Abstract
MicroRNAs (miRNAs) regulate messenger RNA (mRNA) translation in a sequence-specific manner and are emerging as critical regulators of central nervous system (CNS) plasticity. We found hippocampal miRNA level changes following chronic treatment with mood stabilizers (lithium and valproate (VPA)). Several of these miRNAs were then confirmed by quantitative PCR: let-7b, let-7c, miR-128a, miR-24a, miR-30c, miR-34a, miR-221, and miR-144. The predicted effectors of these miRNAs are involved in neurite outgrowth, neurogenesis, and signaling of PTEN, ERK and Wnt/β-catenin pathways. Interestingly, several of these effector coding genes are also genetic risk candidates for bipolar disorder (BPD). We provide evidence that treatment with mood stabilizers increases these potential susceptibility genes in vivo: dipeptidyl-peptidase 10 (DPP10), metabotropic glutamate receptor 7 (GRM7), and thyroid hormone receptor, beta (THRB). Treatment of primary cultures with lithium or VPA lowered levels of miR- 34a and elevated levels of GRM7, a predicted effector of miR-34a. Conversely, miR-34a precursor treatment lowered GRM7 levels and treatment with a miR-34a inhibitor enhanced GRM7 levels. These data confirm that endogenous miR-34a regulates GRM7 levels and supports the notion that miR-34a contributes to the effects of lithium and VPA on GRM7. These findings are the first to demonstrate that miRNAs and their predicted effectors are targets for the action of psychotherapeutic drugs.
Keywords: microRNA (miRNA); lithium, valproate (VPA); GRM7 (metabotropic glutamate receptor 7); DPP10 (dipeptidyl-peptidase 10); THRB (thyroid hormone receptor, beta); bipolar disorder (BPD)
Introduction
Bipolar disorder (BPD) is a common, chronic recurrent mental illness that affects the lives and functioning of many worldwide. A growing number of recent studies indicate that for a majority of patients, outcome is quite poor. High rates of relapse, chronicity, lingering residual symptoms, subsyndromes, cognitive and functional impairment, psychosocial disability, and diminished well-being are unfortunately common occurrences in BPD (Belmaker, 2004; Goodwin and Jamison, 2007). There is thus a clear need to develop improved long-term therapeutics for this devastating illness.
A major impediment in our ability to develop improved therapeutics for BPD is the fact that our understanding of the molecular and cellular underpinnings of the illness is in its infancy. It is clear that a true understanding of the underlying biology of recurrent mood disorders must include an explanation for the predilection to episodic and often profound mood disturbance that can become progressive over time. BPD undoubtedly arises from the complex interaction of multiple susceptibility (and protective) genes and environmental factors. The phenotypic expression of the disease includes not only mood disturbance, but also a constellation of cognitive, motor, autonomic, endocrine, and sleep/wake abnormalities. In recent years, research has highlighted the role of neural circuits and synapses, and the plastic processes controlling their function. Thus, there is a growing appreciation that these illnesses can best be conceptualized as genetically influenced disorders of synapses and circuits, rather than simply as deficits or excesses in individual neurotransmitters (Schloesser et al, 2008).
Furthermore, the therapeutic effects in the treatment of these disorders are seen only after chronic administration, thereby precluding simple mechanistic interpretations based on their acute biochemical effects. This has led to the suggestion that a cascade of downstream gene and protein expression changes is ultimately responsible for their therapeutic effects (Kubota et al, 2006; Pandey and Dwivedi, 2005; Perova et al, 2008). In this context, it is noteworthy that microRNAs (miRNAs) have recently emerged as key regulators of complex temporal and spatial patterns of gene/protein expression changes and, thereby, synaptic and neural plasticity.
miRNAs are encoded in the genomes of all multicellular organisms and can be transcripted from either an intergenic cluster or single genetic regions (Filipowicz et al, 2008; Novina and Sharp, 2004). The transcripts fold into long hairpin RNAs called primary microRNAs (pri-miRNAs) with imperfect internal sequence complementarity (Filipowicz et al, 2008; Novina et al, 2004). Drosha, a nuclear enzyme, cleaves pri-miRNAs into smaller, roughly 70-nucleotide hairpin RNAs termed precursor miRNAs (pre-miRNAs) (Filipowicz et al, 2008; Novina et al, 2004). Pre-miRNAs are exported from the nucleus to the cytoplasm and then cleaved into mature, single-stranded miRNAs, 21–22 nucleotides long, by another nuclease, Dicer (Filipowicz et al, 2008; Novina et al, 2004). A mature miRNA is then assembled into a ribonucleoprotein (miRNP) complex (Novina et al, 2004). This complex most commonly binds to the 3'-untranslated sequences of particular messenger RNAs (mRNAs) through partially complementary sequences, and prevents the mRNAs from being translated into protein (Filipowicz et al, 2008; Novina et al, 2004). In a few cases, the miRNA is exactly or nearly exactly complementary to a site in an mRNA, resulting in mRNA cleavage and degradation similar to that observed with small interfering RNAs (siRNAs) (Filipowicz et al, 2008; Novina et al, 2004). Multiple types of miRNAs can cooperate to suppress translation of a single mRNA. Furthermore, a single type of miRNA can interact with multiple types of miRNAs, thus regulating the protein expression of different genes (Filipowicz et al, 2008; Novina et al, 2004). About 550 human miRNAs have been documented and verified (Griffiths-Jones et al, 2008), some of which are exclusively expressed in neurons (Kim et al, 2004a). miRNAs have recently been shown to play a critical role in regulating a variety of neurobiological processes, including neurogenesis, neurite outgrowth, synaptogenesis, synaptic and neural plasticity, and circadian rhythms (for a review see (Fiore et al, 2008) and (Gao, 2008)).
