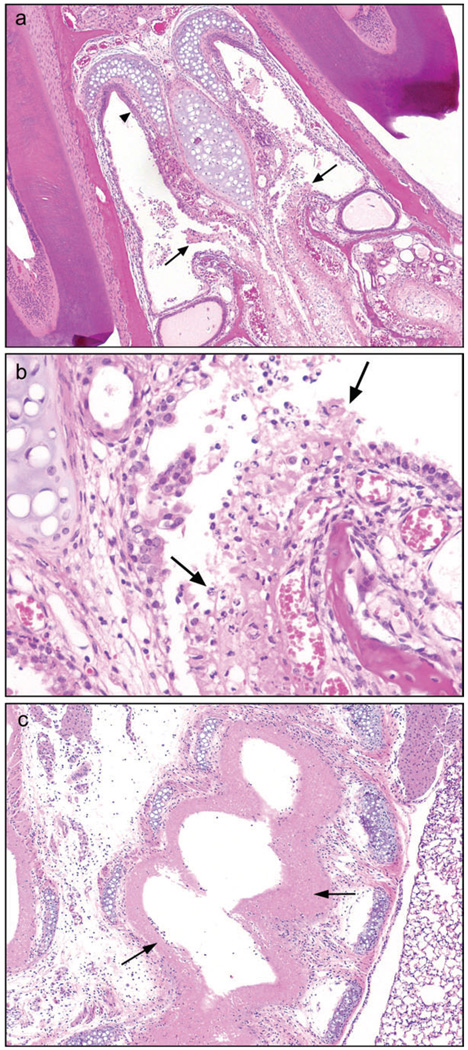
FIG. 1.
Subacute exposure. Inhalation exposure to 400 ppm diacetyl for 4 days (6 h/day) resulted in necrosis of nasal, laryngeal, and mainstem bronchial mucosa. (a) Respiratory epithelium of nasal cavity, Level 1, is inflamed and necrotic (arrows); squamous epithelium (arrowhead) is intact, 4×, hematoxylin and eosin (H&E). (b) Inflammation, necrosis and sloughing of nasal respiratory epithelium, 20×, H&E. (c) Diffuse mucosal necrosis (arrows) of extrapulmonary right mainstem bronchus, 4×, H&E.
In the trachea and major bronchi significant differences were noted between the 200 and 400 ppm mice. In the 200 ppm mice no mucosal necrosis was seen in either the extra- or intrapulmonary bronchi. In contrast, all 400 ppm mice in which trachea (5/5) and extrapulmonary bronchi (12/12) were represented in the sections showed extensive coagulative necrosis of the mucosal epithelium with mild neutrophilic infiltrate (Fig. 1c). These mice were all euthanized early in moribund condition. The submucosal tissue of inflamed airways was edematous and infiltrated by mild to moderate numbers of neutrophils, lymphocytes, and monocytes. In addition, 9 of the 14 mice exposed to 400 ppm also demonstrated coagulative necrosis of the proximal large intrapulmonary bronchi, and in three of these mice degenerative epithelial changes with some loss of cell cohesion were noted in proximal bronchial branches of the mainstem bronchi. No lesions of the bronchioles or of the pulmonary parenchyma were noted in mice subacutely exposed to either 200 or 400 ppm diacetyl.
Intermittent Low Concentration Exposures
In an attempt to decrease the nasal cavity toxicity and increase airway effects, the daily exposure duration was reduced to 1 h/day, 5 days/week and the study duration increased to 2 or 4 weeks. All mice survived to the end of the study with no clinical signs of toxicity. Body and lung weights were not significantly different between controls and exposed mice when evaluated at 2 and 4 weeks (data not shown).
Nasal lesions
The most severe lesions were observed in the nasal cavity. Group scores for nasal lesions were increased in a concentration dependent manner after intermittent exposure for 2 and 4 weeks (Table 2). Chronic active inflammation, suppurative exudate in the nasal cavity, focal areas of epithelial ulceration, and metaplastic and degenerative changes of the respiratory epithelium were noted in the 400 ppm mice after 2 weeks (Fig. 2a). Chronic active inflammation was also present in the 200 and 100 ppm mice at 2 weeks, but suppurative exudate was limited to one of the 200 ppm mice, and no ulceration of the respiratory epithelium was observed. After 4 weeks of exposure, inflammation and suppurative exudate remained at a similar level in the 400 ppm mice, but partial regeneration was suggested by the absence of vacuolar degeneration and ulceration of the epithelium. The most obvious difference between these mice exposed intermittently for 1 h/day to 400 or 200 ppm, and the mice exposed sub-acutely for 6 h/day, was the greatly diminished necrosis and ulceration of the epithelium in the intermittently exposed 400 ppm mice and the absence of necrosis and ulceration in the 200 ppm mice.
TABLE 2.
Nasal Lesions after Intermittent Exposures to Diacetyl
| Lesion | 0 ppm | 100 ppm | 200 ppm | 400 ppm |
|---|---|---|---|---|
| 2-week exposure | ||||
| Inflammation, chronic active | 1/5a (1.0)b | 5/5 (2.0) | 5/5 (3.0) | 5/5 (2.6) |
| Exudate, suppurative | 0/5 | 0/5 | 1/5 (2.0) | 5/5 (2.6) |
| Respiratory epithelium, necrosis, and ulceration | 0/5 | 0/5 | 0/5 | 3/4 (1.7) |
| Respiratory epithelium, metaplasia, squamous | 0/5 | 1/5 (1.0) | 1/5 (1.0) | 4/4 (3.0) |
| Respiratory epithelium, vacuolar degeneration | 0/5 | 0/5 | 2/5 (2.0) | 4/4 (1.5) |
| Olfactory epithelium, vacuolar degeneration | 0/5 | 0/4 | 0/3 | 1/3 (2.0) |
| Olfactory epithelium, metaplasia, respiratory | 0/5 | 0/4 | 0/3 | 1/2 (2.0) |
| Olfactory epithelium, atrophy | 0/5 | 1/4 (1.0) | 0/3 | 2/2 (2.0) |
| Total group score | 1c | 12 | 22 | 73 |
| 4-week exposure | ||||
| Inflammation, chronic active | 0/5 | 2/5 (1.0) | 5/5 (1.0) | 5/5 (2.6) |
| Exudate, suppurative | 0/5 | 0/5 | 1/5 (1.0) | 5/5 (2.2) |
| Respiratory epithelium, necrosis, and ulceration | 0/5 | 0/5 | 0/5 | 0/5 |
| Respiratory epithelium, metaplasia, squamous | 0/5 | 1/5 (1.0) | 5/5 (1.0) | 5/5 (2.4) |
| Olfactory epithelium, metaplasia, respiratory | 0/5 | 0/5 | 0/5 | 3/5 (2.0) |
| Olfactory epithelium, atrophy | 0/5 | 0/5 | 1/5 (1.0) | 5/5 (2.6) |
| Total group score | 0 | 3 | 12 | 55 |
Number of animals with lesion/number evaluated.
