Abstract
Repeated, intermittent exposure to the psychomotor stimulants amphetamine and cocaine induces a progressive and enduring augmentation of their locomotor-activating effects, known as behavioral sensitization, which is accompanied by similarly stable adaptations in the dendritic structure of cortico-striatal neurons. We examined whether repeated exposure to the increasingly abused amphetamine derivative 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) also results in long-lasting behavioral and morphological changes in mesocortical (medial prefrontal cortex) and ventral striatal (nucleus accumbens) neurons. Rats received 2 daily injections of either 5.0 mg/kg (±)-MDMA or saline vehicle, ~6 hr apart, for 3 consecutive days, followed by 4 drug-free days for a total of 3 weeks. Following a 4-week drug-free period, MDMA-pretreated rats displayed behavioral sensitization, as well as large increases in spine density and the number of multiple-headed spines on medium spiny neurons in core and shell subregions of nucleus accumbens. In medial prefrontal cortex, the prelimbic subregion showed increased spine density on distal dendrites of layer V pyramidal neurons, while the anterior cingulate subregion showed a change in the distribution of dendritic material instead. Collectively, our results show that long-lasting locomotor sensitization to MDMA is accompanied by reorganization of synaptic connectivity in limbic-cortico-striatal circuitry. The differential plasticity in cortical subregions, moreover, suggests that drug-induced structural changes are not homogeneous and may be specific to the circuitry underlying long-term changes in drug-seeking and drug-taking behavior.
Keywords: DENDRITIC SPINES, MEDIAL PREFRONTAL CORTEX, MORPHOLOGY, NUCLEUS ACCUMBENS, SENSITIZATION
Repeated, intermittent exposure to a wide range of drugs of abuse is associated with a progressive and enduring augmentation of their locomotor-activating effects (Segal and Mandell, 1974, Stewart and Badiani, 1993). This form of behavioral plasticity, termed sensitization, can persist for months or longer after the last drug exposure (Robinson and Becker, 1986, Paulson et al., 1991). Behavioral sensitization reflects many aspects of drug addiction (Kalivas and Stewart, 1991, Robinson and Berridge, 1993), including the propensity of addicts to relapse, even after long periods of abstinence (Mendelson and Mello, 1996, O’Brien, 1997). Elucidating similarly long-lasting neural adaptations following exposure to drugs of abuse may provide insight into the biological underpinnings of these behavioral changes.
Synaptic transmission in the mesolimbic pathway (the so-called ‘motive circuit’) is altered following repeated exposure to drugs of abuse (Pierce and Kalivas, 1997). Specifically, changes in excitatory limbic cortico-striatal transmission contribute to the expression of behavioral sensitization and addictive behaviors (Tzschentke and Schmidt, 2003, Kalivas, 2004). Indeed, reorganization of dendritic morphology in ventral striatum (nucleus accumbens; NAc) and medial prefrontal cortex (mPFC) is one of the longest lasting forms of drug-induced neural plasticity reported in the drug abuse literature (Robinson and Kolb, 2004). These structural changes parallel those observed following adaptive forms of learning and memory (Yuste and Bonhoeffer, 2001, Lamprecht and LeDoux, 2004), suggesting that drugs of abuse usurp mechanisms normally related to natural rewards (Hyman and Malenka, 2001, Kelley, 2004).
The popular “club” drug 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) is a widely used amphetamine (AMPH) derivative, especially by young adults (Pope et al., 2001, Strote et al., 2002), yet relatively little is known about the drug’s long-term neurobehavioral effects. Similar to AMPH, MDMA increases synaptic levels of monoamines following acute administration (Yamamoto and Spanos, 1988, Gough et al., 1991, Kankaanpaa et al., 1998) primarily via interaction with monoamine reuptake transporters (Nash and Brodkin, 1991, Crespi et al., 1997). MDMA can be distinguished from AMPH, however, based on its more potent serotonin (5-HT)-releasing activity (Crespi et al., 1997, Kankaanpaa et al., 1998). Although most users believe MDMA is a relatively safe drug (Murphy et al., 2006), MDMA administration induces phenomena indicative of exposure to highly addictive drugs, including conditioned place preference (Marona-Lewicka et al., 1996, Meyer et al., 2002), neurobehavioral sensitization (Kalivas et al., 1998, Ball et al., 2006), and drug self-administration (Beardsley et al., 1986, Ball et al., 2007).
We hypothesized that repeated administration of MDMA would result in enduring neurobehavioral changes similar to those that occur following exposure to highly addictive drugs of abuse, such as AMPH and cocaine. Specifically, we assessed 1) behavior to test whether MDMA-induced sensitization was evident one month following cessation of a treatment regimen that approximates human use patterns, and 2) morphology to determine whether the same treatment regimen resulted in long-lasting changes in the dendritic structure of NAc medium spiny neurons and layer V mPFC pyramidal neurons.
Experimental procedures
Animals
Data were collected from adult male Sprague-Dawley rats weighing 250–350 g at the commencement of experiments (first drug administration), which were bred from source animals supplied by Harlan Industries (Indianapolis, IN). Animals were housed individually under standard laboratory conditions (approximately 22°C room temperature; 12-hr light cycle from 7:30 AM to 7:30 PM) with ad libitum access to food and water. All procedures were conducted between 9:00 AM and 5:00 PM, were in compliance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (Academies, 2003), and approved by the Indiana University Institutional Animal Care and Use Committee.
Drug pretreatment
(±)-MDMA was provided by the National Institute on Drug Abuse (NIDA, Research Triangle Park, NC). The dose of 5.0 mg/kg (weight of salt; dissolved in 0.9% saline) was used for all injections during the pretreatment period. This is a dose we have used in previous studies (Ball et al., 2003, Ball and Rebec, 2005, Ball et al., 2006) because it is a relatively low dose, yet induces robust behavioral activation.
