Abstract
Startle reflex studies in rodents indicate that female are more reactive than rats in experimental models of sustained anxiety but not in models of phasic fear (Toufexis, 2007). This study examined evidence for a similar effect in humans. Participants were exposed to three conditions, (1) predictable aversive shocks signaled by a cue, (2) unpredictable shocks, and (3) no shocks. Acoustic startle stimuli were delivered regularly across conditions. Phasic startle potential to the threat cue in the predictable condition was not affected by sex. In contrast, and consistent with basic research, the sustained increase in startle in the predictable and unpredictable conditions was greater in women compared to men. Animal studies suggest that such an effect may be mediated by the effects of sexual dimorphism in limbic structures, including the bed nucleus of the stria terminalis. However, psychosocial factors may also contribute to this effect.
Keywords: fear, anxiety, sex difference, startle, unpredictability
The greater prevalence of mood and anxiety disorders among women compared to men (Kessler et al., 1994) points to a potential sex difference in reactivity to threat. Preclinical studies may provide clues as to the nature of this difference. A recent review focusing on animal models, using the startle reflex as an operational measure of aversive states, compared sex differences in two types of aversive responses, anxiety, a sustained state of distress to uncertain threat, and fear, a phasic defensive response to certain danger (Toufexis, 2007). Contrasting phasic and sustained aversive states was motivated by evidence of a neural differentiation between them, the former relying on the central nucleus of the amygdala (CeA) and the latter on the bed nucleus of the stria terminalis (BNST; Davis, 1998), as well as by an indication of strong sexual dimorphism in the BNST (Allen & Gorski, 1990). Toufexis concluded that BNST-mediated sustained potentiated startle (anxiety), but not cued specific fear-potentiated startle (fear), was increased in female rats compared to males.
The present study examined evidence for a similar sex difference in humans, also using startle as a measure of aversive states. We have distinguished phasic fear to a predictable threat signaled by a cue from the more sustained contextual anxiety state during administration of predictable (certain) and unpredictable (uncertain) shocks (Grillon et al., 2006; Grillon, Baas, Lissek, Smith, & Milstein, 2004a). Based on Toufexis's review (Toufexis, 2007), we hypothesized that relative to men, women would show enhanced contextual-potentiated startle but not increased fear-potentiated startle to a threat cue.
Method
Participants
Participants were 18 men and 18 women with a mean age of 26.6 (SD = 7.2) years and 26.7 (SD = 7.2) years, respectively, who gave written informed consent approved by the NIMH Human Investigation Review Board. Inclusion criteria included (a) no past or current psychiatric disorders as per Structured Clinical Interview for DSM–IV (First, Spitzer, Williams, & Gibbon, 1995), (b) no medical condition that interfered with the objectives of the study as established by a physician (e.g., tachycardia), and (c) no use of illicit drugs or psychoactive medications as per urine screen.
Stimuli and Apparatus
Stimulation and recording were controlled by a commercial system (Contact Precision Instruments, London, Great Britain). The acoustic startle stimulus was a 40-ms duration, 103 dB (A) burst of white noise with a near instantaneous rise time presented binaurally through headphones. The startle reflex was recorded with two 6 mm tin electrodes placed under the left eye. Amplifier bandwidth was set to 30 to 500 Hz with a sampling rate of 1,000Hz. The intensity of the shock was selected based on a shock workup procedure during which participants received three shocks. The 100-ms duration shock did not differ between sex, t(34). It was 4.5 (.6) and 4.7 (24.4) in the women and men, respectively.
Design
The experiment consisted of three different conditions, a no shock condition (N), and two conditions during which shocks were administered either predictably (P), that is, only in the presence of a threat cue, or unpredictably (U; Grillon, Baas, Lissek, Smith, & Milstein, 2004b). Each condition lasted approximately 150 s. In each 150-s condition, an 8-s cue was presented four times. The cues were different geometric colored shapes in the different conditions (e.g., blue square for N, red circle for P). The cues signaled the possibility of receiving an aversive stimulus only in the P condition, but had no signal value in the N and U conditions. Participants were verbally instructed regarding the risk of shock in the different conditions and they were informed of the contingency or lack of contingency between shock and cues in the P and U conditions. In addition, the following instructions were displayed on a computer monitor throughout the experiment: “no shock” (N), “shock only during shape” (P), or “shock at any time” (U). During each predictable and unpredictable condition, one shock was administered, during the cue in the predictable condition and in the absence of the cues in the unpredictable condition. In each N, P, and U condition, six acoustic startle stimuli were delivered, three during intertrial intervals (ITI; i.e., between cues) and one during three of the four cues, 5 to 7 s following cue onset. The threat experiment consisted of two recording blocks with a 5 to 10 min rest between blocks. Each block started with the delivery of six startle stimuli (pretest startle) and consisted of three N, two P, and two U conditions in one of the following two orders: P N U N U N P or U N P N P N U. Each participant was presented with the two orders, with half the participants starting with the P condition. One shock was administered in each individual P and U condition for a total of four shocks in the four P conditions and four shocks in the four U conditions. The shock was delivered 7.5 s following cue onset in the P condition. It was administered either 7 s or 10 s following cue offset in the unpredictable condition. No startle stimuli could follow a shock by less than 10 s.