Numbers in parentheses represent the average severity grades for those animals with the lesions. Severity grading scheme: minimal = 1, mild = 2, moderate = 3, marked = 4.
Total group scores were calculated by multiplying the number of animals with each lesion times the average severity grade for that lesion, and then adding the products obtained for each of these lesions. Group scores were weighted for lesions in which less than five animals were evaluated.
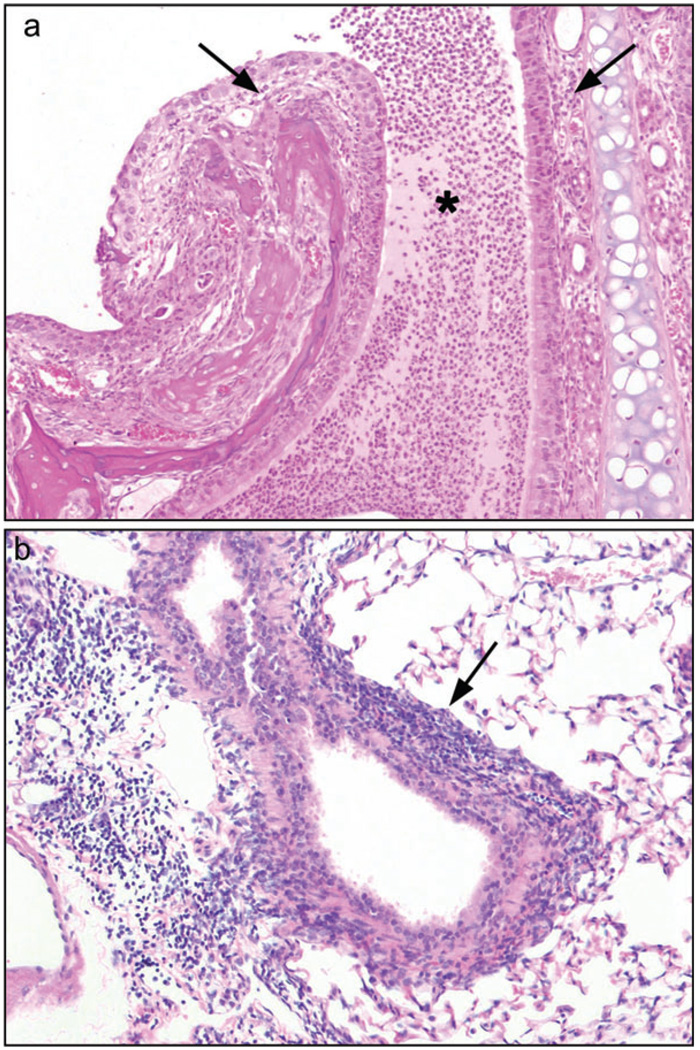
FIG. 2.
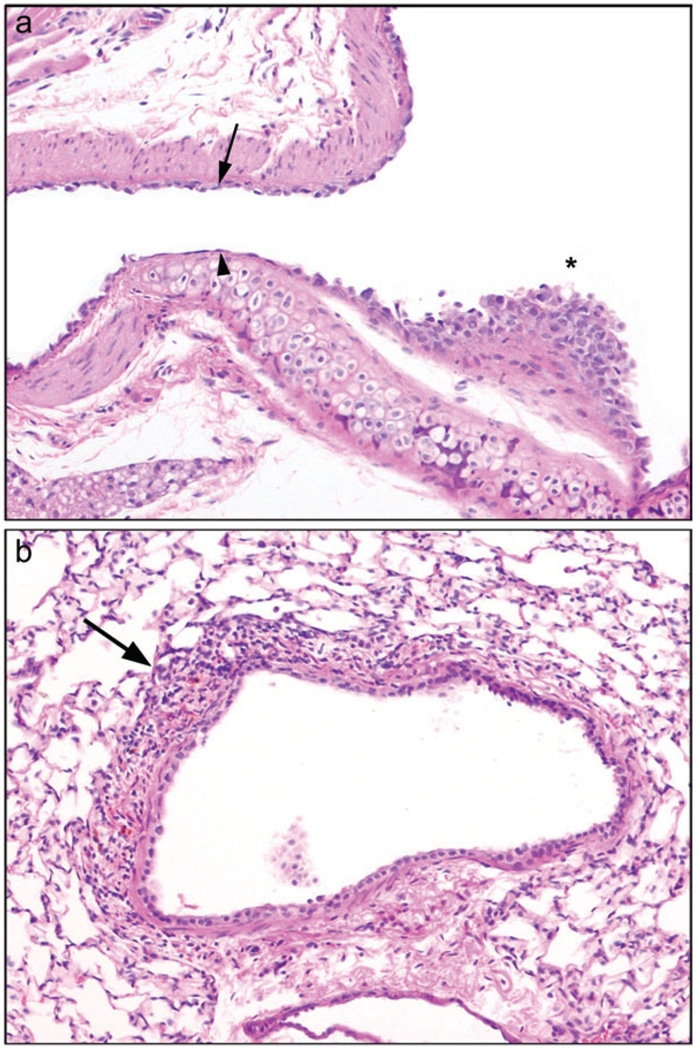
Intermittent low concentration exposure, 1 h/day, 5 days/week for 4 weeks. Diacetyl toxicity for the nasal cavity was reduced by decreasing the exposure duration to 1 h/day for 4 weeks (0, 100, 200, or 400 pm). (a) Acute rhinitis, with inflammatory cells in nasal submucosa (arrows) and suppurative exudate (asterisk), was present in the Level I nasal cavity of mice exposed to 400 ppm, 10×, hematoxylin and eosin (H&E). (b) Moderately severe lymphocytic bronchitis (arrow) was present in the lungs of mice exposed to 400 ppm diacetyl, 10×, H&E.
Lung lesions
Group scores for lung lesions were increased with increasing exposure concentration after exposure for 2 and 4 weeks (Table 3). Peribronchial lymphocytic inflammation of mild to moderate severity was present in all mice exposed to 200 or 400 ppm at both 2 and 4 weeks of exposure (Fig. 2b). Most of the 100 ppm mice also exhibited peribronchial lymphocytic inflammation, of a minimal to mild degree, after 2 and 4 weeks of exposure. Peribronchiolar lymphocytic inflammation was noted in most of the 400 ppm mice after 4 weeks of exposure to diacetyl. No fibrotic lesions of bronchioles, alveolar ducts, or alveolar walls were present in any of the mice in these intermittent exposure regimens.
TABLE 3.
Lung Lesions after Intermittent Exposures to Diacetyl
| Lesion | 0 ppm | 100 ppm | 200 ppm | 400 ppm |
|---|---|---|---|---|
| 2-week exposure | ||||
| Peribronchial lymphocytic inflammation | 0/5a | 4/5 (2.0)b | 5/5 (2.4) | 5/5 (3.0) |
| Peribronchiolar lymphocytic inflammation | 0/5 | 1/5 (1.0) | 0/5 | 1/5 (1.0) |
| Total group score | 0c | 9c | 12 | 16 |
| 4-week exposure | ||||
| Peribronchial lymphocytic inflammation | 0/3 | 2/3 (1.0) | 5/5 (2.4) | 5/5 (3.0) |
| Peribronchiolar lymphocytic inflammation | 0/3 | 0/3 | 1/5 (1.0) | 3/5 (1.0) |
| Total group score | 0 | 3 | 13 | 18 |
Number of animals with lesion/number evaluated.
