Abstract
The growth of the vocal tract (VT) is known to be non-uniform insofar as there are regional differences in anatomic maturation. This study presents quantitative anatomic data on the growth of the oral and pharyngeal portions of the VT from 605 imaging studies for individuals between birth and 19 years. The oral (horizontal) portion of the VT was segmented into lip-thickness, anterior-cavity-length, oropharyngeal-width, and VT-oral, and the pharyngeal (vertical) portion of the VT into posterior-cavity-length, and nasopharyngeal-length. The data were analyzed to determine growth trend, growth rate, and growth type (neural or somatic). Findings indicate differences in the growth trend of segments∕variables analyzed, with significant sex differences for all variables except anterior-cavity-length. While the growth trend of some variables displays prepubertal sex differences at specific age ranges, the importance of such localized differences appears to be masked by overall growth rate differences between males and females. Finally, assessment of growth curve type indicates that most VT structures follow a combined∕hybrid (somatic and neural) growth curve with structures in the vertical plane having a predominantly somatic growth pattern. These data on the non-uniform growth of the vocal tract reveal anatomic differences that contribute to documented acoustic differences in prepubertal speech production.
INTRODUCTION
The development of speech in children is based in part on the maturation of the macroanatomy of the vocal tract (VT), including increases in size, typically expressed as vocal tract length (VTL). During development from infancy to adulthood, the length of the VT increases more than twofold, from approximately 7 to 8 cm in infants to 15 to 18 cm in adult females and males, respectively. Such growth has been characterized to be non-uniform in that the oral and pharyngeal portions of the VT are thought to undergo different growth patterns (Fant, 1960, 1975; Kent and Vorperian, 1995; Fitch and Giedd, 1999). There is longstanding interest in the relative or relational growth of the anterior (oral) portion of the VT, which is in the horizontal plane, versus the posterior (pharyngeal) portion of the VT, which is in the vertical plane. This interest has been due, in part, to understanding the acoustic changes that result from changes in the VT, particularly the differences in the formant frequencies between males and females where the differences cannot be explained by a simple scale factor inversely proportional to the overall VTL (Fant, 1975). Another reason of interest in the differential growth of the oral and pharyngeal portions of the VT has been from an evolutionary perspective. For example, the hypothesis that the elongation of the pharyngeal portion of the VT contributed to the emergence of speech in humans (Lieberman, 1975), and the hypothesis that the permanent shaping of the VT into two tubes—a horizontal oral tube and a vertical pharyngeal tube—which permit the production of quantal vowels (Stevens, 1989), evolved gradually with increased vocalization complexity and frequency (Fitch, 2000, 2002).
The inverse relation between VTL and formant frequencies is well established. As VTL increases during the course of development, formant frequencies decrease (Fant, 1960). By puberty, there are significant differences in VTL between males and females (Fitch and Giedd, 1999). However, the acoustic differences between males and females are non-uniform and thus cannot be explained solely by differences in overall VTL. Fant (1960, 1975), using radiographic data, noted the longer pharynx in adult men compared to women and children, and using a two-tube simplified model (front tube∕oral cavity length and back tube∕pharyngeal cavity length), concluded that such anatomic differences in the oral versus pharyngeal portions of the VT can account for the observed differences in vowel formant frequencies between males and females. Often, the resonant characteristics of children and females are grouped together with the contention that they are similar. This assumption discounts the documented acoustic differences between males and females by the age of 4 years (e.g., Perry et al., 2001), as well as the documented developmental sex differences in the first, second, and third formant frequencies (Vorperian and Kent, 2007). Furthermore, acoustic studies indicate that formant frequencies do not decrease during the first 2 years of life (Robb et al., 1997; Gilbert et al., 1997; Kent and Murray, 1982), a finding that appears to be inconsistent with acoustic theory at first glance since there are documented increases in VTL during this period (Vorperian et al., 1999, 2005). However, it is evident in Fant’s writings (Fant, 1975) that although he used simple tube models to make physiologic-acoustic interpretations, he specified the importance of dimensions other than tube length—specifically laryngeal cavity—and indicated the need for more detailed anatomical studies and calculations. Thus, ultimately it is necessary to have a thorough multi-dimensional understanding of the anatomic development of the acoustic resonator, or the VT, between males and females to help establish anatomic-acoustic correlates during the course of development.
From an evolutionary perspective, the achievement of a length ratio of 1 between the pharyngeal and oral portions of the VT has been postulated to be an anatomic advantage for the emergence of speech (Lieberman, 1975; Lieberman et al. 1992). However, work on articulatory models has contended otherwise and highlighted the importance of auditory feedback and neurocognition (Callan et al. 2000, Menard et al., 2004, 2007; Boe et al., 2007). Irrespective, interest in evolution persists and there is a continuing need for a thorough understanding of the developmental anatomic changes in the VT. Although there have been a select number of radiographic and imaging studies on the anatomic development of the VT (Arens et al., 2002, Fitch and Giedd, 1999; Lieberman et al., 2001; Vorperian et al. 1999, 2005), there is a paucity of detailed quantitative data on sex-specific anatomic development of the VT and its component oral and pharyngeal cavities. As noted above, such information is important for understanding the biologic basis of speech development, and the complex anatomic-acoustic interactions or formant-cavity affiliations. Furthermore, such information would be useful in advancing non-uniform scaling factors for VT or speaker normalization (Vorperian and Kent, 2007). From an anatomic perspective, development of the VT and its constituent cavities can be understood in terms of the growth of the hard and soft tissues that give form to the acoustic conduit. The complex structures of the human head have diverse embryologic structures and tissues of origin (Sadler, 2006; Larsen, 2001; Sperber, 1973) and can be grouped into different schedules of growth and maturation. Scammon (1930) described three general growth schedules of the head and neck region: neural (brain and cranium), somatic (hard and soft tissues of the face), and lymphatic (tonsils and adenoid). This heterogeneity of growth pattern is a major factor to be considered in accounting for the development of the VT.
A primary goal of this study is to characterize the anatomic growth trend and growth rate of the VT and its oral and pharyngeal portions. Also, since different biological structures have sex-specific differences in growth schedule or growth curve type such as the male and female growth charts used clinically to assess the growth of head circumference and body stature (height and weight), a secondary goal of this study is to numerically quantify the sex-specific growth curves of the VTL and its oral and pharyngeal portions as neural or somatic, following Scammon (1930). Distinguishing differences between the neural and somatic growth curves lies in growth trend∕rate and percent growth. Structures with a neural growth curve display a very rapid growth following birth to achieve about 80% of its adult size during early childhood, followed by a slower steady growth until adulthood. Head circumference, a measurement that is mostly in the horizontal plane, follows such a growth curve. The somatic growth curve also displays a very rapid growth following birth, but size achieved during early childhood is barely 25%–40% of adult size. This early phase is followed by a regular and slow growth until maturity except for a brief period of rapid growth during puberty. Body height and facial growth, measurements in the vertical plane, follow this type of growth curve. Vorperian et al. (2005) related the growth curve type of VT structures to the anatomic orientation of the various structures. They reported that structures in the horizontal plane, such as the hard palate, appear to follow a neural growth curve, structures in the vertical plane, such as laryngeal descent, appear to follow a somatic growth curve, and structures oriented in both planes, such as tongue length and VTL, appear to have a hybrid, or a combined or intermediate neural and somatic growth curve. Although Lieberman and McCarthy (1999) and Lieberman et al. (2001) also reported differences in the growth type of the horizontal versus the vertical portions of the VT, they concluded that the growth of the VT has a predominantly skeletal or somatic growth curve.
