Abstract
The cell body or soma in the dosal root ganglion (DRG) is normally excitable and this excitability can increase and persist after an injury of peripheral sensory neurons. In a rat model of radicular pain, an intraforaminal implantation of a rod that chronically compressed the lumbar DRG (“CCD” model) resulted in neuronal somal hyperexcitability and spontaneous activity that was accompanied by hyperalgesia in the ipsilateral hind paw. By the 5th day after onset of CCD, there was a novel upregulation in neuronal expression of the chemokine, monocyte chemoattractant protein-1 (MCP-1 or CCL2) and also its receptor, CCR2. The neurons developed, in response to topically applied MCP-1, an excitatory response that they normally do not have. CCD also activated non-neuronal cells including, for example, the endothelial cells as evidenced by angiogenesis in the form of an increased number of capillaries in the DRG after 7 days. A working hypothesis is that the CCD induced changes in neurons and non-neuronal cells that may act together to promote the survival of the injured tissue. The release of ligands such as CCL2, in addition to possibly activating nociceptive neurons (maintaining the pain), may also act to preserve injured cells in the face of ischemia and hypoxia, for example, by promoting angiogenesis. Thus, somal hyperexcitability, as often said of inflammation, may represent a double edged sword.
Keywords: radicular pain, dorsal root ganglion, neuronal hyperexcitability
The cell body or soma in the dorsal root ganglion (DRG) is normally excitable, and this excitability can increase and persist after an injury of a peripheral nerve or the ganglion. Persistent somal hyperexcitability might amplify or distort trains of action potentials generated from peripheral receptors or cause the generation of spontaneous ectopic discharges from the DRG possibly contributing to novel gene transcription, neurogenic inflammation and leading to novel or enhanced release of inflammatory mediators and neurotransmitters that maintain enhanced pain states and paresthesias.
The enhanced release of inflammatory mediators and neurotransmitters, wherever it occurs, i.e. at nerve terminal endings or within the DRG itself, may alter the activity not only of neighboring neurons but also non-neuronal cells such as glial cells, endothelial cells and immune cells. Glial cells include the Schwann cells along the axon of the neuron, the satellite cells that surround the neuronal cell body in the DRG, and the astrocytes near the central terminals of the neuron in the central nervous system (CNS). Immune cells are represented, for example, by resident mast cells and macrophages in the peripheral nervous system and by microglia in the CNS. Endothelial cells of microvessels are in close proximity to all portions of the primary sensory neuron.
All four cell types, i.e., neurons, glia, immune and endothelial cells, work together as a functional unit; thus, all are likely to be affected by any injury leading to inflammatory or neuropathic pain. This is the case, as summarized in the present review, when the injury occurs in the vicinity of the cell body of the primary sensory neuron as a result of a chronic compression of the DRG (CCD). The injury produces, in the rat, a persistent hyperexcitability of the neuronal somata in the ganglion associated with chronic neuropathic pain behavior. Chronic pain, in this case possibly resulting from somal hyperexcitability, has no beneficial effects for the organism. However, we hypothesize that somal hyperexcitability may contribute beneficially to other biological processes such as those acting to protect and promote the survival of all four cell types in the injured tissue.
1 CCD is an animal model of radicular pain in humans and produces neuropathic pain behavior in the rat
CCD is a model of a type of radicular pain in humans resulting, for example, from a laterally herniated disc or a foraminal stenosis. It was produced in the rat by implanting a stainless steel rod unilaterally into the intervertebral foramen, one rod at L4 and another at L5[1, 2]. This produced, within one day, hyper-reflexive withdrawal of the ipsilateral hind paw to stimuli that normally evoked no withdrawal such as a cool floor or mechanical stroking with a cotton wisp (allodynia). Stimuli that normally evoked a foot withdrawal, namely Von Frey stimuli (with tip diameters of 100 μm) or noxious heat stimuli, now did so at lesser forces and lower temperatures, respectively (hyperalgesia)[2]. The allodynia largely dissipated within two weeks after the onset of CCD whereas the hyperalgesia persisted for more than a month.