Numbers in parentheses represent the average severity grades for those animals with the lesions. Severity grading scheme: minimal = 1, mild = 2, moderate = 3, marked = 4.
Total group scores were calculated by multiplying the number of animals with each lesion times the average severity grade for that lesion, and then adding the products obtained for each of these lesions. Group scores were weighted for lesions in which less than five animals were evaluated.
Intermittent High Concentration Exposures
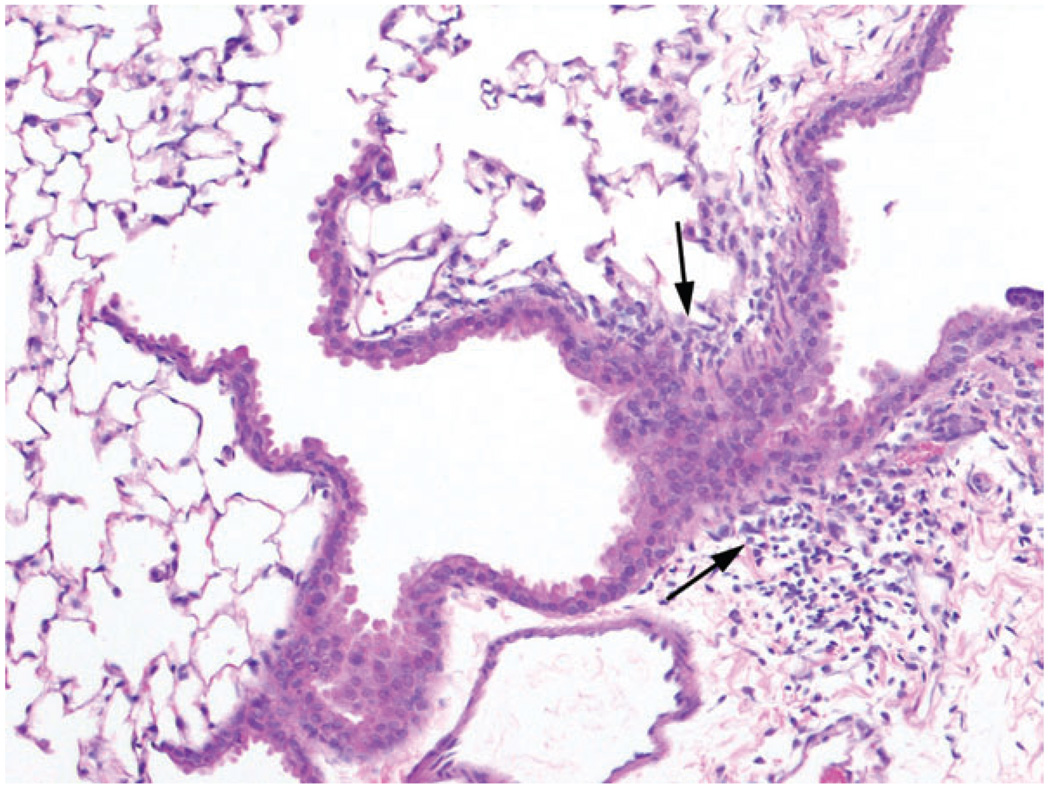
Intermittent exposure of mice to 1200 ppm diacetyl for 15 min twice a day for 2 weeks caused a slight (8%) decrease in body weights relative to controls; however there were no significant differences in lung weights (data not shown). Exposed mice exhibited inflammatory and metaplastic changes within the nasal cavity (Table 4), but the changes were not as severe as those noted after exposure to lower concentrations for 6 h/day. In particular, nasal respiratory epithelial ulceration was present only in minimal foci of three of the five animals, in contrast to the prominent ulceration and necrosis seen in the subacute exposure protocols. Vacuolar degeneration of the nasal respiratory epithelium, and olfactory epithelial atrophy were each present in four of the five animals to a mild degree. Lungs of diacetyl-exposed mice exhibited mild to moderate peribronchial lymphocytic infiltrates, without epithelial lesions. However, the peribronchial lymphocytic infiltrates appeared to extend more frequently into the bronchiolar branches than in the other exposure regimens (Fig. 3). There were no fibrotic lesions of bronchi, bronchioles, or lung.
TABLE 4.
Lesions after Intermittent Exposures to 1200 ppm Diacetyl
| 0 ppm | 1200 ppm | |
|---|---|---|
| Nasal lesions | ||
| Inflammation, chronic active | 0/5a | 5/5 (2.0)b |
| Exudate, suppurative | 0/5 | 2/5 (1.0) |
| Respiratory epithelium, necrosis, and ulceration | 0/5 | 3/5 (1.0) |
| Respiratory epithelium, degeneration, vacuolar | 0/5 | 4/5 (2.0) |
| Respiratory epithelium, metaplasia, squamous | 0/5 | 5/5 (2.6) |
| Olfactory epithelium, metaplasia, respiratory | 0/5 | 1/5 (2.0) |
| Olfactory epithelium, atrophy | 0/5 | 4/5 (2.0) |
| Total group score | 0c | 46 |
| Lung lesions | ||
| Peribronchial lymphocytic inflammation | 0/5 | 5/5 (2.6) |
| Peribronchiolar lymphocytic inflammation | 0/5 | 5/5 (1.6) |
| Total group score | 0 | 21 |
Number of animals with lesion/number evaluated.
Numbers in parentheses represent the average severity grades for those animals with the lesions. Severity grading scheme: minimal = 1, mild = 2, moderate = 3, marked = 4.
Total group scores were calculated by multiplying the number of animals with each lesion times the average severity grade for that lesion, and then adding the products obtained for each of these lesions.
FIG. 3.
Intermittent high concentration exposure (1200 ppm) 15 min two times per day, 5 days/week for 2 weeks. Peribronchiolar lymphocytic inflammation (arrows), preterminal bronchiole, 10×, hematoxylin and eosin.