The hippocampus is one of the brain regions involved in mood regulation. Mood stabilizers such as lithium and valproate (VPA) alter intracellular signaling processes such as PKC (Bebchuk et al, 2000; Birnbaum et al, 2004; Brennan et al, 2007; Chen et al, 1994; Manji et al, 1993), ERK/MAP (Einat et al, 2003; Engel et al, 2008; Hao et al, 2004; Yuan et al, 2001), and the Wnt/β-catenin pathways (Gould et al, 2007; Gould and Manji, 2002; Gould et al, 2008), enhance the expression of Bcl-2 (Chen et al, 1999; Huang et al, 2003; Yuan et al, 2001), BAG1 (Silva et al, 2008; Zhou et al, 2005) and neurotrophin (Angelucci et al, 2003; Einat et al, 2003; Frey et al, 2006; Fukumoto et al, 2001; Jacobsen and Mork, 2004; Walz et al, 2008), promote neuronal remodeling (Silva et al, 2008; Wood et al, 2004; Yuan et al, 2001) and neurogenesis (Chen et al, 2000; Hao et al, 2004; Kim et al, 2004b; Laeng et al, 2004; Son et al, 2003; Yu et al, 2003), and modulate AMPA and NMDA receptor function (Du et al, 2008; Du et al, 2004) in hippocampus. However, the molecular mechanisms through which mood stabilizers deliver this wide range of hippocampal effects are largely unknown. We therefore undertook a series of experiments to determine whether miRNAs represent hippocampal targets for the long-term actions of mood stabilizers.
Materials and Methods
Animals and treatment
All animal treatments, procedures, and care were approved by the National Institute of Mental Health (NIMH) Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (ISBN 0-309-05377-3). Rats were chronically treated with lithium and VPA using a clinically relevant regimen established by our laboratory (Einat et al, 2003). In brief, male Kyoto Wistar rats (starting weight 150–200 g) were housed two to four per cage in a 12-hour light/dark cycle with water and food ad libitum. After a one-week accommodation period, the rats received drug treatments. Control (regular) chow and lithium carbonate- or VPA-containing chows were custom-produced by Bio-Serve (Frenchtown, NJ, USA). Chows were identical with the exception of the drug. Half-dose chows (1.2 g/kg lithium carbonate or 10g/kg VPA) were used in the first week, and full-dose (2.4 g/kg lithium carbonate or 20g/kg VPA) chows for the remaining three weeks. Lithium-treated rats were provided with saline to prevent any potential electrolyte imbalance due to lithium treatment. The rats were euthanized by decapitation, rat brains were dissected on ice, and hippocampi rapidly frozen in dry ice and stored at −80°C until further tissue processing.
miRNA preparation
miRNAs from rat hippocampus were separated with mirVana miRNA Isolation Kit (Ambion, Austin, TX) according to the manufacturer’s manual.
Screening miRNA targets of chronic lithium and VPA treatment
miRNA microarray was used to screen potential targets. The array slides were made in the Microarray CORE Facility of NHGRI, NIH. In brief, mirVana miRNA Probe Set 1564V2, which is a collection of 662 amine-modified DNA oligonucleotides targeting a comprehensive selection of human, mouse, and rat miRNAs, was purchased from Ambion (Austin, TX). These probes were printed on Corning Epoxide Coated Slides (Corning Inc, Corning, NY) in triplicate. miRNA labeling and hybridization were carried out using Ambion’s miRNA Labeling Kit and Bioarray Essential Kit, respectively, per manufacturer’s instructions (Ambion, Austin, TX). miRNA samples were pooled from two animals of the same treatment group for one slide. Samples from control animals and lithium- or VPA-treated animals were measured on the same slide. A second round of array experiments was conducted with another set of pooled samples from different animals to serve as a biological repeat in screening. Assay consistency was analyzed and tested via three replicates of the same miRNA on the slide.
The following criteria were used to select targets for further validation: (1) the detection was reliable, i.e. the difference between the control and drug-treated animal groups were detected with three repeats per group on the same slide, and this difference had a p-value of ≤0.05 (Student’s t-test); (2) the detected difference was biologically repeatable, i.e. similar and significant results were also obtained in the second round of array screening; (3) the expression differences were similar for the lithium and VPA treatment groups, i.e. significant changes occurred in the same direction for both lithium and VPA.
Validation of microarray findings with quantitative real-time PCR (qRT-PCR)
qRT-PCR was carried out using Ambion’s qRT-PCR miRNA detection kit per the manufacturer’s instructions (Ambion, Austin, TX). In brief, hippocampal miRNAs from each individual animal were reverse-transcribed with the corresponding RT primer. The RT product (cDNA) then entered a 40-cycle PCR with the corresponding PCR primers, in which SYBR Green I was used as the signal fluorescence and ROX as the control fluorescence. miRNA reverse transcription was on an Applied Biosystems’ GeneAmp 9700 thermal cycler, and real-time PCR on an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Samples from all treatment groups were processed at the same time to avoid inter-experiment variances.
miRNA effector prediction
Lists of predicted effectors of lithium- and VPA-regulated miRNAs were generated using the TargetScan program (http://www.targetscan.org) (Lewis et al, 2003).
Selection of miRNA effectors for follow-up investigation
The effectors of lithium- and VPA-targeted miRNAs were selected for follow-up investigation based on presumed illness pathophysiology (admittedly in its infancy), as well as from targets implicated in the whole genome association dataset generated by the Wellcome Trust Case Control Consortium (WTCCC) (Consortium, 2007). The WTCCC list contains all genes for which the single nucleotide polymorphisms (SNPs) have strong or moderate (e.g. p-value less than 0.0001) associations with BPD.
Immunoblotting (Western blot)
Immunoblotting was conducted as previously described (Chen et al, 1998), with modifications. In brief, rat hippocampal samples were suspended in ice-cold lysing buffer. The buffer contained 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1% Triton X-100, 150 mM NaCl and 1 mM β-glycerophosphate, as well as the following ingredients added into the buffer immediately before use to the final concentrations of 5 mM DTT: 1% Phosphatase Inhibitor Cocktail I, 1% Phosphatase Inhibitor Cocktail II, and 10% Phosphatase Inhibitor Cocktail (Sigma, St. Louis, MO). Samples were homogenized by sonication (setting 2.5, 1 sec × 10 times, VirSonic ultrasonic cell disrupter (Virtis, Gardiner, NY)). Samples were then centrifuged (15 sec at 14000 × g) to remove undissolvable debris. Protein concentrations were determined using the BCA protein assay kit (Pierce Biotechnology, Rockford, IL).