Separate groups of animals were used for the behavioral and morphological experiments in order to assess morphology following a 4-week drug-free period (rather than subsequent to an MDMA challenge injection, which would be necessary if the same animals were used); however, drug pretreatment was identical for both studies. Rats were divided into two groups and received 2 daily subcutaneous (s.c.) injections of either MDMA (5.0 mg/kg) or saline vehicle (1.0 ml/kg), ~6 hr apart, for 3 consecutive days, followed by 4 drug-free days for a total of 3 weeks. This drug treatment schedule approximates the usual intermittent pattern of human use (Pope et al., 2001, Winstock et al., 2001). Because contextual drug associations are important to the expression of behavioral sensitization to psychomotor stimulants, including MDMA (Ball et al., 2006), the first of the two daily injections during the third week (three injections total) was given in a sound-attenuating, open-field experimental chamber (1.2 m2) where an MDMA challenge injection was later administered in the behavioral study (see Experiment 1 below). Rats were left in the chamber for ~30 min after these injections. All other injections were administered in the home cage. After the last injection of the drug pretreatment regimen, rats remained untreated in their home cages for 4 weeks. This 4-week withdrawal period is similar to that used in seminal studies on the morphological effects of other drugs of abuse (Robinson and Kolb, 1997, 1999a, Robinson and Kolb, b), and allows for the characterization of relatively enduring neurobehavioral changes.
Experiment 1: behavior
Following the 4-week drug-free period, both MDMA- and saline-treated rats (n = 6/group) underwent MDMA challenge injections. Experimental sessions (120 min duration) began when rats were placed in the experimental chamber. After a 10-min habituation period, rats received a saline injection (1.0 ml/kg; s.c.). Twenty min after the saline injection, rats received a challenge injection of MDMA (2.5 mg/kg; s.c.) and were left in the chamber for another 90 min. A white light bulb located at the top of one wall illuminated the chamber, and a video camera mounted above the rat recorded experimental sessions for later analysis.
Behavioral data were analyzed as described previously (Ball et al., 2003). Briefly, a trained observer, blind to treatment conditions, viewed videotapes of experimental sessions and scored locomotion using event-recording software (BEST Collection, Educational Consulting Inc., Las Vegas, NV). The post-MDMA challenge period was divided into three 10-min epochs corresponding to early, middle, and late phases (5–15 min, 35–45 min, and 75–85 min post-challenge, respectively). Because statistical analysis revealed no treatment X time interaction, data from the three phases were collapsed and groups were compared by means of a t-test.
Experiment 2: morphology
At the 4-week withdrawal time point, rats (n = 6 saline and 5 MDMA) were overdosed with urethane and perfused with 0.9% saline. Brains were removed and processed using Glaser and Van der Loos’ modified Golgi-Cox stain (Glaser and Van der Loos, 1981). We have used this modification of the Golgi-Cox method extensively to reliably and quantitatively assess alterations in dendritic arborization in a variety of cortical and subcortical areas due to a variety of manipulations (e.g., Harmon and Wellman, 2003, Churchill et al., 2004, Cook and Wellman, 2004, Works et al., 2004, Izquierdo et al., 2006, Wellman et al., 2007). It is relatively impervious to the types of fixation artifacts that can result in incomplete staining of distal dendritic processes and therefore inconsistencies in numbers of neurons stained across animals using other techniques such as rapid Golgi (see Williams et al., 1978, Buell, 1982, Coleman and Flood, 1987). Briefly, tissue was immersed in Golgi-Cox solution for 10 days. This time was determined in pilot animals by developing test sections at regular intervals and assessing the presence of dendrites trailing off into a series of dots and thus minimizes the number of incompletely stained neurons (see Coleman and Flood, 1987). Brains were then dehydrated, infiltrated with a graded series of celloidins, and embedded in celloidin. Coronal sections were cut at 160 μm on a sliding microtome (Leica Histoslide 2000). Free-floating sections were alkalinized, developed, fixed, dehydrated, mounted, and coverslipped.
Medium spiny projection neurons from NAc core and shell subregions and layer V pyramidal neurons from anterior cingulate (AC) and prelimbic (PL) cortex were analyzed separately (see Fig. 1A). Medium spiny neurons were readily identifiable based on their characteristic morphology (12–20 μm diameter soma, dendrites that taper and become heavily laden with spines; see Fig. 1B, left), which is distinct from the few other cell types in striatum (Wilson and Groves, 1980). Pyramidal neurons within AC and PL were defined by the presence of a distinct, single apical dendrite, 2 or more basilar dendritic trees extending from the base of the soma, and dendritic spines (see Fig. 1B, right). Neurons selected for reconstruction were completely stained (see above), located in the middle third of the section, did not have truncated branches, and were unobscured by neighboring neurons and glia, with dendrites that were easily discriminable by focusing through the depth of the tissue. Numbers of neurons meeting these criteria did not vary significantly across animals or groups. For each region in each animal, 6–10 neurons (~ equal samples from each hemisphere) were drawn; this number yielded a mean (± S.E.M.) within-animal error of 12.18 (± 1.01)% (branch length). For each morphological measure, averages across neurons were calculated for each animal. Thus, data points for each animal represent the mean of all cells analyzed in the specified region. All neurons were drawn at 600X and morphology of dendrites was quantified in three dimensions using a computer-based neuron tracing system (Neurolucida, MBF Bioscience, Williston, VT), with the experimenter blind to condition. To assess overall changes in dendritic length and branching, total length and number of dendritic branches were compared between groups using independent measures t-tests. Differences in amount and location of dendritic material were assessed by counting the number of intersections of dendrites with an overlay of concentric rings centered on the soma (Sholl, 1956). These data were compared using two-way, repeated measures ANOVAs (treatment X distance from soma) plus Bonferroni post-tests.
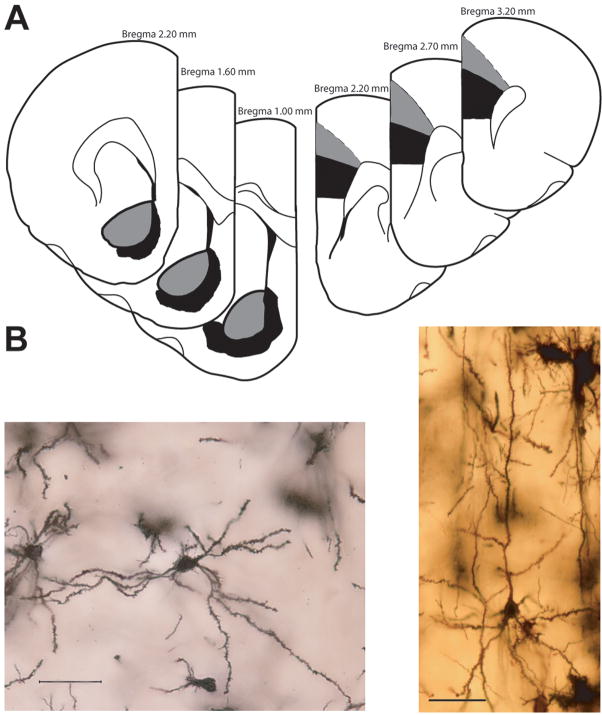
Figure 1.