Following each block, participants were asked to rate retrospectively their level of anxiety/fear during each condition (including during cue and ITI) on a scale from 0 (to at all anxious) to 10 (extremely anxious).
Data Analysis
Peak amplitude of the blink reflex was determined in the 20 to 100-ms time frame following stimulus onset relative to baseline (average baseline EMG level for the 50 ms immediately preceding stimulus onset) and averaged within each condition. The startle magnitude and subjective rating of anxiety/fear data were averaged across conditions and separately for cues and ITI over the two blocks. Because of our a priori hypothesis, we conducted two separate ANOVAs, one for the threat cue and the other for context, as we have done in the past (Grillon et al., 2006; Grillon, Levenson, & Pine, 2007). Responses to the cues were calculated as the difference in startle magnitude or subjective rating between cue and ITI in each threat condition. These difference scores were entered in a Sex (men/women) × Condition (N/P/U) analysis of variance (ANOVA). We predicted a condition main effect due to greater startle and subjective anxiety/fear in the P condition, compared to the N and U conditions, but not Sex × Condition interaction. The contextual data were analyzed using the ITI data in a Sex (men/women) × Condition (N/P/U) ANOVA. We predicted significant condition main effect due a linear increase in startle reactivity and in subjective rating of anxiety/fear from the N to the P to the U (Grillon et al., 2006; Grillon, Levenson, et al., 2007). We also predicted that, due to enhanced contextual anxiety in the women, the slope of this linear trend would be greater in women compared to men, resulting in a linear Sex × Condition interaction (Grillon et al., 2006; Grillon, Levenson, et al., 2007). Alpha was set at .05 for all statistical tests. Greenhouse–Geisser corrections (GG-ε) were used when appropriate.
Results
Startle Magnitude
Figure 1 (top) presents the fear-potentiated startle data, that is, the increase in startle during cue compared to ITI. As expected fear-potentiated was greater in the P condition, when the cue signaled the possibility of a shock, compared to the N and U conditions, F(2, 68) = 14.7, p < .0009, ε = .93. This effect did not differ between sex, F(2, 68) = .8, ns. A restricted analysis of the P condition data confirmed this lack of sex difference, F(1, 34) = .2, ns.
Figure 1.
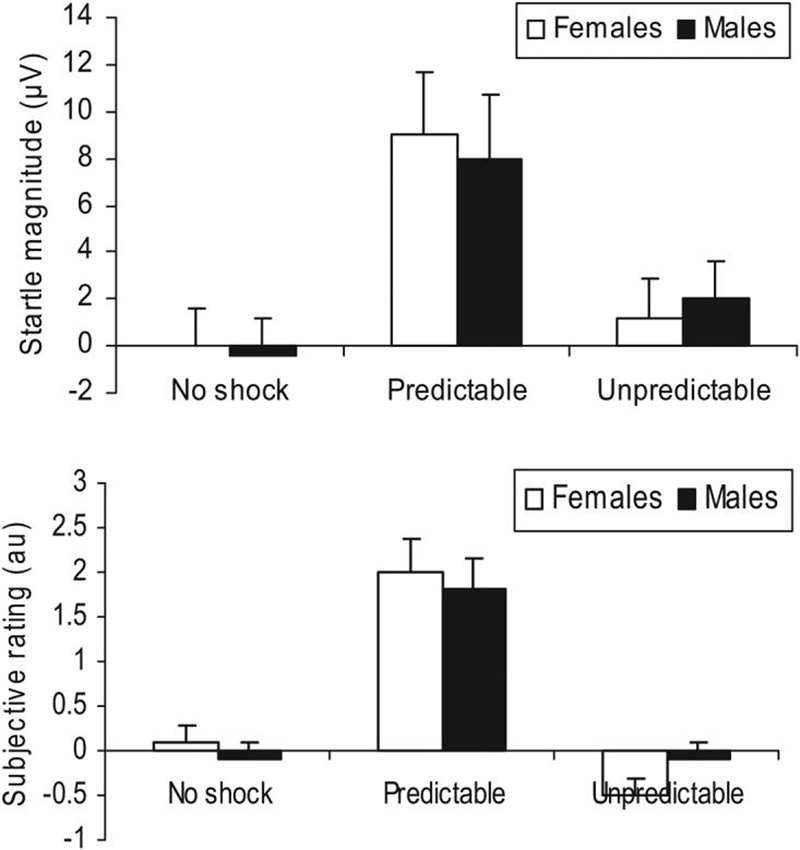
Startle magnitude (top) and subjective report of fear/anxiety in arbitrary unit (au; bottom) during the cue relative to intertrial interval (ITI) in men and women.
