Abstract
Advances in magnetic resonance spectroscopy (MRS) methodology and related analytic strategies allow sophisticated testing of neurobiological models of disease pathology in psychiatric disorders. An overview of principles underlying MRS, methodological considerations and investigative approaches is presented. A review of recent research is presented that highlights innovative approaches applying MRS, in particular 1H MRS, to systematically investigate specific psychiatric disorders, including autism spectrum disorders, schizophrenia, panic disorder, major depression and bipolar disorder.
Background
From its earliest days, psychiatry has sought to relate psychological processes to brain mechanisms. The development and research application of newer imaging modalities is beginning to make this goal feasible. Clinical applications of highly resolved 2-D and 3-D structural magnetic resonance imaging (MRI) techniques have demonstrated intriguing relationships between brain anatomical features and psychiatric disorders1 but, do not directly address mechanisms underlying these relationships. Delineation of structural abnormalities by MRI can help to focus research, but the factors that influence image intensity in conventional MRI are complex and image intensity changes alone cannot be used to infer specific pathological processes. Furthermore, although MRI is sensitive to changes in tissue water characteristics and defining structure at a macroscopic level, it is insensitive to much of cellular level organization.2 Invasive studies directly assessing brain tissue are not generally suited to the study of psychiatric illness. Investigations of peripheral metabolic or endocrinologic processes as an analog for the brain are substantially limited by the isolation of the brain from the bloodstream by the blood-brain barrier.3 Although animal studies using invasive techniques have attempted to bridge this gap, it is difficult to reach conclusions regarding human psychiatric disorders on the basis of animal observations.
Magnetic resonance spectroscopy (MRS) provides a non-invasive method for characterizing chemistry and cellular features in vivo. Prior to the availability of MRI, MRS had been developed and used extensively in vitro for the characterization of chemical samples. In fact, the development of MRI, based on detection of water distribution in tissue, was made possible by innovative extensions of existing MRS technology in liquids and solid state nuclear magnetic resonance techniques.4,5 When applied to living systems, MRS can be used to measure the chemical composition of tissues, characterize certain tissue metabolic processes, and identify unanticipated chemical or metabolic relationships to disease. In brain tissue, concentrations and mobility of MRS-visible low molecular-weight chemicals are measured as spectral peaks and can be used to detect abnormalities in brain regions that appear normal in MRI, as well as to elucidate pathology underlying MRI-visible abnormalities. Numerous studies have utilized MRI to study brain structural abnormalities associated with psychiatric disorders, but increasingly MRS is being applied to characterize tissue-based chemical or metabolic abnormalities in specific psychiatric disorders.
It is beyond the scope of this paper to describe the basic principles of MRS in detail, but comprehensive reference works are available.6,7 As a brief overview, certain atomic nuclei possess magnetic properties due to unpaired nucleons, protons or neutrons, that have potential psychiatric research applications, including hydrogen (1H), phosphorus (31P), lithium (7Li), fluorine (19F) and carbon (13C). It is important to note that these nuclei are nonradioactive and occur naturally, although often in small physiologic concentrations. When placed in a strong magnetic field the unpaired nucleons align their spins either parallel or antiparallel to the axis of the externally applied magnetic field (Bo). Hydrogen, for example, has two spin orientations with slightly different energy levels and spin population numbers. Other nuclei may have more than two spin orientations, or spin states. A difference in population numbers between spin states gives rise to a magnetic moment along the direction of the applied magnetic field. In addition to the vector sum of the population of nuclear spins, each individual spin aligns at some angle with respect to the magnetic field and rotates or “precesses” around it.
For a specific molecule, the MRS signal reflects a unique frequency of precession, the Larmor frequency, wherein nuclei within different chemical functional groups exhibit different resonance frequencies due to the unique magnetic shielding of local molecular electrons. Electron clouds shield each nucleus differently because the cloud is unique to the bond configuration within a particular molecule. Each nucleus experiences a slightly different local magnetic field and resonates at a slightly different frequency. The amount of shift in resonance frequency for a given nucleus in a particular molecule is termed its “chemical shift.” This unique chemical shift experienced by the same nucleus in different molecules is what makes MRS a useful investigative tool.
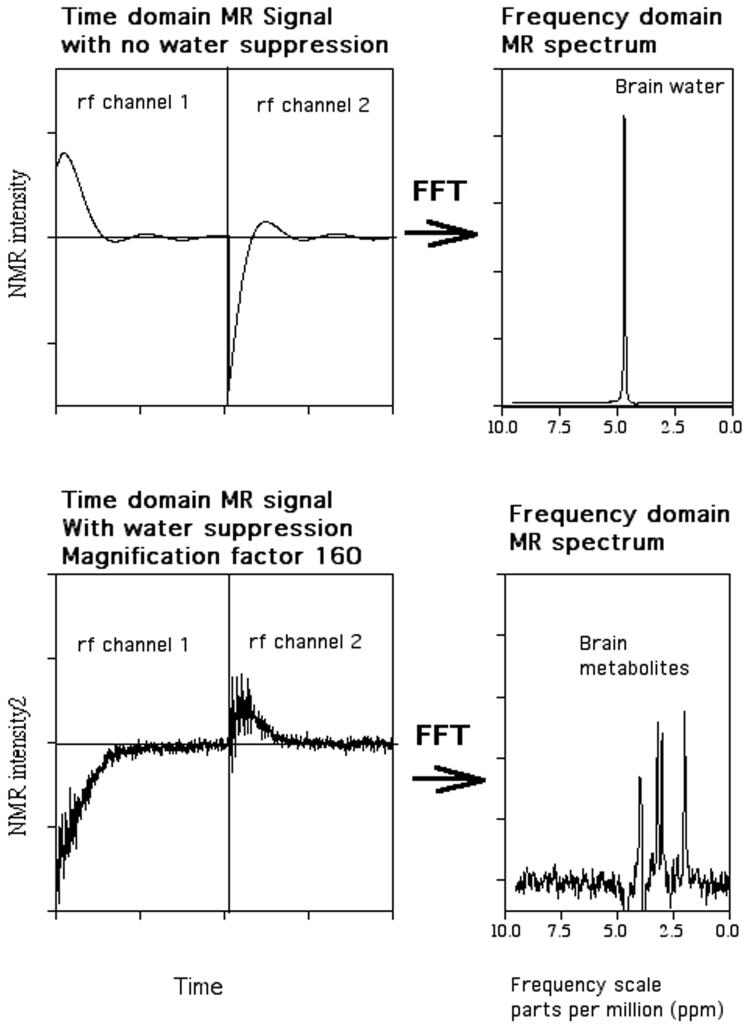
To obtain detectable MRS signals, nuclei at equilibrium in the magnetic field are perturbed by transmitting a radiofrequency (RF) pulse at the Larmor frequency that causes the spins to absorb and re-emit energy (resonate). During the absorption process the nuclear magnetization changes orientation (spin flip) and realigns itself with the magnetic field during re-emission of energy. The MRS signal, referred to as a free-induction decay, is acquired as a waveform over time, digitized and then Fourier transformed to obtain a frequency domain spectrum, shown in Figure 1. As the Larmor frequency for a given molecule is directly proportional to the magnitude of magnetic field strength, frequency measurements are expressed as chemical shift, a dimensionless quantity by convention given in units of parts per million (PPM), to allow standardization between magnets of differing field strengths. MRS metabolite levels are quantified by finding the area under the spectral peak using integration or curve-fitting techniques. Typically, these peak areas are referenced to an internal reference (eg., brain water or another brain chemical) or external phantom (e.g., test-tube placed next to the head) in order to follow/compare chemical levels within and between individuals. Coupling between the nuclei within a molecule gives rise to multiplet patterns (i.e. splitting of spectral lines) that are specific to the chemical bond structure between the nuclei. These multiplet patterns also change with the timing of specific data acquisition parameters, referred to as spectral editing methods,that can be used to reveal complex patterns that provide more specific information about the chemical bond structure and can allow some molecules not otherwise separable, such as GABA, to be measured.
Figure 1.
Time domain free induction decay (left-hand side) and frequency domain Fourier transform (right-hand side) of brain 1H MRS signal. The top and bottom panels demonstrate spectra without and with water suppression, respectively.
MRS applications to investigate 31P, 7Li, 19F and 1H- containing compounds have all been used to pursue psychiatric research questions, for example, 31P MRS investigations of pH regulation in panic disorder8,7Li determination of brain lithium uptake9, 19F MRS investigations of brain pharmacokinetics for fluorine-containing psychotropic drugs (eg, fluoxetine and fluvoxamine)10, and 1H MRS investigations of brain metabolic response to caffeine ingestion and the effects of tolerance.11 Psychiatric research applications using 1H MRS, are becoming more widely applied, in part due to methodological advances, and will be the main focus of this paper.