Immunobloting was performed using amounts of protein demonstrated to be within the linear range for the analysis. Equal amounts of proteins were loaded to 8–16% SDS-PAGE gels and separated by electrophoresis. Proteins were then electrophoretically transferred to nitrocellulose membranes. Nonspecific binding on the nitrocellulose was blocked with Tris Buffered Saline plus Tween−20 (TBST), 5% nonfat dry milk. The antibodies for beta-actin (ACTB) (Cell signaling, Boston, MA), calpain 6 (Abcam, Cambridge, MA), Dipeptidyl peptidase 10 (DPP10) (Abcam, Cambridge, MA), estrogen-related receptor gamma (ESRRG) (Imgenex, San Diego, CA), metabotropic glutamate receptor 7 (GRM7) (Abcam, Cambridge, MA), and thyroid hormone receptor beta (THRB) (Abcam, Cambridge, MA) were diluted according to the manufacturer’s recommendations and, if necessary, further adjusted according to initial test results. The secondary antibodies were horseradish peroxidase (HRP) conjugated anti-rabbit antibody. The immunocomplex was detected with an ECL plus kit (Amersham Biosciences, Piscataway, NJ). Quantitation of the immunoblots was performed by densitometric scanning of the film using the Kodak IS4400CF image analysis system and the corresponding software (Eastman Kodak, Rochester, NY).
Primary hippocampal neuron culture, treatments and transfections
Primary hippocampal neuron cultures were prepared as previously described (Du et al, 2008; Du et al, 2004). The neurons were treated with 1 mM (final concentration) of lithium or VPA for one or five days. RNAs were delivered into neurons using i-Fect reagent according to the manufacturer’s specification. A pilot experiment was conducted to test the dosage of RNAs (10, 20, 30 and 40 nmol/L) as well as incubation time (one, two, and three days) to assess earliest optimal outcomes. Forty nmol/L of RNAs and a two-day incubation period were selected for the rest of experiments. All RNA reagents were from Ambion (Austin, TX) including Pre-miR™ miRNA-34a precursor (PM11030), Pre-miR™ miRNA-34a precursor negative control (AM17110), anti-miR™ miRNA-34a inhibitor (AM11050), and anti-miR™ miRNA-34a inhibitor negative control (AM17010).
Results
Potential hippocampal miRNA targets of chronic lithium and VPA treatment
The microarray screening revealed reliable changes (at least 30 %; t-test, p-value <0.05 for in-slide technical repeats) in hippocampal expression levels of 37 miRNAs in the lithium group (Table S1) and 31 miRNAs in the VPA group (Table S2). These changes were also observed in biological repeat experiments with different samples (that is, samples from different animals treated chronically with lithium or VPA). Among these potential lithium- and VPA-regulated miRNAs, nine were regulated by both lithium and VPA in a similar manner (Table 1).
Table 1.
Findings from miRNA array screening of common hippocampal miRNA targets of chronic treatment with lithium and VPA
| Test | Initial test | Repeated test | ||||||
|---|---|---|---|---|---|---|---|---|
| Lithium | VPA | Lithium | VPA | |||||
| miRNA | fold | P | fold | P | fold | P | fold | P |
| let-7b | 0.4737 | 0.0013 | 0.5395 | 0.0040 | 0.6495 | 0.0377 | 0.5640 | 0.0049 |
| let-7c | 0.4783 | 0.0010 | 0.5403 | 0.0143 | 0.6903 | 0.0083 | 0.5963 | 0.0300 |
| miR-105 | 0.6329 | 0.0013 | 0.5816 | 0.0228 | 0.6722 | 0.0395 | 0.7493 | 0.0312 |
| miR-128a | 0.5831 | 0.0083 | 0.6920 | 0.0108 | 0.7925 | 0.0148 | 0.6909 | 0.0346 |
| miR-24 | 0.5671 | 0.0010 | 0.7024 | 0.0068 | 0.7919 | 0.0277 | 0.7349 | 0.0473 |
| miR-30c | 0.6256 | 0.0060 | 0.6054 | 0.0321 | 0.7197 | 0.0381 | 0.8390 | 0.0060 |
| miR-34a | 0.6186 | 0.0450 | 0.5934 | 0.0079 | 0.7168 | 0.0197 | 0.6054 | 0.0283 |
| miR-221 | 0.5484 | 0.0155 | 0.6581 | 0.0383 | 0.7492 | 0.0024 | 0.7241 | 0.0044 |
| miR-144 | 1.7022 | 0.0167 | 1.6420 | 0.0067 | 1.6902 | 0.0087 | 1.5262 | 0.0398 |
| miR-136 | 1.0037 | 0.7752 | 0.9607 | 0.1456 | 1.0004 | 0.9963 | 1.0296 | 0.8016 |
See Materials & Methods for details about the procedures
Common hippocampal miRNA targets of chronic treatment with lithium and VPA
To validate the screening findings, additional hippocampal samples were obtained from another cohort of rats chronically treated in the same manner. Five individual samples per treatment group were used in the validation experiments. Consistent with the screening results (Table 1), chronic treatment with either lithium or VPA significantly downregulated levels of let-7b, let-7c, miR-128a, miR-24a, miR-30c, miR-34a, and miR-221 (Figs. 1 A-G) and significantly upregulated levels of miR-144 (Fig. 1H). To evaluate the background difference, we tested 5S rRNA levels for lithium, VPA, and control groups (n=5 for each group). There was no difference according to this evaluation (levels were: lithium: 0.9739 ±1841, VPA: 0.9904 ±2078, and control: 1.0000± 0.1333; all values adjusted with control levels and in the mean±SE format). To assess the selectivity of the common effect of lithium and VPA on miRNA expression, the levels of miR-136 were also measured in hippocampal samples from treated animals. As with the screening results (Table 1), the treatments did not significantly alter miR-136 levels (Fig. 1I). The screening findings for miR-105 (Table 1) were not continued further due to the inability to obtain specific and efficient working PCR primers.
Figure 1. Effects of chronic lithium and VPA treatments on hippocampal levels of miRNAs.
Rats (five per group) were treated with lithium or VPA. miRNAs were isolated and qRT-PCR was conducted. Data are presented as percent of control.
(A) Levels of let-7b. Significant group differences were detected (F(2,12)=10.85, p=0.0020, ANOVA; control vs. lithium: p<0.01, control vs. VPA, p<0.01, Bonferroni's Multiple Comparison Test).
(B) Levels of let-7c. Significant group differences were detected (F(2,12)=21.37, p<0.01, ANOVA; control vs. lithium: p<0.01, control vs. VPA, p<0.01, Bonferroni's Multiple Comparison Test).
(C) Levels of miR-128a. Significant group differences were detected (F(2,12)=8.807, p<0.01, ANOVA; control vs. lithium: p<0.05, control vs. VPA, p<0.01, Bonferroni's Multiple Comparison Test).