Figure 2 (top) shows the context-potentiated startle results, that is, startle magnitude during ITI. As reported previously (Grillon et al., 2006; Grillon, Levenson, et al., 2007), startle magnitude increased progressively from the N, to the P, to the U condition, Condition: F(2, 68) = 36.4, p < .0009, ε = .83, partial η2 = .52, observed power = 1; Condition linear trend: F(1, 34) = 47.8, p < .0009, partial η2 = .58, observed power = 1. This effect was greater in women compared to men, Condition × Sex: F(2, 68) = 3.5, p < .04, ε = .83, partial η2 = .09, observed power = .6; Condition × Sex linear trend: F(1, 34) = 4.3, p < .04, partial η2 = .11, observed power = 53.
Figure 2.
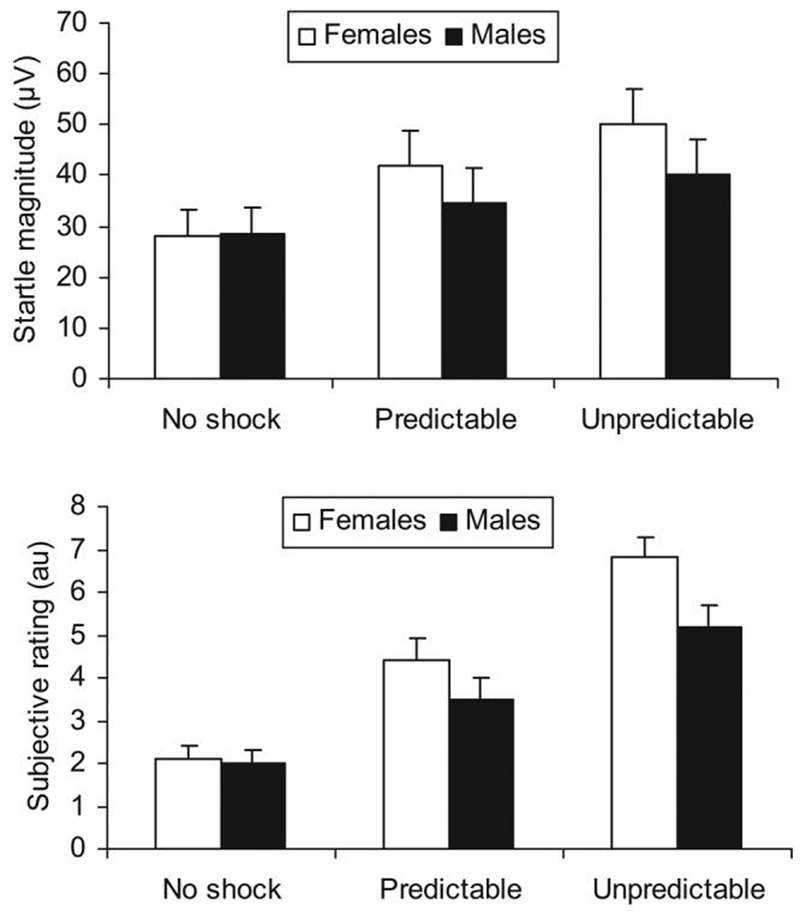
Startle magnitude (top) and subjective report of fear/anxiety in arbitrary unit (au; bottom) during ITI in men and women. Startle magnitude during ITI is a measure of sustained anxiety elicited by the experimental context.
Subjective Ratings
Figure 1 (bottom) presents the changes in subjective rating of anxiety/fear during the cues minus ITI. As expected participants felt more fearful when the cue signaled the possibility of a shock in the P condition, compared to the N and U conditions, F(2, 68) = 45.7, p < .0009, ε = .98. This effect did not differ between sex, F(2, 68) = .9, ns. A restricted analysis of the P condition data confirmed this lack of sex difference, F(1, 34) = .4, ns.