Quantifiable chemicals by 1H MRS include N-acetyl aspartate (NAA, most often measured as the total of NAA + N-acetyl aspartyl glutamate), creatine (Cre; composed of creatine and phosphocreatine), choline (Cho; includes multiple resonances primarily consisting of four membrane/myelin related chemicals: phosphorylethanolamine (PE), phosphorylcholine (PC), glycerophosphorylethanolamine (GPE), and glycerophosphorylcholine(GPC)), myo-Inositol (mI) and lactate (Lac). Glutamate (Glu), gamma-amino butyric acid (GABA), and glutamine (Gln) have complicated peak shapes and resonate at overlapping spectral locations, resulting in the use of GLX as a common description of the combined peaks.12 Though the individual resonances comprising GLX are difficult to separate due to complicated multi-peak shapes and broad baseline components (lipids/macromolecules), Glu can be isolated at higher magnetic field strength since spectral patterns separate with increasing field strength13 but, correction for T2 relaxation is necessary if absolute quantification is required. The extent to which glutamine can be distinguished from glutamate in 1H MRS studies is a current focus of technical development due to the presence of glutamate-glutamine neuronalastrocyte cycles that make this distinction important. Although the only true solution is to use very high field strength (>4T)14, there are recent MR spectroscopic approaches for separating glutamate from glutamine and other spectral editing needs using 2-D J-resolved MRS methods to measure J-coupled metabolites15. Similarly, although time consuming for data acquisition and technically challenging, GABA can be measured using spectral editing techniques.16
NAA, the most prominent 1H MRS spectral peak, is found only in the nervous system.17 Available data support a role for NAA as a sensitive marker for neuronal integrity or neuronal-glial homeostasis. For example, in a study of closed head injury sequelae, NAA levels were reduced in relation to severity of head trauma and predictive of clinical outcome.18 A complex NAA shuttle between neurons and oligodendrites suggests an important role for NAA in synaptic maintenance, myelination, the regulation of cellular osmolarity and neuronal metabolism.19-22 During normal development, and paralleling myelination, NAA levels increase dramatically during the first year of life, variably increase across different brain regions during the next 2 to 3 years, and then gradually plateau in early adulthood.12,23,24 This pattern of change in NAA is thought to reflect neuronal maturation due to increasing synaptic, dendritic and axonal projections. Cre levels also change during development, increasing in the brain from birth until about two years of age.12,23,24 During the first year of life, and also paralleling myelination, 1H MRS measured Cho concentration decreases rapidly as PE and PC decrease; in contrast, smaller magnitude increases of GPE and GPC occur.2,23 In disease states or traumatic brain injury, NAA and Cre generally decrease, whereas Cho levels typically increase, in relation to severity of neuronal injury, resulting from membrane and myelin breakdown adding to the measurable signal.18 The mI resonance, a singlet at 1.5 Tesla that also includes a contribution from syllo-inositol, is an important regulator of brain osmotic balance and also the precursor for phosphoinositides involved in the cellular membrane-based second messenger system.25 Although difficult to detect at rest in the normal brain at 1.5T, Lac has an important role in brain bioenergetics26 and is often elevated in the setting of inborn errors of metabolism or hypoxia, but also under conditions of moderate reduction in cerebral blood flow and/or increased brain metabolism as occurs, for example, in response to hyperventilation or caffeine ingestion.11,27 Small Lac elevations, in conjunction with other chemical alterations, such as GLX elevation, may also reflect subtle impairment of brain bioenergetics, such as a shift in redox state, providing a potential marker for mitochondrial compromise in specific psychiatric disorders, such as bipolar disorder.28 Glu and GABA, the major excitatory and inhibitory neurotransmitter, respectively, are of substantial interest in many clinical conditions, including seizure disorders. 29
MR Spectroscopy Quantification Techniques
A contentious but important topic concerns the optimal approach for analyzing and reporting MRS data. In vivo applications of MRS to quantify brain levels of measurable chemicals, such as NAA, choline and creatine, require the use of specialized software to fit the spectral peaks. At one time, particularly for initial in vitro applications, individual NMR peaks printed on paper were actually cut out and weighed in order to evaluate the relative peak area. An early advance was the use of peak heights as an estimate of chemical levels, initially using a ruler then fitting software that allowed Lorentzian/Gaussian peaks to be fit. Subsequently, the field has advanced so that chemical concentrations can be automatically extracted from MR spectra using sophisticated and well-documented time domain and spectral frequency fitting software packages such as: LCModel 30,31 or Magnetic Resonance User Interface (MRUI)32. These packages also account for the extreme baseline variation that occurs with very short-echo proton spectra of the brain. LCmodel has been directly compared with MRUI to assess the influence of signal-to-noise ratios (SNRs) and different linewidths on the accuracy and precision of the quantification results of proton metabolites found in the brain.33 Both approaches were found to be comparable when applied in their standard configuration (i.e., fitting a spline as a baseline for LCModel, and weighting the first data points for AMARES (part of MRUI) although the more accurate quantification of Glx favored the use of LCModel. Other approaches, such as SAGE (General Electric Medical Systems, Milwaukee, WI) can perform line fitting and have the capability of using time-domain filters, such as apodization and Fourier transformation, but have limited ability to correct for baseline variation.
Reporting of 1H MRS data initially favored the use of metabolite ratios (e.g., NAA/Cre) to adjust for SNR differences between studies. This approach further had the advantage of compensating for partial volume differences in individual voxels, primarily a concern for the CSF component and, to a lesser extent, also grey-white tissue metabolite differences. However, a drawback to this approach is the assumption that the ratio denominator (e.g., Cre) is not affected by the pathological processes under investigation, an assumption often not met. More recent approaches have utilized a separate water scan, using the unsuppressed water peak as an internal reference standard to control for SNR differences across studies. However, this approach requires the acquisition of an additional scan and also entails assumptions regarding the stability of the water peak. Using the water peak as an internal standard further necessitates correction for partial volume effects, frequently not performed in published studies. The LCModel fitting approach allows fitting in relationship to an external reference standard using basis sets consisting of known quantities of individual chemicals being measured, preferably scanned on the same scanner (an electronic basis set library is available for newer scanner platforms), but does not account for scanner drift over time, as the basis set is typically scanned at one point in time, nor individual subject differences in field inhomogeneity. 30,31 This latter approach additionally necessitates correction for partial volume effects. Other factors that can influence metabolite quantification, such as T1 and T2 relaxation differences in specific disease states are currently infrequently addressed but can be diminished using shorter TE.34 In our laboratory, we currently use LCModel for spectral fitting in combination with internal water referencing and partial volume correction to determine metabolite concentrations.
Advances in Magnetic Resonance Spectroscopy
Critical to psychiatric applications, recent technical advances have led to substantial improvement in 1H MRS spatial and temporal resolution. In general, a direct trade-off occurs between spatial and temporal resolution, with increased spatial resolution achieved at the expense of temporal resolution and vice-versa. Spatial or temporal resolution is a function of signal-to-noise ratio (SNR)/unit time and unit volume (i.e. a function of field strength and coil sensitivity, which is also spatially dependent), metabolite concentration, multiplet pattern and spectral resolution. Earlier applications of 1HMRS requiring good temporal resolution for measuring metabolites at low concentration, such as lactate, could necessitate voxel sizes as large as 27cc.35 For non-proton MRS such as 31P MRS, the spatial resolution, even at higher field strength and for longer acquisition times, generally remains very coarse (e.g., typically 8cc voxel size, at best). On the other hand, we and others have shown that SNR increases linearly with field strength and the singlet resonances narrow slightly with increasing field strength.36 As further discussed below, this permits at 3T, for example, the acquisition over 17 minutes (TE= 35 msec) of chemical images with as small as 0.18 cc voxel size (3.4×3.4×15 mm3) using an 8 channel array coil. Generally, improved SNR is achieved with shorter TE that follows from the T2 relaxation curve equation which, however, is not linear in relationship. There are additional measurement sensitivity considerations for singlet resonances, such as NAA, compared to the measurement of multiplets, such as Glu. For instance, in comparison to the above example but instead acquiring data over 8.5 minutes and using a 12 channel array coil with 0.5 cc voxel size, along with a shorter TE (TE= 15msec), we can obtain a similar SNR for NAA whereas to achieve similar sensitivity for Glu it would be necessary to measure for about 8 times as long acquisition time or to use correspondingly larger voxels.
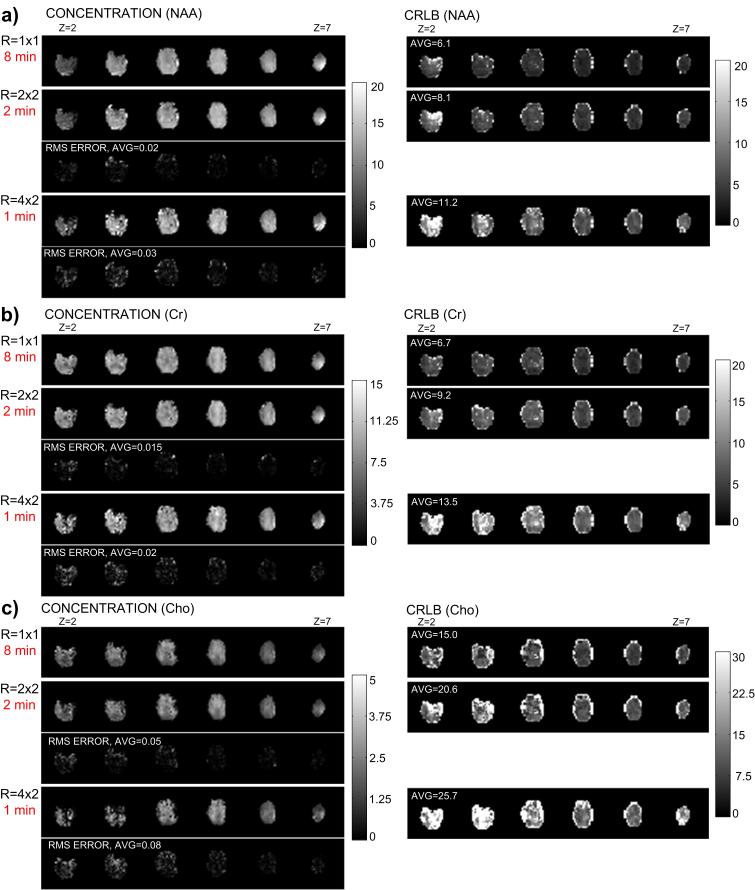
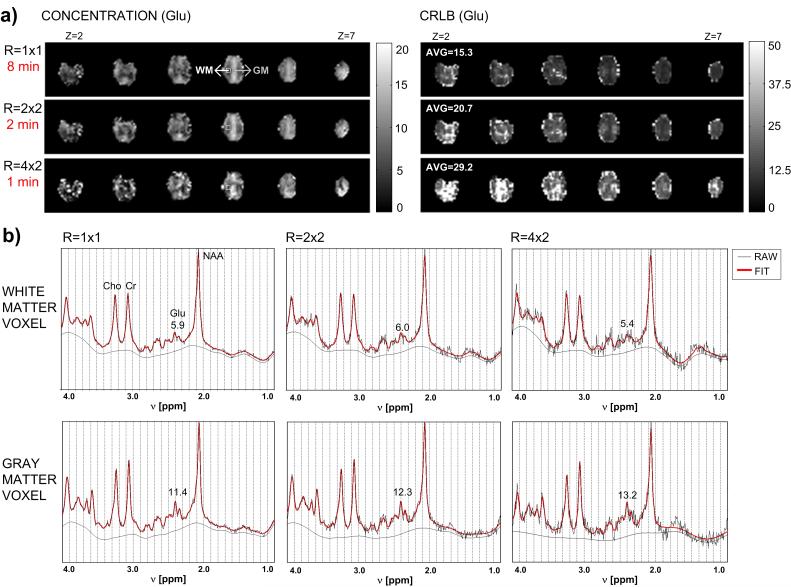
Clinical MRS applications to study psychiatric populations have generally employed single-voxel techniques that acquire chemical information from a single brain area, typically of large volume in order to obtain sufficient SNR, and with variable voxel placement. A substantial advance in MRS applications has been the implementation of spectroscopic imaging techniques having very short acquisition times that can be used to systematically measure regional brain chemistry. Spectroscopic imaging maps chemical signal intensity distributions in human brain, similar to mapping the distribution of water protons for obtaining an MRI. Chemical imaging techniques are particularly suitable for IH MRS, because of the high physiologic concentration of most proton-containing compounds and its greater magnetic sensitivity compared to other nuclei. 6,7 In contrast to the time required for performing repeated single-voxel 1H MRS acquisitions to sample multiple regions, rapid spectroscopic imaging techniques (MRSI), such as proton echo-planar spectroscopic imaging (PEPSI), allows multiple regions to be acquired simultaneously (eg, a 32 × 32 spatial matrix [1024 voxels] at 1.5T, and, more recently, a 64 × 64 spatial matrix [4096 voxels] at 3T) which is clinically advantageous, particularly for studies of agitated or sleeping subjects(eg., children) and for measuring changing metabolic states during a single scanning session, since 2-D and 3-D spatial encoding can be achieved in a reasonable time frame, typically under 5-10 minutes, 11,37,38 Combining PEPSI with recent advances in parallel MRI utilizing RF coil arrays can further accelerate MRSI data acquisition to under a minute for some applications, depending on the field strength, to obtain single-average chemical images of Cho, Cre, NAA, as illustrated in Figure 2, and certain J-coupled metabolites, such as Glu, illustrated in Figure 3, with acceptable spectral quality and localization. 38,39
Figure 2.