(A) Schematic illustration of coronal sections through rat brain (modified from (Paxinos and Watson, 1998)). The portions of NAc (left) and mPFC (right) from which neurons were sampled are shown in gray and black for core and shell (NAc) and AC and PL (mPFC), respectively. Coordinates indicate position anterior to bregma. (B) Digital light micrographs of a Golgi-stained medium spiny neuron in the NAc (left) and mPFC (right) of a saline-treated rat. Four photomicrographs were taken at different Z-levels and merged to increase the number of dendritic branches in focus. Scale bar = 50 μm.
Spine density was also assessed. In AC and PL, separate analyses were performed for apical and basilar dendrites. For apical dendrites, spines were counted on apical trunk, oblique, and horizontal segments separately, as spine density varies across these dendritic compartments (Peters and Kaiserman-Abramof, 1970). Because at least 95% of basilar branches of AC and PL pyramidal cells and NAcc neurons of saline-treated rats were fourth-order or lower, only first through fourth order branches were analyzed. For each region, spines were counted from 8 neurons per rat, sampled in ~ equal numbers from each hemisphere. This number yielded a mean (± S.E.M.) within-animal error of 17.06 (± 0.95)%. For each neuron, one dendritic tree containing at least one first-, second-, and third-order dendrite (for basilar and NAcc dendrites; first-order dendrites were defined as the trunks that emerge directly from the soma) or at least one horizontal segment (for apical dendrites) was chosen. One to two branches at each order [mean (± S.E.M.) length = 26.24 (± 0.68) μm] were drawn and spines counted at 1000X. Spine counts at each branch order began at the branch node. Branches chosen for analysis had ~25 μm segments that were unobscured by overlapping branches in the same plane of focus. Spines were identified based on standard morphological criteria for “mushroom” and “thin” spines (Peters and Kaiserman-Abramof, 1970). Lengths of dendritic segments at each order were recorded, and spine densities (spines/10 μm) for each branch order were calculated. Comparisons between groups were made with two-way, repeated measures ANOVAs (treatment X branch order) followed by Bonferroni post-tests. At the same time spines were counted, multiple-headed spines, defined as spines with two or more heads connected to a common shaft (Comery et al., 1996), also were counted. Independent measures t-tests compared these data.
Results
Experiment 1
MDMA induces behavioral sensitization
As expected, a challenge injection of MDMA caused an increase in locomotion in both saline- and MDMA-pretreated rats. However, MDMA-pretreated rats showed a significantly augmented (sensitized) locomotor response to the MDMA challenge compared to saline-pretreated controls [Fig. 2; t(10) = 2.28, p < 0.05]. Notably, the locomotor response in sensitized rats was nearly twice as large (98% increase) as that of saline-pretreated animals.
Figure 2.
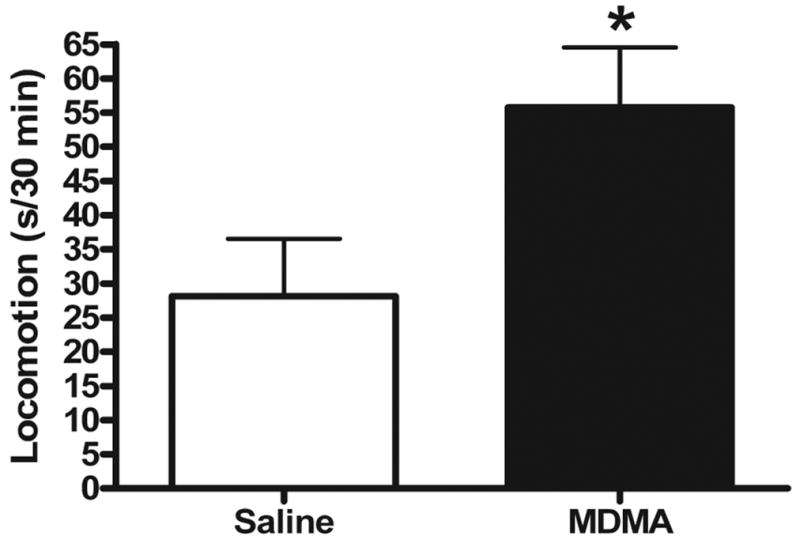
Locomotor response to MDMA challenge injection four weeks following repeated saline or MDMA administration (n = 6/group). Ten-min epochs corresponding to 5–15 min, 35–45 min, and 75–85 min post-challenge were collapsed in each group. MDMA-pretreated rats displayed a sensitized locomotor response to MDMA challenge (p < 0.05). Bars represent mean (+ S.E.M.).
Experiment 2
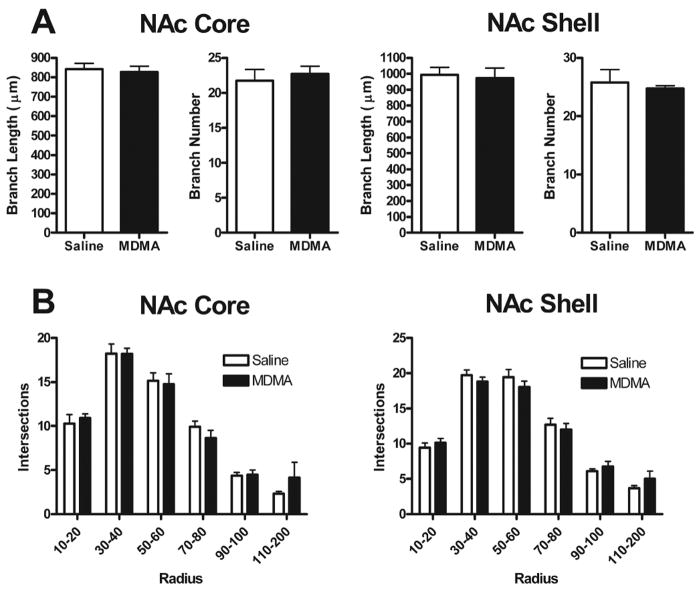
MDMA does not change NAc dendritic length or branching
To assess overall changes in dendritic length and branching, total length and number of dendritic branches were compared. There were no significant group differences for either measure in core or shell (Fig. 3A). To determine if MDMA treatment produced changes in the distribution of dendritic material, a Sholl analysis (Sholl, 1956) was performed, and again revealed no significant differences between treatment groups in either core or shell (Fig. 3B).