(D) Levels of miR-24a. Significant group differences were detected (F(2,12)=14.86, p<0.01, ANOVA; control vs. lithium: p<0.01, Bonferroni's Multiple Comparison Test; control vs. VPA, t(8)=2.108, p=0.07, t-test).
(E) Levels of miR-30c. Significant group differences were detected (F(2,12)=7.104, p<0.01, ANOVA; control vs. lithium: p<0.01, control vs. VPA, p<0.05, Bonferroni's Multiple Comparison Test).
(F) Levels of miR-34a. Significant group differences were detected (F(2,12)=4.633, p<0.01, ANOVA; control vs. lithium: p<0.05, Bonferroni's Multiple Comparison Test; control vs. VPA, p<0.05, Newman-Keuls Multiple Comparison Test).
(G) Levels of miR-221. Significant group differences were detected (F(2,15)=8.360, p<0.01, ANOVA; control vs. lithium: p<0.05, control vs. VPA, p<0.01, Bonferroni's Multiple Comparison Test).
(H) Levels of miR-144. Significant group differences were detected (F(2,15)=6.597, p<0.01, ANOVA; control vs. lithium: p<0.05, Newman-Keuls Multiple Comparison Test; control vs. VPA, p<0.01, Bonferroni's Multiple Comparison Test).
(I) levels of miR-136. No significant group differences were detected (F(2,15)=0.03413, p=0.97, ANOVA). *:p<0.05. #: p<0.10.
Prediction of potential overall effects of lithium and VPA through their common miRNA targets
The list of predicted effectors of validated lithium- and VPA-regulated miRNAs was generated by the TargetScan program. The list contains 1988 total entries and 1654 unique entries (Table S3); differences are due to the fact that some effectors are common effectors of multiple miRNAs. It was noted that no predicted effectors for miR-105 were found in the rat database. The list was uploaded to, and annotated with, the Ingenuity Pathways Analysis System. Several biological processes and signaling pathways were significantly targeted by the effectors on the list (Table 2 and Table 3). Based on the multiple comparison-corrected p-values, the most significant of the targeted neuronal processes included neurogenesis, neurite outgrowth, and brain and nervous system development (Table 2). The most significant of the targeted signaling pathways were the PTEN, axonal guidance, ERK, Wnt/β-catenin, and β-adrenergic signaling pathways (Table 3).
Table 2.
Neuronal function annotation of predicted miRNA targets
| Processes and functions of nervous system | p-value a |
|---|---|
| neurogenesis | 1.52E-11 |
| development of brain | 5.51E-08 |
| development of central nervous system | 2.33E-07 |
| development of nervous system | 2.74E-07 |
| outgrowth of neurites | 4.63E-07 |
| differentiation of neurons | 5.94E-07 |
| growth of neurites | 5.45E-06 |
| development of forebrain | 6.13E-06 |
| development of neurons | 7.56E-06 |
| quantity of central nervous system cells | 9.58E-05 |
| development of hippocampus | 1.12E-04 |
| differentiation of central nervous system cells | 1.29E-04 |
| quantity of brain cells | 4.88E-04 |
| neurological process of mice | 8.10E-04 |
| branching of dendrites | 1.11E-03 |
| development of nervous tissue | 1.26E-03 |
| development of mesencephalon | 1.33E-03 |
| branching of neurites | 1.38E-03 |
| depression of synapse | 1.72E-03 |
| differentiation of granule cells | 2.37E-03 |
| differentiation of brain cells | 3.76E-03 |
| development of nerves | 3.99E-03 |
| guidance of axons | 5.69E-03 |
| quantity of neurons | 6.05E-03 |
| maturation of neurons | 6.05E-03 |
| long term depression of synapse | 6.23E-03 |
| survival of neurons | 7.37E-03 |
| learning of mice | 7.84E-03 |
| neurological process of axons | 8.32E-03 |
| neurological process of neurites | 8.32E-03 |
| proliferation of neurons | 8.86E-03 |
| long-term potentiation of neurons | 1.34E-02 |
| biogenesis of synapse | 1.47E-02 |
| spatial learning of mice | 1.64E-02 |
| survival of neuroglia | 1.83E-02 |
| long-term potentiation of eukaryotic cells | 1.89E-02 |
| development of neurites | 1.90E-02 |
| migration of neurons | 1.90E-02 |
p-value with B-H Multiple testing correction
Table 3.
Pathway annotation of predicted miRNA targets
| Signaling and metabolic pathways | p-value a |
|---|---|
| PTEN Signaling | 2.82E-05 |
| Axonal Guidance Signaling | 2.82E-05 |
| ERK/MAPK Signaling | 6.61E-05 |
| Wnt/β-catenin Signaling | 3.09E-04 |
| Cardiac β-adrenergic Signaling | 3.09E-03 |
| Hypoxia Signaling in the Cardiovascular System | 4.79E-03 |
| Ephrin Receptor Signaling | 6.31E-03 |
| Neuregulin Signaling | 1.00E-02 |
| Integrin Signaling | 1.00E-02 |
| PPARα/RXRα Activation | 1.00E-02 |
| PI3K/AKT Signaling | 1.02E-02 |
| PPAR Signaling | 1.20E-02 |
| Inositol Phosphate Metabolism | 1.41E-02 |
| Actin Cytoskeleton Signaling | 1.51E-02 |
| B Cell Receptor Signaling | 1.51E-02 |
| G-Protein Coupled Receptor Signaling | 1.74E-02 |
| Insulin Receptor Signaling | 1.82E-02 |
| Neurotrophin/TRK Signaling | 1.82E-02 |
| IGF-1 Signaling | 2.95E-02 |
| Notch Signaling | 3.72E-02 |
| Tight Junction Signaling | 3.98E-02 |
| Nicotinate and Nicotinamide Metabolism | 4.68E-02 |
| TGF-β Signaling | 4.68E-02 |
p-value with B-H Multiple testing correction
Common candidates on the miRNA effector and BPD candidate gene lists
To prioritize the follow-up investigation of predicted miRNA effectors, we adapted a “convergent genomic approach” (Bertsch et al, 2005). The WTCCC-reported list of SNPs (p-value < 0.001) was obtained from the supplement of the WTCCC paper; a total of 42 SNP corresponding genes were retrieved from the NCBI database (http://www.ncbi.nlm.nih.gov/SNP/batch.cgi) (Table S4). Comparison of this gene list to the list of predicted effectors of validated lithium- and VPA-regulated miRNAs was done using the Ingenuity Pathways Analysis System. The comparison revealed six common candidates (Table 4).