Subchronic Inhalation Exposures
Survival and body weights
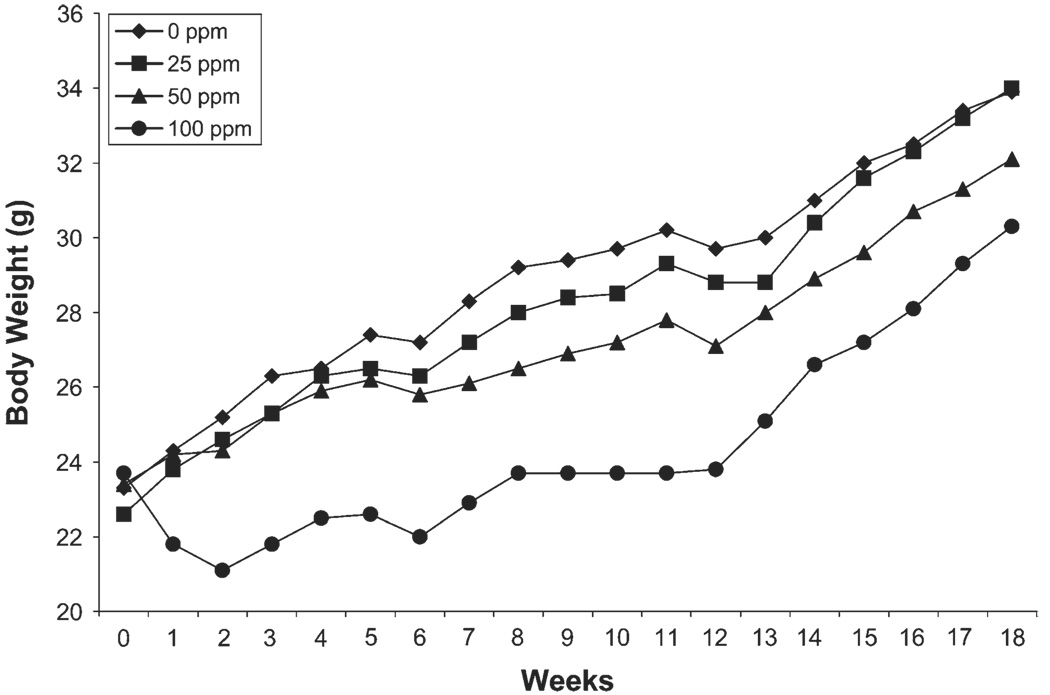
All mice survived the 12-week exposure to 0, 25, 50, or 100 ppm diacetyl 6 h/day, 5 days/week for 12 weeks. Body weights of mice designated for pulmonary function (5 mice/concentration) were measured weekly throughout the 12-week exposure and 6-weeks postexposure. Body weights of mice exposed to 100 ppm diacetyl were significantly less than controls (p < 0.05) throughout the 12-week exposure and the 6-week recovery period (Fig. 4). By 6-weeks postexposure, body weights began to increase but remained significantly less than controls. Body weights of mice exposed to 25 and 50 ppm were not significantly different from controls during the 12-week exposure and the 6-week recovery period.
FIG. 4.
Subchronic exposure. Body weights of mice exposed to diacetyl 6 h/day, 5 days/week for 12 weeks and at 6 weeks after termination of the exposure (week 18). Body weights of mice exposed to 100 ppm were significantly decreased from air-exposed (0 ppm) controls (p < 0.05) throughout the study.
BAL cells
Total cells and cell differentials were evaluated in the BAL fluid of mice after exposure to diacetyl for 6 and 12 weeks. Although there appeared to be a trend of increasing total cell number with increasing diacetyl concentration at both time points, these changes were not statistically significant (data not shown). Similarly, cell differentials in diacetyl-exposed groups were not significantly different from air-exposed controls after 6 and 12 weeks (data not shown).
Protein and LDH activity
Protein was measured in the BAL fluid as an indication of pulmonary vascular leakage caused by diacetyl exposure. Diacetyl exposure did not cause significant changes in BAL fluid protein levels (data not shown). LDH, a cytosolic enzyme, was measured in the BAL fluid as a marker of cell membrane damage. LDH activity (mM activity/min) was significantly increased (p < 0.05) in the BAL fluid from mice exposed for 6 weeks to 50 ppm (11.99 ± 3.72) and 100 ppm (11.04 ± 0.73) relative to controls (6.54 ± 1.34). LDH activity remained significantly increased (p < 0.05) in the 100 ppm group after 12 weeks of exposure.
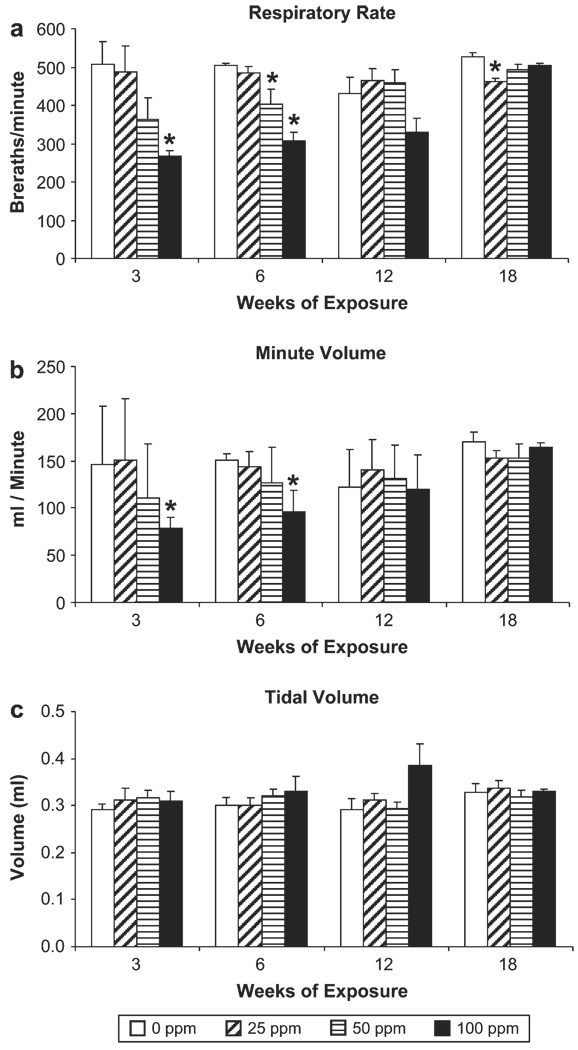
Pulmonary function changes
Pulmonary function was evaluated repetitively in the same mice (five per concentration) after 3,6, and 12 weeks of exposure and 6 weeks after the last exposure (week 18). Respiratory rates in diacetyl-exposed mice appeared to decrease in a concentration dependent manner; and to increase with duration of exposure (Fig. 5a). This trend was present after 3 and 6 weeks of exposure and was no longer apparent by week 12. Statistically significant (p < 0.05) decreases in respiratory rate occurred after exposure to 100 ppm for 3 and 6 weeks and to 50 ppm for 6 weeks. Respiratory rates returned to control levels by 12-weeks of exposure, and by 6-weeks postexposure (week 18) respiratory rates were significantly lower (p < 0.05) than controls only in the 25 ppm group. A similar pattern was observed for minute volume (Fig. 5b). Minute volume was decreased in a concentration dependent manner when measured after 3 and 6 weeks of exposure. Minute volume was significantly (p < 0.05) decreased relative to air-exposed controls only after exposure to 100 ppm for 3 and 6 weeks. Tidal volume in diacetyl-exposed mice was not significantly different from controls at any time point (Fig. 5c). All animals responded to challenge with increasing concentrations of methacholine; however, the responses of diacetyl-exposed mice were not significantly different from responses of air-exposed control mice (data not shown).
FIG. 5.
Subchronic exposure. Pulmonary function (Buxco) was measured in a subgroup of mice after 3, 6, and 12 weeks of exposure and at 6 weeks after termination of the exposures (week 18). *Significantly decreased from airexposed (0 ppm) controls (p < 0.05).