Figure 3.
(A) Mean (+ S.E.M.) branch length and number for NAc core (left) and shell (right) medium spiny neurons in saline- and MDMA-treated rats. No significant differences between groups were observed for either of these measures in either NAc subregion. (B) Mean (+ S.E.M.) intersections of dendrites with 10 μm concentric spheres. There were no significant differences in dendritic organization between saline- and MDMA-treated animals in either NAc core (left) or shell (right).
MDMA increases NAc spine density
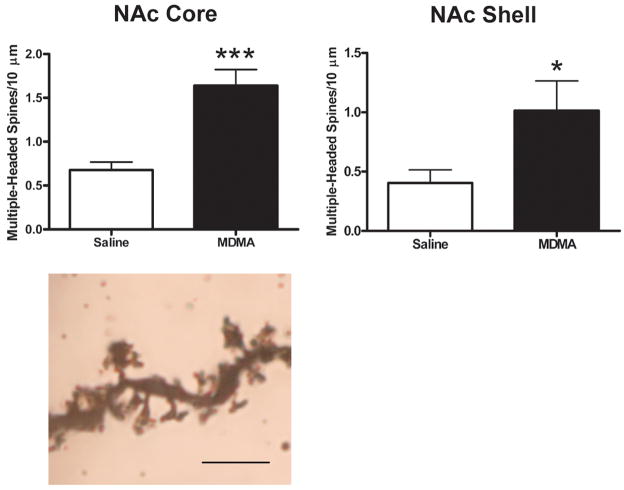
Spine density varied significantly with treatment in both core and shell [main effect of treatment, F(1,9) = 8.31, p < 0.05 and F(1,9) = 19.33, p < 0.01 for core and shell, respectively]. MDMA treatment increased spine density across all branch orders in both regions. These differences were quite large—62%, 65%, 47%, and 24% increases for first-, second-, third-, and fourth-order branches, respectively in core, and 20%, 103%, 51%, and 42% increases, respectively in shell (Fig. 4A). In NAc shell, there also was a significant interaction [F(3,9) = 6.35, p < 0.01], with a larger effect of MDMA treatment on more distal branches (second-, third-, and fourth-order). Additionally, visual inspection suggested that MDMA treatment was associated with a thickening of dendritic segments. Although this effect was not quantified, it is clearly evident in the photomicrographs in Fig. 4B, which were taken at the same magnification. Finally, MDMA treatment resulted in an especially large increase in the overall number of multiple-headed spines in both NAc core and shell [t(9) = 4.97, p < 0.001 and t(9) = 2.4, p < 0.05 for core and shell, respectively; see Fig. 5]. Compared to controls, MDMA-treated rats displayed increases in the density of multiple-headed core and shell spines of 142% and 150%, respectively.
Figure 4.
(A) Mean (+ S.E.M.) spine density on first-, second-, third-, and fourth-order branches of medium spiny neurons in NAc core (left) and shell (right) four weeks after repeated saline or MDMA treatment. MDMA-treated rats displayed consistent increases in spine density across all branch orders, averaging 50% and 54% for NAc core and shell, respectively (main effect of treatment, p < 0.05 and p < 0.01 for core and shell, respectively). In the case of NAc shell, there was a significant interaction (p < 0.01) in that the increases in spine density were larger on second-, third-, and fourth-order branches compared to first-order branches. *** indicates Bonferroni post-test, p < 0.001. (B) Photomicrographs of Golgi-stained dendritic segments from NAc core medium spiny neurons in saline- (left) and MDMA-treated (right) rats. Visual inspection suggested that, in addition to increases in spine density, MDMA treatment was associated with an apparent thickening of dendritic segments. Several photomicrographs were taken at different Z-levels and merged to increase the number of spines in focus. Scale bar = 10 μm.
Figure 5.
Overall density of multiple-headed spines in NAc core (left, top) and shell (right, top) in saline and MDMA-treated rats. MDMA treatment resulted in an especially large increase in the overall number of multiple-headed spines in both NAc core and shell subregions. All points represent mean (+ S.E.M.). * p < 0.05. *** p < 0.001. Bottom: Photomicrograph of Golgi-stained dendritic segment from NAc core in an MDMA-treated rat showing several multiple-headed spines. Several photomicrographs were taken at different Z-levels and merged to increase the number of spines in focus. Scale bar = 5 μm.