METHODS
Subjects
Using imaging studies performed for medical reasons that are considered not to affect growth and development, a total of 605 head or neck imaging studies (307 MRI and 298 CT) were selected for making measurements where the VT structures could be visualized. The imaging studies were from 327 males and 278 females between the ages birth to 19 years. While developing∕acquiring this imaging database, significant effort was directed to select cases representative of the age range with an equivalent number of males and females per age∕year. The weights of the majority of the cases were at the 50th percentile reference growth curves for boys and girls, with all cases falling between the 25th and 95th percentile growth curves as per the National Center of Health Statistics growth charts (Centers for Disease Control and Prevention (CDC), 2000).
Procedures
Image acquisition
The medical imaging studies used for making measurements included both MRI and CT cases with subjects in the supine position. The method for MRI image acquisition has been described previously (Vorperian et al., 1999, 2005). The CT studies of the entire neck and face were obtained using General Electric helical CT scanners. Most young pediatric patients were sedated using either chloral hydrate 50 mg∕kg administered orally, or Propofol, Midazolam, Atropine, or Fentanyl administered intramuscularly (1 mg∕kg), prior to entering the scanner. Once in the scanner, the facial structures of all subjects were placed centrally in the head coil using the laser lights of the GE scanners. The CT scans were obtained using axial 1.25 mm thick slices. The images were obtained from the thoracic inlet, inferiorly, to the top of the orbits, superiorly with a 15–30 cm field of view. The field of view for young pediatric subjects ranged from 15 to 25 cm while those of older pediatric or adult subjects ranged from 25 to 30 cm. The images were reconstructed with a matrix size of 512×512. In-plane image resolution is given by dividing the field of view by the matrix size. Resolution ranged from 0.29 to 0.48 mm for pediatric patients and from 0.48 to 0.58 mm for adult patients. The axial CT scan data were reconstructed using two different algorithms (standard and bone plus) to provide two image sets, one optimized for soft tissue detail (standard algorithm) and one optimized for bone detail (bone algorithm). The axial images were then used to generate multiplanar reformatted images in the sagittal and coronal planes with a 2–3 mm slice thickness. The images were initially stored on a McKesson Horizon Rad Station PACS system. Next, the images were set anonymous using a General Electric Advantage Windows workstation. Then, the entire study was saved in DICOM format for image analysis and data acquisition.
Data acquisition
Data acquisition entailed making measurements of the variables defined below from the midsagittal plane. Midsagittal slice selection for CT studies entailed the use of both the standard and bone algorithms of the same slice to meet the same criteria as used and described previously for MRI studies (Vorperian et al., 1999, 2005) and entails the distinct visualization of cerebral sulci extending to the corpus callosum, also the visualization of the fourth ventricle, the full length of the cerebral aqueduct of Sylvius, the pituitary gland, part of the optic chiasm, the brainstem, and the cervical cord. Of note, image reconstruction using the software EFILM (by Merge eFilm) was implemented if the slice was not in true midline. Anatomic landmarks for making measurements were placed on the midsagittal bone algorithm slice by two researchers independently while visualizing both the bone and standard algorithms of the selected midsagittal slice. The two sets of landmarks were compared, discrepancies resolved using the radiologist’s medical expertise, and a final “master” set of landmarks was used for making measurements (Chung et al., 2008). Given the developmental nature of this study, the use of this landmark placement protocol was necessary as it improved measurement accuracy between 82% and 100% (average 98%) as measured by reduction in error variability. Of note, despite the careful selection of imaging studies where VT structures could be visualized, occasionally, not all anatomic landmarks could be clearly seen. Rather than excluding the entire study in such instances, all the measurements that could be secured using placed landmarks were secured and included in data analysis. The number of imaging studies∕cases per variable are listed in Table 1. Measurements were made using the image measurement software SIGMASCAN PRO by SYSTAT (formerly SPSS and Jandel Scientific) which was calibrated for each case∕slice using the hash scale mark on the CT image∕slice.
Table 1.
Summary of F-test for gender effect. The first two rows specify the number of male and female measurements available and included in the analysis from imaging studies per variable, and number of outliers per variable. The remaining rows reflect the results of the F-test for global sex differences of fits per variable and include the dfs, the F-values, and the p-values of the F-tests (×10−04).
| VTL | VT-V | PCL | NPhL | VT-H | LTh | ACL | OPhW | VT-O | |
|---|---|---|---|---|---|---|---|---|---|
| n males∕ outliers | 274∕4 | 277 ∕5 | 278∕4 | 277∕3 | 316∕4 | 311∕8 | 278∕6 | 269∕7 | 308 ∕4 |
| n females∕ outliers | 222∕7 | 226 ∕2 | 224∕5 | 223∕3 | 263 ∕3 | 270∕2 | 224∕3 | 222∕3 | 261 ∕1 |
| df | 4,476 | 4,487 | 4,484 | 4,485 | 4,563 | 4,562 | 4,484 | 4,472 | 4,555 |
| F-value | 40.38 | 27.13 | 34.29 | 4.93 | 6.38 | 11.74 | 0.03 | 2.53 | 3.29 |
| p-value | <1.0×10−12 | <1.0×10−12 | <1.0×10−12 | 6.619×10−04 | 5.004×10−05 | 3.622×10−09 | 9.980×10−01 | 3.972×10−02 | 1.106×10−02 |
| Significant | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
Variables
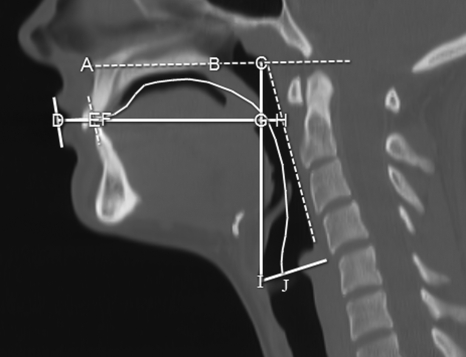
The nine variables used in this study are illustrated in Fig. 1 and are defined below. The data were acquired either via direct distance measurements (centimeters) of the variables from the midsagittal slice or calculated from those direct measurements. The variables included VTL, the curvilinear distance along the midline of the tract starting at the glottis (level of true vocal folds) to the intersection with a line drawn tangentially to the lips (curvilinear distance from points J to D in Fig. 1). Variables in the vertical plane included vocal tract-vertical (VT-V), the vertical distance from the glottis to the palatal plane (the ANS-PNS plane which extends from the anterior nasal spine to the posterior nasal spine; vertical distance from point I to C in Fig. 1). This VT-V distance consisted of two segments: the posterior-cavity length (PCL), the vertical distance of a line drawn from the glottis to the intersection with the end of the oral or anterior-cavity length (ACL) (distance I-to-G in Fig. 1); and the nasopharyngeal-length (NPhL), VT-V minus PCL (distance G-C in Fig. 1). Also, variables in the horizontal plane included vocal tract-horizontal (VT-H), the horizontal distance from a line tangential to lips to the posterior pharyngeal wall (horizontal distance D-to-H in Fig. 1). This VT-H distance consisted of three segments: lip-thickness (LTh), the distance at the level of the stomion between two lines, the first of which is drawn tangential to the anterior aspect and the second to the posterior or buccal aspect of the maxillary and mandibular lips (distance D-to-E in Fig. 1); ACL, the horizontal distance of a line drawn from the lingual incisor (start of the hard palate) to the intersection with the vertical line drawn from the glottis to the A-to-B palatal plane (distance F-to-G in Fig. 1); and the oropharyngeal-width (OPhW), VT-H minus LTh minus ACL (distance G-to-H in Fig. 1). Another horizontal segment calculated included the Vocal Tract-Oral (VT-O), VT-H minus LTh (distance E-to-H in Fig. 1).