4-Hydroxyproline
4-HP, an index of collagen deposition, was measured in lung tissue after collection of BAL fluid. There were no significant differences in 4-HP content between exposed and controls when measured after 6 and 12 weeks of exposure (data not shown).
Histopathology
In the nasal cavity, suppurative rhinitis with chronic active inflammation, foci of respiratory mucosal ulceration and/or necrosis, and moderate squamous metaplasia were noted in the 100 ppm group after 6 and 12 weeks of exposure (Table 5). Olfactory epithelial atrophy was also present in all 100 ppm mice after 6 and 12 weeks, and respiratory metaplasia of the olfactory epithelium was particularly prominent in the 12 week group. The group scores for nasal lesion severity/incidence were concentration dependent at each time point. Group scores were highest at 12-weeks and were diminished at 6-weeks postexposure. Inflammatory changes, metaplasia, and olfactory epithelial atrophy were also present but were decreased in the 50 ppm group, and inflammation and squamous metaplasia were relatively minor in the 25 ppm group.
TABLE 5.
Nasal Lesions after Subchronic Exposure to Diacetyl
| Lesion | 0 ppm | 25 ppm | 50 ppm | 100 ppm |
|---|---|---|---|---|
| 6-week exposure | ||||
| Inflammation, chronic active | 2/5a (1.0)b | 2/4 (1.5) | 4/5 (1.5) | 5/5 (3.8) |
| Exudate, suppurative | 0/5 | 2/4 (1.0) | 4/5 (1.0) | 5/5 (3.8) |
| Respiratory epithelium, necrosis, and ulceration | 0/5 | 0/4 | 2/5 (1.0) | 5/5 (1.2) |
| Respiratory epithelium, metaplasia, squamous | 0/5 | 1/4 (1.0) | 3/5 (2.3) | 5/5 (3.0) |
| Olfactory epithelium, metaplasia, respiratory | 0/5 | 0/4 | 3/5 (1.7) | 1/5 (3.0) |
| Olfactory epithelium, atrophy | 0/5 | 0/4 | 4/5 (2.0) | 5/5 (2.9) |
| Total group score | 2c | 8 | 32 | 77 |
| 12-week exposure | ||||
| Inflammation, chronic active | 1/5 (1.0) | 4/5 (1.3) | 5/5 (1.8) | 5/5 (4.0) |
| Exudate, suppurative | 0/5 | 0/5 | 5/5 (2.2) | 5/5 (3.6) |
| Respiratory epithelium, necrosis, and ulceration | 0/5 | 0/5 | 1/5 (1) | 5/5 (2.6) |
| Respiratory epithelium, metaplasia, squamous | 0/5 | 2/5 (1.0) | 4/5 (1.3) | 5/5 (3.0) |
| Olfactory epithelium, metaplasia, respiratory | 0/5 | 0/5 | 5/5 (2.6) | 5/5 (3.4) |
| Olfactory epithelium, atrophy | 0/5 | 0/5 | 5/5 (1.8) | 5/5 (3.0) |
| Total group score | 1 | 7 | 48 | 98 |
| 6-weeks postexposure | ||||
| Inflammation, chronic active | 0/5 | 2/5 (1.0) | 4/5 (1.3) | 5/5 (2.2) |
| Exudate, suppurative | 0/5 | 0/5 | 0/5 | 3/5 (2.0) |
| Respiratory epithelium, necrosis, and ulceration | 0/5 | 0/5 | 0/5 | 0/5 |
| Respiratory epithelium, metaplasia, squamous | 0/5 | 1/5 (1.0) | 0/5 | 4/5 (1.0) |
| Olfactory epithelium, metaplasia, respiratory | 0/5 | 1/5 (1.0) | 4/5 (1.5) | 4/5 (2.3) |
| Olfactory epithelium, atrophy | 0/5 | 1/5 (1.0) | 3/5 (1.0) | 4/5 (2.0) |
| Total group score | 0 | 5 | 14 | 38 |
Number of animals with lesion/number evaluated.
Numbers in parentheses represent the average severity grades for those animals with the lesions. Severity grading scheme: minimal = 1, mild = 2, moderate = 3, marked = 4.
Total group score calculated by multiplying the number of animals with each lesion times the average severity grade for that lesion, and then adding the products obtained for each of these lesions. Group scores were weighted for lesions in which less than five animals were evaluated.
All mice (5/5) exposed to 100 ppm for 12 weeks exhibited peribronchial lymphocytic infiltrates, accompanied by prominent epithelial changes within the large bronchi. The latter were characterized by a mixed pattern of atrophy, denudation, and regeneration (Fig. 6a), resulting in alternating zones of attenuated cuboidal respiratory epithelial cells, denuded foci covered only by a single layer of spindleoid basal cells, and regenerative foci of epithelial hypertrophy and karyomegaly (Table 6). The lymphocytic inflammation also extended to some of the smaller airways and bronchioles in three of the five mice (Fig. 6b). Peribronchial lymphocytic inflammation was also noted after 12 weeks of exposure in four of the five mice exposed to 50 ppm, and in two of the five mice exposed to 25 ppm, but the inflammation in these animals was only of a minimal to mild degree, and was unaccompanied by epithelial atrophy or denudation. When evaluated 6 weeks after the subchronic exposure, the peribronchial lymphocytic inflammation was essentially unchanged, but epithelial atrophy was diminished, denudation was absent, and regenerative changes were ongoing. Perivascular lymphocytic inflammation was also noted in four of the five mice in the 100 ppm group at 6-weeks postexposure. There were no fibroproliferative lesions in the small airways of diacetyl-exposed mice at either time point.
FIG. 6.
Subchronic exposure. Mice were exposed to 0, 25, 50, 100 ppm diacetyl for 12 weeks (6 h/day, 5 days/week), (a) Mainstem bronchus exhibiting mixed pattern of epithelial atrophy (arrow), denudation (arrowhead), and regenerative hyperplasia with karyomegaly (asterisk). 100 ppm, 10×, hematoxylin and eosin (H&E). (b) Lymphocytic bronchitis was present in some smaller airway branches of the lung. 100 ppm, 10×, H&E.
TABLE 6.