MDMA differentially alters the distribution of dendritic material in mPFC
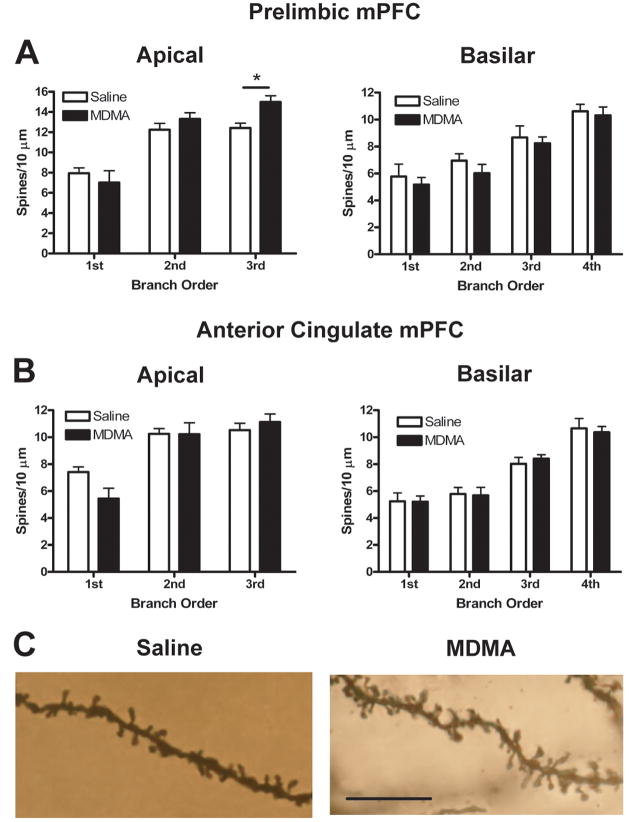
There were no significant group differences in total length or number of dendritic branches in either PL or AC mPFC (Figs. 6A and 6B, top). MDMA treatment, however, altered the distribution of dendritic material for both apical and basilar dendrites in AC, as revealed by a Sholl analysis (treatment-X-radius interaction, F(5,9) = 3.14, p < 0.05 and F(5,9) = 5.49, p < 0.001 for apical and basilar dendrites, respectively). This was manifest generally as an increase in dendritic material distal to the cell body and a decrease in dendritic material proximal to the cell body (Figs. 6B, bottom and 6C). Conversely, in PL the Sholl analysis revealed no significant effect of MDMA on the distribution of dendritic material for either apical or basilar dendrites.
Figure 6.

(A and B, top) Mean (+ S.E.M.) branch length and number for PL and AC layer V pyramidal neurons in saline- and MDMA-treated rats. No significant differences between groups were observed for either of these measures in either mPFC subregion. (A and B, bottom) Mean (+ S.E.M.) intersections of dendrites with 10 μm concentric spheres (data have been summed into 20 μm bins). MDMA treatment rats displayed altered dendritic organization in AC (treatment X radius interaction, p < 0.05 and p < 0.001 for apical and basilar dendrites, respectively), but not PL. * indicates Bonferroni post-test, p < 0.05. (C) Computer-assisted reconstructions of representative AC neurons four weeks after the last saline (left) or MDMA (right) injection. Scale bar = 25 μm.
MDMA differentially alters mPFC spine density
MDMA treatment had no significant effect on basilar spine density in either PL or AC. Apical spine density, however, was altered in a subregion-specific way. Thus, as Fig. 7 shows, spine density increased by 21% on third-order branches in PL and decreased by 27% on first-order branches in AC (treatment-X-branch order interaction, (F(2,9) = 7.93, p < 0.01 and F(2,9) = 5.79, p < 0.05 for PL and AC, respectively).
Figure 7.
Mean (+ S.E.M.) spine density on first- through third-order (left, apical) and first- through fourth-order (right, basilar) branches of layer V pyramidal neurons in PL (A) and AC (B) four weeks after repeated saline or MDMA treatment. MDMA treatment altered spine density in a subregion-specific way such that spine density increased by 21% on third-order apical branches in PL and decreased by 27% on first-order apical branches in AC (treatment X branch order interaction, p < 0.01 and p < 0.05 for PL and AC, respectively). * indicates Bonferroni post-test, p < 0.05. (C) Photomicrographs of Golgi-stained third-order apical dendritic segments from PL neurons in saline- (left) and MDMA-treated (right) rats. Several photomicrographs were taken at different Z-levels and merged to increase the number of spines in focus. Scale bar = 10 μm.
Discussion
Repeated, intermittent exposure to the recreational drug MDMA produced a long-lasting form of behavioral sensitization that was evident 4 weeks after the last drug exposure. In addition, the treatment regimen that produced this behavioral sensitization was associated with equally enduring and differential structural plasticity in NAc and mPFC. Specifically, MDMA-treated animals showed large increases in spine density and the number of multiple-headed spines on medium spiny neurons, the major output neurons in NAc. In PL mPFC, MDMA treatment resulted in increased spine density on distal dendrites of layer V pyramidal neurons, the output neurons in mPFC, while the AC showed a decrease in spine density on proximal dendrites and a change in the distribution of dendritic material. Collectively, these results show that MDMA produces a long-lasting form of behavioral plasticity, and that this behavioral change may be related to reorganization of limbic cortico-striatal synaptic connectivity.
Although it is possible that the ability of psychomotor stimulants to induce changes in spine density is simply secondary to their ability to increase locomotor activity, this possibility is very unlikely. In fact, to control for general locomotor activity, Robinson and Kolb (1999a) compared rats that were allowed access to a running wheel to rats that received either AMPH or cocaine treatment. As opposed to either drug treatment, wheel running experience had no effect on spine density in NAc. Thus, it appears that locomotor activation per se does not induce detectable alterations in spine density when compared to the effects of psychomotor stimulant administration.
MDMA-induced structural plasticity: comparison to other drugs
NAc
Exposure to the psychomotor stimulants cocaine, AMPH, and nicotine increases spine density on medium spiny neurons in NAc (Robinson and Kolb, 1997, 1999a, Brown and Kolb, 2001, Robinson et al., 2001), whereas morphine exposure decreases spine density in this region (Robinson and Kolb, 1999b, Robinson et al., 2002). Thus, our results with MDMA are consistent with a psychomotor stimulant-like effect on spine density in NAc, and the magnitude of this effect is comparable to what has been reported for other drugs in this class. Furthermore, MDMA treatment resulted in significantly larger increases in spine density on second- through fourth-order branches compared to first-order branches in the NAc shell. This result is similar to Li et al.’s (2003) finding that AMPH increases spine density on the distal, but not proximal, dendrites of medium spiny neurons in NAc shell and dorsal striatum.
In addition to changes in overall spine density, MDMA exposure was associated with especially large increases in the number of multiple-headed spines in both the core and shell of NAc, which parallels data on cocaine and AMPH administration (Robinson and Kolb, 1997, 1999a). Although the function of multiple-headed spines is not known, they likely play a key role in synaptic plasticity. For example, both long-term potentiation (Andersen and Soleng, 1998) and kindling (Geinisman et al., 1989) are associated with increases in multiple-headed spines in hippocampus, while rearing in a complex environment results in similar increases in striatum (Comery et al., 1996).