3-D spatial chemical concentration maps at different acquisition times (using SENSE acceleration to reduce the number of phase-encoding steps) are shown on the left-hand side, with corresponding spectral fitting error maps (RMS-ERROR) calculated in comparison to the non-accelerated reconstruction (R=1×1) acquired at 8 minutes for a) NAA, b) Creatine and c) Choline. Cramer-Rao lower bound (CRLB) is shown on the right-hand side for each chemical concentration map. CRLB is the error term resulting from spectral fitting to the LC apriori model used to determine chemical concentration. (Figure provided by Dr. Ricardo Otazo)
Figure 3.
a) 3-D spatial chemical concentration maps and Cramer-Rao lower bound (CRLB) maps for Glutamate (Glu) at different acquisition times using SENSE to reduce the number of phase-encoding steps. CRLB is the error term resulting from spectral fitting to the LC apriori model used to determine chemical concentration. b) Raw absorption mode spectrum (black line) and corresponding LCModel fit (red line) for a gray matter (GM) voxel and a white matter (WM) voxel (voxel locations are indicated in part a). The remaining baseline is given by the smooth black curve. The concentration of Glutamate is given in each example. (Figure provided by Dr. Ricardo Otazo)
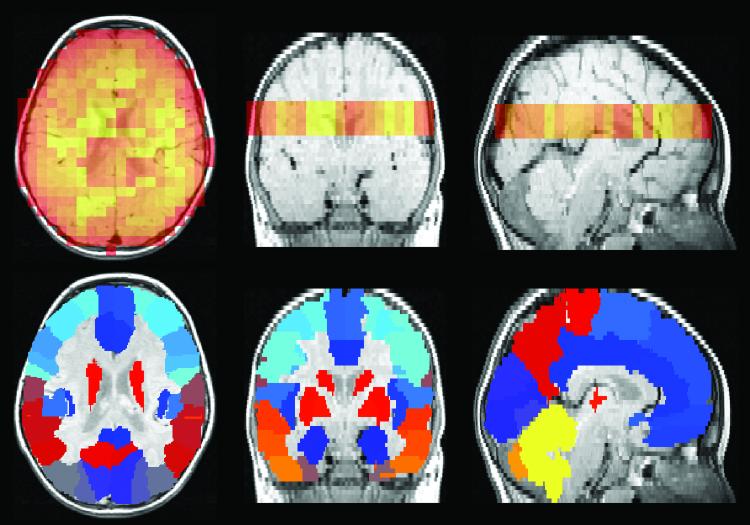
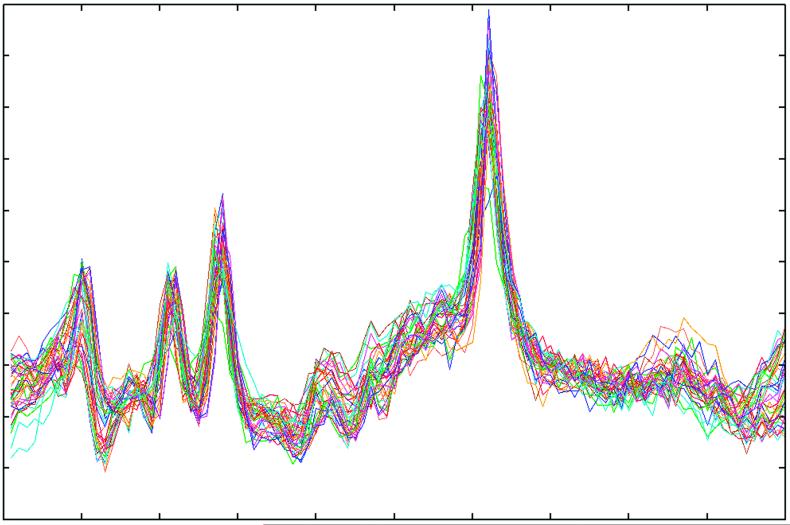
One analytic approach for increasing SNR, complementary to the effects of increasing magnetic field strength of the scanner, capitalizes upon the large number of voxels obtained using MRSI. A single MRSI acquisition can generate thousands of individual spectra, each with a SNR that is dependent upon the voxel size. Through anatomical co-registration of the MRS signal from each voxel and averaging individual spectra together within an anatomically defined brain region or regions, in such a way as to correct for individual phase and frequency shift differences between voxels, substantially improvement in SNR can be achieved. This analytic approach, illustrated using T2-weighted MRI and PEPSI data acquired with a 1.5 Tesla GE scanner from a 3 year old child with autism, is shown in Figures 4-6. PEPSI raw data are first reconstructed using software developed for this purpose (Richards- unpublished data), then individually phased-corrected and shift-corrected using a commercially available spectral fitting program (LCmodel).30,31 Figure 4 demonstrates one step in the process of co-registering PEPSI spectra to the anatomical substrate (detailed at http://www.sph.sc.edu/comd/rorden/mricro.html). A 3-D volume is then created that contains the position of the PEPSI slice within the 3-D MRI structural volume. Figure 5 shows an example data set of superimposed spectra acquired from a specific region of interest (ROI), after filtering out spectral artifact, that are then combined together to obtain a single averaged ROI fitted spectrum, as shown in Figure 5.
Figure 4.
Proton echo-planar spectroscopic image (PEPSI) NAA spatial distribution map shown in orange (top panel) and corresponding anatomical atlas (bottom panel) both of which are overlaid onto a MRI structural image. Each color in the atlas represents a different brain region (http://www.sph.sc.edu/comd/rorden/mricro.html).
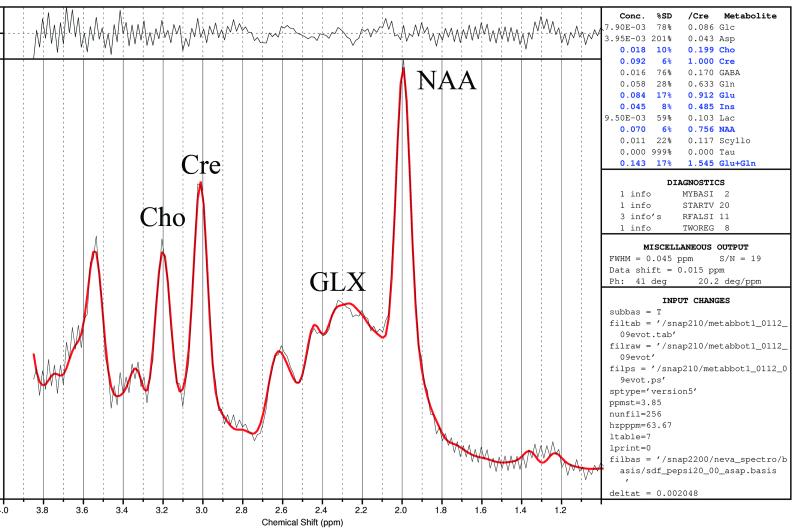
Figure 6.
LC Model fit of averaged spectra acquired from the left frontal lobe. Black line: averaged spectrum. Red line: the LC Model fit. The residuals, calculated as a subtraction of the fit from the average spectrum, are plotted at the top. The table of metabolite concentrations calculated from the fit is to the right.
Figure 5.
Superimposed individual raw spectral acquisitions from the left frontal lobe, after filtering out spectra with artifact. These spectra are then averaged to obtain a single spectrum for this brain region.
Spectral aliasing and localization artifacts, problems commonly encountered with conventional spectroscopic imaging techniques, can be avoided by using modified data acquisition and reconstruction methods.37-39 This approach has increasingly been applied to assess brain chemical/ anatomical relations, but most results have been obtained at long-echo times (eg,TE=272 ms) for reasons of technical feasibility, primarily due to lipid suppression, the need to simplify the spectral pattern by removing multiplet resonances to facilitate quantification, image reconstruction, and acquisition considerations. Very short-echo times (eg, 20 msec) can also be acquired, which along with affording additional mapping of chemicals with short T2’s such as mI, alleviates some of the confounds associated with chemical quantification, as noted above.34,40
Psychiatric applications of MRS
DSM-IV defined axis I psychiatric conditions,41 such as autism disorder, panic disorder, schizophrenia, depression and bipolar disorder (manic-depressive illness), have long been believed to have abnormal neural regulatory mechanisms underlying symptom manifestation. The application of MRS techniques provides the psychiatrist/neuroscientist an opportunity to directly test hypotheses regarding both normal emotional expression and pathophysiologic processes underlying disorders of emotional expression or psychosis. In general, comparison of findings between studies using MRS is challenging due to the small numbers of subjects typically reported with resultant power considerations limiting interpretation of findings, differences in methodology for spectral acquisition, brain region(s) investigated (single-voxel, in particular), and demographic and clinical characteristics of populations studied (e.g., differences in age ranges, clinical subtype, such as bipolar depressed vs euthymic vs hypomanic/manic mood state, medication status, etc). Prior reviews have detailed earlier applications of MRS to investigate psychiatric disorders.42-44 However, the capabilities and potential applications of MRS have rapidly progressed since those articles were written. The intent of this review is to highlight more recent technical advances and research strategies for clinically applying 1H MRS to investigate specific psychiatric disorders.