Table 4.
Common candidates on the miRNA effector and BPD candidate gene lists
| Symbol | Description | miRNAs (context score) | Genetics | ||
|---|---|---|---|---|---|
| miR-128a | miR-24 | miR-34a | p-value | ||
| CAPN6 | Calpain 6 | −0.26 | 9.99E-06 | ||
| DPP10 | Dipeptidyl-peptidase 10 | −0.17 | 1.31E-05 | ||
| ESRRG | Estrogen-related receptor gamma | −0.07 | 2.15E-04 | ||
| FAM126A | Family with sequence similarity 126, member A. | −0.78 | 2.04E-05 | ||
| GRM7 | Metabotropic glutamate receptor 7 | −0.30 | 9.73E-05 | ||
| THRB | Thyroid hormone receptor, beta | −0.31 | 2.16E-04 | ||
The effectors of miRNAs targeted by both lithium and VPA treatments were predicted using TargetScan. Genetic data were originated from the WTCCC whole genome association study. The WTCCC-reported SNPs list (p-value < 0.001) was obtained from the supplement of the WTCCC paper and SNP corresponding gene IDs were retrieved from the NCBI database. Comparison of predicted miRNA effectors and genetic data was done using the Ingenuity Pathways Analysis System.
Effects of chronic lithium and VPA on protein levels of common candidates on the miRNA effector and BPD candidate gene lists
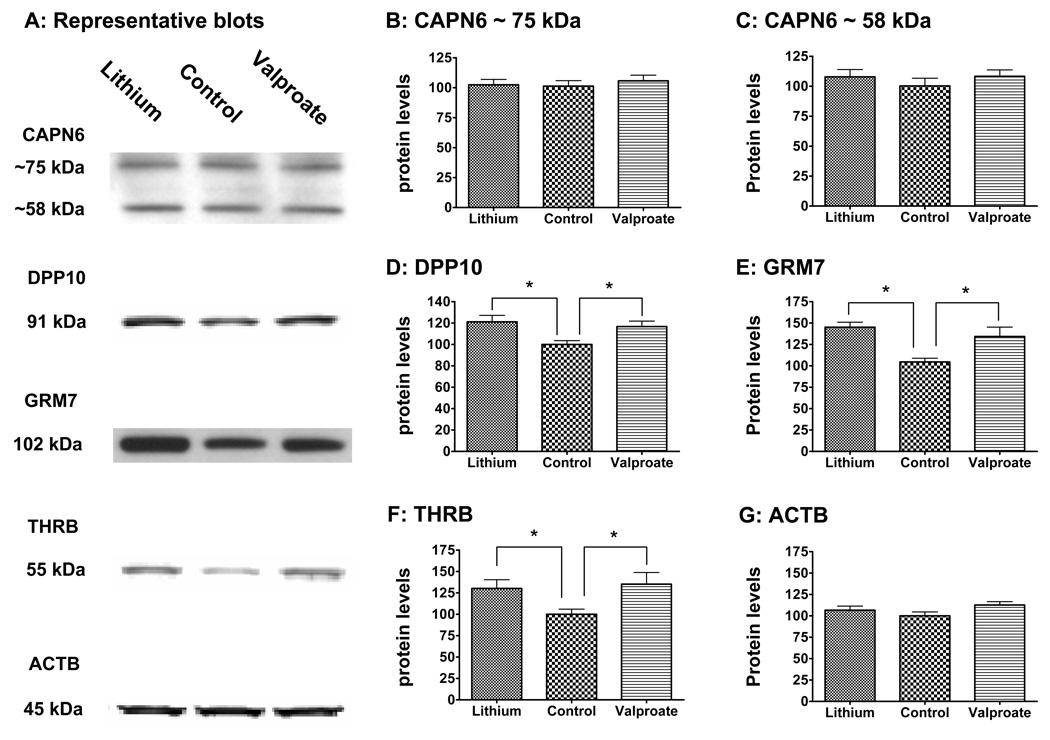
Evaluation of common candidates found on both the miRNA effector and BPD candidate gene lists (Table 4) were undertaken by monitoring hippocampal protein levels following chronic treatment with lithium or VPA (Fig. 2). Consistent with our microarray findings that showed downregulation of miR-128a, miR-24, and miR-34a, which negatively control translations of their effectors, chronic treatment with lithium or VPA significantly upregulated hippocampal protein levels of DPP10, GRM7, and THRB. Chronic treatment with lithium and VPA did not significantly alter hippocampal protein level of CAPN6 (Figs. 2A&B), which is inconsistent with miR-34a downregulation by these agents. In order to ensure consistent sample preparation, β-actin (ACTB) levels were also monitored where no significant differences were found (Figs. 2A&I).
Figure 2. Effects of chronic lithium and VPA treatments on hippocampal levels of selected proteins.
Rats (five or sixteen per group) were treated with lithium or VPA. Hippocampal protein extracts were prepared, and immunoblots of selected proteins and densitometry analysis of the blots were conducted. Data are presented as percent of control.
(A) Representative blots of proteins tested.
(B) Levels of 75 kDa CAPN6. No significant group differences were detected (F(2,12)=0.2553, P=0.78, ANOVA).
(C) Levels of 58 kDa CAPN6. No significant group differences were detected (F(2,12)=0.5858, P=0.57, ANOVA).
(D) Levels of DPP10. Significant group differences were detected (F(2,45)=4.954, p<0.01, ANOVA; control vs. lithium: p<0.01, control vs. VPA, p<0.05, Bonferroni's Multiple Comparison Test).
(E) Levels of GRM7. Significant group differences were detected (F(2,12)=7.710, p<0.01, ANOVA: lithium vs. control: P<0.01, VPA vs. control: p<0.05, Bonferroni's Multiple Comparison Test).
(F) Levels of THRB. Significant group differences were detected (F(2,45)=9.205, p<0.05, ANOVA; control vs. lithium: t(30)=2.553, p<0.02; control vs. VPA, p<0.05, Bonferroni's Multiple Comparison Test).
(G) Levels of ACTB to ensure consistent protein loading. *: p<0.05.