2 CCD produced neuronal hyperexcitability that originated within the DRG
After CCD, DRG neurons of the injured ganglion exhibited characteristics of hyperexcitability. Extracellular electrophysiological recordings of nerve impulse activity in single dorsal root fibers revealed that a subpopulation of both slowly and rapidly conducting types exhibited spontaneous, ectopic discharges. The patterns of such discharge were the same as those obtained by previous investigators from axotomized neurons (tonic, bursting or irregular) except that in this case, the fibers were not axotomized as their conduction velocities could be obtained via electrical stimulation of the sciatic nerve. Thus, axotomy is not required to produce such abnormal spontaneous activity.
Most fiber recordings were obtained from myelinated fibers, in vitro, wherein the sciatic nerve and attached L4 and L5 DRGs and their dorsal roots were placed in a chamber in which the ganglia could be superfused with artificial cerebrospinal fluid (ACSF) at 37 °C[2]. Spontaneous ectopic discharge (SED) occurred in about 9% of the myelinated fiber population. SED originated within the ganglion because it continued after the spinal nerve was cut a few millimeters distal to the DRG but not after the dorsal root was transected just proximal to the ganglion.
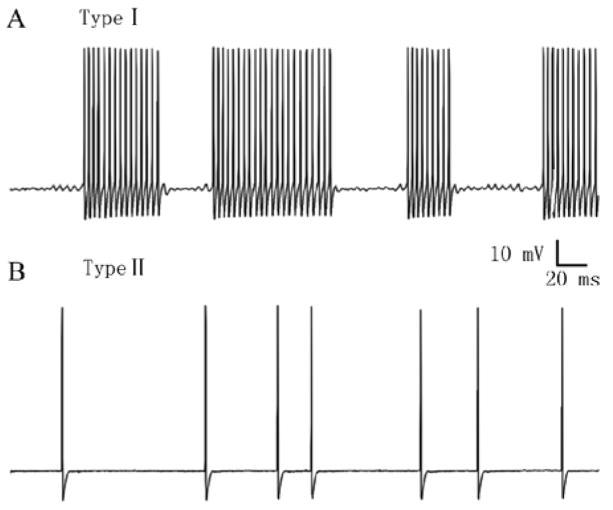
To examine the membrane properties of the hyperexcitable neurons, intracellular recordings were obtained, in vitro, from visualized somata on the surface of the intact DRG superfused with ACSF at 37 °C[3, 4]. Viewed through a microscope these somata were classified as large (> 45 μm diameter), medium (30–45 μm) or small (< 30 μm) in size and typically having, respectively, thickly, thinly myelinated and unmyelinated axons as indicated by measurements of axonal conduction velocity. In comparison with somata of sham operated or unoperated DRGs, those of each size category from formerly compressed ganglia exhibited lowered thresholds for action potential generation (in response to current injection through the recording electrode) and decreased accommodation (more impulses in response to a suprathreshold current pulse). Approximately 10% of the CCD neurons in each size category in the intact DRG exhibited SED. The SED could be classified into one of two types (Fig. 1) according to whether a sub-threshold membrane potential oscillation (SMPO) was present (type I, occurring in approximately 60% of the neurons with SED) or absent (type II, approx. 40%)[5]. Neurons with type I SED had lower current thresholds. The rate of SED for type I neurons, in contrast to that of type II, was higher and could be increased by depolarizing the membrane potential by current injection. Thus, it was hypothesized that SED originated in the soma for type I and in the axon for type II. In support of this, only type I remained after DRG neurons were dissociated (loosing their axons) and placed in culture.
Fig. 1.
In chronically compressed DRG neurons, the spontaneous ectopic discharge can be classified into two types according to the presence (type I) or absence (type II) of a sub-threshold membrane potential oscillation. A: Type I spontaneous activity in a neuron. B: Type II spontaneous activity in another neuron. Both of these neurons had large-diameter somata and were recorded with intracellular (sharp) electrodes from the intact DRG-nerve preparation, in vitro.