Figure 1.
Midsagittal CT image displaying the anatomic landmarks used for making measurements. Measurements include VTL, the curvilinear line extending from points D-to-J. VT-V vertical distance from points I-to-C and consisting of two segments PCL (points I-to-G) and NPhL (points G-to-C). VT-H horizontal distance from points D-to-H, consisting of three line segments: LTh (points D-to-E), ACL (points F-to-G), and OPhW (points G-to-H). Also, the segment VT-O (points E-to-H).
Statistical analysis
Pooling of CT and MRI data
To maximize the data available for analysis, it was desirable to include the data from both CT and MRI studies. To determine if measurements from the CT and MRI studies can be pooled, data were secured from 28 cases that had both MRI and CT studies in less than a 3 month interval and the data were compared using paired t-tests. The measurement discrepancy between CT and MRI were not significant at alpha=0.01 for the variables used in this study. Therefore, the CT and MRI data were combined for increased statistical power.
Analysis of growth trend, rate, and type
The data (distance measurements as a function of age) were plotted to identify growth trends and gender differences (Figs. 2345678910). Following the removal of outliers from the data, as specified in Table 1, two sets of analyses were done. The criterion used for outlier removal was measurements exceeding 2.576σ which gives the probability of less than 0.01 for false removal of data. The first analysis was to characterize sex-specific growth curve trends and its growth rate for each of the nine variables. Based on the model selection framework, various polynomial model fits were performed and the fourth degree model was determined to describe∕fit the data best. Degree 1–3 models were too simplistic to model complex growth patterns. In comparing degree 4 and 5 models, degree 4 was determined to be a better model based on checking the significance of the sum of squared residuals (Rao and Toutenburg, 1999). Sex differences of the fits were assessed using an F-test and the results are summarized in Table 1. The fits are plotted in the left panel of Figs. 2345678910 and the p-values for sex differences are embedded in each figure. The growth rate (cm∕months), plotted in the right panel of Figs. 2345678910, was computed by differentiating the estimated model. The second analysis included an assessment of growth curve type by regressing each variable’s model fit to a neural (N) growth curve, a somatic (S) growth curve, and both N and S growth fits. The head circumference growth curve was used as the basic model for the neural growth curve, and the body height growth curve was used as the basic model for the somatic growth curve (Centers for Disease Control and Prevention (CDC), 2000; Nellhaus, 1968; Vorperian et al., 2007). The numeric quantification results are summarized in Table 2. Also, the percent growth to reach adult size is displayed on the second y-axis in the left panel of Figs. 2345678910 with black outwards tick orientation for males and gray inwards tick orientation for females.
Figure 2.
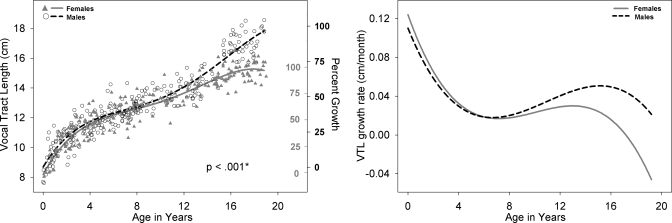
Left panel: VTL development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are significantly different (p<0.001). The second Y-axis reflects the percent growth of adult size for males (black, outwards tick orientation) and females (gray, inwards tick orientation). VTL is defined as the curvilinear distance along the midline of the VT starting at the level of the glottis to the intersection with a line drawn tangentially to the lips. Right panel: Growth rate of VTL, derived from the polynomial fit on left (cm∕months), for males (dashed black line) and females (solid gray line) as a function of age.
Figure 3.
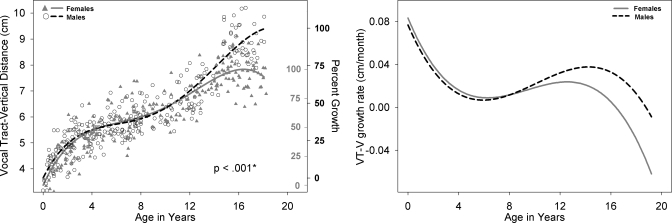
Left panel: VT-V development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are significantly different (p<0.001). The second Y-axis reflects the percent growth of adult size for males (black, outwards tick orientation) and females (gray, inwards tick orientation). VT-V is defined as the vertical distance from the glottis to the palatal plane. Right panel: Growth rate of VT-V, derived from the polynomial fit on left (cm∕months), for males (dashed black line) and females (solid gray line) as a function of age.
Figure 4.
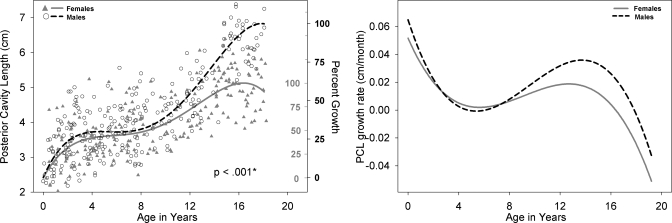
Left panel: PCL development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are significantly different (p<0.001). The second Y-axis reflects the percent growth of adult size for males (black, outwards tick orientation) and females (gray, inwards tick orientation). PCL is defined as the vertical distance of a line drawn from the glottis to the intersection with the end of the oral or anterior-cavity length. Right panel: Growth rate of PCL for males (dashed black line) and females (solid gray line) as a function of age.
Figure 5.
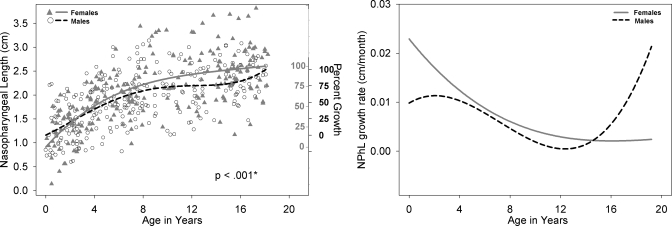
Left panel: NPhL development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are significantly different (p=0.008). The second Y-axis reflects the percent growth for males (black, outwards tick orientation) and females (gray, inwards tick orientation). NPhL is a calculated measurement of VT-V minus PCL. Right panel: Growth rate of NPhL for males (dashed black line) and females (solid gray line) as a function of age.
Figure 6.
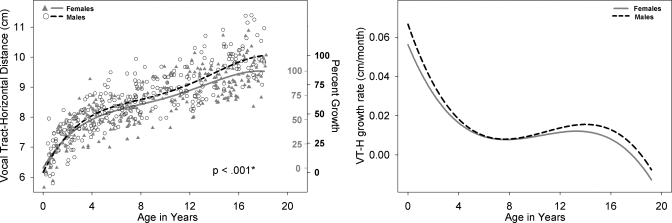
Left panel: VT-H development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are significantly different (p<0.0001).The second Y-axis reflects the percent growth for males (black, outwards tick orientation) and females (gray, inwards tick orientation). VT-H is defined as the horizontal distance from a line tangential to lips to the posterior pharyngeal wall. Right panel: Growth rate of VT-H for males (dashed black line) and females (solid gray line) as a function of age.
Figure 7.
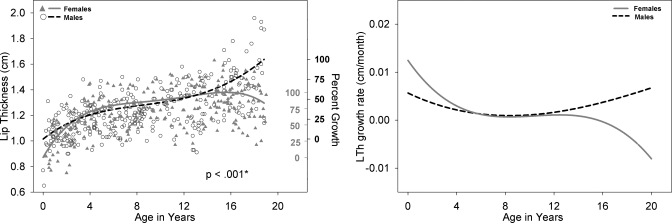
Left panel: LTh development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are significantly different (p<0.001). The second Y-axis reflects the percent growth for males (black, outwards tick orientation) and females (gray, inwards tick orientation). LTh defined as the distance between two lines, the first of which is drawn tangential to the anterior aspect of the maxillary and mandibular lips and the second to the posterior or buccal aspect of the maxillary and mandibular lips. Right panel: Growth rate of LTh for males (dashed black line) and females (solid gray line) as a function of age.