Effects of lithium and VPA on miR-34a and GRM7 levels in primary cultures of hippocampal neurons
To test whether hippocampal cultures are a valid way to mechanistically assess the effects of lithium and VPA on miRNAs and their effectors, these cultures were treated with lithium or VPA for either one or five days. The longer treatment (five days) reduced levels of miR-34a (Figs. 3A&B) and increased levels of GRM7 (Figs. 3C–F).
Figure 3. Effects of chronic lithium and VPA treatments on miR-34a and GRM7 levels in primary culture of hippocampal neurons.
Primary cultures of rat hippocampal neurons were prepared and treated with 1mM of lithium or VPA for one or five days. Levels of miR-34a and GRM7 were measured. Data are presented as percent of control.
(A) Levels of miR-34a after one day of treatment. No significant group differences were detected. (F(2,12)=2.021, p=0.18, ANOVA).
(B) Levels of miR-34a after five days of treatment. Significant group differences were detected. (F(2,12)=22.54, p=0.18, ANOVA; control vs. lithium: p<0.01, control vs. VPA, p<0.01, Bonferroni's Multiple Comparison Test).
(A&B) Levels of 5S rRNA to justify the background difference.
(C&D) Representative blots of GRM7, levels of ACTB to ensure consistent protein loading.
(E) Levels of GRM7 after one day of treatment. No significant group differences were detected. (F(2,12)=1.374, p=0.28, ANOVA).
(F) Levels of GRM7 after five days of treatment. Significant group differences were detected. (F(2,12)=9.307, p<0.01, ANOVA; control vs. lithium: p<0.01, control vs. VPA, p<0.05, Bonferroni's Multiple Comparison Test). *: p<0.05.
Effects of gain or loss of function of miR-34a on GRM7 levels in primary culture of hippocampal neurons
To test whether miR-34a was sufficient to impose negative control on GRM7 protein levels in hippocampal neurons, cultured hippocampal neurons were incubated with Pre-miR™ miRNA-34a precursor (PM11030) and Pre-miR™ miRNA-34a precursor negative control for two days. Pre-miR™ miRNA-34a precursor incubation significantly reduced GRM7 protein levels (Figs. 4A&B). In order to test whether miR-34a and GRM7 co-exist in the same cell and whether endogenous miR-34a is sufficient to control GRM7 protein levels, cultured hippocampal neurons were incubated with antimiR™ miRNA-34a inhibitor and anti-miR™ miRNA-34a inhibitor negative control for two days. The anti-miR™ miRNA-34a inhibitor significantly increased GRM7 protein levels (Figs. 4C&D).
Figure 4. Effects of miR-34a precursor and inhibitor on GRM7 levels in primary culture of hippocampal neurons.
Primary cultures of rat hippocampal neurons were prepared and treated with miR-34a precursor, miR-34a inhibitor, or relative controls for two days. GRM7 levels were measured. Data are presented as percent of control, levels of ACTB to ensure consistent protein loading.
(A&C) Representative blots of GRM7 are shown.
(B) GRM7 levels after treatment with miR-34a precursor. Significant group differences were detected. (t(6)=3.829, p<0.01, unpaired t-test).
(D) GRM7 levels after treatment with miR-34a inhibitor. Significant group differences were detected. (t(6)=2.806, p<0.05, unpaired t-test). *: p<0.05.
Discussion
In this study, we demonstrated for the first time that select miRNAs are regulated by psychotherapeutic drug administration. Furthermore, several of these miRNAs are regulated in concert by the administration of the structurally dissimilar mood stabilizing agents, lithium and VPA. Additionally, using a “convergent genomic approach” (Bertsch et al, 2005), we identified and validated changes in selected downstream targets of the miRNAs that emerged from genome-wide association studies of BPD. miRNAs have now emerged as key regulators of integrated cellular function, and may be particularly important for regulating plastic processes in the brain (Leung and Sharp, 2006; Novina and Sharp, 2004). As we discuss in greater detail below, these findings suggest that some miRNAs and their effectors may contribute to the molecular, cellular, and behavioral actions of lithium and VPA.
Lithium and VPA selectively regulate a selected group of miRNAs in hippocampus
In this paper, the miRNAs identified as targets for the actions of chronic lithium and VPA are known to play diverse and intriguing roles in brain function. A recent report from miRBase—the central online repository for miRNA nomenclature, sequence data, annotation. and target prediction—indicates that there are 533 documented miRNA genes that encode 555 distinct miRNAs in humans (Griffiths-Jones et al, 2008). The microarray used in this study contained probes for 662 sequences for human, mouse, and rat miRNAs. The repeated microarray screening experiments showed that hippocampal expression levels of 37 and 31 miRNAs are altered by chronic treatment with lithium and VPA, respectively (Table S1 and Table S2).
Among all affected miRNAs, nine were common for both treatments (Table 1). The screening findings on eight of these commonly affected miRNAs were further confirmed using quantitative PCR (Fig. 1). These data demonstrate that lithium and VPA regulate a select group of miRNAs in the hippocampus. The miRNAs in this group include let-7b, let-7c, miR-128a, miR-24a, miR-30c, miR-34a, miR-221, and miR-144 (Fig. 1). Future investigation will be needed to reveal the sub-hippocampal regions and cell types involved in these selective effects of lithium and VPA on miRNAs. Whether lithium and VPA induce a similar pattern of miRNA changes in other brain regions related to mood regulation also warrants further microarray investigation. Similarly, it is important to bear in mind that drug-induced changes in gene expression do not necessarily reflect changes in protein expression.
Support from the literature demonstrates that chronic lithium or VPA treatment alters intracellular signaling systems including PKC, ERK/MAPK, PI3K/Akt, and Wnt/b-catenin pathways (see Introduction and references therein). Despite some reports (Fiore et al, 2008; Gao, 2008; Hermeking, 2007), the roles of these pathways in miRNA regulation are largely unknown. With miRNA research still in its infancy and the regulatory mechanisms governing expressions of miRNAs affected by lithium and VPA relatively unknown, investigating the effects of lithium and VPA on these pathways via changes in miRNAs represents an exciting and untapped area of study that holds promise for understanding yet another level of regulatory control and modulation.
miRNAs can contribute to the effects of lithium and VPA by targeting specific proteins
Given that miRNAs directly interfere with mRNA translation and stability, miRNAs have been viewed as the gatekeepers for the expression of many genes (Kosik, 2006; Leung and Sharp, 2006; Novina et al, 2004). It is estimated that up to 30% of all mammalian genes are regulated by miRNAs (Leung et al, 2006). miRNA effectors can be predicted based on the sequences of mRNAs and miRNAs and the strength of their interaction; the accuracy of these predictions is about 70% (Lewis et al, 2003; Martin et al, 2007). In the current study, the TargetScan program was preferred because it predicts effectors in rats in addition to humans and mice; a lower context score reflects higher prediction validity (Lewis et al, 2003).
