Abstract
Pulmonary surfactant lowers surface tension in the lungs. Physiological studies indicate two key aspects of this function: that the surfactant film forms rapidly; and that when compressed by the shrinking alveolar area during exhalation, the film reduces surface tension to very low values. These observations suggest that surfactant vesicles adsorb quickly, and that during compression, the adsorbed film resists the tendency to collapse from the interface to form a three-dimensional bulk phase. Available evidence suggests that adsorption occurs by way of a rate-limiting structure that bridges the gap between the vesicle and the interface, and that the adsorbed film avoids collapse by undergoing a process of solidification. Current models, although incomplete, suggest mechanisms that would partially explain both rapid adsorption and resistance to collapse as well as how different constituents of pulmonary surfactant might affect its behavior.
1. Introduction
The original report that interfacial forces contribute to pulmonary elastic recoil speculated that the lungs might lower surface tension1 (γ) by producing a surfactant (von Neergaard, 1929; von Neergaard, 1976). Subsequent research has confirmed that suggestion. Rather than a single compound, however, pulmonary surfactant is a mixture of constituents, including a distinct set of phospholipids along with small amounts of cholesterol and specific proteins. After synthesis by the alveolar type II pneumocytes, assembly into multilamellar vesicles, and secretion into the liquid layer that lines the alveolus, the mixture of components together acts as a surfactant, adsorbing to the air/liquid interface and forming a film that lowers γ. The function of pulmonary surfactant is essential. Ventilation of lungs that lack adequate surfactant, both in premature babies (Robertson, 1984) and in experimental animals subjected to repeated lavage (Lachmann et al., 1980), damages the alveolocapillary barrier, leading to pulmonary edema, respiratory failure, and death.
This review focuses on the two most fundamental aspects of the surface activity demonstrated by physiological studies, that the surfactant vesicles adsorb rapidly, and that, when compressed by the decreasing alveolar surface area during exhalation, the resulting film reduces γ to exceptionally low levels. These two fundamental characteristics should be interdependent (Clements, 1977). Mechanisms that promote interfacial insertion of surfactants generally also accelerate the reverse process of collapse from the interface, limiting the surface tension that a compressed film can achieve. An understanding of how pulmonary surfactant functions in the lungs will ultimately require insights into the interaction between adsorption and stability during compression, and how constituents of surfactant promote one process without negatively affecting the other. Because the mechanisms by which pulmonary surfactant achieves either function currently remain unclear, in this review, each process will be considered separately.
2. Adsorption
In the lungs, interfacial films form quickly. Following the initial inspiration after birth, when babies first generate the air/water interface, pulmonary mechanics are normal within the initial or first few exhalations (Lachmann et al., 1979), indicating that the surfactant film has formed and is fully functional. In anesthetized adult animals ventilated at a low constant tidal volume, which reduces the effectiveness of the surfactant film (Faridy et al., 1966), a single large-volume inflation that stimulates the type II cells to secrete surfactant vesicles (Hildebran et al., 1981) returns pulmonary mechanics to normal during the subsequent breath (Nicholas et al., 1982). Both observations indicate that the surfactant vesicles must adsorb at least within seconds.
Pulmonary surfactant and classical molecular surfactants adsorb by processes that are distinct, both kinetically and thermodynamically. Classical surfactants adsorb as monomers, the concentration of which determines the final γ reached at equilibrium according to the Gibbs adsorption isotherm (Adamson and Gast, 1997b). At the critical micelle concentration (cmc), the interface reaches its point of saturation, and additional constituents instead aggregate to form micelles. These structures are themselves surface inactive and have essentially no effect on surface tension. Material added above the cmc produces minimal change in either the subphase-concentration of monomer or in the equilibrium γ. For the phospholipids that constitute most of pulmonary surfactant, the cmc's fall within the range of 10−10-10−9 M (Smith and Tanford, 1972; Marsh and King, 1986). In essentially all studies in vitro, and at estimated physiological concentrations, pulmonary surfactant is well above its cmc. Although not rigorously investigated, the final γ in practical experiments is likely to be constant. Factors such as composition and concentration may affect the kinetics of adsorption, but the thermodynamic value of the equilibrium γ is presumably fixed.
The low cmc's for the surfactant phospholipids also affect the kinetics of adsorption. Because concentrations are routinely far above the cmc, the vast proportion of pulmonary surfactant constituents aggregate into vesicles rather than existing as free monomers. The available evidence suggests that these vesicles represent the form in which surfactant constituents adsorb. Pulmonary surfactant can lower γ in discrete increments during adsorption, corresponding to the simultaneous insertion of large groups of molecules (Schürch et al., 1994). Direct microscopic observations have demonstrated intact vesicles inserting into the interface (Sen et al., 1988; Haller et al., 2004). The preponderance of evidence indicates that, rather than individual monomers, the constituents of pulmonary surfactant adsorb together as the components of complete vesicles.
In contrast to the interfacial insertion of molecular surfactants, adsorption of surfactant vesicles more closely resembles the fusion of two bilayers, such as occurs at multiple stages of intracellular trafficking and during the initial entry of an enveloped virus into an infected cell. Both adsorption and fusion involve the approach of a vesicle to another surface, followed by a major rearrangement of an initial bilayer to achieve the final structure. In both cases, the reconfiguration requires that the hydrophobic acyl groups, which in the bilayer are sequestered from the aqueous environment, must transiently have greater exposure to water. For the simplest molecular surfactants, the rate of adsorption can be limited only by transport to the interface (Miller and Kretzschmar, 1991). Vesicular adsorption, like the fusion of two vesicles, instead involves an energy of activation.
In both the fusion of biological bilayers and the adsorption of pulmonary surfactant, specific proteins play a key role. Of the four surfactant proteins (SP), the two collectins, SP-A and SP-D, contribute to innate immunity and perhaps to the extracellular trafficking of pulmonary surfactant rather than to its surface activity. Removal of the collectins by extraction with nonpolar solvents has little effect on biophysical function (Hall et al., 1992b). In contrast, SP-B and SP-C, which are sufficiently hydrophobic to partition with the surfactant lipids during extraction, promote adsorption. Separation of these proteins from the surfactant lipids, whether in the alveolus during ventilation (Wright et al., 1984) or chromatographically in vitro (Takahashi and Fujiwara, 1986; Hawgood et al., 1987; Hall et al., 1994b), greatly reduces the rate at which vesicles lower γ to its final value (~24 mN/m) (Hall et al., 1994a; Wang et al., 1996b).
SP-B is particularly effective in facilitating adsorption (Wang et al., 1996a). This protein is the only constituent of pulmonary surfactant proven to be essential for its biophysical function. Babies with congenital abnormalities (Nogee et al., 1993; Nogee et al., 1994) and genetically modified animals (Clark et al., 1995) that lack SP-B develop injured lungs during ventilation shortly after birth despite normal levels of the surfactant lipids. Ventilation similarly injures the lungs of adult animals if production of SP-B stops (Melton et al., 2003). Although other functions have been suggested for SP-B, its ability to promote the rapid formation of surfactant films represents the most likely explanation of its essential role.
The effect of the proteins, however, emphasizes a fundamental difference between adsorption and vesicular fusion. By lowering γ, adsorption reduces interfacial energy, which provides a thermodynamic force that drives the process. With fusion, the structures of the initial and final bilayers are often equivalent. Progression therefore frequently requires an apparatus constructed with the consumption of energy to drive fusion. For both intracellular vesicles and enveloped viruses, the fusion proteins can provide that apparatus (Lentz et al., 2000; Bonifacino and Glick, 2004; Earp et al., 2005). In contrast, adsorption proceeds perfectly well with a low-energy system formed by self-assembly. Vesicles formed by hydration of extracted surfactant or by construction within type II pneumocytes adsorb at similar rates (Hall et al., 1992b). The rate-limiting kinetic barriers should be comparable for adsorption and fusion, but because of the fundamental energetic driving force, progression of adsorption should require only a catalyst.
The first barrier to the fusion of two bilayers is the "hydration force" that limits their close approach (Creuwels et al., 1993; McIntosh and Simon, 1994; Rand and Parsegian, 2005). These forces result mostly from the undulation of the two surfaces, and seem likely also to affect adsorption by interfering with the approach of a surfactant vesicle either to a clean interface or to a nascent film. Inclusion of anionic phospholipids in the vesicles and calcium in the medium minimizes this barrier for fusion of bilayers (McIntosh and Simon, 1994), and also promotes the initial stages of adsorption (Walters et al., 2000), for which the presence of calcium is essential (Oosterlaken-Dijksterhuis et al., 1991). The parallel behavior has led to the speculation that SP-B might function like many fusion proteins by binding the two surfaces together. Different portions of SP-B might insert into the adsorbing membrane and the nascent film, tethering the two structures and promoting their interaction (Johansson and Curstedt, 1997). Evidence for or against such a mechanism is currently limited.
Once the vesicles reach the interface, their constituents must reorient to form the initial monolayer. Any model of this process must explain two fundamental observations:
Factors that promote adsorption produce a similar effect whether present in the adsorbing vesicle or confined to a preexisting film at the interface. This effect, first demonstrated for the surfactant proteins (Oosterlaken-Dijksterhuis et al., 1991), also occurs for the surfactant phospholipids. Relative to dipalmitoyl phosphatidylcholine (DPPC) alone, which represents the most prevalent constituent of surfactant from most species, the complete set of surfactant phospholipids produces faster adsorption, whether restricted to the vesicles or the interface (Walters et al., 2000). The effect of the phospholipids in the two locations is indistinguishable. These results suggest that a kinetic intermediate, the formation of which limits the rate of adsorption, must be equally accessible from both the vesicle and the interface.
Constituents produce predictable effects on adsorption according to how they affect the curvature of lipid leaflets. The relative cross-sectional areas of the hydrophilic and hydrophobic ends of a lipid determine an effective shape (Israelachvili et al., 1976). The spontaneous curvature of a lipid leaflet, obtained in the absence of applied force, results in turn from the average shape of its constituents (Gruner, 1989). In a bilayer, the individual leaflets fail to express their spontaneous curvature because of the canceling effect of the oppositely oriented, paired leaflet. In the inverted hexagonal (HII) phase, however, individual phospholipid leaflets form cylinders, each with an aqueous core, that stack into a hexagonal array, and that are free to express their spontaneous curvature. By convention, the sign of the curvature for a leaflet with a concave hydrophilic face, as occurs in the HII structures, is considered negative.
In lipid mixtures without the surfactant proteins, factors that promote formation of the HII phase, including both lipids (Yu et al., 1984; Perkins et al., 1996; Biswas et al., 2007) and peptides (Biswas et al., 2005), accelerate adsorption. Although this observation suggests that adsorption of phospholipids, like the fusion of vesicles, proceeds via a structure with negative curvature, adsorption of these model systems and of pulmonary surfactant could proceed along different pathways. Results with lysophosphatidylcholine are therefore particularly important. That compound, which forms positively curved micelles and can prevent formation of HII structures by other lipids, inhibits adsorption of pulmonary surfactant itself (Biswas et al., 2007). Negative curvature should therefore represent an important characteristic of the intermediate structure that limits the rate at which pulmonary surfactant adsorbs.