Autism
The autism spectrum disorders (ASD) are a behavioral syndrome considered to reflect a complex interaction of genetic and environmental influences on altering normal brain development.45, ASD is frequently associated with mental retardation, evident in 30- 70% of individuals, the variability of which may be related to the recognized extent of ASD symptom expression. 46,47 There also is a substantial risk of seizures in ASD that have a bimodal pattern of onset either in the first two years of life or, more typically, as the child enters adolescence, with prevalence rates variously estimated at between 15-38%.48,49
One of the more intriguing and consistent MRI findings, also observed from head circumference (HC) studies, has been strong evidence for cerebral enlargement in ASD, at least during early childhood. The three published MRI investigations to date, that examined preschool-aged children (ages 2-4 years), studied soon after clinical diagnosis of ASD, all found enlarged cerebral volumes, on average 10- 15% increases, excluding cerebrospinal fluid, at this age range.50-52 Numerous factors have been postulated to underlie the brain volume increases in ASD, including theories of accelerated early brain growth50, or a failure of apoptosis and/or synaptic pruning.34
Combining 1H MRSI with MRI can allow hypothesis-driven approaches for a better appreciation of the mechanisms underlying specific anatomical alterations associated with ASD. For example, to account for an approximately 10% cerebral enlargement observed in ASD children at 3-4 years51, PEPSI was used to test an exploratory hypothesis of regionally increased NAA concentrations posited to reflect increased numbers of neurons or denser connections arising from a disturbance of normal neuronal apoptosis or synaptic pruning processes during early development in children with autism.34 In this model, regional chemical concentrations would be increased, and chemical T2 relaxation times estimated from MRSI acquired at 2 widely separated echo times, 20 msec and 272 msec, would be expected to be shortened, as a result of densely packed molecules interacting over time following excitation by the RF pulse. 1H MRS findings instead revealed a pattern of widely distributed regional reductions in NAA, Cho, Cre, and mI concentration, and prolonged chemical T2 relaxation, that were in a direction opposite to what was initially hypothesized. These 1H MRS findings suggest that cellular packing density is not increased among young children with ASD and, thus, do not support models of failed or reduced apoptosis and/or delayed synaptic pruning, nor provide evidence for neuronal “overgrowth” during the preschool years, soon after emergence of clinical symptoms diagnostic for ASD.34 In further analyses of these 1H MRS data, co-registered to segmented tissue maps, regression analytic techniques were used to characterize the compartmental distribution of chemical alterations between white and grey matter. Chemical abnormalities were predominantly observed in gray matter, supporting an altered gray matter cytoarchitecture for the 3-4 year old ASD children compared to age-matched typically developing and developmentally delayed children.53 A subsequent 1H MRSI study of pre-adolescent children with ASD, that used different acquisition techniques but similar regression analytic approaches, also observed reduced NAA in gray matter, but not white matter, compared to typically developing controls.54 Overall, the direction and widespread anatomical distribution of chemical alterations observed, primarily reduced brain chemical concentrations and prolonged chemical T2 relaxation, were not consistent with theoretical models of diffusely increased neuronal packing density in the children with ASD. 34 Thus, 1H MRS findings to date point towards a different explanatory model for structural findings of cerebral enlargement in the 3-4 year old ASD children that speculatively could reflect developmental abnormalities associated with reduced synapse density55 , poorly differentiated cortex56, column density/packing abnormalities57 and/or active inflammatory processes, such as reactive gliosis.58
MRS applications have also been directed towards addressing competing theories of hypoglutamatergic59 or reduced inhibitory balance60 mechanisms in ASD. These theories could support findings of either decreased or elevated Glu, possibly concurrently for different brain regions.61 In support of this, a recent single-voxel 1H MRS report studying an adult sample with ASD found regionally elevated GLX in the right amygdalahippocampal region compared to a control brain region in the parietal lobe.62 13C MRS used to evaluate glutamate-glutamine cycling in patients with intractable temporal lobe epilepsy also demonstrates significant slowing of the cycle and elevated hippocampal Glu levels.29 As proposed by those authors and others63, 64, regionally increased Glu release and impaired clearance might contribute to an ongoing state of increased cerebral excitability/ excitotoxicity that could potentially set up the conditions for seizure generation. This model is of potential clinical relevance to ASD, which is associated with a high incidence of seizure disorders. Although finding of elevated Glu in older individuals with ASD remain to be replicated, in the context of recent findings of lower GLX levels in pre-adolescents with ASD 54, the possibility of GLX levels increasing as these children get older has implications for seizure onset in adolescents with ASD and, as well, for new targeted treatment approaches.65
Schizophrenia
Schizophrenia (SZ) is a prevalent and incapacitating psychiatric disorder occurring in about 1% of the general adult population. Neuroimaging findings from anatomical, metabolic, and receptor studies of SZ support that core symptoms arise from abnormal neurobiological processes underlying the disorder. Most earlier MRS studies of SZ, and a number of the more recent, used 31P MRS to study alterations of membrane phospholipid characteristics, pH, and bioenergetic status.43,66-69 Increasingly, 1H MRS is being used to investigate SZ 70, in particular to characterize regional NAA alterations and the effects of treatment.
Specific brain regions have been implicated in SZ, in particular abnormalities in the structure and function of the frontal lobes and medial temporal lobes (including the hippocampus) that have been well-documented through histological studies 71,72, volumetric analyses 73,74 and activation studies employing functional magnetic resonance imaging.75,76 A recent comprehensive review of 1H MRS studies investigating SZ found the most robust findings were of substantial NAA reductions (> 5%) in frontal lobe and medial temporal lobe gray and white matter, along with similar magnitudes of NAA reduction in the cerebellum and parietal cortex.77 Diffusion tensor imaging (DTI) used in conjunction with 1H MRS to further characterize medial temporal lobe white matter involvement in SZ lends additional support implicating this region with findings of reductions in both NAA concentration and DTI anisotropy in the SZ sample.78 Even convergence of these findings, however, does not lend detailed insight into mechanisms underlying SZ, as brain NAA reductions have been found to be characteristic across a wide range of psychiatric disorders and, hence, are not specific to SZ. However, accruing evidence for specific treatment effects on modulating NAA reductions, discussed below, provides support for a role for NAA alterations in the pathophysiology of SZ. Furthermore, the regional specificity to these NAA findings appears to implicate specific brain regions that may be uniquely involved in the pathophysiology of SZ.
The majority of MRS studies of SZ have focused on chronic adult samples. As a result, there is not a clear consensus on the relationship between stage of disease progression and brain chemical alterations. 77 To address this limitation, one recent study undertook parallel comparisons of an older chronic SZ group compared to age-matched controls and a much younger group of recent onset SZ subjects similarly compared to age-matched young controls with findings of reduced left dorsolateral prefrontal cortex NAA only in the younger SZ group.79 In considering this finding, it is difficult to distinguish effects of disease progression from treatment effects on brain chemical levels. Both cross-sectional assessment of unmedicated compared to medicated patients and longitudinal studies of treatment effects provide evidence that regional NAA reductions observed in untreated SZ patients increase in conjunction with treatment, and probably more so for the atypical antipsychotics in comparison to older generation antipsychotic medications.80-83 One longitudinal treatment study that administered an atypical antipsychotic observed significant NAA increases in SZ patients within 4 weeks from their unmedicated baseline state.81 There also is some evidence for an effect of treatment duration on increasing NAA levels, although this relationship is less well supported.77 In the context of findings indicating NAA increases in conjunction with treatment over the span of weeks, it is difficult to reconcile earlier suggestions that regional NAA levels primarily reflect underlying ncuronal density. More plausibly, NAA reductions in SZ may reflect alterations of neuronal homeostasis, consistent with increasing evidence for NAA’s role in synaptic maintenance, myelination, regulation of cellular osmolarity and neuronal metabolism.19-22
Conventional treatment of SZ is based on a dopaminergic hyperfunction model with typical and atypical antipsychotics antagonists targeting the dopamine D2 receptor.84 However, these medications are not ideal agents and there is substantial variability, typically modest, in treatment response. Alternative models of mechanism underlying SZ have been proposed, in particular one based on evidence for hypoactivity of the NMDA glutamate receptor that has important treatment implications. This glutamatergic model is derived from experiments demonstrating that the NMDA receptor antagonists PCP and ketamine both produce psychotic schizophrenic-like symptoms when administered to healthy individuals or animals. In this model, the blocking of NMDA receptors results in excessive Glu release and consequent over-excitation of post-synaptic neurons.85-90 1H MRS studies of Glu and Gln alterations associated with SZ lend support to the NMDA-hypofunction or glutamatergic model. For example, two complementary studies used 1H MRS to measure Glu and Gln levels in the left anterior cingulate cortex and thalamus. Increased Gln, though not Glu, was found in first episode, never-treated SZ patients whereas in chronically treated SZ patients, lower Glu and Gln levels in the left anterior cingulate cortex, but increased thalamic Gln levels, were found. 91,92 Interpretation of these findings might suggest that early stages of SZ disease progression are associated with increased glutamatergic metabolism which becomes reduced in specific regions during later stages of the illness, perhaps in relationship to treatment effects.
Anxiety Disorders
Anxiety disorders rank among the most prevalent of psychiatric illnesses. Classified among the anxiety disorders, panic disorder (PD) has been extensively investigated in an attempt to link core clinical symptoms to underlying biological mechanisms. There is evidence that PD may be genetically transmitted93,94 and that respiratory dysregulation linked to brain metabolic alterations persist as trait features in the remitted, asymptomatic state.27,95 Individuals with PD are susceptible to having panic attacks precipitated by specific physiological challenges that include intravenous sodium lactate infusion 96-98 , caffeine ingestion99, carbon dioxide (CO2) inhalation100, and sustained hyperventilation (hypocapnea).101 In particular, sodium lactate infusion has been extensively investigated using 1H MRS in an attempt to explain the panic phenomenon. The mechanism(s) underlying lactate-induced panic are considered to be biologically driven. Systematic characterization of coping mechanisms engaged in during MRS lactate studies further reveal that PD subjects have effective coping strategies that allow them a high degree of success in psychologically tolerating the combined stressors of magnet confinement, intravenous lactate infusion and acute panic provocation, counterintuitive to what might be expected if panic were primarily a psychological process.102
One approach applying 1H MRS to study PD has been to capitalize on the ability to acutely induce characteristic panic attacks within the laboratory setting that allows direct investigation of the phenomenon. Intravenous infusion of 0.5 mol/L sodium lactate produces marked physiologic and psychologic symptoms in panic patients but only infrequently in normal control subjects.96 In a series of single-voxel 1H MRS studies, lactate infusion was used as a physiological probe to test theories of abnormal brain metabolism in PD with findings of excess brain lactate increases in the insular cortex region during and post-infusion in comparison to controls.103-105 When the compartmental distribution of Lac increases over time was specifically assessed, abnormal brain Lac increases were determined to be tissue-based and not mediated through CSF, suggesting the involvement of underlying brain metabolic mechanisms.106 As regional brain Lac increases can be detected in response to neuronal activation from sensory stimulation, posited to reflect decoupling of neuronal blood flow and energy requirements107, regionally specific magnitude differences in brain Lac elevation in response to lactate-induced panic should occur if neuronal activation were the underlying mechanism. However, follow-up 1H MRS investigations that used PEPSI (32 × 32 1-cm3 voxel matrix acquired axially at the level of the insular cortex) to assess anatomic and compartmental distributions of Lac response to intravenous lactate infusion instead found diffusely distributed abnormal brain Lac elevations without hemispheric lateralization, nor a discrete anatomical locus for metabolic response to lactate-induced panic.108 Consistent with this observation, persistent abnormal brain lactate rises with lactate re-infusion of PD subjects under treatment with fluoxetine or gabapentin, and who clinically converted to a negative lactate response, indicate that symptom provocation, per se, is not a necessary requirement for abnormal brain Lac increases.105,109 Instead, a role for metabolic and/or neurovascular blood flow dysregulation is suggested by the widely distributed abnormal brain Lac elevations.