In contrast to the effects of MDMA on spine density, MDMA treatment had no effect on dendritic length, branching, or distribution in either the core or shell of NAc. These findings provide an interesting divergence from the effects of other psychomotor stimulants in that AMPH, cocaine, and nicotine all increased dendritic length and/or branching in NAc (Robinson and Kolb, 1997, 1999a, Brown and Kolb, 2001, Robinson et al., 2001). This difference may be due to the pharmacology of MDMA: relative to AMPH and nicotine, MDMA has a potent 5-HT-releasing action (Gough et al., 1991, Kankaanpaa et al., 1998). Cocaine, however, binds with high affinity to 5-HT transporters (Ritz et al., 1990), also resulting in relatively large increases in extracellular 5-HT concentrations (Bradberry et al., 1993, Teneud et al., 1996). Another explanation may relate to differences in drug treatment regimens. Protocols that involved escalating drug doses (Robinson and Kolb, 1997), extended or chronic daily treatments (Robinson and Kolb, 1999a, Brown and Kolb, 2001), or drug self-administration (Robinson et al., 2001) were used in studies that reported changes in dendritic organization. Therefore, a more aggressive treatment regimen of MDMA or simply a different pattern of MDMA administration might produce alterations in dendritic arborization in addition to changes in spine density.
mPFC
Similar to what has been reported in NAc, the mPFC shows increases in both spine density and dendritic branching following exposure to cocaine, AMPH, and nicotine (Robinson and Kolb, 1997, 1999a, Brown and Kolb, 2001, Robinson et al., 2001). In regard to AMPH, these effects are limited mainly to apical dendrites (Robinson and Kolb, 1997, 1999a). We found that MDMA exposure resulted in strikingly different changes in AC versus PL. These subregion-specific effects may be related to differences in afferent connections. For example, PL receives more dopamine (DA) afferents (Sesack et al., 1989, Berendse et al., 1992) and displays different DA dynamics in response to psychostimulants (Hedou et al., 1999, Hedou et al., 2001) compared to AC. Although DA innervation in rat PFC is most dense in the deep layers (V and VI), the increased DA innervation in PL compared to AC is most prominent in the superficial layers of cortex (Van Eden et al., 1987, Kalsbeek et al., 1988). Interestingly, the increases in spine density we report in PL occurred only on the third-order branches of apical dendrites, many of which may extend into the superficial layers of cortex. Moreover, it was recently shown that 6-hydroxydopamine lesions of the ventral tegmental area, which remove most of the DA input to PFC, result in a decrease in spine density in layer V PL pyramidal cells (Wang and Deutch, 2008), further supporting a role for DA in changes in mPFC spine density.
To our knowledge, this is the first comparison of drug-induced alterations in dendritic structure in discrete subregions of mPFC; however, differential drug-induced structural changes have been reported in mPFC compared to other regions of PFC. For example, Robinson and colleagues (Robinson et al., 2002, Crombag et al., 2005) found that rats with AMPH self-administration experience displayed increased spine density on pyramidal cells in mPFC, but decreased spine density in orbital PFC. Also, morphine exposure was associated with decreased spine density in mPFC, but increased spine density in orbital PFC. Our results support and extend these findings by showing that even within closely related regions of mPFC striking differences in drug-induced structural changes can be observed.
A recent study in nonhuman primates showed that a sensitizing regimen of AMPH resulted in a > 3 year reduction in spine density on the apical trunk of dorsolateral PFC (medial PFC in rats) pyramidal neurons (Selemon et al., 2007). These data, along with our results in AC, suggest that pyramidal cell dendrites proximal to the soma may be uniquely prone to reductions in spine density following psychostimulant administration compared to dendrites that are more distal to the soma. Because Selemon et al. (2007) reported no increases in spine density, it is possible that certain morphological changes induced by psychomotor stimulants (e.g., increases in spine density on distal dendrites) are no longer evident following a 3-year drug-free state, whereas other changes (e.g., decreases in spine density on proximal dendrites) are remarkably enduring. While this is an intriguing possibility, more studies are necessary to determine the exact timecourse of morphological changes following abstinence from repeated drug administration.
Behavioral sensitization, cortico-striatal plasticity, and addiction
Behavioral sensitization to cocaine (Bell and Kalivas, 1996, Pierce et al., 1996) and reinstatement of cocaine seeking (Cornish and Kalivas, 2000, Park et al., 2002) both involve increased α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid (AMPA) receptor-mediated transmission in NAc, and previous exposure to AMPH is associated with enhanced reinstatement of drug seeking induced by intra-NAc infusions of AMPA (Suto et al., 2004). Drug-induced increases in spine density, such as those reported here, may be a morphological substrate for this increased transmission at AMPA receptors, a modification that could potentially enhance information flow through relevant neurobehavioral circuits. Because DA functions to increase the signal-to-noise ratio at cortico-striatal synapses (Servan-Schreiber et al., 1990, Rebec, 1999), drug-induced sensitization of DA release (Kalivas et al., 1998, Vanderschuren and Kalivas, 2000) would be expected to decrease the threshold required for a behaviorally relevant stimulus to engage these circuits (Kiyatkin and Rebec, 1996). This could explain how, through repeated exposure, drugs and drug-associated cues come to acquire increased salience and the ability to induce craving and relapse in humans (Jaffe et al., 1989, O’Brien et al., 1992).
Although the functional significance of differential drug-induced structural changes in AC vs. PL is not known, they are interesting in light of previous findings. For example, increased glutamate transmission in NAc during drug seeking appears to be driven by input from mPFC (Park et al., 2002, McFarland et al., 2003). Compared to AC, which projects largely to dorsal parts of striatum, PL (the area in which we report MDMA-induced spine increases) sends efferents to NAc (Sesack et al., 1989, Berendse et al., 1992). There also is evidence of mPFC involvement in the development and expression of behavioral sensitization to both cocaine and MDMA (Tzschentke and Schmidt, 2000, Ramos et al., 2005). Moreover, discrete subregion-specific lesions of mPFC showed that only PL (not AC or infralimbic cortex) was involved in cocaine-induced behavioral sensitization (Tzschentke and Schmidt, 1999, 2000). Finally, glutamate transmission in the ventral tegmental area, the source of mesolimbic DA, regulates both behavioral sensitization to psychostimulants and cocaine seeking (Vanderschuren and Kalivas, 2000, Sun et al., 2005); PL sends approximately three times as many efferents to the ventral tegmental area as does AC (Geisler et al., 2007). Thus, the MDMA-induced increases in mPFC spine density appear to be located in a region strategically positioned to regulate drug-seeking and drug-taking behavior.