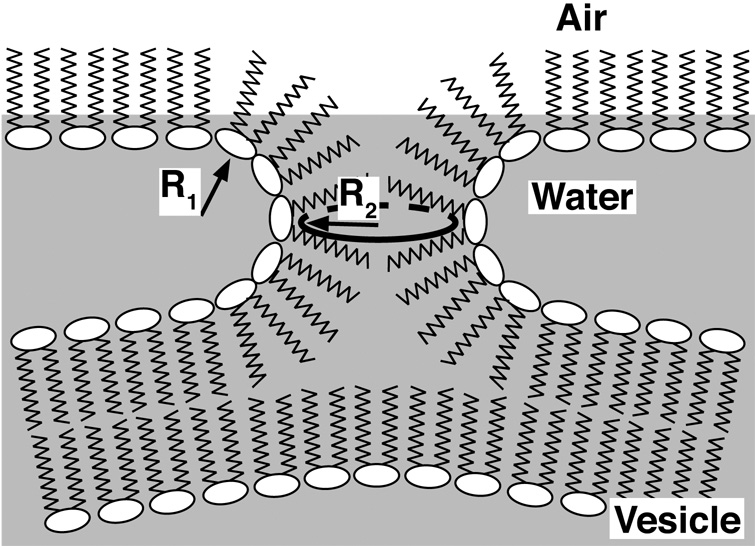
These observations concerning effects from the interface and the vesicle along with the role of curvature suggest a model in which adsorption proceeds by way of a structure that is directly analogous to the stalk-intermediate suggested as a key initial step in the fusion of two bilayers (Chernomordik and Kozlov, 2003). The negatively curved stalk would bridge the gap between the adsorbing vesicle and the air/liquid interface (Fig. 1). The model makes a number of predictions that fit with additional experimental results:
The importance of curvature suggests that the energy of bending represents a major component of the rate-limiting activation barrier. The effect of the proteins supports that possibility. The largest component of the free energy barrier that limits the rate of adsorption is entropic (King and Clements, 1972; Schram and Hall, 2001), consistent with a process that involves the exposure of hydrophobic acyl groups to the aqueous environment and the unfavorable entropy that provides the basis of the hydrophobic effect (Tanford, 1973). The hydrophobic surfactant proteins, however, accelerate the initial fall in γ by reducing the enthalpy of activation (Schram and Hall, 2001), consistent with a decrease in the energy of bending.
- Factors that reduce the energy of bending by any mechanism should promote adsorption. For curvatures (c) defined in terms of radii (R) according to c ≡ 1/R (Fig. 1), the energy per unit area, f, for limited bending of a thin continuous layer from its spontaneous curvature, co, to a new configuration with curvatures c1 and c2 along the two principal axes, is given by
where κ and κG are the elastic moduli of simple (splay) and Gaussian (saddle-splay) bending, respectively (Helfrich, 1973; Zimmerberg and Kozlov, 2006). In addition to factors that shift co toward the net curvature (c1 + c2) of the stalk, components that lower the rigidity of the leaflets, expressed by κ and κG, and allow them to deviate more easily from their spontaneous curvatures should also decrease the energy of bending. Cholesterol and the surfactant phospholipids other than DPPC, both of which should increase flexibility, increase rates of adsorption (Wang et al., 1996b). By lowering the energy barrier that limits the rate at which constituents transfer between adjacent structures and the interface, factors that promote adsorption should similarly facilitate desorption. Alveolar films undergo compression when surface area decreases during exhalation. When the density of the film exceeds an equilibrium value, constituents tend to collapse from the interface into adjacent bilayers (Amrein et al., 1997; Galla et al., 1998; Lipp et al., 1998; Schief et al., 2003). The hydrophobic surfactant proteins promote adsorption of constituents into the interface at high γ, and facilitate collapse from the interface at low γ (Lhert et al., 2007).
Figure 1. Structure of the hypothetical kinetic intermediate that limits the rate of adsorption.
R1 and R2 indicate the two principal radii of curvature for the stalk that connects the adsorbing vesicle with the nascent interfacial monolayer. These radii in turn define the principal curvatures (c), according to c ≡ 1/R. Because the standard frame of reference originates at the phosphate group and projects along the acyl tail, R1 and c1 have negative values. Modified with permission from (Walters et al., 2000; Schram and Hall, 2001).
Although the stalk-model fits with these several experimental observations, significant uncertainties remain, particularly concerning how the hydrophobic proteins achieve their effect. The extent to which the proteins alter either spontaneous curvature or the flexibility of simple (splay) bending is unknown. Because the stalk has two curvatures that have opposite signs, negative Gaussian curvature, (c1·c2), also represents a prominent feature (Fig. 1). Previous considerations suggested that κG should be small, but more recent evidence indicates that its magnitude could be significant (Siegel, 2005; Siegel, 2006). Effects by the proteins on co, κ or κG therefore seem equally possible.
The structure of the hydrophobic surfactant proteins has also provided only limited guidance concerning the molecular mechanisms by which they might alter any of these variables. Structural information on the functionally essential SP-B dimer has so far been restricted largely to predictions based on the sequence of the monomeric peptide, and on the homology of that sequence with the saposin-family of proteins, some of which have yielded detailed structures (Ahn et al., 2003; Bruhn, 2005; Hawkins et al., 2005; John et al., 2006). This information has not yet prompted specific proposals concerning how the proteins would stabilize the stalk-intermediate, or how they would accelerate adsorption via other hypothetical pathways.
Studies on the adsorption of pulmonary surfactant have generally suffered from the indirect methods used to detect adsorbed material. Most experiments have monitored adsorption by measuring γ, assuming that the equilibrium relationship between interfacial concentration and γ also holds during adsorption. This approach may be inadequate to explain kinetic anomalies observed during studies from several groups (Notter et al., 1983; Oosterlaken-Dijksterhuis et al., 1991; Schram and Hall, 2001), and in particular to follow the formation of adsorbed multilayers. Electron micrographs showed long ago that at least portions of the alveolar films are multilamellar (Hills, 1988; Schürch et al., 1995), but these static images provide no insight concerning how the structures form. Studies in vitro show that surfactant monolayers, when compressed by the decrease in surface area that occurs during exhalation, collapse from the interface into multilayers, and suggest that the multiple layers adjacent to the alveolar interface could form by collapse (Amrein et al., 1997; Galla et al., 1998; Schief et al., 2003). Recent studies using neutron reflection demonstrate that multilamellar films can also form by adsorption (Follows et al., 2007). These structures could be functionally significant. Detection of these and other structures not predicted by the simplest models of adsorption may require methods other than measurements of γ.
One final issue concerning the formation of the surfactant film is the relative importance of adsorption and respreading. Early studies with compressed monolayers, in the absence of subphase vesicles, showed that collapsed material has different tendencies, determined by composition, to respread into an expanding interface (Notter, 1984). This observation raises the possibility that during inhalation, constituents routinely enter the alveolar film by respreading as well as adsorption. The beneficial effect of free fatty acids on respreading (Tanaka et al., 1986) led to inclusion of palmitic acid in one of the first therapeutic surfactants. Subsequent evidence that collapsed bilayers can remain continuous with the interfacial monolayer (Amrein et al., 1997; Galla et al., 1998; Lipp et al., 1998; Schief et al., 2003) emphasizes that respreading requires no fusion for reinsertion into the surface, and that it represents a process distinct from adsorption by surfactant vesicles.
Determining the relative importance of respreading and adsorption in vivo is difficult. Although the initial formation of the film during the first breath after birth can occur only by adsorption, no comparable circumstance exists that would only allow respreading. In vitro experiments favor adsorption. Although both processes could contribute to the behavior of cyclically compressed adsorbed films, the strong dependence on subphase concentration (e.g., (Otis et al., 1994; Krueger and Gaver, 2000)), which should affect adsorption but not respreading, argues that adsorption represents the predominant process by which constituents enter the interface.
Adsorption and respreading, however, may be mechanistically comparable. Their compositional dependence is similar, with the hydrophobic proteins, unsaturated phospholipids, and cholesterol all producing better respreading as well as faster adsorption (Wang et al., 1995; Wang et al., 1996b). The bending-energy of a negatively curved kinetic intermediate might well represent the rate-limiting determinant for both processes.
3. Stability of the interfacial film
Adsorption of surfactant vesicles reduces γ to ~24 mN/m. In the lungs, alveolar γ reaches much lower values. When the adsorbed films are compressed by the decreasing alveolar surface area during exhalation, γ decreases to <5 mN/m, and perhaps to values below 1 mN/m (Fisher et al., 1970; Horie and Hildebrandt, 1971; Valberg and Brain, 1977; Wilson, 1981; Schürch, 1982; Smith and Stamenovic, 1986). Alveolar films also sustain these low γ for prolonged periods in static lungs after compression stops (Horie and Hildebrandt, 1971; Schürch, 1982). At the end of exhalation, when the high curvature of the small alveoli should maximize the contribution of interfacial forces to elastic recoil and the tendency to deflate the lungs, films of pulmonary surfactant come close to eliminating γ.
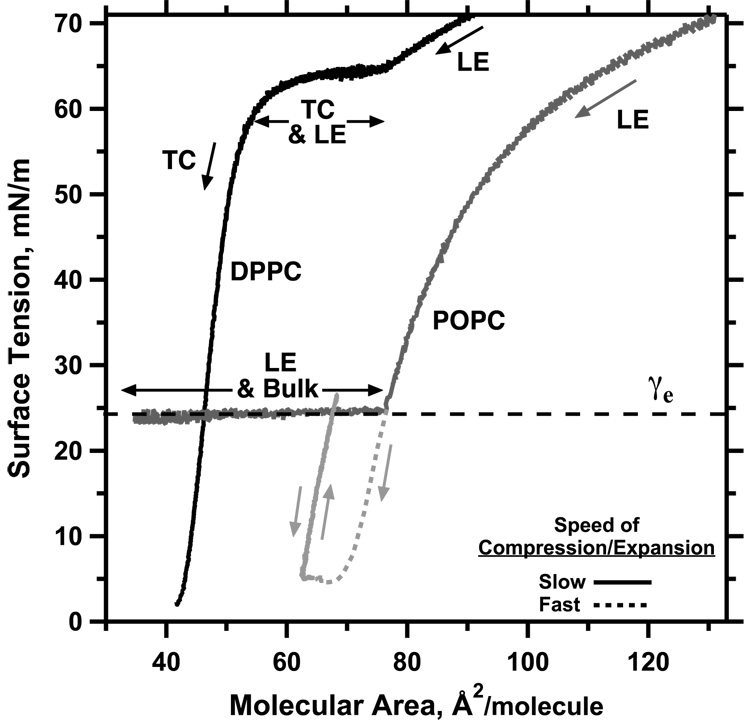
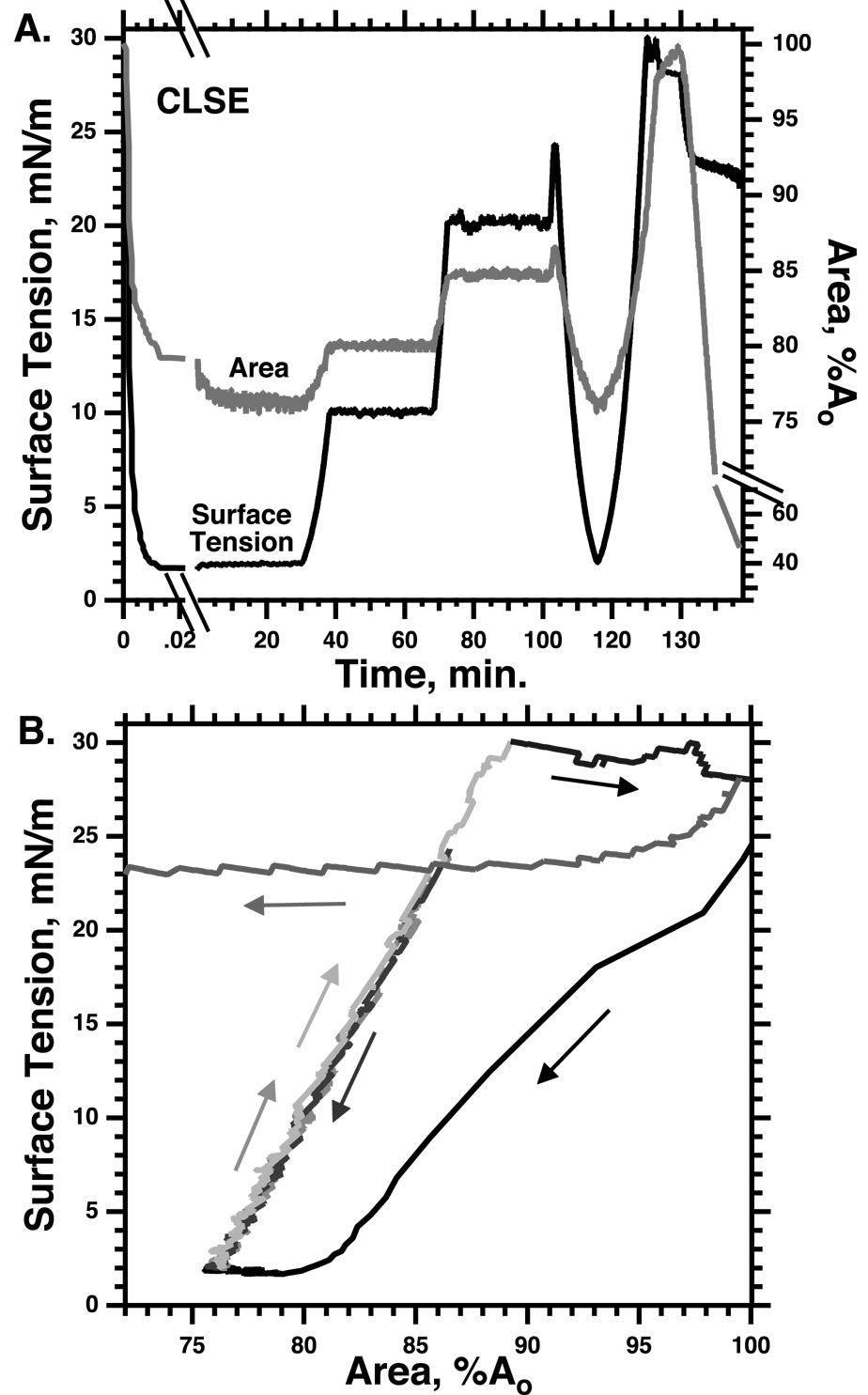
The ability of the compressed surfactant films to reach and sustain γ well below the values achieved during adsorption indicates that the films must have specific physical characteristics. Phospholipids added to an air/water interface in vitro, either by adsorption from the subphase or by direct deposition at the surface, initially lower γ by increasing the surface concentration of a monomolecular film. At ~24 mN/m, however, additional directly deposited material no longer affects the monolayer, and instead forms a new three-dimensional phase. The γ of a compressed film faces the same limitation (Fig. 2). During quasi-static compressions, decreasing the area of a monolayer lowers γ only to ~24 mN/m, at which point constituents collapse from the interface to form a 3D smectic liquid-crystal (e.g. POPC in Fig. 2). At this equilibrium spreading tension (γe), defined by the coexistence of the 2D film and the 3D phase (Gaines, 1966), further decreases in area enlarge the collapsed phase, but produce no further increase in the density of the monolayer or decrease in γ. The prolonged low γ in static lungs, well below γe, indicates that the alveolar films somehow avoid the phase transition of collapse, and that they are therefore metastable.