One thousand six hundred fifty-four unique effectors were predicted for miRNAs affected by treatment with both lithium and VPA in hippocampus (Table S3). The “convergent genomic approach” was used to prioritize the investigation on the predicted effectors. Six candidates were common to both the predicted miRNA effectors and the BPD risk gene lists (Table 4). Notably, and given the limitations of genetic association studies and miRNA prediction, such prioritizing does not mean that the common candidates are the genes responsible for BPD, or that other effectors on the predicted effector list are not important.
Given the limitations of available working antibodies, four of the six common candidates could be investigated. As predicted from lithium- and VPA-induced downregulation of miR-128a, miR-24a and miR-34a, which negatively control translations of their effectors, chronic treatment with lithium or VPA significantly upregulated hippocampal protein levels of DPP10, GRM7, and THRB (Fig. 2). The data suggest that miRNAs contribute to the effects of lithium and VPA on the protein levels of certain effector genes. The accuracy in determining these effector genes in biological systems may be due to the effector prediction software as well as considering differential cell localizations of miRNA and its predicted effectors. This may help explain why chronic treatment with lithium and VPA did not significantly alter hippocampal protein levels of CAPN6 (Figs. 2A&B).
In addition, a series of experiments were conducted in primary culture of hippocampal neurons in order to assess the role of miRNAs in lithium- and VPA-induced protein level changes. As with the in vivo experiments, long-term, but not short-term, treatment with lithium and VPA downregulated miR-34a levels and elevated GRM7 protein levels (Fig. 3). These data validate hippocampal culture as a useful way to investigate the mechanisms underlying the effects of lithium and VPA on miR-34a levels and GRM7 expression. Pre-miR™ miRNA-34a precursor incubation significantly reduced GRM7 protein levels in primary hippocampal culture (Figs. 4A&B), confirming that miR-34a is sufficient to impose negative control on GRM7 protein levels. The anti-miR™ miRNA-34a inhibitor significantly increased GRM7 protein levels in primary hippocampal culture (Figs. 4C&D), demonstrating that miR-34a and GRM7 co-exist, at least in cultured hippocampal neurons, and that endogenous miR-34a is sufficient to control GRM7 protein levels. Additional studies are needed to further confirm the role of miR-34a in induction of GRM7 protein levels by lithium and VPA treatments. These studies may include double in situ hybridization or immunohistochemistry experiments with probes for miR-34a and GRM7 in addition to treatment experiments using miR-34a knockout (KO) animals, in order to determine whether these animals are resistant to some of the behavioral and biochemical effects of either lithium or VPA.
Plausible implications of the current findings for future studies
Mood stabilizers are known to produce a variety of molecular, cellular, and behavioral effects. These include direct interactions of mood stabilizers with lithium-sensitive-magnesium-dependent phosphatases, GSK-3, HDAC, and enzymes involved in GABA production (for review see (Gould et al, 2004)). These effects also include alterations in signaling systems such as the ERK/MAPK, PKC, PI3K/Akt and Wnt/b-catenin pathways and in neuronal processes such as neurite outgrowth, neurogenesis, and neuronal structure remodeling (see Introduction and references therein). Although the step-by-step molecular mechanisms underlying the signaling and morphological effects are not well understood, gene function modulation through both transcriptional regulation and post-translational modification has been suggested as a plausible mechanism.
The unique feature of miRNA-mediated gene regulation is that a single miRNA can negatively control the expression of multiple genes in cytosol; thus they are ideally placed at the nexus of concerted temporal and spatial regulation of gene expression. Recent data also suggest that the effectors of a given miRNA are concentrated in one or few biological functional processes (Hobert, 2008; Kosik, 2006; Leung et al, 2006; Novina et al, 2004). The function and pathway analysis described here revealed that the effectors of common miRNA targets of lithium and VPA were significantly represented in neurogenesis, neurite outgrowth, brain and nervous system development, and the signaling pathways PTEN, axonal guidance, ERK/MAPK, and Wnt/β-catenin (Table 2&Table 3). These prediction results are surprisingly consistent with the known cellular and signaling actions of lithium and VPA. Considering this consistency, the potential contribution of miRNAs to the known cellular and intracellular effects of lithium and VPA should, at the very least, not be ignored in future studies.
GRM7 has emerged from a recent whole genome association study as a candidate gene for BPD (2007). GRM7 is a predicted effector of miR-34a where endogenous miR- 34a is sufficient to control GRM7 levels, at least in cultured hippocampal neurons (Fig. 4). These results, showing that miR-34a is downregulated, lead to the predicted up-regulation of its effector GRM7 in both in vivo (Fig. 2) and cultured hippocampal neurons by lithium and VPA (Fig. 3). GRM7 (also known as mGluR7) KO mice show less immobility time in the forced swim and tail suspension tests, and less anxiety-like (or more risk-taking-like) behavioral displays in the light-dark box, elevated plus maze, staircase, and stress-induced hyperthermia tests, as well as more activity when first exposed to a novel environment (Cryan et al, 2003). Lithium and VPA have been found to alleviate excessive behavioral excitement in several genetically modified strains of mice assessed using some of the same or similar behavioral paradigms (Engel et al, 2008; Roybal et al, 2007; Shaltiel et al, 2008). Taken together, these data raise the possibility that lithium and VPA produce at least some of their behavioral effects through mechanisms that include modulation of protein levels through miRNAs such as miR-34a.
In summary, the data from this study demonstrate, for the first time, that a small group of miRNAs and their predicted effectors are regulated by the administration of the mood stabilizers lithium and VPA. These findings suggest that some miRNAs and their effectors may contribute to the molecular, cellular, and behavioral actions of lithium and VPA. Further investigation on neuronal and behavioral functions of the miRNAs specified in this study might lead to new insights into the pathophysiology of mood disorders, and to novel targeted therapies for BPD.
Supplementary Material
Acknowledgments
We thank Ioline Henter for providing outstanding editorial assistance. This work was supported by the Intramural Program of the NIMH-NIH.