Figure 8.
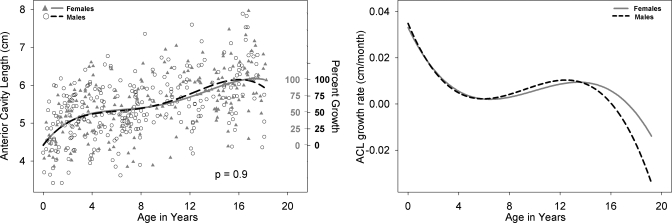
Left panel: ACL development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are not significantly different (p=0.9). The second Y-axis reflects the percent growth for males (black, outwards tick orientation) and females (gray, inwards tick orientation). ACL is defined as the horizontal distance from the beginning of the hard palate to the intersection with the vertical line drawn from the glottis to the palatal plane. Right panel: Growth rate of ACL for males (dashed black line) and females (solid gray line) as a function of age
Figure 9.
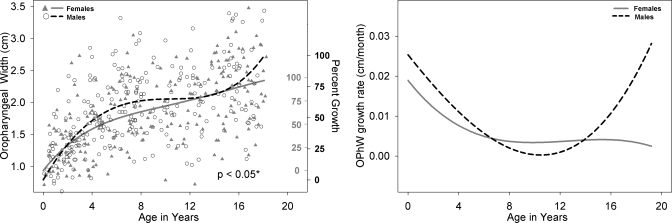
Left panel: OPhW development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are significantly different (p<0.01). The second Y-axis reflects percent growth for males (black, outwards tick orientation) and females (gray, inwards tick orientation). OPhW is calculated using the measurements of VT-H minus LTh minus ACL. Right panel: Growth rate of OPhW for males (dashed black line) and females (solid gray line) as a function of age.
Figure 10.
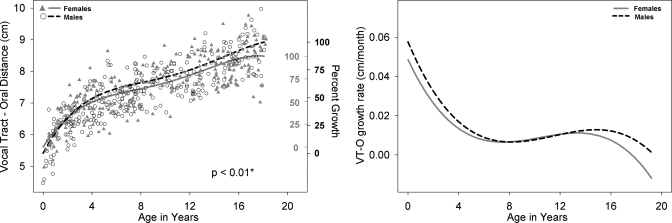
Left panel: VT-O development for males (black open circle) and females (shaded gray triangles) with growth curve using a fourth degree polynomial fit for males (dashed black line) and females (solid gray line). Male versus female fits are significantly different (p=0.03). The second Y-axis reflects percent growth for males (black, outwards tick orientation) and females (gray, inwards tick orientation). VT-O is a calculated using the measurements VT-H minus LTh. Right panel: Growth rate of VT-O for males (dashed black line) and females (solid gray line) as a function of age.
Table 2.
Numeric quantification in percent of the regression of sex-specific polynomial model fit per variable with the neural and somatic growth curves.
| Variable | Sex | % somatic | % neural | Numeric quantification |
|---|---|---|---|---|
| VTL | Female | 88 | 12 | Somatic∕neural |
| Male | 100 | 0 | Somatic | |
| VT-V | Female | 98 | 2 | Somatic |
| Male | 99 | 1 | Somatic | |
| PCL | Female | 100 | 0 | Somatic |
| Male | 97 | 3 | Somatic | |
| NPhL | Female | 89 | 11 | Somatic∕neural |
| Male | 91 | 9 | Somatic∕neural | |
| VT-H | Female | 60 | 40 | Somatic∕neural |
| Male | 76 | 24 | Somatic∕neural | |
| LTh | Female | 15 | 85 | Neural∕somatic |
| Male | 90 | 10 | Somatic∕neural | |
| ACL | Female | 61 | 39 | Somatic∕neural |
| Male | 90 | 10 | Somatic∕neural | |
| OPhW | Female | 75 | 25 | Somatic∕neural |
| Male | 39 | 61 | Neural∕somatic | |
| VT-O | Female | 61 | 39 | Somatic∕neural |
| Male | 67 | 33 | Somatic∕neural |
RESULTS
Growth trend and growth rate
All available measurements of the nine variables from the 605 cases between birth and age 19 years are plotted for males and females in the left panel of Figs. 210, with sex-specific fits—using the fourth degree polynomial model—and a p-value reflecting the outcome of the F-test for sex differences which were significant (p<0.05) for all variables except ACL. Table 1 includes for each of the nine variables the total number of male∕female measurements available for analysis, the number of outliers per variable for each sex, the degrees of freedom (dfs), the F-value, and the p-value. The growth trend for all the variables displayed in Figs. 2345678910 (left panel) reflects an overall nonlinear growth trend throughout the first 18 years of life, as noted by the general increases in measurements for both males and females. Of note, the negative growth noted past age 17 for select variables is mostly due to the nature of the data set, which is cross-sectional rather than longitudinal, and the insufficient number of measurements past age 17, which affect the fit at extreme ages adversely—a boundary limitation of regression fits∕models. As noted above, the F-test for sex differences was significant for all variables except ACL (p=0.9). While the visual display of the fits for most structures shows large sex differences past age 12, there are some structures that show somewhat large sex differences at an earlier age [such as PCL (Fig. 4) and NPhL (Fig. 5)]. Furthermore, during early childhood, the fits reflect slight sex differences for most structures where typically females are smaller than males except for OPhW (Fig. 9) and VT-O (Fig. 10) where the female fit displays a slightly larger value than the male fit. Also, during childhood, some sex differences appear to emerge and then dissolve [e.g., OPhW (Fig. 9)]. This appears to be related to growth rate (Figs. 2345678910, right panel) and also growth type, as addressed below. Figures 2345678910 (left panel) also include a second Y-axis documenting the percent growth to reach the mature adult size. The growth percentages on this second y-axes indicate that some structures reach the adult size sooner [e.g., VT-H (Fig. 6)] than others [e.g., VT-V (Fig. 3)]. Such findings have implication for growth type and are further discussed below.
Figures 2345678910 (right panel) show the growth rates (cm∕months) for each of the nine variables, where growth rate decreases during approximately the first 8 years of life. This is then followed by an increase in growth rate during approximately ages 8–14 years, and then a decrease in growth rate after age 16 for most variables except for NPhL, OPhW, and LTh in males (Figs. 579, right panel) where there is an apparent increase in growth rate after age 12 particularly for NPhL and OPhW (Figs. 59). As noted above, the polynomial fits (left panel) reflect sexual dimorphism emerging after age 12 for most variables; however, the fits for some variable, such as PCL (Fig. 4), NPhL (Fig. 5), and OPhW (Fig. 9) show prepubertal sex differences. Such differences are also evident in the growth rate figures of those variables, with more distinct demarcation of the age where differences in growth rate emerge. Thus, the growth rate figures may serve to detect the emergence of differences in growth trend. For example, while sex differences in VTL (Fig. 2, left panel) become apparent after age 12, the growth rate figure (Fig. 2, right panel) validates the emergence of such differences by age 8.