Figure 2. Phase behavior and metastability of phospholipid monolayers.
After spreading of films containing dipalmitoyl phosphatidylcholine (DPPC) or 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) at an air/water interface, area was changed at slow or fast rates (20 or 300 Å2/(molecule·min), respectively) at 26°C in the direction indicated by the single-headed arrows. Labels indicate the presence of liquid-expanded (LE), tilted-condensed (TC), and collapsed (bulk) phases detected microscopically in separate experiments. Horizontal double-headed arrows indicate regions of coexisting phases. POPC, which reaches the γe of 24 mN/m in the LE phase, has minimal effect on γ during further slow compression because of collapse (dark grey line). DPPC films, which at this temperature form the TC phase at γ ≈ 65 mN/m, sustain γ < γe without evidence of collapse above γ = 3 mN/m (black line). If compressed fast enough to reach low γ, supercompressed LE POPC transforms to a metastable film that also resists collapse over the full range of γ between γe and at least 5 mN/m (light grey lines). Modified with permission from (Yan et al., 2007).
The ability to avoid collapse indicates that films behave like solids. The defining characteristic of a solid is the inability to flow, with a viscosity that is immeasurably high (Halliday and Resnick, 1966; Goodstein, 1975; Barber and Loudon, 1989; Debenedetti, 1996a; Allen and Thomas, 1999). Resistance to collapse indicates that films are solid according to three criteria:
Films defined as solid by their two-dimensional shear viscosities avoid collapse. Two-dimensional films, like three dimensional materials, exist at specific thermodynamic conditions in different structural phases (Knobler, 1990; Kaganer et al., 1999). Films with disordered structures, such as phospholipid monolayers in the liquid-expanded (LE) phase, have low two-dimensional viscosities, and collapse readily at γe (e.g. POPC in Fig. 2). In the tilted-condensed (TC) phase, which has a highly ordered structure, approaching that of a two-dimensional crystal, shear viscosities are immeasurably high, and the films resist collapse (e.g. DPPC in Fig. 2). These prior measurements of 2D shear viscosities have established the correlation that fluid films readily collapse, and solid films do not.
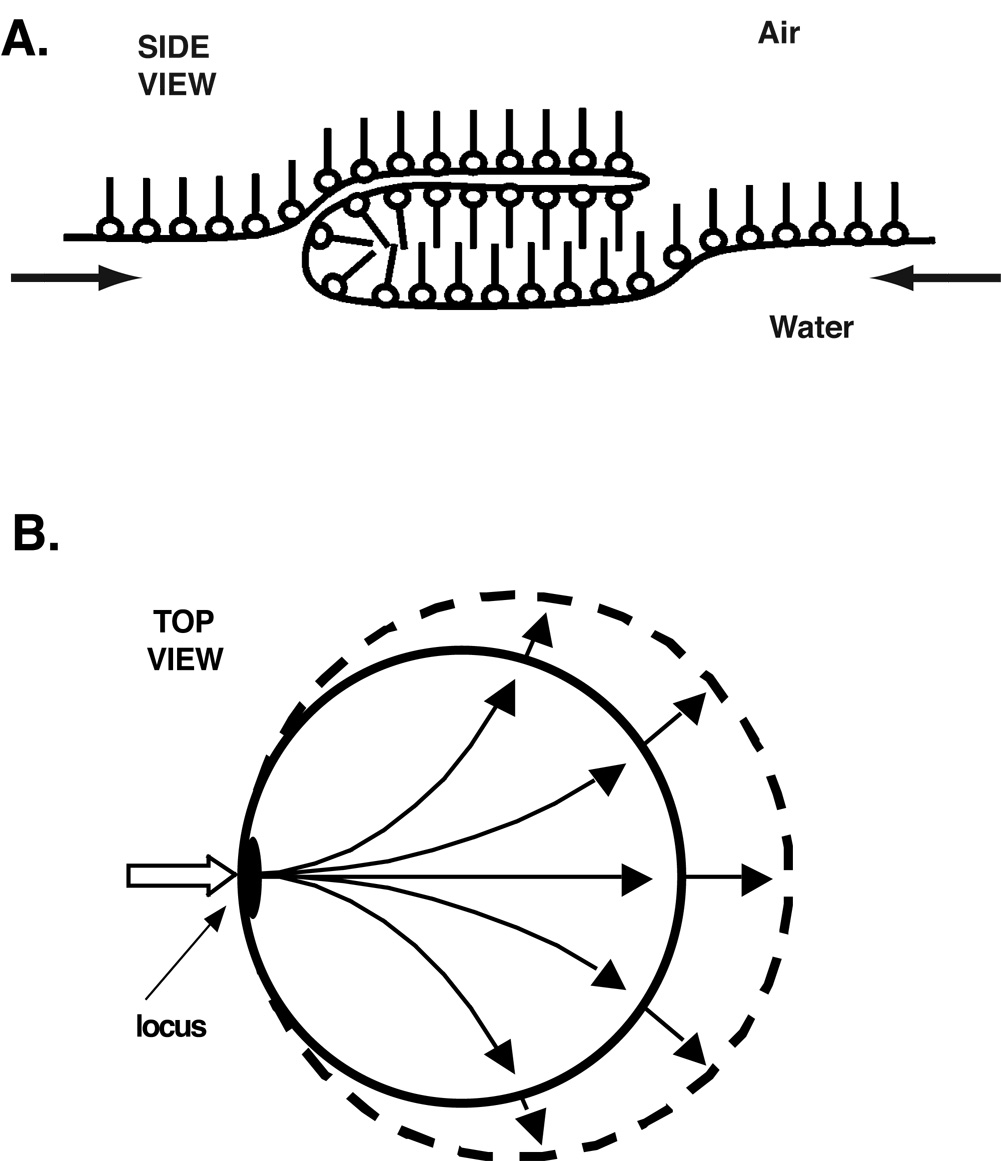
The specific mechanism by which phospholipids leave the interface indicates that resistance to collapse reflects a viscous film. In contrast to most phase transitions, in which constituents move individually across the boundary between the two phases, compounds that undergo liquid-crystalline collapse instead flow as a continuous sheet from the interfacial monolayer to the collapsed smectic liquid-crystal (Xue et al., 1992; de Mul and Mann, 1994; Friedenberg et al., 1994; Fang et al., 1997; de Mul and Mann, 1998; Schief et al., 2003) (Fig. 3). The avoidance of collapse in the lungs indicates that the alveolar film resists this flow, and that its viscosity must be high, characteristic of a solid.
-
Resistance to collapse by any mechanism defines the films as viscous. Movement of constituents from the interface into the third dimension, regardless of the process by which it occurs, represents a flow, which can define a viscosity (Rapp and Gruler, 1990). At the lowest γ, where the driving force for collapse is greatest, the absence of collapse in the alveolus again demonstrates the high viscosity that defines a solid film.
Two kinds of solid monolayers can replicate the behavior of alveolar films. The characteristics of the two films suggest that each is analogous to a 3D solid, specifically crystal and glass. Physiological mechanisms, however, that would generate either film remain unclear.
Figure 3. Mechanism of liquid-crystalline collapse.
Microscopic images show that constituents move as a continuous sheet from the monolayer to a stacked smectic liquid-crystal through a locus of restricted area (Schief et al., 2003). Adapted with permission from (Rugonyi et al., 2005).
3.1 Quasicrystalline monolayers and the classical model
Relative to other biological lipids, pulmonary surfactant contains an unusually high content of phospholipids with two saturated fatty acids. DPPC represents ~35–45% (mol:mol) of the surfactant phospholipids from most species (Kahn et al., 1995; Postle et al., 2001). The absence of acyl double bonds, and the kinks that they produce, has important structural and functional consequences. In contrast to the other surfactant phospholipids, DPPC at 37°C forms monolayers that convert from LE to TC structures above γe (Crane et al., 1999), and that, below γe, replicate the slow rates of collapse observed in the lungs (Fig. 2). The classical model of pulmonary surfactant concludes that the metastability of the alveolar film indicates that it must be TC (Watkins, 1968; Clements, 1977; Bangham et al., 1979).
That conclusion about structure has direct implications concerning composition. In films containing the surfactant phospholipids or model lipid mixtures, compression of the initially LE film forms a coexisting TC phase that contains >95% DPPC (Hildebran et al., 1979; Discher et al., 1999b; Yan and Hall, 2006). The TC phase can accommodate essentially no phospholipids other than DPPC. Rather than a mixture of constituents, the functional film of the classical model would contain effectively pure DPPC. A key feature of the classical model is therefore that, relative to the initial material secreted by the type II pneumocyte, the functional film must be substantially enriched in DPPC.
The change in composition could occur either by selective adsorption of DPPC or by selective exclusion of the other compounds. Because constituents of pulmonary surfactant insert collectively into the interface as the components of complete vesicles, a kinetic basis for selective adsorption has seemed unlikely. A thermodynamic basis might originate in differences among the γe for the various phospholipids, which would allow compounds with lower γe to continue adsorbing after other constituents have reached their point of saturation. The limited available data, however, argue against such a process. Phosphatidylcholines in the disordered state share a common γe of ~24 mN/m (Lee et al., 2001), but compounds in ordered structures, such as DPPC at 37°C, spread minimally, and reach γe just below γ for a clean interface (Horn and Gershfeld, 1977; Lee et al., 2001). Adsorption based on the γe of the individual constituents would allow the phospholipids other than DPPC to continue entering the interface long after insertion of DPPC stopped, selecting against, rather than for, DPPC.