Disclosure/Conflict of Interest
We would like to acknowledge the support of the Intramural Research Program of the National Institute of Mental Health. The author(s) declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Angelucci F, Aloe L, Jimenez-Vasquez P, Mathe AA. Lithium treatment alters brain concentrations of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in a rat model of depression. Int J Neuropsychopharmacol. 2003;6(3):225–231. doi: 10.1017/S1461145703003468. [DOI] [PubMed] [Google Scholar]
- Bebchuk JM, Arfken CL, Dolan-Manji S, Murphy J, Hasanat K, Manji HK. A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch Gen Psychiatry. 2000;57(1):95–97. doi: 10.1001/archpsyc.57.1.95. [DOI] [PubMed] [Google Scholar]
- Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351(5):476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- Bertsch B, Ogden CA, Sidhu K, Le-Niculescu H, Kuczenski R, Niculescu AB. Convergent functional genomics: a Bayesian candidate gene identification approach for complex disorders. Methods. 2005;37(3):274–279. doi: 10.1016/j.ymeth.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306(5697):882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Brennan AR, Yuan P, Dickstein DL, Rocher AB, Hof PR, Manji H, et al. Protein kinase C activity is associated with prefrontal cortical decline in aging. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Manji HK, Hawver DB, Wright CB, Potter WZ. Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J Neurochem. 1994;63(6):2361–2364. doi: 10.1046/j.1471-4159.1994.63062361.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75(4):1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Yuan PX, Jiang YM, Huang LD, Manji HK. Lithium increases tyrosine hydroxylase levels both in vivo and in vitro. J Neurochem. 1998;70(4):1768–1771. doi: 10.1046/j.1471-4159.1998.70041768.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72(2):879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Consortium WTCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci. 2003;17(11):2409–2417. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- Du J, Creson TK, Wu LJ, Ren M, Gray NA, Falke C, et al. The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J Neurosci. 2008;28(1):68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24(29):6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23(19):7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2008 Jan 29; doi: 10.1038/sj.mp.4002135. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Fiore R, Siegel G, Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim Biophys Acta. 2008 Jan 14; doi: 10.1016/j.bbagrm.2007.12.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Cereser KM, Martins MR, Valvassori SS, Reus GZ, et al. Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci. 2006;79(3):281–286. doi: 10.1016/j.lfs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacol (Berl) 2001;158(1):100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- Gao FB. Posttranscriptional control of neuronal development by microRNA networks. Trends Neurosci. 2008;31(1):20–26. doi: 10.1016/j.tins.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar and Recurrent Unipolar Disorders. second ed. New-York: Oxford University Press; 2007. [Google Scholar]
- Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32(10):2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. The Wnt signaling pathway in bipolar disorder. Neuroscientist. 2002;8(5):497–511. doi: 10.1177/107385802237176. [DOI] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Picchinis AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189(1):117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004;9(8):734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36(Database issue):D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24(29):6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12(5):414–418. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319(5871):1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- Huang X, Wu DY, Chen G, Manji H, Chen DF. Support of retinal ganglion cell survival and axon regeneration by lithium through a Bcl-2-dependent mechanism. Invest Ophthalmol Vis Sci. 2003;44(1):347–354. doi: 10.1167/iovs.02-0198. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 2004;1024(1–2):183–192. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, Church GM, et al. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci U S A. 2004a;101(1):360–365. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, et al. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem. 2004b;89(2):324–336. doi: 10.1046/j.1471-4159.2004.02329.x. [DOI] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7(12):911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Kubota M, Kasahara T, Nakamura T, Ishiwata M, Miyauchi T, Kato T. Abnormal Ca2+ dynamics in transgenic mice with neuron-specific mitochondrial DNA defects. J Neurosci. 2006;26:12314–12324. doi: 10.1523/JNEUROSCI.3933-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng P, Pitts RL, Lemire AL, Drabik CE, Weiner A, Tang H, et al. The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. J Neurochem. 2004;91(1):238–251. doi: 10.1111/j.1471-4159.2004.02725.x. [DOI] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. Function and localization of microRNAs in mammalian cells. Cold Spring Harb Symp Quant Biol. 2006;71:29–38. doi: 10.1101/sqb.2006.71.049. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Manji HK, Etcheberrigaray R, Chen G, Olds JL. Lithium decreases membrane-associated protein kinase C in hippocampus: selectivity for the alpha isozyme. J Neurochem. 1993;61(6):2303–2310. doi: 10.1111/j.1471-4159.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]
- Martin G, Schouest K, Kovvuru P, Spillane C. Prediction and validation of microRNA targets in animal genomes. J Biosci. 2007;32(6):1049–1052. doi: 10.1007/s12038-007-0106-0. [DOI] [PubMed] [Google Scholar]
- Novina CD, Sharp PA. The RNAi revolution. Nature. 2004;430(6996):161–164. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y. Focus on protein kinase A and protein kinase C, critical components of signal transduction system, in mood disorders and suicide. Int J Neuropsychopharmacol. 2005;8:1–4. doi: 10.1017/S1461145704004936. [DOI] [PubMed] [Google Scholar]
- Perova T, Wasserman MJ, Li PP, Warsh JJ. Hyperactive intracellular calcium dynamics in B lymphoblasts from patients with bipolar I disorder. Int J Neuropsychopharmacol. 2008;11:185–196. doi: 10.1017/S1461145707007973. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104(15):6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33(1):110–133. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008 Mar 11; doi: 10.1038/mp.2008.20. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, et al. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience. 2008;152(3):656–669. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, et al. Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J Neurochem. 2003;85(4):872–881. doi: 10.1046/j.1471-4159.2003.01725.x. [DOI] [PubMed] [Google Scholar]
- Walz JC, Frey BN, Andreazza AC, Cereser KM, Cacilhas AA, Valvassori SS, et al. Effects of lithium and valproate on serum and hippocampal neurotrophin-3 levels in an animal model of mania. J Psychiatr Res. 2008;42(5):416–421. doi: 10.1016/j.jpsychires.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004;101(11):3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IT, Kim JS, Lee SH, Lee YS, Son H. Chronic lithium enhances hippocampal long-term potentiation, but not neurogenesis, in the aged rat dentate gyrus. Biochem Biophys Res Commun. 2003;303(4):1193–1198. doi: 10.1016/s0006-291x(03)00494-7. [DOI] [PubMed] [Google Scholar]
- Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276(34):31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G, et al. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005;25(18):4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.