Earlier work that used 1H MRS single-voxel techniques to measure regional insular cortex lactate changes during regulated hyperventilation (to a end-tidal pCO2 of 20mm Hg over 20 minutes), found more rapid, prolonged and greater brain Lac rises in asymptomatic, medication-treated PD subjects who were previously reactive to lactate-infusion, a group specifically studied in order to evaluate underlying trait features of the disorder.27 Observations of greater brain Lac increases and delayed end-tidal CO2 recovery in PD subjects during hyperventilation, and increased hyperventilation and excessive brain lactate rises during lactate-infusion, implicate altered abnormal acid-base regulation. From this indirect evidence for abnormal pH compensation, a follow-up 31P MRS investigation sought to directly characterize brain pH response to regulated hyperventilation in PD.8 Competing explanatory models, were tested, positing either that PD subjects would demonstrate greater alkalosis to hyperventilation, implicating elevated Lac as directly compensatory, or reduced or blunted alkalosis, implicating a disproportionate lactate response to alkalosis induced by hypocapnea. The physiological response pattern observed for PD subjects, wherein a substantially greater decrease in end-tidal pCO2 was not accompanied by a similar magnitude of brain alkalotic response (pH increase), supports a model of exaggerated buffering in PD that could be accounted for by excess Lac production.8 In this model, delayed end-tidal pCO2 recovery following hyperventilation, a clinical hallmark of PD, would reflect delayed pH normalization in the recovery phase due to excess brain Lac, that also maintains pathological hyperventilation.
Affective disorders
The affective disorders, in particular bipolar disorder (BD) and major depressive disorder (MDD), are chronic psychiatric illnesses, though exhibiting an episodic clinical course, that carry substantial health care and overall societal burdens. A growing MRS literature supports the presence of brain metabolic alterations in relationships to mood state. Initial MRS studies applied 31P MRS to investigate abnormalities of brain bioenergetic status and phospholipid metabolism. A meta-analysis of 8 31P-MRS studies provides support for state-specific alterations of phospholipid membranes and high energy phosphates in BD that primarily reflect increased phosphomonoester levels and decreased phosphocreatinine levels in the depressed state.110 More generally, findings of decreased nucleotide triphosphate (primarily comprised of adenosine triphosphate) provide evidence for abnormal brain energy metabolism in mood disorders.111,112 Metabolic aberrations may be intrinsic to the underlying pathophysiology of BD since brain intracellular pH determined by 31P-MRS is found to be decreased in medication-free BD patients in both manic and depressed mood states, as well as in euthymic BD patients stabilized with lithium treatment113 In this context, findings of decreased frontal lobe intracellular pH observed in BD patients.would be consistent with lactate acidosis, hypothesized to reflect underlying mitochondrial dysfunction.114
1H-MRS investigations of the affective disorders have generally applied single voxel techniques to test hypotheses of Cho alterations. Evidence for both regional elevations115-117 and decreased Cho levels118-120 have been reported in association with depressed mood state. Differences in clinical populations evaluated, medication status, spectral acquisition parameters or brain regions measured could all have contributed to these variable findings. In a 1H MRS study that sought to address some of these concerns, 2-D PEPSI was used to study unmedicated BD subjects, predominantly in a depressed or mixed-mood state and never treated, and chemical measures were acquired at two echo-times (20/272 milliseconds) in order to account for possible effects of altered T2 relaxation on chemical quantification.28 No tissue-specific CHO alterations were found in relationship to either BD diagnosis or mood state, nor was there evidence for tissue-specific or regional NAA reductions that also has been occasionally reported in BD121 Instead, using regression analytic techniques that allow more sensitivity for assessing chemical alterations between brain tissue compartments (grey and white matter), BD subjects were observed to exhibit elevated gray matter Lac and GLX. These chemical alterations, indicative of alterations in cellular energy metabolism with a redox shift from oxidative phosphorylation toward glycolysis, were posited to reflect a generalized pattern of compromised mitochondrial metabolism in BD, consistent with prior work indicating alterations of pH regulation and mitochondrial genetic loading in this patient population. In conjunction with these considerations, recent in vivo evidence also supports an association between elevated brain Lac and GLX levels and cyanide-induced inhibition of mitochondrial oxidative metabolism.122 Phospholipid membrane alterations found in association with BD also may interfere with mitochondrial function, just as mitochondrial dysfunction can alter phospholipid metabolism.123 Although the demonstration of causal relationships remains challenging, treatment intervention using medications with differing therapeutic profiles provides a strategic approach for systematically investigating subtle alterations of energy metabolism in BD.
Considerations regarding the possible role of mitochondrial compromise in BD have important treatment implications. For example, therapeutic properties of lithium are hypothesized, in part, to be mediated through bcl-2 elevation, a neuroprotective protein that also has mitochondrial membrane-stabilizing effects.124 A longitudinal treatment study of the above described medication-free BP subjects evaluated 1H MRS changes over time in response to treatment with lithium carbonate compared to valproic acid, the two most commonly used mood stablizers.125 In comparison to valproic acid, lithium significantly decreased gray matter GLX concentrations. The magnitude of gray matter GLX decrease was related at the trend level to serum lithium levels, supporting a relationship to drug effects. Lactate changes were not demonstrated for either lithium or valproic acid treatment groups, which may reflect SNR factors as the study was conducted at 1.5T. In the context of gray matter GLX elevations observed in unmedicated BD subjects at baseline, reductions in GLX during lithium treatment may reflect partial normalization of altered redox bioenergetic state.28
1H MRS investigations of MDD have begun to investigate GABA, implicated in the disorder and made possible by recent technical advances in acquisition methods.16 Initial studies reported decreased GABA levels in the occipital cortex of unmedicated MDD subjects, a brain region not implicated in MDD but evaluated due to technical feasibility issues with measuring GABA levels from other brain regions, of greater theoretical interest.126,127 Subsequent studies demonstrated effects of SSRI treatment and ECT on raising occipital GABA levels in MDD patients.128,129 More recent work, addressing some of the technical considerations for regional localization, found that GABA levels, reported as a metabolite ratio in relationship to CRE, were reduced by a similar magnitude in both occiput and anterior cingulate cortex in unmedicated, remitted MDD subjects.130 The magnitude of GABA reduction in these remitted subjects was approximately one fifth of the reduction reported for acutely ill MDD subjects.126 It is uncertain whether this reflects a changing clinical status with normalization of GABA level after recovery from depression or differences in quantification approach from earlier studies. The basis for reduced GABA measured by 1H MRS has been posited to reflect alterations of glial cell activity.127 However, the significance of GABA changes in the occipital lobe is unclear since findings from histopathological studies of glial cell counts and density, as well as from neuroimaging studies, do not implicate the occipital cortex in MDD.131,132 In consideration of this, a recent study reported reduced GABA in the dorsomedial/dorsal anterolateral prefrontal cortex in unmedicated MDD subjects, a region where glial cell abnormalities have been reported.133
Limitations and Future Developments of MRS
Magnetic resonance spectroscopy has the potential to greatly advance our understanding of basic mechanisms responsible for psychiatric disorders. However, a number of technological issues continue to limit the scope of MRS research capabilities and widespread clinical applications. Initially, the small bore size of magnets available for spectroscopy confined in vivo brain studies to animals, or human infants with a small head circumference. Larger bore magnets are now the standard for clinical MRS studies but these are often of relatively low magnetic field strength (typically 1.5 Tesla [T)). Magnetic field strength is an important factor that influences SNR, spatial localization, and acquisition time, which can substantially impact psychiatric research applications of MRS. Fortunately, an increasing number of imaging centers are now utilizing higher field strength magnets, up to 9.4 T, although field strengths past 3T are not yet approved for clinical use by the Food and Drug Administration. Theoretically, a higher magnetic field should proportionately increase the SNR of MRS. Practically, this relationship is not necessarily linear due to technical problems that mount as field strength increases.134 Nonetheless, recent studies using advanced MRS and MRSI technology clearly demonstrate the linear increase in SNR with field strength of singlet resonances36 and significantly increased spectral resolution that permits quantification of a large number of chemicals at high field.135,136
The recent development of large scale array coils provides significant increases in sensitivity that are particularly advantageous for MRS and MRSI applications.137 SNR can be additionally enhanced by increasing acquisition time, although the improvement is proportional only to the square root of the acquisition time. For example, in order to double the SNR ratio, the signal must be acquired for four times as long. This substantially increased acquisition time can introduce serious issues regarding toleration in clinical patient populations. The use of higher field strength is thus an important consideration for successful applications of MRS and MRSI in psychiatric research due to the small metabolic signal differences between patient groups and healthy controls. These considerations for 1H MRS pertain even more to MRS applications using other nuclei, which have lower measurement sensitivity. Although existing clinical MRI systems are usually adaptable for MRS, this can entail additional costs for implementation, as well substantial training for acquisition. The adaptation of MRS to nuclei other than proton also requires additional equipment for RF production and reception.
Current MRS applications essentially provide a snapshot of metabolite pool size rather than of pool kinetics. Changes in turnover rate are of potentially greater clinical interest, since changes in pool size generally occur only in response to a severe metabolic insult such as a hypoxic event. In this regard, burgeoning interest in the use of 13C MRS in vivo, to investigate turnover of 13C labeled metabolic substrates, can provide useful information regarding subtle metabolic differences in pool turnover rate.138,139 The chemical shift range for 13C MRS, about 20-fold greater than for IH MRS, makes it particularly applicable to measuring metabolic changes. However, widespread utilization of 13C MRS for clinical research awaits the availability of inexpensive 13C-Iabeled compounds in sufficient quantity to be detectable when administered in vivo. Moreover, the MRS sensitivity of 13C, as well as for other hetero-nuclear MRS applications, is sufficiently low that adequate source of substrate signal is not the only problem to overcome. This is exemplified by a study of investigating carbohydrate metabolism in rats in which it was necessary to administer to the rats the equivalent of l lb of 13C glucose in a 125lb human in order to obtain interpretable spectra.140 Fortunately the development of detection methods, in addition to higher field strength, that rely on the enhanced sensitivity of the 1H nucleus, in particular heteronuclear double-resonance techniques, can substantially enhance the sensitivity of MRS measurements (for example, up to 11-fold depending upon 13C position in the molecule of interest for 13C MRS studies).138 Hyperpolarized MRI of 13C molecules also is an emerging technology that provides several orders of magnitude increase in sensitivity, but the life time of this 13C hyperpolarization is tens of seconds and even shorter in tissue. Currently, this technology holds great promise for cancer imaging and may perhaps be applicable to probing changes in metabolic flux rates of compounds that are relevant to psychiatric research.141,142
There are also cautionary notes regarding patient acceptance and safety issues. Currently, in vivo applications of MRS often necessitate lengthy patient examinations, lasting up to several hours for some types of acquisition. It is frequently difficult to remain motionless for the time required to complete a study. Although confinement in the scanner can engender extreme claustrophobic reactions which can be amplified by the loud, intermittent knocking sounds and vibrations that occur within the magnet during signal acquisition, this may be difficult to predict for specific patient populations. For example, panic subject undergoing a lactate infusion intended to precipitate panic have remarkably evolved coping skills that, perhaps counter-intuitively, allow them to successfully adapt to the scanner environment.102
The increasing availability of higher field scanners and the refinement of pulse sequences permit shorter acquisition times and, thus, enhance clinical acceptance. However, there are lingering questions regarding the long-term health effects of magnetic field exposure. It should be noted that most safety research has been directed towards the study of chronic exposure to low-frequency electromagnetic fields, such as those found near high-voltage power transmission wires, or in relationship to alternating magnetic fields rather than the static magnetic field generally associated with MRI and MRS. There have been no long term side effects or health problems linked to MRI or MRS. Sporadic patient reports of transient muscular, visual, gustatory or cognitive disturbances are difficult to distinguish from the psychological or physiological manifestations of claustrophobia or anxiety, but may arise from acute induction effects. For example, self-reports of enhancement of mood state in depressed BD subjects undergoing MRSI acquisition on a 1.5T clinical scanner may be related to electrical fields induced by gradient switching, similar to the effects of transcranial magnetic stimulation on enhancing mood but without the associated risk for discomfort or seizure.143
In addition to concerns regarding certain technical limitations, health effects, and patient acceptance, there are a number of problems in interpreting clinical data generated by MRS brain studies. A considerable proportion of the available literature must be considered descriptive, since MRS findings may be based on only a few case studies. As with any new medical technology, initial reports often fail to adequately address potential methodological problems, such as patient and control group selection criteria, which can substantially bias results. Comparison between studies may be difficult due to changing MRS protocols, as new signal acquisition techniques and more powerful magnets become available. Determination of the area under the spectral peak for molecules in low physiologic concentrations or with overlapping spectral resonances is prone to considerable interpretation. The optimal method for quantification of metabolite concentrations also remains controversial, as discussed above. Moreover, MRS and MRSI are intrinsically low-resolution techniques that rely heavily on MRI-based partial volume correction of the different tissue types and relaxation time constants of water and metabolites, in particular with the large voxel size at clinical field strengths. Small errors in tissue segmentation can have significant effects on metabolite quantification. These problems are compounded by a dearth of “gold standards” for spectral quantification that requires tightly constrained spectral models, which depend on prior knowledge about the spectral composition of the tissue under investigation.