Most investigators have instead focused on the possibility of selective exclusion (Watkins, 1968; Clements, 1977; Bangham et al., 1979). The existence of coexisting phases with distinct compositions and rates of collapse within the surfactant films would provide the basis for that selection. Films containing binary mixtures of dioleoyl phosphatidylcholine (DOPC) with DPPC provide a simple model system that demonstrates the expected behavior. The binary mixtures separate at γ > γe into coexisting LE and TC phases. When held at γ just below γe, collapse of the LE regions converts the mixture to a film that is TC and that contains only DPPC (Yan and Hall, 2006).
Results with model systems, however, point out that rates of collapse for fluid (LE) and solid (TC) phases are less distinct than previously thought. Fluid films, which were originally thought to collapse "essentially instantaneously" (Smith and Berg, 1980), in fact fold from the surface at finite rates that depend on composition and the specific experimental conditions. In contrast to the DOPC-DPPC films held at constant γ (Yan and Hall, 2006), in monolayers containing the complete set of surfactant phospholipids, continuous slow compression to low γ produces minimal change in the relative areas of the solid and fluid phases (Piknova et al., 2001). These experiments at 23°C leave open the possibility that at physiological temperatures, the rates of collapse for the two phases could still be sufficiently different to produce the proposed selective exclusion. The studies indicate, however, that results with model systems must be interpreted with increasing caution as they deviate progressively further from physiological compositions and conditions.
Selective exclusion seems unlikely to convert a monolayer containing the full set of surfactant-constituents to a TC film during physiological compressions. Three observations contribute to that conclusion:
The content of DPPC, although high in pulmonary surfactant relative to other biological phospholipids, is no greater than ~35–45% in most species (Kahn et al., 1995; Postle et al., 2001), and substantially lower in a few others (Lang et al., 2005). Even if constituents collapsed individually rather than collectively as regions of a film, formation of a TC monolayer would require at least a 55–65% reduction in area before an alveolar film would become capable of sustaining γ below 24 mN/m. This extent of compression would occur only during exhalation following the largest possible sigh that extends to total lung capacity (Bachofen et al., 1987).
-
The process by which phospholipids collapse would require phase separation as a basis for selective exclusion. In contrast to some other lipids, such as fatty acids, which move to the collapsed phase as individual constituents (Vollhardt and Retter, 1991; Vollhardt et al., 1991; Vollhardt and Retter, 1992; Retter and Vollhardt, 1993; Vollhardt et al., 1993), the phospholipids flow from the interface as regions of the monolayer (Schief et al., 2003). Selective exclusion would therefore require separation of the film into regions with distinct compositions and different tendencies to collapse.
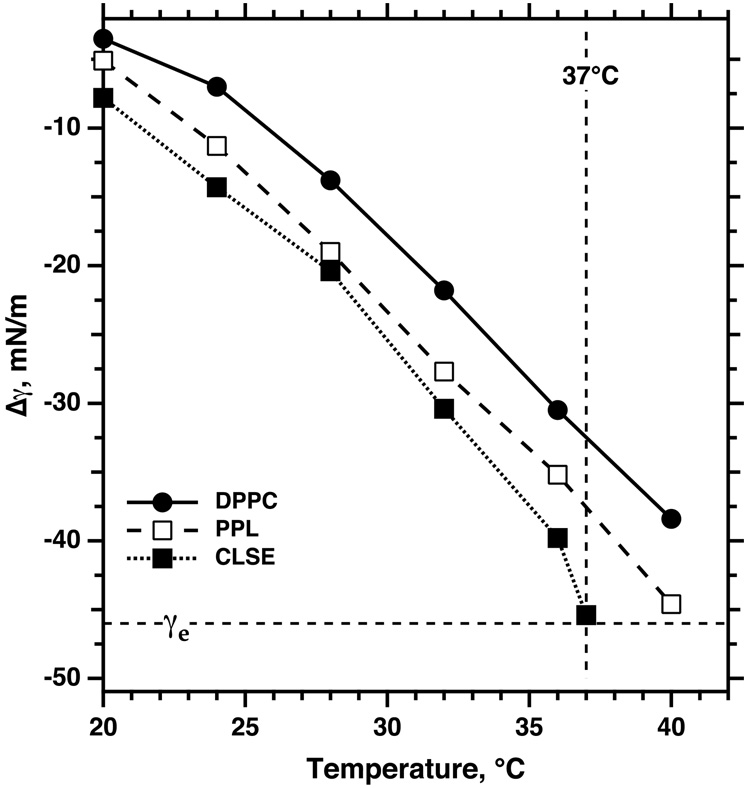
A separation of phases does occur within surfactant monolayers, but when collapse begins at 37°C, the LE phase occupies almost the entire interface (Discher et al., 1996). This minimal extent of coexistence reflects the effects of temperature and composition. To achieve the LE-TC phase transition, films of pure DPPC require compression to lower γ when temperatures are higher (Fig. 4) (Crane et al., 1999; Discher et al., 1999a). Additional constituents of pulmonary surfactant other than DPPC further delay the separation of phases (Fig. 4) (Discher et al., 1996; Discher et al., 1999a). Consequently, during continuous compression of extracted calf surfactant at 37°C, the coexisting phases first separate at γ just above γe, directly before the onset of collapse (Fig. 4) (Discher et al., 1996). If the LE phase collapsed completely and the other phase not at all, exclusion of the LE phase would require a decrease in area by ~96%
The coexistence in films containing the complete set of surfactant constituents is fluid-fluid rather than solid-fluid. This behavior, and the comparable process in bilayers (Bernardino de la Serna et al., 2004), reflects the presence of cholesterol. In films containing only the complete set of surfactant phospholipids, microscopy demonstrates an LE phase surrounding irregular TC domains that retain their shape at least for hours. Because the interfacial tension between the domains and the surrounding film should minimize the length of the interface, the failure of the domains to adopt a circular shape indicates that at least one phase is rigid. With the full complement of surfactant lipids, however, which includes cholesterol as well as the phospholipids, the domains instead have circular contours that can undergo rapid reconfiguration (Discher et al., 1999a; Discher et al., 2002), demonstrating that they have the fluidity of the liquid-ordered (LO) phase. Although not studied extensively, fluid LO films, like the LE phase, apparently collapse readily just below γe (Schief et al., 2003). The absence of solid regions in a surfactant monolayer eliminates the proposed basis by which selective exclusion could produce a TC film.
Figure 4. Temperature-dependence of the γ at which phase separation begins in monomolecular films related to pulmonary surfactant.
Monolayers containing extracted calf surfactant (calf lung surfactant extract, CLSE), the complete set of purified phospholipids (PPL) obtained chromatographically from CLSE, or DPPC were spread at an air/water interface and monitored microscopically during slow compression at specific temperatures. Symbols indicate the point at which microscopy first detected distinct domains in the initially homogeneous film. Because the γ of water, γo, varies with temperature, results are expressed in terms of the extent to which the film changes γ, given by Δγ = γ − γo. Adapted with permission from (Discher et al., 1999a).
In light of these considerations, the mechanisms by which physiological processes could produce the compositional refinement necessary to yield the TC film of the classical model are unclear.
3.3 Supercompressed fluid monolayers
Until recently, perhaps the strongest evidence favoring the classical model was the absence of any well defined film other than the TC monolayer that had the metastability observed in the lungs. Studies have now established, however, that if compressed fast enough to reach low γ, well below γe, fluid films can also become metastable (Fig. 2,5).
Figure 5. Transformation of surfactant monolayers at low γ.
Monomolecular films of extracted calf surfactant (calf lung surfactant extract, CLSE) at 37°C were compressed at >100% Ao/sec from an initial area (Ao) established at γ = 25 mN/m. After reaching γ < 5 mN/m, area remained essentially constant, indicating the absence of collapse, during incubation at the lowest γ, and after expansion to γ of 10 and 20 mN/m. Subsequent recompression and reexpansion without hysteresis similarly indicates the absence of collapse as long as γ remains below 25 mN/m. Expansion to 30 mN/m, at which γ remains roughly constant while area increases during respreading of the extracted surfactant, restores the ability of the film to collapse during slow compression. Modified with permission from (Crane and Hall, 2001).
3.3.1 Formation
The ability of rapidly compressed fluid films to reach low γ was not a complete surprise. Changes in the area of a film alter γ according to its viscoelasticity and the rate of compression. Under quasi-static conditions, the elasticity of a film provides the fundamental relationship, in the absence of collapse, between γ and the area of a static interface. During a dynamic compression, the response of γ also includes viscous effects. Above γe, surface viscosity reflects the rate at which compressed constituents can relax to their lowest energy configuration within the interface, changing surface area or γ or both. Below γe, relaxation also includes the effects of collapse into the third dimension. The relative rates of compression and viscoelastic relaxation determine how γ changes during a dynamic compression (Kato, 1990). If films are compressed more slowly than they can relax by collapse from the interface, the fluid films will fail to lower γ below γe. Fast compressions that exceed the rate of relaxation, however, will achieve lower γ. Because the driving force for collapse increases at γ progressively further below γe, relaxation should be faster at lower γ A sufficiently rapid compression should nonetheless reduce γ to very low values.
Unlike alveolar films in static lungs, however, as soon as compression stops, a film that retained its original fluid characteristics would return to its equilibrium density by collapse of constituents from the interface. Supercompressed fluid monolayers unexpectedly behave like the alveolar films. After compression to γ < 5 mN/m, supercompressed films, rather than relaxing back to γe, instead sustain those low γ for prolonged periods (Fig. 5) (Crane and Hall, 2001; Smith et al., 2003; Smith et al., 2004). Although a disparity between the characteristic times of compression and relaxation could explain how fluid films reach low γ (Kato, 1990), their subsequent slow collapse after compression requires that their fundamental properties have changed.
The supercompressed fluid films would nonetheless be physiologically irrelevant if they recovered their ability to collapse when expanded back to γe. In static lungs, alveolar films resist collapse not only at the lowest γ, but, when the lungs expand during inhalation, over the full range of γ below γe (Horie and Hildebrandt, 1971). The supercompressed fluid films replicate that behavior (Fig. 2,5). They retain their slow rates of relaxation despite return, during expansion, to conditions at which they originally collapsed (Crane and Hall, 2001; Smith et al., 2003; Smith et al., 2004) (Fig. 2,5). When held at high γ for sufficient durations, the supercompressed films eventually recover their original behavior (Fig. 5), but at γ just above γe, films containing only phospholipids retain their resistance to collapse for days (Smith et al., 2004). In the lung, if compressed fast enough to reach low γ during the exhalation after a sigh, a fluid monolayer would become transformed at low γ to a structure that resists collapse over the full range of γ below γe. During tidal breathing, the film would retain its metastability through repeated cycles of expansion and compression (Fig. 2,5).
3.3.2 Analogy to supercooled liquids
The defining characteristic of fluid films transformed by supercompression is their slow rate of relaxation. Although viscoelastic relaxation below γe could occur either by collapse or by effects within the monolayer, experimental results indicate that collapse is much more important. Following extensive, fast compressions from high γ to values just above γe, where collapse does not occur, area changes <2% (Smith et al., 2003; Rugonyi et al., 2005), indicating that viscoelastic relaxation within the interface is minimal. The much larger changes in area following compression to γ just below γe must reflect collapse. The slow collapse rates at low γ, however, also indicates a viscous film (Rapp and Gruler, 1990). Slow relaxation then indicates that at low γ, the viscosity of the fluid film must increase dramatically.
This characteristic of the supercompressed fluid films suggests that they fit within the spectrum of jammed systems (Liu and Nagel, 1998). Assemblies of granular (Richard et al., 2005) and colloidal particles (Pusey and van Megen, 1986), when compacted sufficiently, can retain an initial disordered structure, but acquire a markedly increased resistance to flow. Three dimensional liquids, if cooled quickly to sufficiently low temperatures, similarly become trapped in the metastable structure of a glass (Debenedetti, 1996b). The high viscosities induced by the low temperatures prevent the constituents from moving to their equilibrium location on a crystalline lattice, and despite temperatures well below the freezing point, they retain the disordered structure of the original liquid. Because of the high viscosities, the metastable liquids lose the ability to flow, and despite their amorphous structures, they acquire the rheological characteristics of a solid.