Growth type: Neural or somatic
The assessment of growth type (neural, somatic, or hybrid), as defined by Scammon (1930), has to be based on percent growth [as marked on the second Y-axes of the polynomial fit (Figs. 2345678910, left panel] and Table 2 which quantifies numerically the regression of each fit with a neural growth curve and a somatic growth curve. Table 2 (last column) shows that most of the variables have a hybrid or a combined neural and somatic growth type except for LTh in females and OPhW in males. As seen in Fig. 7 (left panel), LTh growth in females approaches the mature value by age 6, an indication of a predominantly neural growth curve. Also, the numeric quantification in Table 2 indicates that for each variable, males and females have different growth types. For example OPhW is 61% neural for males and 75% somatic for females. However, in general, structures in the vertical plane appear to have a predominantly somatic growth pattern.
DISCUSSION
Current findings
This study quantifies the non-uniform growth of the oral and pharyngeal portions of the VT structures in males and females during approximately the first 2 decades of life. While it has been known for decades that growth is non-uniform, the data presented here are without precedent in that they provide information on sex-specific anatomic development of VTL and of segments within the oral and pharyngeal portions of the VT. Furthermore, this quantification is detailed in that it specifies the growth trend and growth rate, as well as the growth type (neural versus somatic growth curves as defined by Scammon, 1930) for each of those various segments for males and females during the course of development. These major findings are highlighted and discussed in Secs. 4A1, 4A2, followed by a discussion on implications for speech acoustics and other ramifications.
Growth trend and growth rate
For all nine variables, the growth trend is somewhat rapid during the first few years of life with overall growth continuing until maturity (Figs. 2345678910, left panel). However, this rapid growth during the first 4–6 years of life differs for structures in the oral versus the pharyngeal portions of the VT. As seen on the second∕right y-axes in Figs. 2345678910, the variables in the oral region, which are in the horizontal plane, approximate the mature adult size sooner than the variables in the pharyngeal region, which are in the vertical plane. These findings were expected based on the reports by Lieberman and McCarthy (1999), Lieberman et al. (2001), and Vorperian et al. (2005) on growth types, and the implications are discussed further below in Sec. 4A2. Also, such differences in the growth schedule of structures in the oral∕horizontal versus pharyngeal∕vertical portions of the VT are to be expected given their diverse embryologic origins. Specifically, the embryologic structure∕tissue type for the oral cavity is the stomodeum∕neural crest-ectoderm, whereas for the pharynx, it is the foregut∕endoderm and splanchnic mesoderm (Sadler, 2006). Again, this issue is addressed further in Sec. 4A2. As for the negative growth trend past age 17, as noted in Sec. 3, this is not an accurate representation of growth trend but a limitation of the model∕regression fit at the extreme age due to the nature of the cross-sectional data set that has limited data points past age 17.
There are significant sex differences in the growth trend of eight of the nine variables studied (ACL not significant), with the majority of the variables displaying distinct sexual dimorphism after age 12 (Figs. 2345678910, left panel). However, as noted in the Sec. 3, there are some variables that display prepubertal sexual dimorphism at much earlier ages such as VT-H (Fig. 6) where differences are evident by age 4 and persist until adulthood, and other variables such as VTL (Fig. 2), PCL (Fig. 4), NPhL (Fig. 5), and OPhW (Fig. 9) where sex differences are either localized to specific ages or fluctuate during the course of development and appear to be related to growth rate differences between males and females. This is discussed further in the following paragraph.
Since growth rate differences between males and females are evident (Figs. 2345678910, right panel), ideally the interpretation of sex differences in growth trend fits (Figs. 2345678910, left panel) should be made in conjunction with growth rate curves because differences in growth rate between males and females can easily mask differences in growth trend. The variables NPhL (Fig. 5) and OPhW (Fig. 9) demonstrate this point clearly. Such an observation indicates that to assess whether prepubertal sex differences are being masked by differences in growth rate, it is necessary to implement more localized or smaller age range comparisons between males and females rather than comparisons across all ages (birth to 19) as was done in this study. In addition, it seems that such localized age group comparison between males and females should ideally use groups smaller than a 6 year span since Vorperian et al. (2005) did not identify sexual dimorphism of any VT structures between the ages of birth to approximately 6 years. However, Lieberman et al. (2001) have noted sex differences in the growth of oropharyngeal-width (the distance from the posterior pharyngeal wall to the posterior margin of oral cavity) with growth being slightly larger in males between the ages of 1.75 and 4.75 years. It is reasonable to expect the identification of prepubertal sexual dimorphism in VT structures given that the growth charts (height, weight, and head circumference) used clinically are sex-specific (Centers for Disease Control and Prevention (CDC), 2000) and the knowledge that there is a strong correlation between VTL and body size (both height and weight; Bennett, 1981; Fitch and Giedd, 1999). This issue is discussed further under Sec. 4B.
Growth rate, past age 16 (Figs. 2345678910, right panel), decreases or levels off for all structures except for the variables NPhL and OPhW in males where growth rate increases. This warrants assessment of whether growth in the oro-naso-pharyngeal portion persists beyond approximately the first 2 decades of life. This point is addressed further under Sec. 4B.
Growth type: Neural or somatic
The distinguishing difference between the neural and somatic growth curves lies not only in growth trend but also percent growth (Scammon, 1930). As expected and discussed above, structures in the oral region reached maturity sooner than structures in the pharyngeal region implying a neural growth type for VT structures in the oral∕horizontal region and somatic growth type for VT structures in the pharyngeal∕vertical region. Numeric quantification of growth type for the nine variables studied, as summarized in Table 2, supports the following conclusions. Most of the nine variables studied appear to follow either a predominantly somatic growth type or a hybrid∕combined somatic and neural growth curves for both males and females. Structures in the vertical plane or the pharyngeal region follow a predominantly somatic growth type for both males and females. The numeric quantification confirms findings reported by Lieberman and McCarthy (1999), Lieberman et al. (2001), and Vorperian et al. (2005). Structures in the horizontal plane or the oral region, however, follow a hybrid somatic∕neural or neural∕somatic growth type. The numeric quantification does not match the expectations of a predominantly neural growth type except for the variable LTh in females and OPhW in males. Interestingly, this latter finding on OPhW is consistent with the findings of Lieberman et al. (2001) of the growth of oropharyngeal-width being slightly larger in males between the ages of 1.75 and 4.75 years. The variables in the oral∕horizontal region also seem to display sex differences in growth type; particularly distinct sex differences are noted for the variables LTh and OPhW. For example, the variable OPhW is predominantly neural (61%) in males with percent growth of adult size at about 70% by age 6, whereas it is predominantly somatic (75%) in females with percent growth of adult size at about 50% by age 6. As noted above, such sex-specific differences in growth type have also been reported by Lieberman et al. (2001) and further highlight the need to take into account growth rate differences when making interpretations of growth pattern and growth type. VTL, which is a variable in both planes, follows a predominantly somatic growth curve in females (88%) and a purely somatic growth curve in males (100%). Thus, multiple factors are at play and ultimately determine the growth type of VT structures. While structure orientation was a useful general guide, it is necessary to consider other factors, such as differences in the growth rate between males and females and the embryologic origin of the various VT structures—such as the palate, mandible, and tongue—that border the oral and pharyngeal cavities.