Conclusions
This review has highlighted both technical advances and more recent research strategies for psychiatric applications of MRS. Technically, MRS is a very difficult and complex tool to use properly. A firm footing that combines theoretical and practical knowledge of MRS is vital for successful research applications and valid interpretation of results. Despite these caveats, careful application of MRS offers a unique opportunity to noninvasively study the pathophysiology underlying psychiatric disorders. The ability to bridge mind and brain becomes increasingly possible in conjunction with progressive advances in the development and research applications of MRS and allied imaging modalities.
The ultimate goals for research and clinical applications of MRS are to more fully understand mechanisms underlying pychopathology and to identify relationships between alterations in brain chemical makeup or metabolic homeostasis and symptom expression. The potential to identify specific brain biomarkers for more informed and targeted treatment interventions, and as indices of treatment response separate from symptom abatement that may lag behind, further encourages the pursuit of this important objective. The application of MRS also has the potential to identify more homogeneous clinical populations with increased opportunity to identify specific genetic relationships to disease. Although MRS has yet to fully realize this potential, promising efforts have been made to begin to link 1H MRS findings to putative brain mechanisms underlying particular psychiatric disorders and modulation of MRS identified abnormalities in response to specific treatment interventions.
Acknowledgments
This work was supported by 2RO1 MH50579, 2PO1 HD 35465 and U54 MH066399 through the National Institutes of Health.
References
- 1.Andreasen NC. Brain imaging: applications in psychiatry. Science. 1988;239:1381–1388. doi: 10.1126/science.3279509. [DOI] [PubMed] [Google Scholar]
- 2.Ross B, Michaelis T. Clinical applications of magnetic resonance spectroscopy. Magn Reson Med. 1994;10:191–247. [PubMed] [Google Scholar]
- 3.Rapaport S. Blood-Brain Barrier in Physiology and Medicine. Raven Press; New York: 1976. [Google Scholar]
- 4.Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 5.Mansfield P, Grannell PK. NMR diffraction in solids? J Phys C: Solid State Phys. 1973;6:422–426. [Google Scholar]
- 6.Gadian DF. Nuclear magnentic resonance and its applications to living systems. Oxford University Press; New York: 1982. [Google Scholar]
- 7.Cohen SM. Physiological NMR Spectroscopy: from Isolated Cells to Man. Ann NY Acad Sci. 1987;508:1–537. [PubMed] [Google Scholar]
- 8.Friedman SD, Mathis CM, Hayes C, Renshaw P, Dager SR. Brain pH response to hyperventilation in panic disorder: preliminary evidence for altered acid-base regulation. Am J Psychiatry. 2006;163(4):710–5. doi: 10.1176/ajp.2006.163.4.710. [DOI] [PubMed] [Google Scholar]
- 9.Renshaw PF, Wicklund S. In vivo measurement of lithium in humans by nuclear magnetic resonance spectroscopy. Biol Psychiatry. 1988;23:465–475. doi: 10.1016/0006-3223(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 10.Strauss WL, Unis AS, Cowan C, Dawson G, Dager SR. 19F measurement of brain fluvoxamine and fluoxetine in pediatric patients treated for pervasive developmental disorders. Am J Psychiatry. 2002;159:755–760. doi: 10.1176/appi.ajp.159.5.755. [DOI] [PubMed] [Google Scholar]
- 11.Dager SR, Layton ME, Strauss W, Richards TL, Heide A, Friedman SD, Artru AA, Hayes CE, Posse S. Human brain metabolic response to caffeine and the effects of tolerance. Am J Psychiatry. 1999;156:229–237. doi: 10.1176/ajp.156.2.229. [DOI] [PubMed] [Google Scholar]
- 12.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 13.Schubert F, Gallinat J, Seifert F, Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage. 2004;21:1762–1771. doi: 10.1016/j.neuroimage.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Pfeuffer J, Tkác I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain. J Magn Reson. 1999;141(1):104–20. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chen AP, Crane JC, Chang SM, Vigneron DB, Nelson SJ. Three-dimensional J-resolved H-1 magnetic resonance spectroscopic imaging of volunteers and patients with brain tumors at 3T. Magn Reson Med. 58(5):886–92. doi: 10.1002/mrm.21415. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Rothman DL, Brown P. In vivo GABA editing using a novel doubly selective multiple quantum filter. Magn Reson Med. 2002;47(3):447–54. doi: 10.1002/mrm.10104. [DOI] [PubMed] [Google Scholar]
- 17.Tallan HH, Moore S, Stein WH. N-acetyl-l-aspartic acid in brain. J Biol Chem. 1956;219:257–264. [PubMed] [Google Scholar]
- 18.Friedman SD, Brooks WM, Jung RE, Chiulli SJ, Sloan JH, Montoya BT, Hart BL, Yeo RA. Quantitative proton MRS predicts outcome after traumatic brain injury. Neurology. 1999;52:1384–1391. doi: 10.1212/wnl.52.7.1384. [DOI] [PubMed] [Google Scholar]
- 19.Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989;13:23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- 20.Coyle JT, Schwarcz R. Mind glue: implications of glial cell biology for psychiatry. Arch Gen Psychiatry. 2000;57:90–93. doi: 10.1001/archpsyc.57.1.90. [DOI] [PubMed] [Google Scholar]
- 21.Baslow MH. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem. 2000;75:453–459. doi: 10.1046/j.1471-4159.2000.0750453.x. [DOI] [PubMed] [Google Scholar]
- 22.Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- 23.van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology. 1990;176:509–515. doi: 10.1148/radiology.176.2.2164237. [DOI] [PubMed] [Google Scholar]
- 24.Huppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz N. Magnetic resonance in preterm and term newborns: 1H-spectroscopy in developing human brain. Pediatr Res. 1991;30:574–578. doi: 10.1203/00006450-199112000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Moore CM, Breeze JL, Kukes TJ, Rose SL, Dager SR, Cohen BM, Renshaw PF. Effects of myo-inositol ingestion on human brain myo-inositol levels: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 1999;45:1197–1202. doi: 10.1016/s0006-3223(98)00249-2. [DOI] [PubMed] [Google Scholar]
- 26.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dager SR, Strauss WL, Marro KI, Richards TL, Metzger GD, Artru AA. Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. Am J Psychiatry. 1995;152:666–672. doi: 10.1176/ajp.152.5.666. [DOI] [PubMed] [Google Scholar]
- 28.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 29.Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43:703–710. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- 30.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 31. http://s-provencher.com/pages/lcmodel.shtml
- 32. http://carbon.uab.es/mrui
- 33.Kanowski M, Kaufmann J, Braun J, Bernarding J, Tempelmann C. Quantitation of simulated short echo time 1H human brain spectra by LCModel and AMARES. Magn Reson Med. 2004;51(5):904–12. doi: 10.1002/mrm.20063. [DOI] [PubMed] [Google Scholar]
- 34.Friedman SD, Shaw DWW, Artru AA, et al. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology. 2003;60:100–107. doi: 10.1212/wnl.60.1.100. [DOI] [PubMed] [Google Scholar]
- 35.Dager SR, Marro KI, Richards TL, Metzger G. Localized Magnetic Resonance Spectroscopy Measurement of Brain Lactate During Intravenous 0.5 M Sodium L-Lactate Infusion in Healthy Volunteers. Life Sciences. 1992;51:973–985. doi: 10.1016/0024-3205(92)90404-d. [DOI] [PubMed] [Google Scholar]
- 36.Otazo R, Mueller B, Ugurbil K, Wald L, Posse S. Signal-to-noise ratio and spectral linewidth improvements between 1.5 and 7 Tesla in proton echo-planar spectroscopic imaging. Magn Reson Med. 2006;56(6):1200–10. doi: 10.1002/mrm.21067. [DOI] [PubMed] [Google Scholar]
- 37.Posse S, Dager SR, Richards TL, et al. In vivo measurement of regional brain metabolic response to hyperventilation using functional proton echo-planar spectroscopic imaging (PEPSI) Magn Reson Med. 1997;37:858–865. doi: 10.1002/mrm.1910370609. [DOI] [PubMed] [Google Scholar]
- 38.Lin FH, Tsai SY, Otazo R, Caprihan A, Wald LL, Belliveau JW, Posse S. Sensitivity-encoded (SENSE) proton echo-planar spectroscopic imaging (PEPSI) in the human brain. Magn Reson Med. 2007;57(2):249–57. doi: 10.1002/mrm.21119. [DOI] [PubMed] [Google Scholar]
- 39.Posse S, Otazo R, Caprihan A, et al. Proton Echo Planar Spectroscopic Imaging of J-Coupled Resonances in Human Brain at 3 and 4 Tesla. Magn Reson Med. 2007;58(2):236–44. doi: 10.1002/mrm.21287. [DOI] [PubMed] [Google Scholar]
- 40.Posse S, Schuknecht B, Smith ME, van Zijl PC, Herschkowitz N, Moonen CT. Short echo time proton MR spectroscopic imaging. J Comput Assist Tomogr. 1993;17:1–14. doi: 10.1097/00004728-199301000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Diagnostic and statistical manual of mental disorders. 4th American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 42.Lock T, Abou-Saleh MT, Edwards RHT. Psychiatry and the new magnetic resonance era. Br J Psychiatry. 1990;157:38–55. [PubMed] [Google Scholar]
- 43.Dager SR, Steen RG. Applications of magnetic resonance spectroscopy to the investigation of neuropsychiatric disorders. Neuropsychopharmacology. 1992;6:249–266. [PubMed] [Google Scholar]
- 44.Lyoo IK, Renshaw PF. Magnetic resonance spectroscopy: current and future applications in psychiatric research. Biol Psychiatry. 2002;51:195–207. doi: 10.1016/s0006-3223(01)01313-0. [DOI] [PubMed] [Google Scholar]
- 45.DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawson G, Munson J, Estes A, Osterling J, McPartland J, Toth K, Carver L, Abbott R. Neurocognitive function and joint attention ability in young children with autism spectrum disorder versus developmental delay. Child Dev. 2002;73:345–358. doi: 10.1111/1467-8624.00411. [DOI] [PubMed] [Google Scholar]
- 47.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162(6):1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- 48.Volkmar FR, Nelson DS. Seizure disorders in autism. J Am Acad Child Adolesc Psych. 1990;29:127–129. doi: 10.1097/00004583-199001000-00020. [DOI] [PubMed] [Google Scholar]