The supercompressed fluid films are analogous to supercooled liquids in several respects:
both systems avoid a phase transition (crystallization for the supercooled liquid, and collapse for the supercompressed surfactant film) and are, by definition, metastable;
both achieve the metastable state by undergoing rapid changes in thermodynamic conditions;
under equilibrium conditions, before reaching the phase transition, decreases in temperatures for the 3D liquids and in γ for the 2D fluid films both produce higher viscosities;
in both cases, deviation of conditions progressively further from equilibrium initially produces the expected progressive increase in the rate of the phase transition, which then surprisingly passes through a maximum value and decreases (Rugonyi et al., 2004) (Fig. 6);
both acquire the resistance to flow that defines a solid;
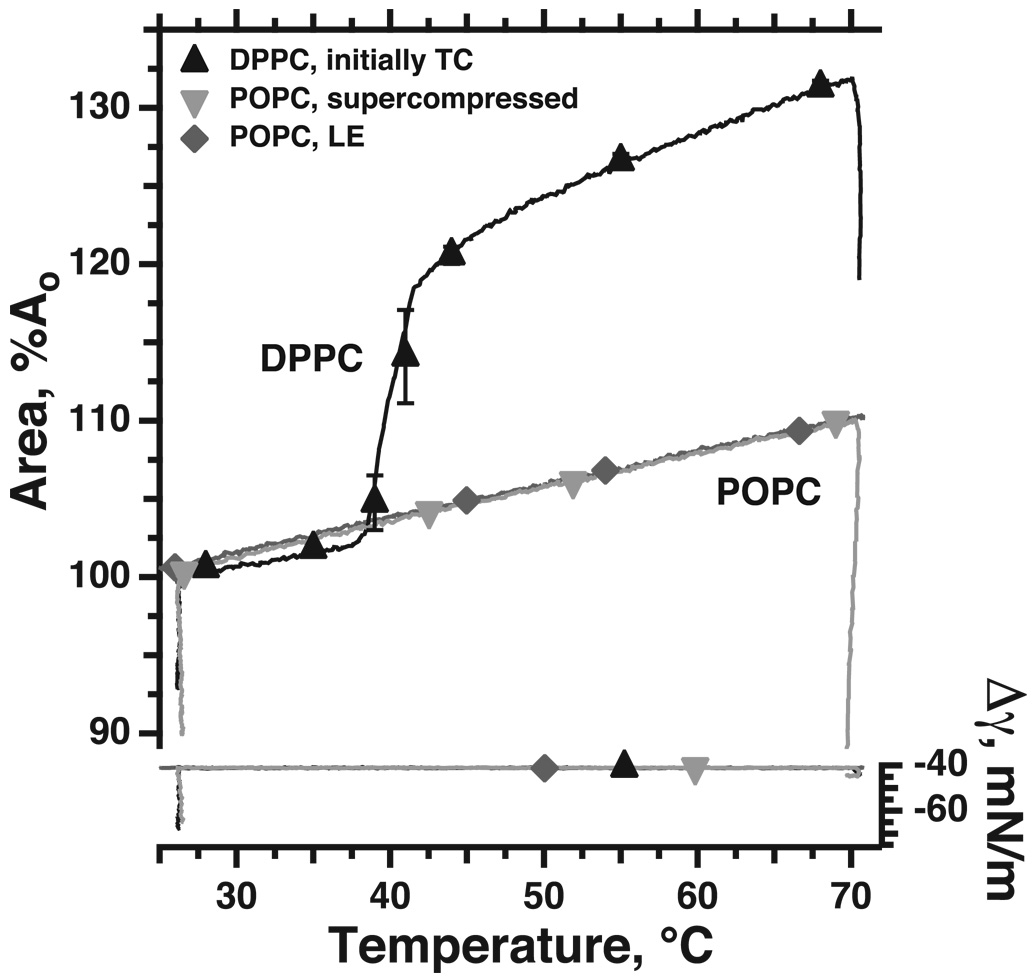
the change in rheological characteristics occurs for both systems apparently without a major change in structure. For the supercompressed films, results obtained during melting demonstrate this issue most clearly. When a TC film melts to a LE monolayer, the difference between the two structures dramatically expands the molecular area over a narrow range of temperatures (DPPC in Fig. 7). Supercompressed fluid films also melt during heating, as indicated by their recovered ability to collapse (Fig. 7, POPC, inverted triangles, Δγ on right axis). Heating of a supercompressed film, however, increases area only to the same extent as a heated fluid monolayer that has not been supercompressed (Fig. 7, POPC, upright and inverted triangles, left axis). These results suggest that despite their distinctly different tendencies to collapse, the structures of the initially fluid film before and after supercompression are similar (Fig. 7) (Smith et al., 2003).
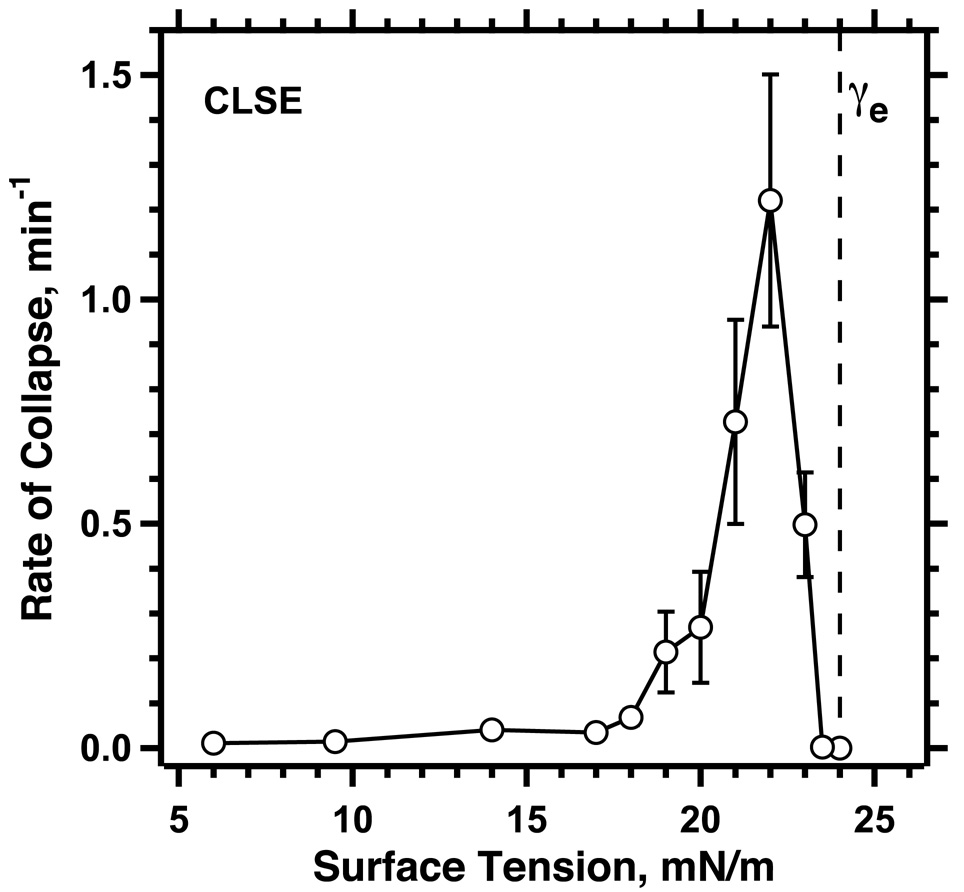
Figure 6. Rates of collapse at different γ < γe.
Monomolecular films of CLSE were compressed rapidly (32 min−1) at 37°C to specific γ, which were then held constant. Within a few seconds after reaching the constant γ, area decreased along a simple exponential function of time, the time-constant of which provided the rate of collapse. Symbols indicate mean ± S.D. Modified with permission from (Lhert et al., 2007)
Figure 7. Variation of area during the melting of TC and supercompressed fluid films.
Monomolecular films contained: TC DPPC; LE POPC not exposed to low γ; or LE POPC after supercompression to γ < 5 mN/m and return to γ = 30 mN/m. Because the γ of the clean air/water interface, γo, varies as a function of temperature, the films were held during heating at constant Δγ = γ − γo rather than at constant γ. Between 26 and 70°C, TC DPPC and supercompressed POPC both melted from a structure that sustained low γ without collapse (decrease in area with accompanying decrease in Δγ at 26°) to a structure that collapsed at Δγ = −45 mN/m (despite extensive decrease in area at 70°, Δγ reached only −45 mN/m), as expected for a fluid film. Melting of DPPC from the TC to the LE phase at 39–41°C abruptly expanded the film because of the larger molecular area for the LE phase. In contrast, during melting of the supercompressed POPC film, area expanded only to the same extent as POPC that had never reached low γ, indicating that the molecular areas for the films with and without supercompression are similar. Symbols indicate mean ± S.D. Modified with permission from (Smith et al., 2003).
Unlike the TC film of the classical model, the metastability of a supercompressed fluid film requires no compositional change. The characteristics established with supercompressed monolayers of extracted surfactant (Fig. 5) also occur for fluid films containing a single constituent, for which composition is fixed (Fig. 2) (Crane and Hall, 2001; Smith et al., 2003). A model of the alveolar film as a supercompressed fluid would avoid the question raised but unanswered by the classical model of how composition changes at the interface.
The supercompressed fluid model, however, does present a problem that is absent from the classical model. To become metastable, a fluid film must reach low γ, which requires compression faster than a certain threshold rate (Rugonyi et al., 2004). γ decreases below γe only if compression exceeds the rate at which the film can collapse from the interface. If compression and collapse occur at equal rates, γ will remain constant. Because the fastest collapse occurs at a γ only slightly below γe (Fig. 6) (Smith et al., 2003; Lhert et al., 2007), compression at a series of progressively faster rates initially produces little change in γ. As soon as compression exceeds the fastest rate of collapse, however, γ will continue decreasing to low values. The variation of collapse with γ (Fig. 6) predicts the sharply defined threshold rate of compression required to reach low γ that occurs for fluid films of both individual phospholipids (Smith et al., 2003) and the complete mix of extracted pulmonary surfactant (Crane and Hall, 2001; Lhert et al., 2007).
The immediate physiological question is whether ventilation normally generates the required threshold rate of compression. The threshold established in vitro for extracted calf surfactant seems surprisingly fast (Crane and Hall, 2001; Lhert et al., 2007). Information is limited concerning the rate at which alveolar area changes following a sigh, but in excised lungs, compressions much slower than the rates required in vitro produce functional films. After inflation from a degassed state, excised lungs have normal mechanics during the first slow deflation (Bermel et al., 1984; Hall et al., 1992a). These results suggest that in situ, compressions much slower than the threshold rates established in vitro can generate low γ and metastable films. If the alveolar film is a supercompressed fluid, then mechanisms must exist that reduce the threshold rate required in vitro to reach the low γ at which the monolayers become metastable.
The analogy to three-dimensional liquids suggests possible factors that might promote formation of the metastable films:
Minimized effects of contaminants: Contaminating dust particles disrupt formation of 3D glass during supercooling by providing heterogeneous nuclei that promote crystallization. The problem can be minimized by dividing the liquid into droplets that greatly outnumber the contaminating particles (Turnbull, 1949). Most of the liquid is then removed from the effects of the contaminant, allowing it to supercool more readily to form glass. In 2D films, which are notoriously susceptible to contamination (McConlogue and Vanderlick, 1997), a coexisting solid TC phase can divide the fluid LE phase into separated domains, analogous to 3D droplets. The fluid phase does collapse more slowly when present as separated domains than as a continuous structure (Yan and Hall, 2006). The effect, however, is sufficiently minor to be physiologically insignificant.
γe: Constituents added to 3D liquids can lower their freezing points and narrow the range through which the system must be cooled without crystallization to reach the temperature of the glass-transition. Additional constituents in 2D films might similarly facilitate transformation by supercompression if they reduce γe. The multiple phospholipids in pulmonary surfactant do produce a small shift in the onset of collapse relative to a single fluid phospholipid, but to a higher rather than lower γ, making transformation more difficult rather than easier (Yan et al., 2005).