Electrophysiological recordings were obtained from dissociated DRG neurons in acute or one day culture under the same conditions as in the intact ganglion, in vitro (intracellular recording, superfusion with ACSF at 37 °C)[6]. The overall incidence of SED was higher for CCD than for control neurons after one day culture (about 10 % vs 2%), approximately the same as that obtained in the intact DRG. The current thresholds of medium and large sized neurons (both acute and one day culture) were similar to those obtained from intact DRGs, in vitro, and significantly lower for CCD than for control rats. But the current thresholds of small sized neurons from control DRGs were significantly lower after dissociation (acute or one day) than they were in the intact ganglia and about the same as those obtained from dissociated CCD neurons. Thus, the characteristics of CCD induced somal hyperexcitability in medium and large neurons in the intact DRG were preserved after dissociation and probably intrinsic to the soma and not dependent on factors released from neighboring cells. CCD effects on small-sized neurons in the intact DRG were more difficult to determine after dissociation due to the hyperexcitability-inducing effects of the dissociation process itself.
Further experiments were carried out to investigate the contribution of different ionic currents to CCD-induced excitability. Whole-cell patch-clamp recordings were obtained from acutely dissociated, cutaneous medium-sized neurons, retrogradely dye-labeled from the hind paw. To date, the following currents have been investigated: The hyperpolarization-activated current (Ih)[7]; tetrodotoxin-sensitive and insensitive sodium current (TTX-S Na+ and TTX-R Na+, respectively), the fast-inactivating K+ current (Ka), and the sustained current, Kdr[8]. In comparison with control, CCD significantly increased the current density and rate of activation of Ih, shifted the steady-state activation curves for TTX-S Na+ current in a hyperpolarizing direction, enhanced peak TTX-R Na+ current and reduced Ka current. Since Ih activation provides a depolarizing current to the neuron, an increase of Ih can enhance neuronal excitability. Taken together, these changes may contribute to the decrease in current threshold and/or accommodation that occur after CCD. The initial signals and cellular pathways causing these effects are under study.
3 CCD enhanced the responses of DRG neurons to inflammatory mediators
We tested whether DRG neurons become more excitable to inflammatory mediators after the CCD injury. An inflammatory “soup” (IS) consisting of bradykinin, serotonin, prostaglandin E2 and histamine (each 10−6 mol/L) was topically applied either to neuronal somata in the intact DRG or to those that had been acutely dissociated. Either IS or one of its components is known to excite subpopulations of cutaneous neurons in vivo[9]. We found that IS produced a small depolarization of the resting potential of most neurons in both control and CCD ganglia including those in each size category and with nociceptive and non-nociceptive properties[4]. Interestingly, the magnitude of response to IS was not significantly different between CCD and control neurons. But because CCD neurons were more excitable than controls they were more likely to exhibit action potential discharges. In addition, IS reduced accommodation in CCD neurons but not in control neurons. Because the excitatory effects of IS were found not only in the intact DRG but also in dissociated neurons that were not in the immediate proximity of satellite cells or other neurons, they are likely intrinsic to the neuron.
More surprising was the finding that 5 days after CCD many neurons exhibited a novel upregulation of an inflammatory mediator and its receptor, namely the chemokine, monocyte chemoattractant protein-1 (MCP-1 or CCL2) and also its receptor, CCR2[10]. MCP-1 is known for its ability to recruit monocytes to sites of hypoxia, ischemia, trauma infection and toxin exposure and is implicated in many inflammatory diseases such as multiple sclerosis, rheumatoid arthritis and atherosclerosis[11]. MCP-1-immunoreactivity, while not detectable in unoperated or sham-operated DRGs was readily apparent at 5 days in approximately 29% of the neurons (somata) in the compressed L4 and L5 DRGs and in about 21% of neurons in the adjacent, non-compressed ganglia (L3 and L6). These were predominantly neurons of small and medium size but included some of large diameter as well. MCP-1 immunoreactivity was not detectable in non-neuronal cells in the compressed or non-compressed DRGs. In situ hybridization for CCR2 mRNA revealed an upregulation of label of 41% to 49% of the neuronal cell bodies (small, medium and large sized) in the compressed DRGs and also with slightly lesser percentages in neurons in the adjacent non-compressed ganglia. CCR2 mRNA label was readily apparent in non-neuronal cells (thought to be primarily satellite glia) both in the compressed, and, to a lesser extent, in the non-compressed DRGs.