Finally, it is noteworthy that although Golgi staining has been used extensively by other investigators to reliably and quantitatively assess alterations in dendritic arborization and spine density following exposure to drugs of abuse [for review see (Robinson and Kolb, 2004)], the technique provides no information regarding the way in which the electrophysiology of cells and circuits is altered. Thus, while it is parsimonious to suggest that an increase in spine density corresponds to an increase in excitatory drive in relevant circuits, this is not necessarily the case. As noted by Robinson and Kolb (2004), opposite changes in structure (e.g., spine density increases vs. decreases) could have the same effect on cell signaling depending on how the synapses are arranged around the altered postsynaptic dendrite. For example, behavioral sensitization to psychomotor stimulants is associated with increases in spine density in NAc and mPFC, but exposure to morphine, which produces decreases in spine density in these regions (Robinson and Kolb, 1999b, Robinson et al., 2002), also can result in behavioral sensitization. The morphological differences may be related to differences in the acute neurochemical effects of the drugs; for example, acute administration of cocaine and AMPH increases striatal glutamate release (Reid et al., 1997), whereas morphine has the opposite effect (Enrico et al., 1998). However, because both classes of drugs can induce behavioral sensitization, it is likely that changes in synaptic connectivity in multiple brain regions and circuits interact to drive changes in behavior that are specific to the particular pharmacology of the drug in question.
Conclusion
It is likely that the neuronal alterations underlying behavioral sensitization are important in the transition from recreational drug use to addiction and the propensity toward relapse during abstinence (Robinson and Berridge, 1993, De Vries et al., 1998, Vezina et al., 2002). Given that the MDMA-induced structural adaptations that we report are similar to those that occur following exposure to other addictive psychostimulants, our results, combined with growing behavioral evidence, suggest that, like these other drugs of abuse, MDMA use may lead to compulsive drug-seeking and drug-taking behavior.
Acknowledgments
This work was supported by National Institutes of Health grant DA 02451 (G.V.R.) and National Research Service Award DA 020209 (K.T.B.). MDMA was generously provided by the National Institute on Drug Abuse. The authors wish to thank Mitchell Lloyd for dendritic reconstructions, Ann Shively for behavioral scoring, and Faye Caylor for editorial assistance.
Abbreviations
- AC
anterior cingulate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid
- AMPH
amphetamine
- DA
dopamine
- MDMA
(±)3-4,methylenedioxymethamphetamine
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- PL
prelimbic
- s.c
subcutaneous
- 5-HT
serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kevin T. Ball, Department of Psychology, Bloomsburg University of Pennsylvania, Bloomsburg, PA, USA
Cara L. Wellman, Department of Psychological and Brain Sciences and Program in Neuroscience, Indiana University, Bloomington, IN, USA
Emma Fortenberry, Department of Psychological and Brain Sciences and Program in Neuroscience, Indiana University, Bloomington, IN, USA.
George V. Rebec, Department of Psychological and Brain Sciences and Program in Neuroscience, Indiana University, Bloomington, IN, USA.
References
- Academies NRCotN. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- Andersen P, Soleng AF. Long-term potentiation and spatial training are both associated with the generation of new excitatory synapses. Brain Res Rev. 1998;26:353–359. doi: 10.1016/s0165-0173(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Ball KT, Budreau D, Rebec GV. Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Res. 2003;994:203–215. doi: 10.1016/j.brainres.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Ball KT, Budreau D, Rebec GV. Context-dependent behavioural and neuronal sensitization in striatum to MDMA (ecstasy) administration in rats. Eur J Neurosci. 2006;24:217–228. doi: 10.1111/j.1460-9568.2006.04885.x. [DOI] [PubMed] [Google Scholar]
- Ball KT, Rebec GV. Role of 5-HT2A and 5-HT2C/B receptors in the acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on striatal single-unit activity and locomotion in freely moving rats. Psychopharmacology. 2005;181:676–687. doi: 10.1007/s00213-005-0038-z. [DOI] [PubMed] [Google Scholar]
- Ball KT, Walsh KM, Rebec GV. Reinstatement of MDMA (ecstasy) seeking by exposure to discrete drug-conditioned cues. Pharmacol Biochem Behav. 2007;87:420–425. doi: 10.1016/j.pbb.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Self-administration of methylenedioxymethamphetamine (MDMA) by rhesus monkeys. Drug Alcohol Depend. 1986;18:149–157. doi: 10.1016/0376-8716(86)90047-5. [DOI] [PubMed] [Google Scholar]
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacology. 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. Cocaine and cocaethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J Neurochem. 1993;60:1429–1435. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Brown RW, Kolb B. Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res. 2001;899:94–100. doi: 10.1016/s0006-8993(01)02201-6. [DOI] [PubMed] [Google Scholar]
- Buell SJ. Golgi-Cox and rapid Golgi methods as applied to autopsied human brain tissue: widely disparate results. J Neuropath Exp Neur. 1982;41:500–507. doi: 10.1097/00005072-198209000-00003. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Tharp JA, Wellman CL, Sengelaub DR, Garraghty PE. Morphological correlates of injury-induced reorganization in primate somatosensory cortex. BMC Neurosci. 2004;5:43. doi: 10.1186/1471-2202-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging. 1987;8:521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Comery TA, Stamoudis CX, Irwin SA, Greenough WT. Increased density of multiple-head dendritic spines on medium-sized spiny neurons of the striatum in rats reared in a complex environment. Neurobiol Learn Mem. 1996;66:93–96. doi: 10.1006/nlme.1996.0049. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi D, Mennini T, Gobbi M. Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- Enrico P, Mura MA, Esposito G, Serra P, Migheli R, De Natale G, Desole MS, Miele M, Miele E. Effect of naloxone on morphine-induced changes in striatal dopamine metabolism and glutamate, ascorbic acid and uric acid release in freely moving rats. Brain Res. 1998;797:94–102. doi: 10.1016/s0006-8993(98)00371-0. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Morrell F, deToledo-Morrell L. Perforated synapses on double-headed dendritic spines: a possible structural substrate of synaptic plasticity. Brain Res. 1989;480:326–329. doi: 10.1016/0006-8993(89)90201-1. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Gough B, Ali SF, Slikker W, Jr, Holson RR. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on monoamines in rat caudate. Pharmacol Biochem Behav. 1991;39:619–623. doi: 10.1016/0091-3057(91)90137-q. [DOI] [PubMed] [Google Scholar]