Hydrophobic proteins: Specific constituents could also promote supercompression by stabilizing the film. Several studies with model lipids under nonphysiological conditions have suggested that the hydrophobic surfactant proteins significantly slow collapse (Cochrane and Revak, 1991; Longo et al., 1993; Lipp et al., 1996). With monolayers containing the complete set of surfactant lipids at 37°C, however, the presence of the hydrophobic proteins causes collapse that is slightly faster rather than slower (Lhert et al., 2007).
A final factor not suggested by supercooled liquids that might slow collapse is the presence of additional layers formed during adsorption (Follows et al., 2007). This possibility has yet to be tested directly. Adjacent layers formed by collapse rather than adsorption fail to produce stabilization. Monolayers of extracted surfactant and related lipids collapse to generate bilayer disks that appear just as frequently above previously collapsed disks as above the initial monolayer (Amrein et al., 1997; Galla et al., 1998; Schief et al., 2003). Additional layers, at least in these circumstances, provide no obvious deterrent to subsequent collapse.
The disparity between the threshold rates of compression required in vitro and in situ therefore remains unexplained. Like the TC films of the classical model, mechanisms by which the supercompressed fluid films might form in the alveolus are unclear.
3.4 Temperature-dependence
One feature that might distinguish which model better represents the alveolar film is temperature-dependence. A solid film that melts to a fluid structure during heating loses its resistance to collapse. The γ of an alveolar film in a heated lung would then rise to γe, and the interfacial component of the recoil forces that tend to deflate static lungs would increase. Correspondence of the melting-temperature for one solid monolayer (TC or supercompressed), but not the other, with temperatures at which pulmonary mechanics change would provide strong evidence for one of the models.
The melting-temperatures for the two kinds of solid monolayers are distinct (Yan et al., 2007). At γ < γe, supercompressed extracted surfactant melts significantly before TC DPPC, at lower temperatures that correspond well to changes in the mechanics of heated lungs from rats (Clements and Trahan, 1963; Clements, 1967; Clements, 1977). Unfortunately, however, lungs from cats and rabbits have pulmonary mechanics with different temperature-dependence (Horie et al., 1974; Inoue et al., 1981; Inoue et al., 1982). Because the pulmonary surfactant from the different species has an equivalent composition, the different physiological results are unexplained. Which form of solid film better represents the alveolar structure remains unresolved (Yan et al., 2007).
3.5 Role of subphase constituents
The inability to explain how either metastable monolayer could form suggests that the experiments in vitro lack some crucial element of the alveolar environment. The most obvious candidate is the presence of surfactant vesicles in the subphase. Both models of the metastable film are based on results with monolayers formed by directly depositing material in immiscible volatile solvents at the surface of a liquid that contains no vesicles. The resulting films microscopically resemble the monolayers formed initially during adsorption (Nag et al., 1998), and with low concentrations of vesicles, the behavior during compression is also similar. With high concentrations, however, adsorbed films become more stable (Schürch et al., 1989). Although direct comparisons of spread monolayers and films adsorbed from high subphase concentrations have not yet been published, the impressive performance at higher concentrations supports the possibility that material in the subphase provides an essential contribution to the film's stability.
The two models of the metastable film each suggest possible explanations for the contribution of the subphase material. The classical model predicts that the additional compounds provide a pool of excess DPPC that might, in response to some unknown driving force, exchange with interfacial constituents to produce the enrichment in DPPC necessary to form a TC film. The model of the supercompressed fluid instead predicts that by some unknown mechanism, the adjacent material slows collapse, and either allows physiological compressions to reach the low γ at which films become metastable, or produces a multilayered structure that is itself inherently resistant to collapse.
4. Conclusions
Fifty years after the existence of pulmonary surfactant was first demonstrated (Pattle, 1955; Clements, 1957), the behavior of the alveolar film is reasonably well documented. Recent evidence, however, has challenged well-established convictions concerning the basis of that behavior. Multilamellar films formed by adsorption suggest that the functional structure in the alveolus may contain more than a monolayer. The metastability of supercompressed LE films suggests that a compositional change at the interface with a major enrichment in DPPC may be unnecessary to yield a functional film. These observations, along with methods that, in a few cases, have come close to replicating the behavior of the alveolar film in vitro (Schürch et al., 1989), are yielding new models concerning the mechanistic basis of surfactant function. These models will guide studies that may provide a better understanding of the extent that pulmonary surfactant, which represents a fundamental element of pulmonary physiology, contributes to pathophysiology, and whether the remarkable success of therapeutic surfactants in the treatment of premature infants (Guyer et al., 1997) might extend to other disorders.
Acknowledgements
Supported by grants from the American Lung Association of Oregon, the Northwest Affiliate of the American Heart Association, and the National Institutes of Health (HL 03502, 54209, and 60914).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript uses the practice implicit in most physiological papers of defining surface tension as the force/length that tends to contract an interface between two bulk phases, with or without an intervening film. This definition differs subtly from the thermodynamic definition of surface tension as free energy/area (Adamson and Gast, 1997a), and extends to nonequilibrium systems (Bangham, 1992; Bangham, 1995).
References
- Adamson AW, Gast AP. Physical Chemistry of Surfaces. New York: Wiley; 1997a. p. 4. [Google Scholar]
- Adamson AW, Gast AP. Physical Chemistry of Surfaces. New York: Wiley; 1997b. pp. 71–74. [Google Scholar]
- Ahn VE, Faull KF, Whitelegge JP, Fluharty AL, Prive GG. Crystal structure of saposin B reveals a dimeric shell for lipid binding. Proc. Natl. Acad. Sci. U.S.A. 2003;100:38–43. doi: 10.1073/pnas.0136947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SM, Thomas EL. The Structure of Materials. J. New York: Wiley; 1999. p. 31. [Google Scholar]
- Amrein M, von Nahmen A, Sieber M. A scanning force and fluorescence light microscopy study of the structure and function of a model pulmonary surfactant. Eur. Biophys. J. Biophys. Lett. 1997;26:349–357. doi: 10.1007/s002490050089. [DOI] [PubMed] [Google Scholar]
- Bachofen H, Schürch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J. Appl. Physiol. 1987;62:1878–1887. doi: 10.1152/jappl.1987.62.5.1878. [DOI] [PubMed] [Google Scholar]
- Bangham AD, Morley CJ, Phillips MC. The physical properties of an effective lung surfactant. Biochim. Biophys. Acta. 1979;573:552–556. doi: 10.1016/0005-2760(79)90229-7. [DOI] [PubMed] [Google Scholar]
- Bangham AD. "Surface tension" in the lungs. Nature. 1992;359:110. doi: 10.1038/359110a0. [DOI] [PubMed] [Google Scholar]
- Bangham AD. "Surface tensions" in the lung. Biophys. J. 1995;68:1630–1633. doi: 10.1016/S0006-3495(95)80339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DJ, Loudon R. An Introduction to the Properties of Condensed Matter. Cambridge [England]: Cambridge University Press; 1989. pp. 21–23. [Google Scholar]
- Bermel MS, McBride JT, Notter RH. Lavaged excised rat lungs as a model of surfactant deficiency. Lung. 1984;162:99–113. doi: 10.1007/BF02715636. [DOI] [PubMed] [Google Scholar]
- Bernardino de la Serna J, Perez-Gil J, Simonsen AC, Bagatolli LA. Cholesterol rules: direct observation of the coexistence of two fluid phases in native pulmonary surfactant membranes at physiological temperatures. J. Biol. Chem. 2004;279:40715–40722. doi: 10.1074/jbc.M404648200. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Rananavare SB, Hall SB. Effects of gramicidin-A on the adsorption of phospholipids to the air-water interface. Biochim. Biophys. Acta. 2005;1717:41–49. doi: 10.1016/j.bbamem.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SC, Rananavare SB, Hall SB. Differential effects of lysophosphatidylcholine on the adsorption of phospholipids to an air/water interface. Biophys. J. 2007;92:493–501. doi: 10.1529/biophysj.106.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Bruhn H. A short guided tour through functional and structural features of saposin-like proteins. Biochem. J. 2005;389:249–257. doi: 10.1042/BJ20050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JA. Surface tension of lung extracts. Proc. Soc. Exp. Biol. Med. 1957;95:170–172. doi: 10.3181/00379727-95-23156. [DOI] [PubMed] [Google Scholar]
- Clements JA, Trahan HJ. Effect of temperature on pressure-volume characteristics of rat lungs. Fed. Proc. 1963;22:281. [Google Scholar]
- Clements JA. The alveolar lining layer. In: De Reuck AVS, Porter R, editors. Development of the Lung. Boston: Little, Brown and Company; 1967. pp. 202–228. [Google Scholar]
- Clements JA. Functions of the alveolar lining. Am. Rev. Respir. Dis. 1977;115(6 part 2):67–71. doi: 10.1164/arrd.1977.115.S.67. [DOI] [PubMed] [Google Scholar]
- Cochrane CG, Revak SD. Pulmonary surfactant protein B (SP-B):structure-function relationships. Science. 1991;254:566–568. doi: 10.1126/science.1948032. [DOI] [PubMed] [Google Scholar]
- Crane JM, Putz G, Hall SB. Persistence of phase coexistence in disaturated phosphatidylcholine monolayers at high surface pressures. Biophys. J. 1999;77:3134–3143. doi: 10.1016/S0006-3495(99)77143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Hall SB. Rapid compression transforms interfacial monolayers of ulmonary surfactant. Biophys. J. 2001;80:1863–1872. doi: 10.1016/S0006-3495(01)76156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuwels LA, Demel RA, van Golde LMG, Benson BJ, Haagsman HP. Effect of acylation on structure and function of surfactant protein C at the air-liquid interface. J. Biol. Chem. 1993;268:26752–26758. [PubMed] [Google Scholar]
- de Mul MNG, Mann JA. Multilayer formation in thin-films of thermotropic liquid-crystals at the air-water interface. Langmuir. 1994;10:2311–2316. [Google Scholar]
- de Mul MNG, Mann JA. Determination of the thickness and optical properties of a Langmuir film from the domain morphology by Brewster angle microscopy. Langmuir. 1998;14:2455–2466. [Google Scholar]
- Debenedetti PG. Metastable Liquids: Concepts and Principles. Princeton, N.J: Princeton University Press; 1996a. p. 9. [Google Scholar]
- Debenedetti PG. Metastable Liquids: Concepts and Principles. Princeton, N.J: Princeton University Press; 1996b. [Google Scholar]