Figure 2 (bottom) shows the subjective rating in the absence of the cues. Levels of anxiety increased progressively from the N, to the P, to the U condition, Condition: F(2, 68) = 86.6, p < .0009, ε = .92, partial η2 = .72, observed power = 1; Condition linear trend: F(1, 34) = 161.2, p < .0009, partial η2 = .83, observed power = 1. This effect was greater in women compared to men, Condition × Sex: F(2, 68) = 2.8, p < .07, ε = .92, partial η2 = .08, observed power = .53; Condition × Sex linear trend: F(1, 34) = 5.2, p < .03, partial η2 = .13, observed power = .6.
Discussion
The present results found no sex difference in fear-potentiated startle to a threat cue, but did find greater contextual-potentiated startle magnitude among women compared to men. This sex difference was paralleled by similar results in subjective ratings of fear and anxiety. These findings are consistent with Toufexis' (2007) conclusion following a review of defensive responses in rodents based on startle. They are also in agreement with ethological studies showing that female rats are more anxious than male rats in situations involving potential threat (cat odor), as opposed to actual and present threat (cat) (Blanchard, Shepherd, De Padua CaroBrez, & Blanchard, 1991), suggesting that these findings may be generalizable to other models of anxiety.
It is unclear whether this sex difference is due to biological or psychosocial factors. Touxefis's(2007) review focused on sex hormones. Less anxiety in men could be due to the inhibitory effect of testosterone on anxiety (Toufexis, 2007) or to women's ovarian hormones, such as estrogen and progesterone, which affect different aspects of fear and anxiety. In ovariectomized rats, progesterone, but not estrogen, reduces corticotrophin-releasing factor-induced potentiation of startle, suggesting that progesterone is inhibitory in this model of sustained anxiety (Toufexis, 2007). However, in another model of anxiety not based on startle, the open-field test, estrogen-treated but not progesterone-treated ovariectomized rats show reduced defensive responses (Hiroi & Neumaier, 2005). These results point to a complicated effect of ovarian hormones on anxiety, possibly depending on the nature of the test. Among other biological factors, genetic mechanisms unrelated to hormone effects may underlie sexual differentiation in behavior and brain function. For example, the BNST, a region involved in the sustained potentiation of startle is sexually dimorphic (Allen & Gorski, 1990).
Although biological mechanisms could potentially explain the sex difference found in this study, various psychosocial factors (e.g., developmental, cognitive, emotional) should also be considered. These include the influence of maternal behavior on affective reactions, which can differ for male and female offspring (Burnham & Harris, 1992; Reid, 1994). Sex difference in anxiety and in coping strategies may also underlie the present findings (Cole & Sapp, 1988; Mak, Blewitt, & Heaven, 2004). Women tend to have higher levels of anxiety sensitivity than men (Peterson & Reiss, 1992; Stewart, Conrod, Gignac, & Pihl, 1998). Anxiety sensitivity is defined as the tendency to be fearful of anxiety-related sensation. It is possible that this tendency to catastrophize can manifest itself more easily during long (context) compared to short (cue) threat periods. Factors that have been shown to modulate startle, such as negative affectivity, could mediate the present sex difference in contextual anxiety. Although evidence for gender differences in negative affectivity is inconsistent, women tend to score higher on negative affectivity than men (Jex, Adams, & Ehler, 2002; Watson, Clark, & Tellegen, 1988). Of course, biological and psychosocial factors may interact; sex differences in threat appraisal contribute to sex differences in HPA-axis responses to stressors (Rasmusson & Friedman, 2002).
Results should be interpreted in the context of the strengths and limitations of this study. A major strength was that this study relied on previous research in animal suggesting sex difference in reactivity to threat (Toufexis, 2007). The major limitation was that the menstrual phase of the women was not assessed. A previous study did not find that the phase of the menstrual cycle affected the affective modulation of startle to emotional pictures in healthy women (Epperson et al., 2007). Whether potentiated startle to shock threats is affected by menstrual cycle is unknown and should be investigated in future studies.