In response to topically applied MCP-1, many CCD neurons, unlike control neurons, exhibited an excitatory response such as a depolarization of the membrane potential (sometimes leading to action potentials), or, in response to current injection from the recording electrode during MCP-1 application, a decrease in current threshold and an increase in action potential duration. Patch-clamp recordings from acutely dissociated CCD neurons suggested that the MCP-1 induced depolarization was mediated by a non-specific cation conductance whereas the prolongation of the action potential may have resulted from a decreased, sustained, voltage-dependent K+ current[12]. We hypothesize that action potentials elicited in CCD neurons containing MCP-1 may result in the release of MCP-1 as a neurotransmitter, not only in the central and peripheral nerve endings, but also within the DRG where it may act in a cellular autocrine or paracrine manner to increase neuronal, somal hyperexcitability.
Presently, one can only speculate as to the mechanisms by which MCP-1 and CCR2 are upregulated. In the compressed DRG, perhaps factors such as tumor necrosis factor alpha (TNFα), commonly associated with nerve injury and also with MCP-1 induction, may play a role[13]. As for the adjacent, uncompressed ganglia, perhaps their cell bodies are activated by chemical factors released locally from injured DRGs, or their central terminals are activated by chemicals released from adjacent terminals of injured afferents or from activated spinal glia. Activation of the central terminals of these uninjured afferents might result in retrograde abnormal nerve impulse activity in the form of dorsal root reflexes[14] or the transport of cytokines resulting in MCP-1/CCR2 upregulation in uninjured DRGs[10].
4 CCD activated immune cells
The recruitment of immune cells was indicated in the compressed ganglia by the presence of neutrophils but only within the first 24 h (Fletcher White, unpublished observations). This was followed in a few days by an influx of macrophages (labeled by the marker, ED-1) that became numerous by postoperative day 5 in the compressed DRG[10]. The macrophages were observed in the vicinity of neuronal cell bodies of all sizes. In contrast there were few macrophages in the adjacent, non-compressed ganglia (L3 and L6) or in the ganglia of sham-operated and naive rats.
5 CCD activated satellite glia in the DRG
Satellite glia cells (SGCs) in the DRG may play a role in cellular responses to ischemia, injury and inflammation. Relatively little is known about the function of the SGCs in the DRG. Immunostaining for glial fibrillary acidic protein (GFAP) revealed that the SGCs surrounding each neuronal cell body in the DRG begin to show signs of activation within 6 h of the onset of CCD, reaching a peak within 3 days and disappearing by the end of the second week[15]. These results raise the possibility that SGCs in the DRG are involved in the early immune/inflammatory response to compression and may contribute to the neuronal excitability and pain behavior in CCD animals. For example, the time course of GFAP activation corresponded roughly to the time course of allodynia after CCD. However, any causal relationship between these events has yet to be determined.
Whole-cell patch-clamp recordings were obtained form individual SGCs in the intact DRG, in vitro. The patch pipette was filled with Lucifer Yellow to test for the presence of coupling from one cell to the next. In control ganglia, the dye was typically confined to a single SGC and only occasionally spread to an adjacent glial cell associated with the same neuron, presumably though a gap function connection. But 7 days after CCD, there was a 3-fold increase in coupling between SGCs associated with the same neuron resulting in a decrease in input resistance and an increase in capacitance[16]. Under carbenoxylone, which blocked gap junctions and the spread of the dye, a cesium-sensitive, inwardly-rectifying potassium current, Kir, was isolated. SGCs of CCD ganglia had significantly lower Kir than those of naive DRGs (Haijun Zhang, unpublished observations). It is possible that this decrease may contribute to a lesser capacity to take up potassium released by neurons during intense firing after CCD thereby contributing to an increased excitability. The increased coupling between glia may serve to distribute neurotransmitter substances released by neurons to neighboring neurons and endothelial cells of the capillaries. A similar increase in functional coupling occurs between SGDs in the DRG after a peripheral nerve injury[17].