- Discher BM, Maloney KM, Schief WR, Jr, Grainger DW, Vogel V, Hall SB. Lateral phase separation in interfacial films of pulmonary surfactant. Biophys. J. 1996;71:2583–2590. doi: 10.1016/S0006-3495(96)79450-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher BM, Maloney KM, Grainger DW, Sousa CA, Hall SB. Neutral lipids induce critical behavior in interfacial monolayers of pulmonary surfactant. Biochemistry. 1999a;38:374–383. doi: 10.1021/bi981386h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher BM, Schief WR, Vogel V, Hall SB. Phase separation in monolayers of pulmonary surfactant phospholipids at the air-water interface: composition and structure. Biophys. J. 1999b;77:2051–2061. doi: 10.1016/S0006-3495(99)77046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher BM, Maloney KM, Grainger DW, Hall SB. Effect of neutral lipids on coexisting phases in monolayers of pulmonary surfactant. Biophys. Chem. 2002;101:333–345. doi: 10.1016/s0301-4622(02)00191-6. [DOI] [PubMed] [Google Scholar]
- Earp LJ, Delos SE, Park HE, White JM. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 2005;285:25–66. doi: 10.1007/3-540-26764-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JY, Knobler CM, Yokoyama H. Layer growth in collapsed liquid crystal monolayers studied by scanning force microscopy. Physica A. 1997;244:91–98. [Google Scholar]
- Faridy EE, Permutt S, Riley RL. Effect of ventilation on surface forces in excised dogs' lungs. J. Appl. Physiol. 1966;21:1453–1462. doi: 10.1152/jappl.1966.21.5.1453. [DOI] [PubMed] [Google Scholar]
- Fisher MJ, Wilson MF, Weber KC. Determination of alveolar surface area and tension from in situ pressure-volume data. Respir. Physiol. 1970;10:159–171. doi: 10.1016/0034-5687(70)90080-0. [DOI] [PubMed] [Google Scholar]
- Follows D, Tiberg F, Thomas RK, Larsson M. Multilayers at the surface of solutions of exogenous lung surfactant: Direct observation by neutron reflection. Biochim. Biophys. Acta. 2007;1768:228–235. doi: 10.1016/j.bbamem.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Friedenberg MC, Fuller GG, Frank CW, Robertson CR. Formation of bilayer disks and 2-dimensional foams on a collapsing expanding liquid-crystal monolayer. Langmuir. 1994;10:1251–1256. [Google Scholar]
- Gaines GL. Insoluble Monolayers at Liquid-Gas Interfaces. New York: Interscience Publishers; 1966. pp. 144–151. [Google Scholar]
- Galla HJ, Bourdos N, von Nahmen A, Amrein M, Sieber M. The role of pulmonary surfactant protein C during the breathing cycle. Thin Solid Films. 1998;329:632–635. [Google Scholar]
- Goodstein DL. States of Matter. Englewood Cliffs, NJ: Prentice-Hall; 1975. p. 229. [Google Scholar]
- Gruner SM. Stability of lyotropic phases with curved interfaces. J. Phys. Chem. 1989;93:7562–7570. [Google Scholar]
- Guyer B, Martin JA, MacDorman MF, Anderson RN, Strobino DM. Annual summary of vital statistics--1996. Pediatrics. 1997;100:905–918. doi: 10.1542/peds.100.6.905. [DOI] [PubMed] [Google Scholar]
- Hall SB, Lu RZ, Venkitaraman AR, Hyde RW, Notter RH. Inhibition of pulmonary surfactant by oleic acid: mechanisms and characteristics. J.Appl. Physiol. 1992a;72:1708–1716. doi: 10.1152/jappl.1992.72.5.1708. [DOI] [PubMed] [Google Scholar]
- Hall SB, Venkitaraman AR, Whitsett JA, Holm BA, Notter RH. Importance of hydrophobic apoproteins as constituents of clinical exogenous surfactants. Am. Rev. Respir. Dis. 1992b;145:24–30. doi: 10.1164/ajrccm/145.1.24. [DOI] [PubMed] [Google Scholar]
- Hall SB, Hyde RW, Notter RH. Changes in subphase aggregates in rabbits injured by free fatty acid. Am. J. Respir. Crit. Care Med. 1994a;149:1099–1106. doi: 10.1164/ajrccm.149.5.8173747. [DOI] [PubMed] [Google Scholar]
- Hall SB, Wang Z, Notter RH. Separation of subfractions of the hydrophobic components of calf lung surfactant. J. Lipid Res. 1994b;35:1386–1394. [PubMed] [Google Scholar]
- Haller T, Dietl P, Stockner H, Frick M, Mair N, Tinhofer I, Ritsch A, Enhorning G, Putz G. Tracing surfactant transformation from cellular release to insertion into an air-liquid interface. Am. J. Physiol. 2004;286:L1009–L1015. doi: 10.1152/ajplung.00342.2003. [DOI] [PubMed] [Google Scholar]
- Halliday D, Resnick R. Physics. New York: Wiley; 1966. p. 423. [Google Scholar]
- Hawgood S, Benson BJ, Schilling J, Damm D, Clements JA, White RT. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28–36 in surfactant lipid adsorption. Proc. Natl. Acad. Sci. U.S.A. 1987;84:66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins CA, de Alba E, Tjandra N. Solution structure of human saposin C in a detergent environment. J. Mol. Biol. 2005;346:1381–1392. doi: 10.1016/j.jmb.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. 1973;28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Hildebran JN, Goerke J, Clements JA. Pulmonary surface film stability and composition. J. Appl. Physiol. 1979;47:604–611. doi: 10.1152/jappl.1979.47.3.604. [DOI] [PubMed] [Google Scholar]
- Hildebran JN, Goerke J, Clements JA. Surfactant release in excised rat lung is stimulated by air inflation. J. Appl. Physiol. 1981;51:905–910. doi: 10.1152/jappl.1981.51.4.905. [DOI] [PubMed] [Google Scholar]
- Hills BA. The Biology of Surfactant. New York: Cambridge University Press; 1988. pp. 222–235. [Google Scholar]
- Horie T, Hildebrandtz J. Dynamic compliance, limit cycles, and static equilibria of excised cat lung. J. Appl. Physiol. 1971;31:423–430. doi: 10.1152/jappl.1971.31.3.423. [DOI] [PubMed] [Google Scholar]
- Horie T, Ardila R, Hildebrandt J. Static and dynamic properties of excised cat lung in relation to temperature. J. Appl. Physiol. 1974;36:317–322. doi: 10.1152/jappl.1974.36.3.317. [DOI] [PubMed] [Google Scholar]
- Horn LW, Gershfeld NL. Equilibrium and metastable states in lecithin films. Biophys. J. 1977;18:301–310. doi: 10.1016/S0006-3495(77)85615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Inoue C, Hildebrandt J. Temperature and surface forces in excised rabbit lungs. J. Appl. Physiol. 1981;51:823–829. doi: 10.1152/jappl.1981.51.4.823. [DOI] [PubMed] [Google Scholar]
- Inoue H, Inoue C, Hildebrandt J. Temperature effects on lung mechanics in air- and liquid-filled rabbit lungs. J. Appl. Physiol. 1982;53:567–575. doi: 10.1152/jappl.1982.53.3.567. [DOI] [PubMed] [Google Scholar]
- Israelachvili JN, Mitchell DJ, Ninham BW. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc., Faraday Trans. 1976;II72:1525–1568. [Google Scholar]
- Johansson J, Curstedt T. Molecular structures and interactions of pulmonary surfactant components. Eur. J. Biochem. 1997;244:675–693. doi: 10.1111/j.1432-1033.1997.00675.x. [DOI] [PubMed] [Google Scholar]
- John M, Wendeler M, Heller M, Sandhoff K, Kessler H. Characterization of human saposins by NMR spectroscopy. Biochemistry. 2006;45:5206–5216. doi: 10.1021/bi051944+. [DOI] [PubMed] [Google Scholar]
- Kaganer VM, Möhwald H, Dutta P. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 1999;71:779–819. [Google Scholar]
- Kahn MC, Anderson GJ, Anyan WR, Hall SB. Phosphatidylcholine molecular-species of calf lung surfactant. Am. J. Physiol. 1995;13:L567–L573. doi: 10.1152/ajplung.1995.269.5.L567. [DOI] [PubMed] [Google Scholar]
- Kato T. What is the characteristic time of measurement of π-A isotherms? Necessity of a constant strain rate of compression of insoluble monolayers for π-A measurements. Langmuir. 1990;6:870–872. [Google Scholar]
- King RJ, Clements JA. Surface active materials from dog lung. III. Thermal analysis. Am. J. Physiol. 1972;223:727–733. doi: 10.1152/ajplegacy.1972.223.3.727. [DOI] [PubMed] [Google Scholar]
- Knobler CM. Seeing phenomena in flatland: studies of monolayers by fluorescence microscopy. Science. 1990;249:870–874. doi: 10.1126/science.249.4971.870. [DOI] [PubMed] [Google Scholar]
- Krueger MA, Gaver DP. A theoretical model of pulmonary surfactant multilayer collapse under oscillating area conditions. J. Colloid Interf. Sci. 2000;229:353–364. doi: 10.1006/jcis.2000.7029. [DOI] [PubMed] [Google Scholar]
- Lachmann B, Grossmann G, Nilsson R, Robertson B. Lung mechanics during spontaneous ventilation in premature and fullterm rabbit neonates. Respir. Physiol. 1979;38:283–302. doi: 10.1016/0034-5687(79)90055-0. [DOI] [PubMed] [Google Scholar]
- Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesth. Scand. 1980;24:231–236. doi: 10.1111/j.1399-6576.1980.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Lang CJ, Postle AD, Orgeig S, Possmayer F, Bernhard W, Panda AK, Jurgens KD, Milsom WK, Nag K, Daniels CB. Dipalmitoylphosphatidylcholine is not the major surfactant phospholipid species in all mammals. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005;289:R1426–R1439. doi: 10.1152/ajpregu.00496.2004. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim DH, Needham D. Equilibrium and dynamic interfacial tension measurements at microscopic interfaces using a micropipet technique. 2. Dynamics of phospholipid monolayer formation and equilibrium tensions at water-air interface. Langmuir. 2001;17:5544–5550. [Google Scholar]
- Lentz BR, Malinin V, Haque ME, Evans K. Protein machines and lipid assemblies: current views of cell membrane fusion. Curr. Opin. Struct. Biol. 2000;10:607–615. doi: 10.1016/s0959-440x(00)00138-x. [DOI] [PubMed] [Google Scholar]
- Lhert F, Yan W, Biswas SC, Hall SB. Effects of hydrophobic surfactant proteins on collapse of pulmonary surfactant monolayers. Biophys. J. 2007;93:4237–4243. doi: 10.1529/biophysj.107.111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp MM, Lee KYC, Zasadzinski JA, Waring AJ. Phase and morphology changes in lipid monolayers induced by SP-B protein and its amino-terminal peptide. Science. 1996;273:1196–1199. doi: 10.1126/science.273.5279.1196. [DOI] [PubMed] [Google Scholar]
- Lipp MM, Lee KYC, Takamoto DY, Zasadzinski JA, Waring AJ. Coexistence of buckled and flat monolayers. Phys. Rev. Lett. 1998;81:1650–1653. [Google Scholar]
- Liu AJ, Nagel SR. Jamming is not just cool any more. Nature. 1998;396:21–22. [Google Scholar]
- Longo ML, Bisagno AM, Zasadzinski JA, Bruni R, Waring AJ. A function of lung surfactant protein SP-B. Science. 1993;261:453–456. doi: 10.1126/science.8332910. [DOI] [PubMed] [Google Scholar]
- Marsh D, King MD. Prediction of the critical micelle concentrations of mono- and di-acyl phospholipids. Chem. Phys. Lipids. 1986;42:271–277. doi: 10.1016/0009-3084(86)90086-1. [DOI] [PubMed] [Google Scholar]
- McConlogue CW, Vanderlick TK. A close look at domain formation in DPPC monolayers. Langmuir. 1997;13:7158–7164. [Google Scholar]
- McIntosh TJ, Simon SA. Hydration and steric pressures between phospholipid bilayers. Annu. Rev. Biophys. Biomol. Struct. 1994;23:27–51. doi: 10.1146/annurev.bb.23.060194.000331. [DOI] [PubMed] [Google Scholar]
- Melton KR, Nesslein LL, Ikegami M, Tichelaar JW, Clark JC, Whitsett JA, Weaver TE. SP-B deficiency causes respiratory failure in adult mice. Am. J.Physiol. 2003;285:L543–L549. doi: 10.1152/ajplung.00011.2003. [DOI] [PubMed] [Google Scholar]
- Miller R, Kretzschmar G. Adsorption-kinetics of surfactants at fluid interfaces. Adv. Coll. Interface Sci. 1991;37:97–121. [Google Scholar]
- Nag K, Perez-Gil J, Ruano ML, Worthman LA, Stewart J, Casals C, Keough KM. Phase transitions in films of lung surfactant at the air-water interface. Biophys. J. 1998;74:2983–2995. doi: 10.1016/S0006-3495(98)78005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas TE, Power JH, Barr HA. The pulmonary consequences of a deep breath. Respir. Physiol. 1982;49:315–324. doi: 10.1016/0034-5687(82)90119-0. [DOI] [PubMed] [Google Scholar]