Acoustic implications
The growth trend, growth rate and growth type quantification from medical imaging studies, as specified above, are part of the biological basis of speech development. The noted sex-specific differences in the growth of the oral and pharyngeal portions of the VT can have profound acoustic implications. One important aspect is relating anatomic growth patterns to developmental changes in speech acoustics, including male∕female differences. A potential limitation for the comparison of the two modalities is that the anatomic data are from imaging studies with subjects in the supine position at rest, whereas all developmental speech acoustic data are from participants in the upright position. The gravitational effect on airway patency is well documented when subjects are in supine during quiet breathing where the pharyngeal region volume is reportedly reduced in supine as opposed to upright (e.g., Eckmann et al., 1996). Also, differences in tongue behavior during speech production in upright and supine have been reported by Stone et al. (2007) using ultrasound imaging, as well as differences in both the soft tissues and the rigid structures (mandible and larynx) during vowel production in upright and supine have been reported by Kitamura et al. (2005) using open-type MRI. However, Stone et al. (2007) did not identify any significant phoneme effects, and no differences in the first and second formant values. Thus, it is reasonable to conclude that albeit postural differences between the two modalities, it is a worthwhile venture to hypothesize developmental anatomic-acoustic correlates given the uniqueness of the developmental anatomic data set of VT structures and its quantification that are presented in this paper.
Acoustic theory states that as the VT lengthens with age, formant frequencies decrease (Fant, 1960). Current findings on VTL growth trend (Fig. 2, left panel) show a nonlinear growth pattern with significant sex differences. Although the test of sex differences utilized here is a global test (i.e., takes into account all ages), the polynomial model growth fits show sex differences emerging at about age 12, confirming previous reports on significant sexual dimorphism in VTL after age 11 years (Fitch and Giedd, 1999) or 13.75 years (Lieberman et al., 2001). The fits also show a rapid increase in VTL during early childhood that is about 2 cm during the first 2 years of life, a finding that is consistent with previous findings from a much smaller pool of imaging studies (Vorperian et al., 2005). Such anatomic findings are not consistent with two reports on acoustic data. The first acoustic observation is that formant frequencies remain unchanged, i.e., do not decrease, during the first 2 years of life (Buhr, 1980; Gilbert et al., 1997; Kent and Murray, 1982; Robb et al., 1997). The second acoustic observation is that acoustic differences between males and females are present by age 4 (Perry et al., 2001). Thus, the quest is to identify other developmental anatomic findings of VT structures that might explain those two acoustic findings.
Regarding the first point that there are no reported decreases in formant frequencies despite increase in VTL, current findings indicate that there is rapid growth of OPhW which implicates volumetric changes∕increases in the pharyngeal region. Thus, it is possible to postulate that there is an interplay between VTL and volume. As Fant (1975) has noted, VT dimensions to consider in physiologic-acoustic interpretations in addition to oral and pharyngeal tube lengths include laryngeal cavity dimensions. Additional research to quantify developmental changes in length versus volume in the VT, particularly in the hypopharyngeal and laryngopharyngeal regions during the first two years of life, is warranted.
Regarding the second point of acoustic differences being present by age 4 (Perry et al., 2001), although the VTL growth fits show slight differences between males and females until sexual dimorphism becomes apparent around age 12, the anatomic differences do not appear to be related to the reported acoustic differences between males and females that are present by age 4. However, it seems reasonable to hypothesize that such male∕female acoustic differences are related to differences in the development of the oral and pharyngeal portions of the VT. Visual inspection of the growth fits of all the other VT variables examined in this paper (Figs. 345678910, left panel) indicate anatomic sex differences of select variables, some localized to specific ages that warrant a closer or localized examination in the future. Specifically, the variables LTh, PCL, and NPhL during the first 2 years of life; also, the variables OPhW and VT-O, from about age 4 years to 12 years, which are larger in males than in females, warrant detailed analysis of anatomic differences between males and females.
While it is premature to establish anatomic-acoustic correlates from the data presented here, additional localized assessments of those variables in the midsagittal plane would not only assist in deciphering anatomic-acoustic correlates but also guide future research efforts in studying multidimensional growth of the nasopharyngeal region (Vorperian et al. 2005; Vorperian and Kent, 2007). The vowel acoustic space development data summarized by Vorperian and Kent (2007) indicate that the F1–F3 vowel quadrilateral dispersions is greater than F1–F2 dispersion and that they appear to be sensitive to age and speaker sex differences. According to Fant’s (1975) two-tube model, the pharyngeal cavity length is affiliated with the second formant, and the oral cavity length is affiliated with the third formant. Indeed, if the noted differences in VT-H growth, specifically the segments OPhW and VT-O, are confirmed to be significantly different between males and females, then this can explain, at least in part, the documented prepubertal acoustic differences between males and females where there are no significant differences in VTL, i.e., before age 11 (Fitch and Giedd, 1999). Of note, although the two-tube model is simplistic and ignores cross modes in the transfer function of the VT, it is nonetheless a model with a good first approximation and a reasonable approach to begin establishing hypotheses on anatomic-acoustic correlates that may be tested using various more complex modeling approaches (e.g., Motoki, 2002; Menard et al., 2004, 2007; Boe et al., 2007).
A final acoustic observation is the noted decrease in formant frequencies in the aging population which has been attributed to the lengthening of the VT (Benjamin, 1997; Endres et al., 1971; Linville and Fisher, 1985). Xue and Hao’s (2003) findings, using acoustic reflection technology, reflected changes in VT volume but not VTL for both genders; also they noted increases in oral cavity length and volume, again for both genders. The assessment of anatomic change in VT structures using imaging studies may help address whether growth persists past age 18 where most VT structures are presumed to have reached their mature size. In particular, it will be worthwhile to study the growth trend of structures in the oro-naso-pharyngeal region since current findings reflect increases in the growth rate of NPhL and OPhW in males past the age of presumed maturity.
Other ramifications
The quantification on the development of the oral and pharyngeal portions of the VT presented here can be used in a multitude of ways. For one, it can be used in developmental articulatory models including sex-specific developmental models to test predictions on anatomic-acoustic correlates as specified above. To date, modeling efforts have estimated or approximated the growth of the VTL, oral cavity length, and pharyngeal cavity length (e.g., Goldstein, 1980; Martland et al., 1996; Callan et al. 2000; Menard et al. 2004, 2007; Boe et al. 2007). However, most of those modeling efforts are not sex-specific, and some are based on assumptions of growth types that appear not to be valid based on current findings. For example, the predictions of Martland et al. (1996) are based on the assumption that the growth of the oral and the pharyngeal tracts follow the neural and somatic growth types, respectively. However, present findings indicate that while the assumption of somatic growth type of the pharyngeal region is mostly valid, the assumption on the growth of the oral region is not since present findings show the growth of this region to follow a combined somatic∕neural growth type with apparent sex differences (females, 60∕40% and males, 76∕24% males). One implication from having detailed anatomic quantification on the non-uniform growth of the VT is that the data can be used in establishing scaling factors for normalization (age and sex differences in the acoustic properties of speech), a long standing issue in acoustic phonetics and speech technology.
Another ramification is promoting our understanding of the anatomic bases of motor adjustment during development, including speech development. To date, the noted high degree of variability, from acoustic and physiologic studies, in children’s performance has been linked to the maturation of the neuromuscular control or speech motor control (Eguchi and Hirsh, 1969; Tingley and Allen, 1975, Smith et al., 1983; Sharkey and Folkins, 1985; Smith, 1994; Kent, 1976, 1992; Kent and Forner, 1980; Lee et al., 1999; Walsh and Smith, 2002; Wohlert and Smith, 2002). However, it is important not to discount the role of anatomic change in neuromuscular control. For example, this study reflects a rapid growth rate in the pharyngeal region during approximately the ages 8–14 particularly in males. It is within this age range, specifically ages 10–14, that there are reports on high levels of childhood asphyxiation by food, particularly in males, (Baker et al., 1992). Thus, it is reasonable to relate rapid anatomic growth to limited neuromuscular control.