6 CCD activated endothelial cells in the DRG
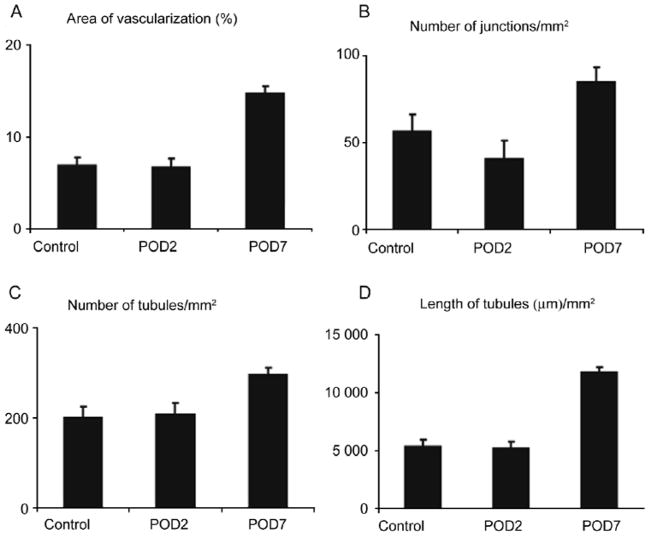
The DRG has an extensive network of capillaries connecting with blood vessels from both the peripheral nerve and dorsal root to provide the blood supply to the neuronal cell bodies. The endothelial cells of the capillaries are in close proximity to the SGCs which immediately surround every neuronal soma within the DRG. The endothelial cells are activated by CCD as evidenced by angiogenesis in the form of an increased number of capillaries in the DRG. Compared with naive, control ganglia, the area of vascularization, the number of vascular junctions, and the lengths of capillaries all significantly increased within compressed DRGs 7 days, but not 2 days, after the onset of CCD[18] (Fig. 2, also, Xiaofeng Mei, unpublished observations). These results suggest that chronic compression can induce angiogenesis within the DRG possibly in response to persistent ischemia/hypoxia and inflammation. The mechanisms underlying angiogenesis require further study, including the measurement of hypoxia and related growth factors such as vascular endothelial growth factor (VEGF).
Fig. 2.
Chronic compression induced angiogenesis in the DRG. Compared with naive, control ganglia (control), the area of vascularization (A), number of vascular junctions (B), number of capillaries (C), and total lengths of capillaries (D) all significantly increased within compressed DRGs 7 days (POD7), but not 2 days (POD2) after CCD surgery. Each bar provides a mean±SEM.
7 Hypothesis: Neuronal hyperexcitability may facilitate cell survival in the DRG
In addition to their role as sensory organs, primary sensory neurons have secretory, trophic functions. A sub-population of nociceptive afferents that is capsaicin sensitive is capable of releasing transmitter chemicals centrally that can sensitize second order neurons (“central sensitization” responsible for secondary hyperalgesia)[19, 20] and peripherally (e.g. via dorsal root reflexes) to produce flare and edema (neurogenic inflammation)[21]. It seems possible that sensory neurons also transduce and release neurotransmitters and other chemical messengers from the cell body within the DRG as evidenced by studies of underlying mechanisms of neurogenic inflammation in dissociated DRG neurons in vitro[21]. Indirect evidence for neurogenically mediated chemical release and transduction, in vivo, is provided by the phenomenon of cross-depolarization whereby activity in a stimulated set of afferents leads to the depolarization of neighboring, unstimulated neurons in the DRG[22].
The CCD-induced hyperexcitability of DRG neurons may enhance the capacities of the neuronal somata to sense as well as to release chemical mediators in the ganglion. There is an increased incidence of spontaneous, ectopic discharges; and, silent neurons are more likely to exhibit action potentials in response to inflammatory chemicals. MCP-1, upregulated in DRG neurons after CCD, may be transported to, and released at distal and proximal nerve endings where it might act as a neuromodulator or as an attractant of immune cells. MCP-1 may also be released in the DRG.
The upregulation of MCP-1 may enhance the survival of injured cells in the DRG after CCD. Its appearance 5 days after onset of CCD is correlated with the onset of angiogenesis. MCP-1 is well known as an angiogenic chemokine. In human aortic endothelial cells, MCP-1 induced vascular endothelial growth factor-A (VEGFA) gene expression and VEGFA secretion which, in turn, caused MCP-1 to be secreted[23]. Both MCP-1 and VEGF induce proliferation and migration of vascular endothelial cells. VEGF is present in a subpopulation of DRG neurons[24] and may contribute to CCD induced angiogenesis. MCP-1 may have other protective features as well. For example, MCP-1 protects human neurons and astrocytes from the toxic effects of glutamate[25] and protects cardiac myocytes from hypoxia-induced apoptosis[26].