- 49.Giovanardi R, Posar A, Parmeggiani A. Epilepsy in adolescents and young adults with autistic disorder. Brain Dev. 2000;22:102–168. doi: 10.1016/s0387-7604(99)00124-2. [DOI] [PubMed] [Google Scholar]
- 50.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 51.Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 52.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 53.Friedman SD, Shaw DW, Artru AA, Dawson G, Petropoulos H, Dager SR. Gray and white matter brain chemistry in young children with autism. Arch Gen Psychiatry. 2006;63:786–794. doi: 10.1001/archpsyc.63.7.786. [DOI] [PubMed] [Google Scholar]
- 54.DeVito TJ, Drost DJ, Neufeld RW, Rajakumar N, Pavlosky W, Williamson P, Nicolson R. Evidence for cortical dysfunction in autism: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 2007;61(4):465–73. doi: 10.1016/j.biopsych.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Mukaetova-Ladinska EB, Arnold H, Jaros E, Perry R, Perry E. Depletion of MAP2 expression and laminar cytoarchitectonic changes in dorsolateral prefrontal cortex in adult autistic individuals. Neuropathol Appl Neurobiol. 2004;30:615–623. doi: 10.1111/j.1365-2990.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 56.Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 57.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 58.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 59.Carlsson ML. Hypothesis: is infantile autism a hypoglutamatergic disorder? Relevance of glutamate serotonin interactions for pharmacotherapy. J Neural Transm. 1998;105:525–535. doi: 10.1007/s007020050076. [DOI] [PubMed] [Google Scholar]
- 60.Belmonte MK, Cook EH, Jr., Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powell SB, Levitt PR, Perry EK, Jiang YH, DeLorey TM, Tierney E. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- 61.Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment.Retard.Dev.Disabil.Res.Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- 62.Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, Ambery F, McAlonan GM, Murphy KC, Murphy DG. In vivo 1H-magnetic resonance spectroscopy study of amygdala-hippocampal and parietal regions in autism. Am J Psychiatry. 2006;163(12):2189–92. doi: 10.1176/appi.ajp.163.12.2189. [DOI] [PubMed] [Google Scholar]
- 63.During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- 64.Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Arch Neurol. 2001;58:365–370. doi: 10.1001/archneur.58.3.365. [DOI] [PubMed] [Google Scholar]
- 65.Niederhofer H. Glutamate antagonists seem to be slightly effective in psychopharmacologic treatment of autism. J Clin Psychopharmacol. 2007 Jun;27(3):317–8. doi: 10.1097/01.jcp.0000270082.30500.69. [DOI] [PubMed] [Google Scholar]
- 66.Volz HR, Riehemann S, Maurer I, Smesny S, Sommer M, Rzanny R, Holstein W, Czekalla J, Sauer H. Reduced phosphodiesters and high-energy phosphates in the frontal lobe of schizophrenic patients: a (31)P chemical shift spectroscopic-imaging study. Biol Psychiatry. 2000;47:954–961. doi: 10.1016/s0006-3223(00)00235-3. [DOI] [PubMed] [Google Scholar]
- 67.Jensen JE, Miller J, Williamson PC, Neufeld RW, Menon RS, Malla A, Manchanda R, Schaefer B, Densmore M, Drost DJ. Focal changes in brain energy and phospholipid metabolism in first-episode schizophrenia: 31P-MRS chemical shift imaging study at 4 Tesla. Br J Psychiatry. 2004;184:409–415. doi: 10.1192/bjp.184.5.409. [DOI] [PubMed] [Google Scholar]
- 68.Jensen JE, Miller J, Williamson PC, Neufeld RW, Menon RS, Malla A, Manchanda, Schaefer B, Densmore M, Drost DJ. Grey and white matter differences in brain energy metabolism in first episode schizophrenia: 31PMRS chemical shift imaging at 4 Tesla. Psychiatry Res. 2006;146:127–135. doi: 10.1016/j.pscychresns.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Smesny S, Rosburg T, Nenadic I, Fenk KP, Kunstmann S, Rzanny R, Volz HP, Sauer H. Metabolic mapping using 2D 31P-MR spectroscopy reveals frontal and thalamic metabolic abnormalities in schizophrenia. Neuroimage. 2007;35(2):729–737. doi: 10.1016/j.neuroimage.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 70.Abbott C, Bustillo J. What have we learned from proton magnetic resonance spectroscopy about schizophrenia? A critical update. Curr Opin Psychiatry. 2006;19(2):135–139. doi: 10.1097/01.yco.0000214337.29378.cd. [DOI] [PubMed] [Google Scholar]
- 71.Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- 72.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–820. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 73.Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedman L, Findling RL, Kenny JT, Swales TP, Stuve TA, Jesberger JA, Lewin JS, Schulz SC. An MRI study of adolescent patients with either schizophrenia or bipolar disorder as compared to healthy control subjects. Biol Psychiatry. 1999;46:78–88. doi: 10.1016/s0006-3223(98)00351-5. [DOI] [PubMed] [Google Scholar]
- 75.Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philosophical Transactions of the Royal Society of London B. Biol Sciences. 1996;351:1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- 76.Yurgelun-Todd DA, Waternaux CM, Cohen BM, Gruber SA, English CD, Renshaw PF. Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. Am J Psychiatry. 1996 Feb;153(2):200–5. doi: 10.1176/ajp.153.2.200. [DOI] [PubMed] [Google Scholar]
- 77.Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30(11):1949–62. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 78.Tang CY, Friedman J, Shungu D, Chang L, Ernst T, Stewart D, Hajianpour A, Carpenter D, Ng J, Mao X, Hof PR, Buchsbaum MS, Davis K, Gorman JM. Correlations between Diffusion Tensor Imaging (DTI) and Magnetic Resonance Spectroscopy (1H MRS) in schizophrenic patients and normal controls. BMC Psychiatry. 2007;19:7–25. doi: 10.1186/1471-244X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stanley JA, Vemulapalli M, Nutche J, Montrose DM, Sweeney JA, Pettegrew JW, MacMaster FP, Keshavan MS. Reduced N-acetyl-aspartate levels in schizophrenia patients with a younger onset age: a single-voxel 1H spectroscopy study. Schizophr Res. 2007;93(13):23–32. doi: 10.1016/j.schres.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fannon D, Simmons A, Tennakoon L, O'Céallaigh S, Sumich A, Doku V, Shew C, Sharma T.Selective deficit of hippocampal N-acetylaspartate in antipsychotic-naive patients with schizophrenia Biol Psychiatry 2003. 15;546587–598. [DOI] [PubMed] [Google Scholar]
- 81.Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, et al. The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia Biol Psychiatry 2001. 2001;4939–46. [DOI] [PubMed] [Google Scholar]
- 82.Braus DF, Ende G, Weber-Fahr W, Demirakca T, Henn FA. Favorable effect on neuronal viability in the anterior cingulate gyrus due to long-term treatment with atypical antipsychotics: an MRSI study. Pharmacopsychiatry. 2001;34:251–253. doi: 10.1055/s-2001-18037. [DOI] [PubMed] [Google Scholar]
- 83.Braus DF, Ende G, Weber-Fahre W, Demirakca T, Tost H, Henn FA. Functioning and neuronal viability of the anterior cingulate neurons following antipsychotic treatment: MR-spectroscopic imaging in chronic schizophrenia. Eur Neuropsychopharm. 2002;12:145–152. doi: 10.1016/s0924-977x(02)00003-2. [DOI] [PubMed] [Google Scholar]
- 84.Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr, Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem. 2006;6(8):771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- 85.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 86.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neuroscience. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neuroscience. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]; Am J Psychiatry. 2003;160:2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 88.Hasegawa M, Kinoshita H, Amano M, Hasegawa T, Kameyama T, Nabeshima T. MK-801 increases endogenous acetylcholine release in the rat parietal cortex: a study using brain microdialysis. Neuroscience Letters. 1993;150:53–56. doi: 10.1016/0304-3940(93)90106-u. [DOI] [PubMed] [Google Scholar]
- 89.Giovannini MG, Camilli F, Mundula A, Pepeu G. Glutamatergic regulation of acetylcholine output in different brain regions: a microdialysis study in the rat. Neurochemistry International (Oxford) 1994;25:23–26. doi: 10.1016/0197-0186(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 90.Kim SH, Price MT, Olney JW, Farber NB. Excessive cerebrocortical release of acetylcholine induced by NMDA antagonists is reduced by GABAergic and _2-adrenergic agonists. Molecular Psychiatry. 1999 doi: 10.1038/sj.mp.4000529. [DOI] [PubMed] [Google Scholar]
- 91.Theberge J, Al-Semaan Y, Menon P, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 92.Theberge J, Bartha R, Drost D, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 93.Torgersen S. Genetic factors in anxiety disorders. Arch Gen Psychiatry. 1983;40(10):1085–9. doi: 10.1001/archpsyc.1983.01790090047007. [DOI] [PubMed] [Google Scholar]
- 94.Crowe RR. The genetics of panic disorder and agoraphobia. Psychiatr Dev. 1985;3(2):171–85. [PubMed] [Google Scholar]
- 95.Wilhelm FH, Gerlach AL, Roth WT. Slow recovery from voluntary hyperventilation in panic disorder. Psychosom Med. 2001;63(4):638–49. doi: 10.1097/00006842-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 96.Pitts FN, Jr., McClure JN., Jr. Lactate metabolism in anxiety neurosis. N Engl J Med. 1967;277(25):1329–36. doi: 10.1056/NEJM196712212772502. [DOI] [PubMed] [Google Scholar]
- 97.Liebowitz MR, Fyer AJ, Gorman JM, Dillon D, Appleby IL, Levy G, Anderson S, Levitt M, Palij M, Davies SO, et al. Lactate provocation of panic attacks. I. Clinical and behavioral findings. Arch Gen Psychiatry. 1984;41(8):764–70. doi: 10.1001/archpsyc.1984.01790190038004. [DOI] [PubMed] [Google Scholar]
- 98.Dager SR, Cowley DS, Dunner DL. Biological markers in panic states: lactate-induced panic and mitral valve prolapse. Biol Psychiatry. 1987;22(3):339–59. doi: 10.1016/0006-3223(87)90152-1. [DOI] [PubMed] [Google Scholar]
- 99.Uhde TW, Bouilenger JP. Caffeine model of panic. In: Lerer I, Gershon S, editors. New Directions in Affective Disorders. Springer-Verlag; New York: 1989. pp. 410–413. [Google Scholar]