Furthermore, as Boe et al. (2007) pointed out, knowledge of the size of the oral and pharyngeal cavities throughout ontogeny not only help understand speech acquisition processes in infants but also assist in understanding phylogeny and the evolution of speech. More recently, Fitch (2000, 2002) noted that many mammals dynamically create a two-tube VT during loud vocalizations where the larynx is lowered into the pharyngeal cavity resulting in a long and well-defined pharyngeal tube. He therefore hypothesized that the evolution of the human speech apparatus with a descended larynx arose gradually with increased vocalization complexity and frequency. Undoubtedly, such a viewpoint places more emphasis on understanding the growth of the oral cavity and specially the pharyngeal cavity, and less emphasis on the ratio of the horizontal and vertical parts of the VT.
CONCLUSION
This study quantifies the non-uniform growth of the VT in terms of both regional differences (oral versus pharyngeal portions of the VT) and sex differences in the growth of those regions. The growth quantification of nine variables in the oral and pharyngeal regions during approximately the first 2 decades of life in males and females indicate that structures in the oral region reach the mature size sooner than those in the pharyngeal region. Structures in the oral region follow a combined somatic and neural growth curve, whereas structures in the pharyngeal region follow a predominantly somatic growth curve as defined by Scammon (1930). While findings confirm global sex differences for most structures, the growth trend and growth rate figures reflect not only postpubertal sexual dimorphisms for most structures but also prepubertal sex differences at particular ages for select structures. This warrants localized assessment of sex differences as a means to explore the anatomic basis of the observed acoustic differences between males and females during early childhood.
ACKNOWLEDGMENTS
This work was supported in part by NIH Research Grant Nos. R03 DC4362 (Anatomic Development of the Vocal Tract: MRI Procedures) and R01 DC6282 (MRI and CT Studies of the Developing Vocal Tract), from the National Institute of Deafness and other Communicative Disorders (NIDCD). Also, by a core Grant No. P-30 HD03352 to the Waisman Center from the National Institute of Child Health and Human Development (NICHHD). We thank Celia Choih for assistance with placing the anatomic landmarks and making the necessary measurements and Katelyn J. Kassulke for assistance with data entry and figures.
Portions of this paper were presented in 2007 at the 154th meeting of the Acoustical Society of America in New Orleans, LA.
References
- Arens, R., McDonough, J., Corbin, A., Hernandez, M., Maislin, G., Schwab, R., and Pack, A.(2002). “Linear dimensions of the upper airway structure during development: Assessment by magnetic resonance imaging,” Am. J. Respir. Crit. Care Med. 165, 117–122. [DOI] [PubMed] [Google Scholar]
- Baker, S. P., O’Neill, B., Ginsburg, M. J., and Li, G. (1992). The Injury Fact Book, 2nd ed. (Oxford University Press, New York: ). [Google Scholar]
- Benjamin, B. J. (1997). “Speech production of normally aging adults,” Semin. Speech Lang. 18, 135–141. [DOI] [PubMed] [Google Scholar]
- Bennett, S. (1981). “Vowel formant frequency characteristics of preadolescent males and females,” J. Acoust. Soc. Am. 10.1121/1.385343 69, 231–238. [DOI] [PubMed] [Google Scholar]
- Boe, L., Heim, J., Honda, K., Maeda, S., Badin, P., and Abry, C. (2007). “The vocal tract of newborn humans and Neanderthals: Acoustic capabilities and consequences for the debate on the origin of language. A reply to Liberman (2007a),” J. Phonetics 35, 564–581. [Google Scholar]
- Buhr, R. D. (1980). “The emergence of vowels in an infant,” J. Speech Lang. Hear. Res. 23, 73–94. [DOI] [PubMed] [Google Scholar]
- Callan, D. E., Kent, R. D., Guenther, F. H., and Vorperian, H. K. (2000). “An auditory-feedback-based neural network model of speech production that is robust to developmental changes in the size and shape of the articulatory system,” J. Speech Lang. Hear. Res. 43, 721–736. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2000). National Center for Health Statistics Clinical Growth Charts, http://www.cdc.gov/growthcharts/ (Last viewed November, 2006).
- Chung, D., Chung, M. K., Durtschi, R. B., Gentry, L. R., and Vorperian, H. K. (2008). “Measurement consistency from magnetic resonance images,” Acad. Radiol. 15, 1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann, D. M., Glassenberg, R., and Gavriely, N. (1996). “Acoustic reflectometry and endotracheal intubation,” Anesth. Analg. (Baltimore) 83, 1084–1089. [DOI] [PubMed] [Google Scholar]
- Eguchi, S., and Hirsh, I. J. (1969). “Development of speech sounds in children,” Acta Oto-Laryngol. 257, 1–51. [PubMed] [Google Scholar]
- Endres, W., Bamback, W., and Flosser, G. (1971). “Voice spectrograms as a function of age, voice disguise, and voice imitation,” J. Acoust. Soc. Am. 10.1121/1.1912589 49, 1842–1848. [DOI] [PubMed] [Google Scholar]
- Fant, G. (1975). “A note on vocal tract size factors and non-uniform F-pattern scalings,” Speech Transm. Lab. Q. Prog. Status Rep. 4, 22–30. [Google Scholar]
- Fant, G. (1960). Acoustic Theory of Speech Production (Mouton, The Hague: ). [Google Scholar]
- Fitch, W. T. (2002). “Comparative vocal production and the evolution of speech: Reinterpreting the descent of the larynx,” in The Transition to Language, edited by Wray A. (Oxford University Press, Oxford: ), pp. 21–45. [Google Scholar]
- Fitch, W. T. (2000). “The evolution of speech: A comparative review,” Trends Cogn. Sci. 10.1016/S1364-6613(00)01494-7 4, 258–267. [DOI] [PubMed] [Google Scholar]
- Fitch, W. T., and Giedd, J. (1999). “Morphology and development of the human vocal tract: A study using magnetic resonance imaging,” J. Acoust. Soc. Am. 10.1121/1.427148 106, 1511–1522. [DOI] [PubMed] [Google Scholar]
- Gilbert, H. R. Robb, M. P., and Chen, Y. (1997). “Formant frequency development: 15 to 36 months,” J. Voice 10.1016/S0892-1997(97)80003-3 11, 260–266. [DOI] [PubMed] [Google Scholar]
- Goldstein, U. G. (1980). “An articulatory model for the vocal tracts of growing children,” Ph.D. thesis, MIT, Cambridge, MA. [Google Scholar]
- Kent, R. D. (1992). “The biology of phonological development,” Phonological Development: Models, Research, Implications, edited by Ferguson C. A., Menn L., and Stoel-Gammon C. (York, Timonium, MD: ), pp. 65–90. [Google Scholar]
- Kent, R. D. (1976). “Anatomical and neuromuscular maturation of the speech mechanism: Evidence from acoustic studies,” J. Speech Hear. Res. 19, 421–447. [DOI] [PubMed] [Google Scholar]
- Kent, R. D., and Forner, L. L. (1980). “Speech segment durations in sentence recitations by children and adults,” J. Phonetics 12, 157–68. [Google Scholar]
- Kent, R. D., and Murray, A. D. (1982). “Acoustic features of infant vocalic utterances at 3, 6, and 9 months,” J. Acoust. Soc. Am. 10.1121/1.388089 72, 353–65. [DOI] [PubMed] [Google Scholar]
- Kent, R. D., and Vorperian, H. K. (1995). “Anatomic development of the craniofacial-oral-laryngeal systems: A review,” J. Med. Speech-Lang. Pathol. 3, 145–190. [Google Scholar]
- Kitamura, T., Takemoto, H., Honda, K., Shimada, Y., Fujimoto, I., Syakudo, Y., Masaki, S., Kuroda, K., Oku-uchi, N., and Senda, M. (2005). “Difference in vocal tract shape between upright and supine postures: Observation by an open-type MRI scanner,” Acoust. Sci. & Tech. 26, 465–468. [Google Scholar]
- Larsen, W. (2001). Human Embryology (Churchill Livingstone, Philadelphia, PA: ). [Google Scholar]
- Lee, S., Potamianos, A., and Narayanan, S. (1999). “Acoustics of children’s speech: Developmental changes of temporal and spectral parameters,” J. Acoust. Soc. Am. 10.1121/1.426686 105, 1455–1468. [DOI] [PubMed] [Google Scholar]
- Lieberman, P. (1975). On the Origins of Language (Macmillan, New York: ). [Google Scholar]
- Lieberman, D. E., McCarthy, R. C., Hiiemae, K. M., and Palmer, J. B. (2001). “Ontogeny of postnatal hyoid and larynx descent in humans,” Arch. Oral Biol. 10.1016/S0003-9969(00)00108-4 46, 117–128. [DOI] [PubMed] [Google Scholar]
- Lieberman, D. E., and McCarthy, R. C. (1999). “The ontogeny of cranial base angulation in humans versus chimpanzees and its implications for reconstructing pharyngeal dimensions,” J. Hum. Evol. 36, 487–517. [DOI] [PubMed] [Google Scholar]
- Lieberman, P., Laitman, J. T., Reidenberg, J. S., and Gannon, P. S. (1992). “The anatomy, physiology, acoustics, and perception of speech: Essential elements in analysis of the evolution of human speech,” J. Hum. Evol. 23, 447–467. [Google Scholar]
- Linville, S. E., and Fisher, H. B. (1985). “Acoustic characteristics of perceived versus actual vocal age in controlled phonation by adult females,” J. Acoust. Soc. Am. 10.1121/1.392452 78, 40–8. [DOI] [PubMed] [Google Scholar]
- Martland, P., Whiteside, S. P., Beet, S. W., and Baghai-Ravary, L. (1996). “Estimating child and adolescent formant frequency values from adult data,” Proceedings from ICSLP '96, The Fourth International Conference on Spoken Language, IEEE, Philadelphia, PA, pp. 626–629.