We hypothesize that somal hyperexcitability, in addition to its negative role in contributing to neuropathic pain, may make a positive contribution by facilitating the release of ligands like MCP-1 which act to preserve injured cells in the face of ischemia and hypoxia, for example, by promoting angiogenesis. Thus, somal hyperexcitability, as often said of inflammation, may represent a double edged sword.
Acknowledgments
This work was supported by National Institute of Health grant NS14624.
References
- 1.Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 2.Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82:3347–3358. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- 3.Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]
- 4.Ma C, Greenquist KW, LaMotte RH. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol. 2006;95:2098–2107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- 5.Ma C, Sun JH, Tan Z, LaMotte RH. Two types of spontaneous activity in neurons of the chronically compressed dorsal root ganglion. Soc Neurosci Abstr. 2004 doi: 10.1523/JNEUROSCI.3699-07.2007. Program No 298.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma C, LaMotte RH. Enhanced excitability of dissociated primary sensory neurons after chronic compression of the dorsal root ganglion in the rat. Pain. 2005;113:106–112. doi: 10.1016/j.pain.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Yao H, Donnelly DF, Ma C, LaMotte RH. Upregulation of the hyperpolarization cation current after chronic compression of the dorsal root ganglion. J Neurosci. 2003;23:2069–2074. doi: 10.1523/JNEUROSCI.23-06-02069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan ZY, Donnelly DF, LaMotte RH. Effects of a chronic compression of the dorsal root ganglion on voltage-gated Na+ and K+ currents in cutaneous afferent neurons. J Neurophysiol. 2006;95:1115–1123. doi: 10.1152/jn.00830.2005. [DOI] [PubMed] [Google Scholar]
- 9.Lang E, Novak A, Reeh PW, Handwerker HO. Chemosensitivity of fine afferents from rat skin in vitro. J Neurophysiol. 1990;63:887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- 10.White FA, Sun JH, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, LaMotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charo IF, Peters W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation. 2003;10:259–264. doi: 10.1038/sj.mn.7800191. [DOI] [PubMed] [Google Scholar]
- 12.Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- 13.Subang MC, Richardson PM. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur J Neurosci. 2001;13:521–528. doi: 10.1046/j.1460-9568.2001.01425.x. [DOI] [PubMed] [Google Scholar]
- 14.Peng YB, Wu J, Willis WD, Kenshalo DR. GABAA and 5-HT3 receptors are involved in dorsal root reflexes: possible role in periaqueductal gray descending inhibition. J Neurophysiol. 2001;86:49–58. doi: 10.1152/jn.2001.86.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Mei XF, Ma C, White FA, LaMotte RH. Upregulation of glial fibrillary acidic proteinin satellite glia cells in the chronically compressed dorsal root ganglion. Soc Neurosci Abstr. 2005 Program No. 511.22. [Google Scholar]
- 16.Zhang H, Donnelly DF, Ma C, Tan Z, LaMotte RH. Chronic compression of the rat dorsal root ganglion causes increased coupling of satellite glial cells but no change in the kinetics of evoked membrane currents. Soc Neurosci Abstr. 2005 Program No. 511.21. [Google Scholar]
- 17.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 18.LaMotte RH, Mei X, Zhang P, Ma C. Angiogenesis in the chronically compressed dorsal root ganglion. Soc Neurosci Abstr. 2007 Program No. 285.17. [Google Scholar]
- 19.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 20.LaMotte RH, Shain CN, Simone DA, Tsai E. Neurogenic hyperalgesia: Psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 21.Richardson JD, Vasko MR. The cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 22.Amir R, Devor M. Chemically-mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1–induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 24.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eugenin EA, Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;5:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 26.Tarzami ST, Calderon TM, Deguzman A, Lopez L, Kitsis RN, Berman JW. MCP-1/CCL2 protects cardiac myocytes from hypoxia-induced apoptosis by a Gαi-independent pathway. Biochem Biophys Res Commun. 2005;335:1008–1016. doi: 10.1016/j.bbrc.2005.07.168. [DOI] [PubMed] [Google Scholar]