- Harmon KM, Wellman CL. Differential effects of cholinergic lesions on dendritic spines in frontal cortex of young adult and aging rats. Brain Res. 2003;992:60–68. doi: 10.1016/j.brainres.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Hedou G, Feldon J, Heidbreder CA. Effects of cocaine on dopamine in subregions of the prefrontal cortex and their efferents to subterritories of the nucleus accumbens. Eur J Pharmacol. 1999;372:143–155. doi: 10.1016/s0014-2999(99)00218-6. [DOI] [PubMed] [Google Scholar]
- Hedou G, Homberg J, Feldon J, Heidbreder CA. Expression of sensitization to amphetamine and dynamics of dopamine neurotransmission in different laminae of the rat medial prefrontal cortex. Neuropharmacology. 2001;40:366–382. doi: 10.1016/s0028-3908(00)00174-x. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Rapid dendritic retraction in medial prefrontal neurons and impaired fear extinction following exposure to uncontrollable stress. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, White SR. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology. 1998;18:469–479. doi: 10.1016/S0893-133X(97)00195-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RMCWP, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Lillsunde P, Seppala T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE. Reinforcing effects of certain serotonin-releasing amphetamine derivatives. Pharmacol Biochem Behav. 1996;53:99–105. doi: 10.1016/0091-3057(95)00205-7. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. New Engl J Med. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Meyer A, Mayerhofer A, Kovar K-A, Schmidt WJ. Rewarding effects of the optical isomers of 3,4-methylenedioxy-methylamphetamine (‘Ecstasy’) and 3,4-methylenedioxy-ethylamphetamine (‘Eve’) measured by conditioned place preference in rats. Neurosci Lett. 2002;330:280–284. doi: 10.1016/s0304-3940(02)00821-2. [DOI] [PubMed] [Google Scholar]
- Murphy PN, Wareing M, Fisk J. Users’ perceptions of the risks and effects of taking ecstasy (MDMA): a questionnaire study. J Psychopharmacol. 2006;20:447–455. doi: 10.1177/0269881106063270. [DOI] [PubMed] [Google Scholar]
- Nash JF, Brodkin J. Microdialysis studies on 3,4-methylenedioxymethamphetamine-induced dopamine release: effect of dopamine uptake inhibitors. J Pharmacol Exp Ther. 1991;259:820–825. [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann NY Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Ionescu-Pioggia M, Pope KW. Drug use and life style among college undergraduates: a 30-year longitudinal study. Am J Psychiat. 2001;158:1519–1521. doi: 10.1176/appi.ajp.158.9.1519. [DOI] [PubMed] [Google Scholar]
- Ramos M, Goñi-Allo B, Aguirre N. Ibotenic acid lesions of the medial prefrontal cortex block the development and expression of 3,4-methylenedioxymethamphetamine-induced behavioral sensitization in rats. Behav Brain Res. 2005;160:304–311. doi: 10.1016/j.bbr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Rebec GV. Stimulants and motor-related striatal neuronal activity. In: Miller R, Wickens JR, editors. Conceptual advances in brain research, brain dynamics and the striatal complex. Reading, UK: Gordon and Breach Science Publishers; 1999. pp. 51–63. [Google Scholar]
- Reid MS, Hsu K, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: Studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999a;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999b;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Begovic A, Goldman-Rakic PS, Castner SA. Amphetamine sensitization alters dendritic morphology in prefrontal cortical pyramidal neurons in the non-human primate. Neuropsychopharmacology. 2007;4:919–931. doi: 10.1038/sj.npp.1301179. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sholl DA. The organization of the cerebral cortex. London: Methuen; 1956. [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Strote J, Lee JE, Wechsler H. Increasing MDMA use among college students: results of a national survey. J Adolescent Health. 2002;30:64–72. doi: 10.1016/s1054-139x(01)00315-9. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Teneud LM, Baptista T, Murzi E, Hoebel BG, Hernandez L. Systemic and local cocaine increase extracellular serotonin in the nucleus accumbens. Pharmacol Biochem Behav. 1996;53:747–752. doi: 10.1016/0091-3057(95)02087-x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specificlesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Differential effects of discrete subarea-specific lesions of the rat medial prefrontal cortex on amphetamine- and cocaine-induced behavioural sensitization. Cereb Cortex. 2000;10:488–498. doi: 10.1093/cercor/10.5.488. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Hoorneman EMD, Buijs RM, Matthijssen MAH, Geffard M, Uylings HBM. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience. 1987;22:849–862. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-D, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Murphy DL, Lesch K-P, Holmes A. Genetic inactivation of the serotonin transporter causes impairment of stress-coping and extinction-recall and medial prefrontal cortex abnormalities. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Ferrante RJVSCJ. The Golgi rapid method in clinical neuropathology: the morphological consequences of suboptimal fixation. J Neuropath Exp Neur. 1978;37:13–33. doi: 10.1097/00005072-197801000-00002. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Griffiths P, Stewart D. Drugs and the dance music scene: a survey of current drug use patterns among a sample of dance music enthusiasts in the UK. Drug Alcohol Depend. 2001;64:9–17. doi: 10.1016/s0376-8716(00)00215-5. [DOI] [PubMed] [Google Scholar]
- Works SJ, Wilson RE, Wellman CL. Age-dependent effect of cholinergic lesion on dendritic morphology in rat frontal cortex. Neurobiol Aging. 2004;25:963–974. doi: 10.1016/j.neurobiolaging.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Spanos LJ. The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. Eur J Pharmacol. 1988;148:195–203. doi: 10.1016/0014-2999(88)90564-x. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Ann Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]