Lung Lesions after Subchronic Exposure to Diacetyl
| Lesion | 0 ppm | 25 ppm | 50 ppm | 100 ppm |
|---|---|---|---|---|
| 6-week exposure | ||||
| Peribronchial lymphocytic inflammation | 0/5a | 3/5 (1.0)b | 5/5 (1.6) | 5/5 (2.6) |
| Bronchial epithelial atrophy and denudation | 0/5 | 0/5 | 1/5 (1.0) | 5/5 (2.8) |
| Bronchial epithelial regeneration | 0/5 | 0/5 | 0/5 | 5/5 (2.8) |
| Peribronchiolar lymphocytic inflammation | 2/5 (1.0) | 0/5 | 1/5 (1.0) | 3/5 (1.0) |
| Total group score | 2c | 3 | 10 | 44 |
| 12-week exposure | ||||
| Peribronchial lymphocytic inflammation | 0/5 | 2/5 (1.0) | 4/5 (1.5) | 5/5 (2.6) |
| Bronchial epithelial atrophy and denudation | 0/5 | 0/5 | 0/5 | 5/5 (3.0) |
| Bronchial epithelial regeneration | 0/5 | 0/5 | 0/5 | 5/5 (3.0) |
| Peribronchiolar lymphocytic inflammation | 0/5 | 0/5 | 0/5 | 3/5 (1.3) |
| Total group score | 0 | 2 | 6 | 47 |
| 6-weeks postexposure | ||||
| Peribronchial lymphocytic inflammation | 3/5 (1.3) | 1/5 (2.0) | 4/5 (1.8) | 5/5 (2.8) |
| Bronchial epithelial atrophy | 0/5 | 0/5 | 0/5 | 3/5 (1.0) |
| Bronchial epithelial regeneration | 0/5 | 0/5 | 0/5 | 5/5 (2.0) |
| Peribronchiolar lymphocytic inflammation | 0/5 | 0/5 | 0/5 | 2/5 (1.0) |
| Total group score | 4 | 2 | 7 | 29 |
Number of animals with lesion/number evaluated.
Numbers in parentheses represent the average severity grades for those animals with the lesions. Severity grading scheme: minimal = 1, mild = 2, moderate = 3, marked = 4.
Total group score calculated by multiplying the number of animals with each lesion times the average severity grade for that lesion, and then adding the products obtained for each of these lesions.
Oropharyngeal Aspiration
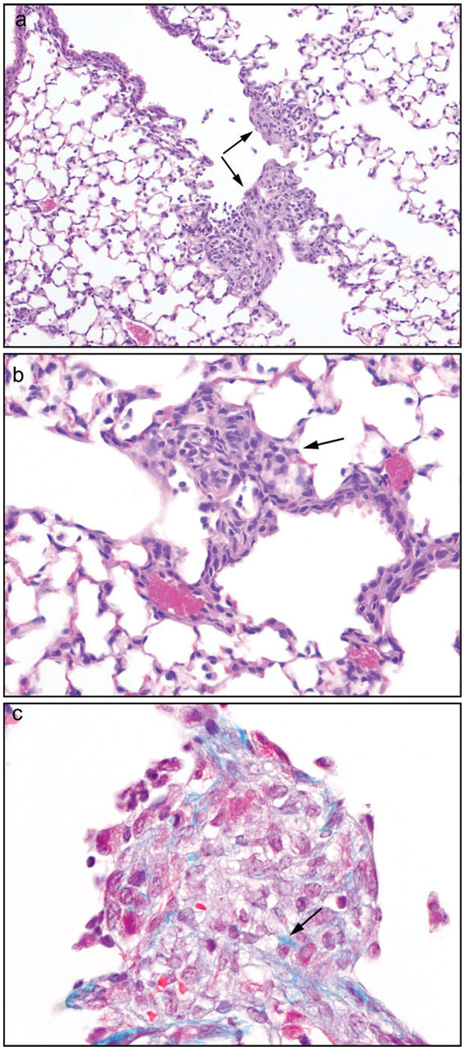
Because of the susceptibility of the nasal cavity to inhaled diacetyl vapors, mice were administered diacetyl by oropharyngeal aspiration. Mice (n = 5 per group) received 0, 100, 200, or 400 mg/kg and were euthanized 4 days later. Two of five mice in the 400 mg/kg group died 2 days after aspiration: there were no deaths in the 0, 100, and 200 mg/kg dose groups. When evaluated 4 days after aspiration, foci of fibrohistiocytic proliferation with little or no inflammation were present at the junction of the terminal bronchioles and alveolar ducts in the three remaining mice treated with 400 mg/kg (Fig. 7a), and in one of the five mice that received 200 mg/kg diacetyl. Most of the lesions were centered on one side of the proximal portion of the alveolar ducts, partially obliterating the ductal lumens. The fibrohistiocytic proliferation sometimes extended into and thickened the adjacent alveolar walls. The lesions were syncytial in appearance, and were composed of plump, rounded to ovoid histiocytic cells, elongate, spindle-shaped fibroblasts, and other cells with features intermediate between the two (Fig. 7b). Other than the histiocytes, some of the lesions contained no inflammatory cells, whereas others contained a few lymphocytes, and in one lesion a few eosinophils were also noted. Apoptotic bodies were sometimes present. With the Masson’s trichrome stain a few lightly staining blue fibers were noted in some foci, suggestive of early collagen deposition (Fig. 7c). Mild to moderate respiratory epithelial hyperplasia, with karyomegaly, scattered apoptotic bodies, and occasional mitoses, was present in the large bronchi of the three mice receiving 400 mg/kg. No fibrohistiocytic lesions were observed in the control or 100 mg/kg dose groups.
FIG. 7.
Oropharyngeal aspiration. Diacetyl was administered by a single oropharyngeal aspiration. (a) Focal fibrohistiocytic lesions with minimal inflammation were observed at the junction of the terminal bronchiole and alveolar duct (arrows) at 4 days after treatment with 400 mg/kg diacetyl, 10×, hematoxylin and eosin (H&E). (b) The fibrohistiocytic lesions were usually composed of a mixture of spindle cells and histiocyte-like cells (arrow), 20×, H&E. (c) Masson’s trichrome stain for collagen. Within this fibrohistiocytic focus, a few blue fibers suggestive of collagen may be seen. 40×, H&E.
DISCUSSION
Inhalation exposure of mice to diacetyl affected primarily the nose and upper airways with minimal effects in the distal airways. Subacute exposure to 200 or 400 ppm diacetyl, 6 h/day for 5 days caused moderately severe necrosis in the nasal cavity and larynx; mucosal necrosis was also present in the proximal large bronchi of mice exposed to 400 ppm. Similar lesions were reported for rats after a 6 h acute exposure to butter flavoring containing 285–371 ppm diacetyl (Hubbs et al., 2002). These two studies demonstrate that the upper respiratory tract is a primary target of inhaled diacetyl in both rodent species. In rodents, the nasal cavity has been shown to be the primary target site for other volatile ketones such as methylvinyl ketone (Morgan et al., 2000), ethylvinyl ketone (Morgan et al., 2001), and 2-cyclohexene-l-one (Cunningham et al., 2001), as well as other direct acting gaseous chemicals such as chlorine (Jiang et al., 1983), formaldehyde, ammonia (Broderson et al., 1976), acetaldehyde (Kruysse et al., 1975), and acrolein (Feron et al., 1978).
Although nasal irritation was reported by some microwave popcorn workers, the primary site of injury was the distal airways (Kreiss et al., 2002). The human nasal cavity may be less susceptible because it is less efficient in removing inhaled reactive chemicals than the rodent nasal cavity (Morgan and Monticello, 1990). Relative to the rodent nasal cavity, the human nasal cavity is shorter, has fewer turbinates, and a smaller surface area for removal of reactive chemicals (Phalen, 1984). In addition, rodents are obligate nose-breathers, whereas humans are nasal and oral breathers, although oral breathing may be more prevalent in exposed workers with nasal irritation and congestion. Oral breathing circumvents the protective scrubbing action of the nasal passages potentially allowing higher concentrations of diacetyl to reach the bronchioles. Because the epithelium of the bronchioles lacks a protective layer of mucus it may be more susceptible to reactive chemicals like diacetyl.