This study has several implications. Practically, the results points to the need to consider participant sex when studying fear and anxiety. It validates the present experimental model based on the cross-species startle reflex to elucidate mechanisms of sex differences in anxiety. At a theoretical level, the findings suggest that women are less able to adapt to contextual threat and unpredictability. Unpredictability is a central variable in theories of mood and anxiety that determines individual susceptibility to anxiety (Foa, Zimbarg, & Rothbaum, 1992; Mineka & Kihlstrom, 1978). The present findings are significant in that they add to a host of data consistently pointing to the role of contextual anxiety in pathological anxiety. Contextual anxiety, not cued fear, is elevated in clinical anxiety (Grillon, Ameli, Goddard, Woods, & Davis, 1994; Grillon & Morgan, 1999; Pole, Neylan, Best, Orr, & Marmar, 2003) and is reduced by the benzodiazepine alprazolam (Grillon et al., 2006). Risks for anxiety disorders include a family history of anxiety disorders, prior stress, and being a woman. These three risk factors have now been linked to contextual anxiety. Contextual anxiety is increased in nonaffected daughters of parents with anxiety disorders (Grillon, Dierker, & Merikangas, 1998). Prior stress increases contextual anxiety (Grillon, Duncko, Covington, Kopperman, & Kling, 2007). The elevated sensitivity of women to contextual anxiety may contribute to the higher risk of anxiety disorders in women compared to men. Future studies examining relations between sustained anxiety, hormones, and phases of the menstrual cycle in women as well as coping strategies are warranted.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Mental Health. C. Grillon reports no competing interests.
References
- Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. Journal of Comparative Neurology. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Shepherd JK, De Padua Carobrez A, Blanchard RJ. Sex effects in defensive behavior, baseline differences and drug interactions. Neuroscience Biobehavioral Review. 1991;15:461–8. doi: 10.1016/s0149-7634(05)80132-0. [DOI] [PubMed] [Google Scholar]
- Burnham DK, Harris MB. Effects of real gender and labeled gender on adults' perceptions of infants. The Journal of Genetic Psychology. 1992;153:165–183. doi: 10.1080/00221325.1992.10753711. [DOI] [PubMed] [Google Scholar]
- Cole T, Jr., Sapp GL. Stress, locus of control, and achievement of high school seniors. Psychological Reports. 1988;63:355–359. doi: 10.2466/pr0.1988.63.2.355. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C. Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32:2190–2198. doi: 10.1038/sj.npp.1301351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RI, Williams JBW, Gibbon M. Structured Clinical Interview for DSM–V (SCID) American Psychiatric Association; Washington, DC: 1995. [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder, an animal model. Psychological Bulletin. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods S, Davis M. Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry. 1994;35:431–439. doi: 10.1016/0006-3223(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, et al. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescents offspring at risk for anxiety disorder. Biological Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Duncko R, Covington MF, Kopperman L, Kling MA. Acute stress potentiates anxiety in humans. Biological Psychiatry. 2007;62:1183–1186. doi: 10.1016/j.biopsych.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans, a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf war veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behavioral Brain Research. 2005;66:93–100. doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Jex S, Adams GA, Ehler ML. Assessing the role of negative affectivity in occupational stress research: Does gender make a difference? In: Nelson DL, Burke JB, editors. Gender work stress and health. American Psychological Association; Washington, DC: 2002. pp. 71–84. [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM–III–R psychiatric disorders in the United States. Archives General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Mak AS, Blewitt K, Heaven PCL. Gender and personality influences in adolescent threat and challenge appraisals and depressive symptoms. Personality and Individual Differences. 2004;36:1483–1496. [Google Scholar]
- Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events. A new perspective on experimental neurosis. Journal of Abnormal Psychology. 1978;87:256–271. doi: 10.1037//0021-843x.87.2.256. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Reiss S. Anxiety sensitivity index manual. 2nd ed. International Diagnostic Systems; Worthington, OH: 1992. [Google Scholar]
- Pole N, Neylan TC, Best SR, Orr SP, Marmar CR. Fear-potentiated startle and posttraumatic stress symptoms in urban police officers. Journal of Traumatic Stress. 2003;16:471–479. doi: 10.1023/A:1025758411370. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Friedman MJ. The neurobiology of PTSD in women. In: Kimerling R, Ouimette PC, Wolfe J, editors. Gender and PTSD. Guilford; New York: 2002. pp. 43–75. [Google Scholar]
- Reid GM. Maternal sex-stereotyping of newborns. Psychological Reports. 1994;75:1443–1450. doi: 10.2466/pr0.1994.75.3f.1443. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Conrod PJ, Gignac ML, Pihl RO. Selective processing biases in anxiety-sensitive men and women. Cognition Emotion. 1998;12:105–133. [Google Scholar]
- Toufexis D. Region- and sex-specific modulation of anxiety behaviours in the rat. Journal of Neuroendocrinology. 2007;19:461–473. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]