- 100.Woods SW, Charney DS, Loke J, Goodman WK, Redmond DE, Jr., Heninger GR. Carbon dioxide sensitivity in panic anxiety. Ventilatory and anxiogenic response to carbon dioxide in healthy subjects and patients with panic anxiety before and after alprazolam treatment. Arch Gen Psychiatry. 1986;43(9):900–9. doi: 10.1001/archpsyc.1986.01800090090013. [DOI] [PubMed] [Google Scholar]
- 101.Maddock RJ, Carter CS, Gietzen DW. Elevated serum lactate associated with panic attacks induced by hyperventilation. Psychiatry Res. 1991;38(3):301–11. doi: 10.1016/0165-1781(91)90020-p. [DOI] [PubMed] [Google Scholar]
- 102.Nazemi H, Dager SR. Coping strategies of panic and control subjects undergoing lactate infusion during MRI confinement. Compr Psychiatry. 2003;44:190–197. doi: 10.1016/S0010-440X(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 103.Dager SR, Marro KI, Richards TL, Metzger G. Localized Magnetic Resonance Spectroscopy Measurement of Brain Lactate During Intravenous 0.5 M Sodium L-Lactate Infusion in Healthy Volunteers. Life Sciences. 1992;51:973–985. doi: 10.1016/0024-3205(92)90404-d. [DOI] [PubMed] [Google Scholar]
- 104.Dager SR, Marro KI, Metzger GD, Richards TL. Preliminary applications of magnetic resonance spectroscopy to investigate lactate-induced panic. Am J Psychiatry. 1994;151:57–63. doi: 10.1176/ajp.151.1.57. [DOI] [PubMed] [Google Scholar]
- 105.Dager SR, Richards T, Strauss WL, Artru A. Single-Voxel 1H MRS Investigation of Brain Metabolic Changes During Lactate-Induced Panic. Psych Research/Neuroimaging. 1997;76:89–99. doi: 10.1016/s0925-4927(97)00066-8. [DOI] [PubMed] [Google Scholar]
- 106.Friedman SD, Dager SR, Richards TL, Petropoulos H, Posse S. Modeling Brain Compartmental Lactate Response to Metabolic Challenge: A Feasibility Study. Psych Research/Neuroimaging. 2000;98(1):55–66. doi: 10.1016/s0925-4927(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 107.Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt neurochemical basis of functional imaging. Proc Natl Acad Sci U S A. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dager SR, Friedman SD, Heide A, Layton ME, Richards T, Artru A, Strauss W, Hayes C, Posse S. Two-dimensional proton echo-planar spectroscopic imaging of brain metabolic changes during lactate-induced panic. Arch Gen Psychiatry. 1999;56:70–77. doi: 10.1001/archpsyc.56.1.70. [DOI] [PubMed] [Google Scholar]
- 109.Layton ME, Friedman SD, Dager SR. Brain Metabolic Changes During Lactate-Induced Panic: Effects of Gabapentin Treatment. Depression and Anxiety. 2001;14:251–254. doi: 10.1002/da.1076. [DOI] [PubMed] [Google Scholar]
- 110.Yildiz A, Sachs GS, Dorer DJ, Renshaw PF. 31P nuclear magnetic resonance spectroscopy findings in bipolar illness: a meta-analysis. Psychiatry Res. 2001;106:181–191. doi: 10.1016/s0925-4927(01)00082-8. [DOI] [PubMed] [Google Scholar]
- 111.Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–118. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- 112.Volz HP, Rzanny R, Riehemann S, May S, Hegewald H, Preussler B, Hubner G, Kaiser WA, Sauer H. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci. 1998;248:289–295. doi: 10.1007/s004060050052. [DOI] [PubMed] [Google Scholar]
- 113.Kato T, Murashita J, Kamiya A, Shioiri T, Kato N, Inubushi T. Decreased brain intracellular pH measured by 31P-MRS in bipolar disorder: a confirmation in drug-free patients and correlation with white matter hyperintensity. Eur Arch Psychiatry Clin Neurosci. 1998;248:301–306. doi: 10.1007/s004060050054. [DOI] [PubMed] [Google Scholar]
- 114.Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2000;2:180–190. doi: 10.1034/j.1399-5618.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- 115.Charles HC, Lazeyras F, Krishnan KRR, Boyko OB, Payne M, Moore D. Brain choline in depression: in vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1121–1127. doi: 10.1016/0278-5846(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 116.Hamakawa H, Kato T, Murashita J, Kato N. Quantitative proton magnetic resonance spectroscopy of the basal ganglia in patients with affective disorders. Eur Arch Psychiatry Clin Neurosci. 1998;248:53–58. doi: 10.1007/s004060050017. [DOI] [PubMed] [Google Scholar]
- 117.Steingard RJ, Yurgelun-Todd DA, Hennen J, Moore JC, Moore CM, Vakili K, Young AD, Katic A, Beardslee WR, Renshaw PF. Increased orbitofrontal cortex levels of choline in depressed adolescents as detected by in vivo proton magnetic resonance spectroscopy. Biol Psychiatry. 2000;48:1053–1061. doi: 10.1016/s0006-3223(00)00942-2. [DOI] [PubMed] [Google Scholar]
- 118.Renshaw PF, Lafer B, Babb SM, Fava M, Stoll AL, Christensen JD, Moore CM, Yurgelun-Todd DA, Bonello CM, Pillay SS, Rothschild AJ, Nierenberg AA, Rosenbaum JF, Cohen BM. Basal ganglia choline levels in depression and response to fluoxetine treatment: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 1997;41:837–843. doi: 10.1016/S0006-3223(96)00256-9. [DOI] [PubMed] [Google Scholar]
- 119.Sonawalla SB, Renshaw PF, Moore CM, Alpert JE, Nierenberg AA, Rosenbaum JF, Fava M. Compounds containing cytosolic choline in the basal ganglia: a potential biological marker of true drug response to fluoxetine. Am J Psychiatry. 1999;156:1638–1640. doi: 10.1176/ajp.156.10.1638. [DOI] [PubMed] [Google Scholar]
- 120.Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. The hippocampus in patients treated with electroconvulsive therapy: a proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry. 2000;57:937–943. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- 121.Frye MA, Thomas MA, Yue K, Binesh N, Davanzo P, Ventura J, O’Neill J, Guze B, Curran JG, Mintz J. Reduced concentrations of N-acetylaspartate (NAA) and the NAA-creatine ratio in the basal ganglia in bipolar disorder: a study using 3-Tesla proton magnetic resonance spectroscopy. Psychiatry Res. 2007 Apr 15;154(3):259–65. doi: 10.1016/j.pscychresns.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 122.Clausen T, Zauner A, Levasseur JE, Rice AC, Bulluck R. Induced mitochondrial failure in the feline brain: implications for understanding acute post-traumatic metabolic events. Brain Res. 2001;908:35–48. doi: 10.1016/s0006-8993(01)02566-5. [DOI] [PubMed] [Google Scholar]
- 123.Modica-Napolitano JS, Renshaw PF. Ethanolamine and phosphoethanolamine inhibit mitochondrial bioenergetic function in vitro: implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol Psychiatry. 2004;55:273–277. doi: 10.1016/s0006-3223(03)00784-4. [DOI] [PubMed] [Google Scholar]
- 124.Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, Gray NA, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 125.Friedman SF, Dager SR, Parow A, Hirashima F, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. Lithium and Valproic Acid Treatment Effects on Brain Chemistry in Bipolar Disorder. Bio Psychiatry. 2004;56(5):340–348. doi: 10.1016/j.biopsych.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 126.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical γ -aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 127.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 128.Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 129.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159(4):663–5. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 130.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, Matthews PM, Cowen PJ. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2007;11:1–6. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 131.Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 132.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 133.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 134.Becker ED, Fisk CL. NMR: physical principles and current status as a biomedical technique. Ann N Y Acad Sci. 1987;508:1–9. [Google Scholar]
- 135.Vaughan T, DelaBarre L, Snyder C, Tian J, Akgun C, Shrivastava D, Liu W, Olson C, Adriany G, Strupp J, Andersen P, Gopinath A, van de Moortele PF, Garwood M, Ugurbil K. 9.4T human MRI: preliminary results. Magn Reson Med. 2006 Dec;56(6):1274–82. doi: 10.1002/mrm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mangia S, Tkac I, Gruetter R, Van De Moortele PF, Giove F, Maraviglia B, Ugurbil K. Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magn Reson Imaging. 2006 May;24(4):343–8. doi: 10.1016/j.mri.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 137.Wald LL, Moyher SE, Day MR, Nelson SJ, Vigneron DB. Proton spectroscopic imaging of the human brain using phased array detectors. Magn Reson Med. 1995 Sep;34(3):440–5. doi: 10.1002/mrm.1910340322. [DOI] [PubMed] [Google Scholar]
- 138.Rothman DL, Behar KL, Hetherington HP, Den Hollander JA, Bendall MR, Petroff OAC, Shulman RG. IH_ observe/13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc Natl Acad Sci USA. 1985;82:1633–1637. doi: 10.1073/pnas.82.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Henry PG, Adriany G, Deelchand D, Gruetter R, Marjanska M, Oz G, Seaquist ER, Shestov A, Ugurbil K. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn Reson Imaging. 2006 May;24(4):527–39. doi: 10.1016/j.mri.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 140.Ross BD, Higgins RJ, Boggan JE, Willis JA, Knittel B, Unger SW. Carbohydrate metabolism of the rat C6 glioma. An in vivo 13C and in vitro IH magnetic resonance spectroscopy study. NMR Biomed. 1988;1:20–2. doi: 10.1002/nbm.1940010105. [DOI] [PubMed] [Google Scholar]
- 141.Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006 Nov 15;66(22):10855–60. doi: 10.1158/0008-5472.CAN-06-2564. [DOI] [PubMed] [Google Scholar]
- 142.Golman K, Zandt R, Thaning M. Real-time metabolic imaging. Proc Natl Acad Sci U S A. 2006;103(30):11270–5. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rohan M, Parow A, Stoll AL, Demopulos C, Friedman S, Dager S, Hennen J, Cohen BM, Renshaw PF. Low-field magnetic stimulation in bipolar depression using an MRI-based stimulator. Am J Psychiatry. 2004 Jan;161(1):93–8. doi: 10.1176/appi.ajp.161.1.93. [DOI] [PubMed] [Google Scholar]