Because of the various exposure scenarios present in microwave popcorn plants we investigated the potential respiratory toxicity resulting from several diacetyl exposure concentration–time profiles relevant to occupational exposures. We evaluated the effects of intermittent exposure to the same concentrations used in the subacute studies. Mice were exposed for 1 h/day to relatively low diacetyl concentrations up to 400 ppm for up to 4 weeks. This exposure regimen greatly reduced the necrosis and ulceration in the nasal cavity that was observed after 6 h daily exposures. Although intermittent exposure to low diacetyl concentrations did not produce fibrotic lesions in the small airways, almost all exposed mice had mild to moderate peribronchial lymphocytic inflammation.
We also exposed mice to 1200 ppm diacetyl for 15 min, twice a day for 2 weeks to simulate the intermittent high concentration exposures experienced by flavoring mixers when opening the tanks of heated oil and butter flavorings. A peak concentration of 1200 ppm diacetyl was selected based upon measurements of 1232 ppm diacetyl inside a butter flavoring holding tank at a microwave popcorn plant (Kreiss et al., 2002). The short exposure duration (15 min) resulted in less injury to the nasal epithelium than observed in subacute 6 h/day exposures. Interestingly, intermittent high concentration exposure to diacetyl produced peribronchiolar accumulations of lymphocytes in the distal airways of all mice. Although this lesion was also observed after intermittent exposure to low diacetyl concentrations, the peribronchiolar lymphocytic infiltrates of the latter group were not present in all mice and were minimal in severity when present.
The highest prevalence of respiratory symptoms and airways obstruction was diagnosed in microwave popcorn workers with more than 12 months experience (Kanwal et al., 2006). To address these longer exposures, we conducted subchronic inhalation exposures to diacetyl. Lymphocytic bronchitis and bronchiolitis, similar to that observed during intermittent exposures, were observed in mice exposed to diacetyl for 12 weeks. The presence of the peribronchiolar lymphocyte accumulations, a potential precursor lesion, and the lack of endobronchiolar fibrotic lesions suggest that longer exposure duration (intermittent or constant) may be necessary for development of airway obstruction in mice.
Although the lymphocytic bronchitis and bronchiolitis were distinct diacetyl-related effects, their significance with respect to obstructive airway disease is not clear at present. Lymphocytes contribute to the epithelial injury in lung transplant patients and lymphocytic bronchiolitis is a known risk factor for the development of OB in the lung transplant population (Sharples et al., 2002). In addition, lymphocytic inflammation has been previously reported in a rat model of toxin-induced airway obliteration that used a continuous infusion of papaverine (Svetlecic et al., 2004). Additional studies are underway to compare and contrast the mechanisms of transplant-related versus toxin-induced OB, and to determine how differing etiologies, mechanisms, and morphologic pathways ultimately lead to OB.
Based upon the results of these studies, the maximum tolerated dose (6 h daily exposures) for diacetyl in mice was estimated to be between 50 and 100 ppm. Exposure of mice to diacetyl concentrations greater than 100 ppm resulted in morbidity and mortality. Body weights of mice exposed to 100 ppm were significantly less than controls for the duration of the 12-week study and nasal lesions were moderate to marked in severity after 6 and 12 weeks exposure. A no-observed effect level was not determined in these studies. Subchronic exposure to the lowest diacetyl exposure concentration (25 ppm) caused lesions in the nasal cavity consisting of minimal to mild chronic inflammation (four of the five mice), minimal squamous metaplasia of the respiratory epithelium (two of the five mice) in the nose, and minimal to mild lymphocytic bronchitis (two of the five mice). These nasal lesions were relatively minor and may be specific to obligate nasal-breathing species. It is also possible that the nasal lesions would be more severe in workers if they were exposed to a constant high concentration of diacetyl for 6 h/day as mice were in this study.
Subchronic inhalation exposure to diacetyl resulted in concentration-related decreases in respiratory rate and minute volume. Shallow breathing (decreased minute volume) can result in an increased deposition in the nasopharyngeal and tracheobronchial regions, and decreased deposition in the pulmonary regions of the lung (Snipes, 1989). These changes in pulmonary function were transient and returned to control levels by 12 weeks of exposure and remained unchanged at 6-weeks postexposure. The transient nature of these respiratory effects indicates that they were likely a result of irritation and congestion of the nasal cavity. We did not observe an increase in airway reactivity to methacholine challenge in mice exposed to diacetyl vapors relative to airexposed controls when evaluated after exposure for 3, 6, or 12 weeks. Fedan et al. (2006) reported that in isolated, perfused guinea pig tracheal preparations, intraluminal treatment with diacetyl increased the reactivity to methacholine by 10-fold. This increased airway hyperreactivity was attributed to increased epithelial permeability caused by diacetyl toxicity. The absence of hyperreactivity in the current study was unexpected especially in the 100 ppm group where diacetyl caused hypertrophy and desquamation of the bronchial epithelium. It is possible that disruption of epithelium in the more distal, smaller bronchioles may be necessary to cause measurable changes in airway reactivity.
Absorption and reaction of inhaled diacetyl in the nasal cavity likely protected the distal airways of mice from exposure to toxic concentrations of diacetyl. Oropharyngeal aspiration was used to bypass the nasal cavity, and provide a higher dose of diacetyl to the airways. Diacetyl administered by oropharyngeal aspiration resulted in fibrohistiocytic lesions in the distal airways of mice. Fibrohistiocytic foci were present at the junction of the terminal bronchioles and alveolar ducts with minimal inflammation in a small number of treated animals. The bronchiole–alveolar junction is the site of greatest lung injury for many inhaled toxicants (Barry et al., 1985; Chang et al., 1988) in part because the protective liquid layer lining the airways decreases in thickness as the airways become smaller. Interestingly, these fibrohistiocytic lesions developed in only 4 days after a single dose of diacetyl suggesting that diacetyl may be highly fibrogenic in the small airways. Although these early lesions did not obstruct the small airways, studies are in progress to determine if they may progress to OB with repeated aspirations and/or with increased time after exposure.
In summary, subacute exposures of mice to diacetyl indicated that most inhaled diacetyl resulted in epithelial injury in the nasal cavity and upper airways thereby protecting the bronchioles from toxic concentrations of diacetyl. Intermittent and subchronic exposures to occupationally relevant diacetyl concentrations caused lymphocytic bronchitis and bronchiolitis in mice. Longer inhalation studies are planned to evaluate whether lymphocytic bronchiolitis is a precursor lesion to OB. Administration of diacetyl by oropharyngeal aspiration demonstrated that if a sufficiently high concentration of diacetyl reaches the distal airways it can cause endobronchiolar fibrohistiocytic lesions suggestive of early stages of OB in a short time frame. Although oropharyngeal aspiration is a nonphysiological route of administration, this route could be useful in developing an animal model for studying the pathogenesis of OB and may be relevant to the lower airways exposures that occur in humans. Collectively, the findings of severe epithelial injury, lymphocytic bronchiolitis and fibro-histiocytic lesions in mice exposed to varying concentrations of diacetyl support the hypothesis that workplace exposures to diacetyl contribute to the development of OB in humans.