- Nogee LM, de Mello DE, Dehner LP, Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. New Engl. J. Med. 1993;328:406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- Nogee LM, Garnier G, Dietz HC, Singer L, Murphy AM, deMello DE, Colten HR. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J. Clin. Invest. 1994;93:1860–1863. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter RH, Finkelstein JN, Taubold RD. Comparative adsorption of natural lung surfactant, extracted phospholipids, and artificial phospholipid mixtures to the air-water interface. Chem. Phys. Lipids. 1983;33:67–80. doi: 10.1016/0009-3084(83)90009-9. [DOI] [PubMed] [Google Scholar]
- Notter RH. Surface chemistry of pulmonary surfactant: the role of individual components. In: Robertson B, van Golde LMG, Batenburg JJ, editors. Pulmonary Surfactant. Amsterdam: Elsevier; 1984. pp. 17–64. [Google Scholar]
- Oosterlaken-Dijksterhuis MA, Haagsman HP, van Golde LMG, Demel RA. Interaction of lipid vesicles with monomolecular layers containing lung surfactant proteins SP-B or SP-C. Biochemistry. 1991;30:8276–8281. doi: 10.1021/bi00247a024. [DOI] [PubMed] [Google Scholar]
- Otis DR, Jr, Ingenito EP, Johnson M. Dynamic surface tension of surfactant TA: experiments and theory. J. Appl. Physiol. 1994;77:2681–2688. doi: 10.1152/jappl.1994.77.6.2681. [DOI] [PubMed] [Google Scholar]
- Pattle RE. Properties, function and origin of the alveolar lining layer. Nature. 1955;175:1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- Perkins WR, Dause RB, Parente RA, Minchey SR, Neuman KC, Gruner SM, Taraschi TF, Janoff AS. Role of lipid polymorphism in pulmonary surfactant. Science. 1996;273:330–332. doi: 10.1126/science.273.5273.330. [DOI] [PubMed] [Google Scholar]
- Piknova B, Schief WR, Vogel V, Discher BM, Hall SB. Discrepancy between phase behavior of lung surfactant phospholipids and the classical model of surfactant function. Biophys. J. 2001;81:2172–2180. doi: 10.1016/S0006-3495(01)75865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle AD, Heeley EL, Wilton DC. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp. Biochem.Physiol. A Mol. Integr. Physiol. 2001;129:65–73. doi: 10.1016/s1095-6433(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Pusey PN, van Megen W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature. 1986;320:340–342. [Google Scholar]
- Rand RP, Parsegian VA. The forces between interacting bilayer membranes and the hydration of phospholipid assemblies. In: Yeagle P, editor. The Structure of Biological Membranes. Boca Raton, Fla: CRC Press; 2005. pp. 201–242. [Google Scholar]
- Rapp B, Gruler H. Phase transitions in thin smectic films at the air-water interface. Phys. Rev. A. 1990;42:2215–2218. doi: 10.1103/physreva.42.2215. [DOI] [PubMed] [Google Scholar]
- Retter U, Vollhardt D. Formation of lenticular nuclei from an insoluble monolayer at the air/water interface: a model. Langmuir. 1993;9:2478–2480. [Google Scholar]
- Richard P, Nicodemi M, Delannay R, Ribiere P, Bideau D. Slow relaxation and compaction of granular systems. Nature Materials. 2005;4:121–128. doi: 10.1038/nmat1300. [DOI] [PubMed] [Google Scholar]
- Robertson B. Pathology and pathophysiology of neonatal surfactant deficiency ("respiratory distress syndrome," "hyaline membrane disease") In: Robertson B, Van Golde LMG, Batenburg JJ, editors. Pulmonary Surfactant. Amsterdam: Elsevier Science Publishers; 1984. pp. 383–418. [Google Scholar]
- Rugonyi S, Smith ECC, Hall SB. Transformation diagrams for the collapse of a phospholipid monolayer. Langmuir. 2004;20:10100–10106. doi: 10.1021/la049081t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugonyi S, Smith EC, Hall SB. Kinetics for the collapse of trilayer liquid-crystalline disks from a monolayer at an air-water interface. Langmuir. 2005;21:7303–7307. doi: 10.1021/la0471083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schief WR, Antia M, Discher BM, Hall SB, Vogel V. Liquid-crystalline collapse of pulmonary surfactant monolayers. Biophys. J. 2003;84:3792–3806. doi: 10.1016/S0006-3495(03)75107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram V, Hall SB. Thermodynamic effects of the hydrophobic surfactant proteins on the early adsorption of pulmonary surfactant. Biophys. J. 2001;81:1536–1546. doi: 10.1016/S0006-3495(01)75807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürch S. Surface tension at low lung volumes: dependence on time and alveolar size. Respir. Physiol. 1982;48:339–355. doi: 10.1016/0034-5687(82)90038-x. [DOI] [PubMed] [Google Scholar]
- Schürch S, Bachofen H, Goerke J, Possmayer F. A captive bubble method reproduces the in situ behavior of lung surfactant monolayers. J. Appl. Physiol. 1989;67:2389–2396. doi: 10.1152/jappl.1989.67.6.2389. [DOI] [PubMed] [Google Scholar]
- Schürch S, Schürch D, Curstedt T, Robertson B. Surface activity of lipid extract surfactant in relation to film area compression and collapse. J. Appl. Physiol. 1994;77:974–986. doi: 10.1152/jappl.1994.77.2.974. [DOI] [PubMed] [Google Scholar]
- Schürch S, Qanbar R, Bachofen H, Possmayer F. The surface-associated surfactant reservoir in the alveolar lining. Biol. Neonate. 1995;67 Suppl. 1:61–76. doi: 10.1159/000244207. [DOI] [PubMed] [Google Scholar]
- Sen A, Hui S-W, Mosgrober-Anthony M, Holm BA, Egan EA. Localization of lipid exchange sites between bulk lung surfactants and surface monolayer: freeze fracture study. J. Colloid Interface Sci. 1988;126:355–360. [Google Scholar]
- Siegel DP. The relationship between bicontinuous inverted cubic phases and membrane fusion. In: Lynch ML, Spicer PT, editors. Bicontinuous Liquid Crystals. Boca Raton: Taylor & Francis; 2005. pp. 59–98. [Google Scholar]
- Siegel DP. Determining the ratio of the Gaussian curvature and bending elastic moduli of phospholipids from Q(II) phase unit cell dimensions. Biophys. J. 2006;91:608–618. doi: 10.1529/biophysj.106.085225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EC, Crane JM, Laderas TG, Hall SB. Metastability of a supercompressed fluid monolayer. Biophys. J. 2003;85:3048–3057. doi: 10.1016/S0006-3495(03)74723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EC, Laderas TG, Crane JM, Hall SB. Persistence of metastability after expansion of a supercompressed fluid monolayer. Langmuir. 2004;20:4945–4953. doi: 10.1021/la036150f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Stamenovic D. Surface forces in lungs. I. Alveolar surface tension-lung volume relationships. J. Appl. Physiol. 1986;60:1341–1350. doi: 10.1152/jappl.1986.60.4.1341. [DOI] [PubMed] [Google Scholar]
- Smith R, Tanford C. The critical micelle concentration of L-α-dipalmitoylphosphatidylcholine in water and water-methanol solutions. J. Mol. Biol. 1972;67:75–83. doi: 10.1016/0022-2836(72)90387-7. [DOI] [PubMed] [Google Scholar]
- Smith RD, Berg JC. The collapse of surfactant monolayers at the air-water interface. J. Colloid Interf. Sci. 1980;74:273–286. [Google Scholar]
- Takahashi A, Fujiwara T. Proteolipid in bovine lung surfactant: its role in surfactant function. Biochem. Biophys. Res. Comm. 1986;135:527–532. doi: 10.1016/0006-291x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Takei T, Aiba T, Masuda K, Kiuchi A, Fujiwara T. Development of synthetic lung surfactants. J. Lipid Res. 1986;27:475–485. [PubMed] [Google Scholar]
- Tanford C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes. New York: Wiley; 1973. [Google Scholar]
- Turnbull D. The subcooling of liquid metals. J. Appl. Phys. 1949;20:817. [Google Scholar]
- Valberg PA, Brain JD. Lung surface tension and air space dimensions from multiple pressure-volume curves. J. Appl. Physiol. 1977;43:730–738. doi: 10.1152/jappl.1977.43.4.730. [DOI] [PubMed] [Google Scholar]
- Vollhardt D, Retter U. Nucleation in insoluble monolayers. 1. Nucleation and growth model for relaxation of metastable monolayers. J. Phys. Chem. 1991;95:3723–3727. [Google Scholar]
- Vollhardt D, Retter U, Siegel S. Nucleation in insoluble monolayers. II: Constant pressure relaxation of octadecanoic acid monolayers. Thin Solid Films. 1991;199:189–199. [Google Scholar]
- Vollhardt D, Retter U. Nucleation in insoluble monolayers. 3. Overlapping effect of the growing centers. Langmuir. 1992;8:309–312. [Google Scholar]
- Vollhardt D, Ziller M, Retter U. Generalized nucleation-growth-collision theory for the formation of lenticular centers from insoluble monolayers. Langmuir. 1993;9:3208–3211. [Google Scholar]
- von Neergaard K. Neue auffassungen über einen grundbegriff der atemmechanik. Die retractionskraft der lunge, abhängig von der oberflaechenspannung in den alveolen. Z. Gesamte. Exp. Med. 1929;66:373–394. [Google Scholar]
- von Neergaard K. New notions on a fundamental principle of respiratory mechanics: the retractile force of the lung, dependent on the surface tension in the alveoli. In: Comroe JH, editor. Pulmonary and Respiratory Physiology. Stroudsburg, Pa: Dowden (distributed by Wiley); 1976. pp. 214–234. [Google Scholar]
- Walters RW, Jenq RR, Hall SB. Distinct steps in the adsorption of pulmonary surfactant to an air-liquid interface. Biophys. J. 2000;78:257–266. doi: 10.1016/S0006-3495(00)76589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hall SB, Notter RH. Dynamic surface activity of films of lung surfactant phospholipids, hydrophobic proteins, and neutral lipids. J. Lipid Res. 1995;36:1283–1293. [PubMed] [Google Scholar]
- Wang Z, Gurel O, Baatz JE, Notter RH. Differential activity and lack of synergy of lung surfactant proteins SP-B and SP-C in interactions with phospholipids. J. Lipid Res. 1996a;37:1749–1760. [PubMed] [Google Scholar]
- Wang Z, Hall SB, Notter RH. Roles of different hydrophobic constituents in the adsorption of pulmonary surfactant. J. Lipid Res. 1996b;37:790–798. [PubMed] [Google Scholar]
- Watkins JC. The surface properties of pure phospholipids in relation to those of lung extracts. Biochim. Biophys. Acta. 1968;152:293–306. doi: 10.1016/0005-2760(68)90037-4. [DOI] [PubMed] [Google Scholar]
- Wilson TA. Relations among recoil pressure, surface area, and surface tension in the lung. J. Appl. Physiol. 1981;50:921–930. doi: 10.1152/jappl.1981.50.5.921. [DOI] [PubMed] [Google Scholar]
- Wright JR, Benson BJ, Williams MC, Goerke J, Clements JA. Protein composition of rabbit alveolar surfactant subfractions. Biochim. Biophys. Acta. 1984;791:320–332. doi: 10.1016/0167-4838(84)90343-1. [DOI] [PubMed] [Google Scholar]
- Xue JZ, Jung CS, Kim MW. Phase transitions of liquid-crystal films on an air-water interface. Phys. Rev. Lett. 1992;69:474–477. doi: 10.1103/PhysRevLett.69.474. [DOI] [PubMed] [Google Scholar]
- Yan W, Piknova B, Hall SB. The collapse of monolayers containing pulmonary surfactant phospholipids is kinetically determined. Biophys. J. 2005;89:306–314. doi: 10.1529/biophysj.105.060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Hall SB. Distribution of coexisting solid and fluid phases alters the kinetics of collapse from phospholipid monolayers. J. Phys. Chem. B. 2006;110:22064–22070. doi: 10.1021/jp056989z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Biswas SC, Laderas TG, Hall SB. The melting of pulmonary surfactant monolayers. J. Appl. Physiol. 2007;102:1739–1745. doi: 10.1152/japplphysiol.00948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S-H, Harding PGR, Possmayer F. Artificial pulmonary surfactant. Potential role for hexagonal HII phase in the formation of a surface-active monolayer. Biochim. Biophys. Acta. 1984;776:37–47. [Google Scholar]
- Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]