- Menard, L., Schwartz, J., and Boe, J. (2004). “Role of vocal tract morphology in speech development: Perceptual targets and sensorimotor maps for synthesized French vowels from birth to adulthood,” J. Speech Lang. Hear. Res. 10.1044/1092-4388(2004/079) 47, 1059–1080. [DOI] [PubMed] [Google Scholar]
- Menard, L., Schwartz, J., Boe, L., and Aubin, J. (2007). “Articulatroy-acoustic relationships during vocal tract growth for French vowels: Analysis of real data and simulations with an articulatory model,” J. Phonetics 10.1016/j.wocn.2006.01.003 35, 1–19. [DOI] [Google Scholar]
- Motoki, K. (2002). “Three-dimensional acoustic field in vocal-tract,” Acoust. Sci. Technol. 23, 207–212. [Google Scholar]
- Nellhaus, G. (1968). “Head circumference from birth to eighteen years: Practical composite international and interracial graphs,” Pediatrics 41, 106–114. [PubMed] [Google Scholar]
- Perry, T. L., Ohde, R. N., and Ashmead, D. H. (2001). “The acoustic bases for gender identification from children’s voices,” J. Acoust. Soc. Am. 10.1121/1.1370525 109, 2988–98. [DOI] [PubMed] [Google Scholar]
- Rao, C. R., and Toutenburg, H. (1999). Linear Models: Least Squares and Alternatives (Springer-Verlag, New York: ). [Google Scholar]
- Robb, M. P., Chen, Y., and Gilbert, H. (1997). “Developmental aspects of formant frequency and bandwidth in infants and toddlers, ” Folia Phoniatr Logop 49, 88–95. [DOI] [PubMed] [Google Scholar]
- Sadler, T. W. (2006). Medical Embryology (Lippincott Williams & Wilkins, Philadelphia: ). [Google Scholar]
- Scammon, R. E. (1930). “The measurement of the body in childhood,” edited by Harris J. A., Jackson C. M., Patterson D. G., and Scammon R. E., The Measurement of Man (University of Minnesota Press, Minneapolis: ), pp. 173–215. [Google Scholar]
- Sharkey, S. G., and Folkins, J. W. (1985). “Variability of lip and jaw movements in children and adults: Implications for the development of speech motor control,” J. Speech Hear. Res. 28, 8–15. [DOI] [PubMed] [Google Scholar]
- Smith, B. L. (1994). “Effects of experimental manipulations and intrinsic contrasts on relationships between duration and temporal variability in children’s and adult’s speech,” J. Phonetics 22, 155–175. [Google Scholar]
- Smith, B. L., Sugarman, M. D., and Long, S. H. (1983). “Experimental manipulation of speaking rate for studying temporal variability in children’s speech,” J. Acoust. Soc. Am. 10.1121/1.389860 74, 744–749. [DOI] [PubMed] [Google Scholar]
- Sperber, G. H. (1973). Craniofacial Embryology (Henry Ling Ltd., Dorchester, Great Britain: ). [Google Scholar]
- Stevens, K. N. (1989). “On the quantal nature of speech,” J. Phonetics 17, 3–45. [Google Scholar]
- Stone, M., Stock, G., Bunin, K., Kumar, K., Epstein, M., Kambhamettu, C., Li, M., Parthasarathy, V., and Prince, J. (2007). “Comparison of speech production in upright and supine position,” J. Acoust. Soc. Am. 10.1121/1.2715659 122, 532–541. [DOI] [PubMed] [Google Scholar]
- Tingley, B. M., and Allen, G. D. (1975). “Development of speech timing control in children,” Child Dev. 10.2307/1128847 46, 186–194. [DOI] [Google Scholar]
- Vorperian, H. K., Durtschi, R. B., Wang, S., Chung, M. K., Zeigert, A. J., and Gentry, L. R. (2007). “Estimating head circumference from imaging studies: An improved method,” Acad. Radiol. 14, 1102–1107. [DOI] [PubMed] [Google Scholar]
- Vorperian, H. K., and Kent, R. D. (2007). “Vowel acoustic space development in children: A synthesis of acoustic and anatomic data,” J. Speech Lang. Hear. Res. 10.1044/1092-4388(2007/104) 50, 1510–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorperian, H. K., Kent, R. D., Lindstrom, M. J., Kalina, C. M., Gentry, L. R., and Yandell, B. S. (2005). “Development of vocal tract length during early childhood: A magnetic resonance imaging study,” J. Acoust. Soc. Am. 10.1121/1.1835958 117, 338–350. [DOI] [PubMed] [Google Scholar]
- Vorperian, H. K., Kent, R. D., Gentry, L. R., and Yandell, B. S. (1999). “Magnetic resonance imaging procedures to study the concurrent anatomic development of the vocal tract structures: Preliminary results,” Int. J. Pediatr. Otorhinolaryngol. 49, 197–206. [DOI] [PubMed] [Google Scholar]
- Walsh, B., and Smith, A. (2002). “Articulatory movements in adolescents: Evidence for protracted development of speech motor control processes,” J. Speech Lang. Hear. Res. 10.1044/1092-4388(2002/090) 45, 1119–1133. [DOI] [PubMed] [Google Scholar]
- Wohlert, A. B., and Smith, A. (2002). “Developmental change in variability of lip muscle activity during speech,” J. Speech Lang. Hear. Res. 45, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Xue, S. A., and Hao, G. J. (2003). “Changes in the human vocal tract due to aging and the acoustic correlates of speech production: A pilot study,” J. Speech Lang. Hear. Res. 46, 689–701. [DOI] [PubMed] [Google Scholar]