ACKNOWLEDGMENTS
All studies were conducted at the NIEHS inhalation facility under contract to Alion Science Technologies, Inc., Research Triangle Park, NC. We acknowledge the technical assistance of R. Bousquet, R. Boone, C. Colegrove, L. Crawford, N. Gage, P. Gage, O. Gamble, T. Godwin, M. Goods, M. Holland, A. Miller, H. Milligan, R. O’Connor, S. Philpot, H. Price, P. Rydell, C. Shines, G. Taylor, B. Yeaman, and P. Yongue.
FUNDING
Intramural Research program of the NIEHS.
REFERENCES
- Arora NS, Aldrich TK. Bronchiolitis obliterans from a burning automobile. South Med. J. 1980;73:507–510. doi: 10.1097/00007611-198004000-00028. [DOI] [PubMed] [Google Scholar]
- Barry BE, Miller FJ, Crapo JD. Effects of inhalation of 0.12 and 0.25 ppm ozone on the proximal alveolar region of juvenile and adult rats. Lab. Invest. 1985;53:692–704. [PubMed] [Google Scholar]
- BASF. Study on the acute inhalation toxicity LC50 of diacetyl FCC as a vapor in rats, 4-h exposure. Ludwigshafen: FRG, BASF Aktiengesellschaft; 1993. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broderson JR, Lindsey JR, Crawford JE. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am. J. Pathol. 1976;85:115–130. [PMC free article] [PubMed] [Google Scholar]
- Chang LY, Mercer RR, Stockstill B, Miller FJ, Graham JA, Ospital JJ, Crapo JD. Effects of low levels of NO2 on terminal bronchiolar cells and its relative toxicity compared to O3. Toxicol. Appl. Pharmacol. 1988;96:451–464. doi: 10.1016/0041-008x(88)90005-1. [DOI] [PubMed] [Google Scholar]
- Colley J, Gaunt IF, Lansdown AB, Grasso P, Gangolli SD. Acute and short-term toxicity of diacetyl in rats. Food Cosmet. Toxicol. 1969;7:571–580. doi: 10.1016/s0015-6264(69)80460-8. [DOI] [PubMed] [Google Scholar]
- Cunningham ML, Price HC, O’Connor RW, Moorman MP, Mahler JF, Nold JB, Morgan DL. Inhalation toxicity studies of the alpha, beta-unsaturated ketones: 2-Cyclohexene-l-one. Inhal. Toxicol. 2001;13:25–36. doi: 10.1080/713856767. [DOI] [PubMed] [Google Scholar]
- Fedan JS, Dowdy JA, Fedan KB, Hubbs AF. Popcorn worker’s lung: in vitro exposure to diacetyl, an ingredient in microwave popcorn butter flavoring, increases reactivity to methacholine. Toxicol. Appl. Pharmacol. 2006;215:17–22. doi: 10.1016/j.taap.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Feron VJ, Kruysse A, Til HP, Immel HR. Repeated exposure to acrolein vapour: Subacute studies in hamsters, rats and rabbits. Toxicology. 1978;9:47–57. doi: 10.1016/0300-483x(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J. Appl. Physiol. 2001;90:1111–1117. doi: 10.1152/jappl.2001.90.3.1111. [DOI] [PubMed] [Google Scholar]
- Furihata C, Yoshida S, Matsushima T. Potential initiating and promoting activities of diacetyl and glyoxal in rat stomach mucosa. Jpn. J. Cancer Res. 1985;76:809–814. [PubMed] [Google Scholar]
- Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, et al. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol. Appl. Pharmacol. 2002;185:128–135. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- Jiang XZ, Buckley LA, Morgan KT. Pathology of toxic responses to the RD50 concentration of chlorine gas in the nasal passages of rats and mice. Toxicol. Appl. Pharmacol. 1983;71:225–236. doi: 10.1016/0041-008x(83)90339-3. [DOI] [PubMed] [Google Scholar]
- Kanwal R, Kullman G, Piacitelli C, Boylstein R, Sahakian N, Martin S, Fedan K, Kreiss K. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J Occup. Environ. Med. 2006;48:149–157. doi: 10.1097/01.jom.0000194152.48728.fb. [DOI] [PubMed] [Google Scholar]
- King T. Bronchiolitis. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman's Pulmonary Diseases and Disorders. New York: McGraw-Hill; 1998. p. 825.p. 825.p. 847. [Google Scholar]
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N. Engl. J. Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- Kruysse A, Feron VJ, Til HP. Repeated exposure to acetaldehyde vapor. Studies in Syrian golden hamsters. Arch. Environ. Health. 1975;30:449–452. doi: 10.1080/00039896.1975.10666748. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Price HC, O’Connor RW, Seely JC, Ward SM, Wilson RE, Cunningham MC. Upper respiratory tract toxicity of inhaled methylvinyl ketone in F344 rats and B6C3F1 mice. Toxicol. Sci. 2000;58:182–194. doi: 10.1093/toxsci/58.1.182. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Ward SM, Wilson RE, Price HC, O’Connor RW, Seely JC, Cunningham ML. Inhalation toxicity studies of the alpha,beta-unsaturated ketones: ethyl vinyl ketone. Inhal. Toxicol. 2001;13:633–658. doi: 10.1080/08958370152409883. [DOI] [PubMed] [Google Scholar]
- Morgan KT, Monticello TM. Airflow, gas deposition, and lesion distribution in the nasal passages. Environ. Health Perspect. 1990;85:209–218. doi: 10.1289/ehp.85-1568327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdyke DL. Monographs on fragrance raw materials. Food Cosmet. Toxicol. 1979;17(Special Issue V):695–923. [PubMed] [Google Scholar]
- Phalen R. Inhalation Studies: Foundations and Techniques. Boca Raton, FL: CRC Press; 1984. [Google Scholar]
- Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: A systematic review of recent publications. J Heart Lung Transplant. 2002;21:271–281. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- Snipes MB. Long-term retention and clearance of particles inhaled by mammalian species. Crit. Rev. Toxicol. 1989;20:175–211. doi: 10.3109/10408448909017909. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Shimkin MB, Kniazeff AJ, Weisburger JH, Weisburger EK, Gori GB. Test for carcinogenicity of food additives and chemotherapeutic agents by the pulmonary tumor response in strain A mice. Cancer Res. 1973;33:3069–3085. [PubMed] [Google Scholar]
- Svetlecic J, Molteni A, Herndon B. Bronchiolitis obliterans induced by intratracheal papaverine: A novel animal model. Lung. 2004;103(1):169–180. doi: 10.1007/s00408-003-1049-3. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]