Abstract
Prepulse inhibition of startle (PPI) is a measure of sensorimotor gating, a pre-conscious regulator of attention. PPI is impaired in adults with schizophrenia and several other neuropsychiatric disorders associated with attentional abnormalities. The core feature of ADHD involves deficits in attention and, like schizophrenia, ADHD is associated with dysregulation of cortical–striatal circuits and dopamine transmission. Therefore, PPI may be disrupted in ADHD. While ADHD persists into adulthood in approximately half the children with ADHD, there has not been any published report of PPI in ADHD adults. In this study, PPI was measured in a sample of ADHD adults and compared to a sample of healthy comparison (HC) subjects. Twenty unmedicated adults with ADHD (11 inattentive subtype, 9 combined subtype) and 17 HC subjects were administered an eyeblink startle PPI paradigm. The PPI of ADHD adults was not significantly different from that of HC subjects in any of the PPI conditions. There was no significant effect of ADHD subtype nor of gender. The lack of PPI deficits in ADHD adults has important implications and suggests that, despite the presence of PPI dysregulation in a large number of disparate neuropsychiatric disorders, it is not a general feature of all neuropsychiatric disorders with attention abnormalities. Furthermore, the attentional deficiency in ADHD may have a neurobiological substrate somewhat distinct from schizophrenia and other neuropsychiatric disorders that are associated with PPI deficits. This distinction may be related to a relative sparing of pre-conscious attentional functions in ADHD compared to other disorders with PPI impairment.
Keywords: ADHD, PPI, Sensorimotor gating, Startle, Attention, Inhibition
1. Introduction
Sensorimotor gating is a normal physiological process mediated by the central nervous system which allows an organism to filter or “gate” irrelevant or intrusive cognitive, sensory or motor processes. Disruption of sensorimotor gating is thought to lead to abnormalities in attention-related information processing, which are observed in a number of neuropsychiatric illnesses.
The most widely used method of measuring sensorimotor gating is prepulse inhibition (PPI) of the startle reflex. PPI is the normal suppression of the startle response to a sudden intense stimulus when that startling stimulus is preceded by a weaker stimulus (prepulse) in a very short time period (30–500 ms), and can be tested across a number of species. In humans, PPI has been shown to exhibit good test-reliability, suggesting that it is a stable marker of underlying neural substrates and processes (Abel et al., 1998). PPI in humans is shown to be significantly heritable with over 50% of the PPI variation attributable to genetic factors (Anokhin et al., 2003). Intact PPI is considered to reflect a physiological marker of the integrity of pre-conscious mechanisms of attention, e.g. the gating of extroceptive and introceptive stimuli (Braff and Light, 2004; Perry et al., 1999).
Significantly reduced PPI is observed in a number of neuropsychiatric disorders, including Schizophrenia (Braff et al., 1978; Perry et al., 2002), Huntington’s Disease (Swerdlow et al., 1995), Tourette’s Disorder (Swerdlow et al., 2001), Obsessive Compulsive Disorder (Swerdlow et al., 1993), Bipolar Mania with Psychosis (Perry et al., 2001), and Autism, (McAlonan et al., 2002; Perry et al., 2007) all of which are associated with deficient attention regulation. Indeed, one of the criticisms of PPI as a useful marker of neuropsychiatric pathology is the large number of diverse neuropsychiatric disorders in which PPI deficits have been observed, making it difficult to ascertain the specific cognitive abnormalities that may be linked to impaired PPI. Cortico-striato-pallido-thalamic circuitry has been implicated in PPI. Dopamine in particular has been shown to exert a strong regulatory influence on PPI, and it has been suggested that PPI can serve as a measure of individual dopamine function (Feifel, 1999; Swerdlow et al., 2003).
ADHD is a disorder that affects 8–12% of children (Spencer et al., 2007). A core feature of ADHD is the inability to volitionally regulate attention and to inhibit distraction of attention by irrelevant stimuli (Spencer et al., 2007). Accordingly, individuals with ADHD have structural abnormalities in brain areas that are thought to regulate attention and executive function (Seidman et al., 2006). Similar to PPI, ADHD is thought to be highly heritable, with a large genetic contribution (Biederman, 2005; Staller and Faraone, 2007). The symptoms of ADHD are thought to be due to dysregulation of cortico-striatal circuits that overlap with those implicated in the regulation of PPI (Biederman, 2005; Staller and Faraone, 2007). In particular, dopamine function has been implicated in the pathophysiology underlying ADHD (Biederman, 2005; Staller and Faraone, 2007). Additionally, PPI is an inhibitory brain process and it has been proposed that deficiency in inhibitory processes is the core dysfunction in ADHD (Barkley, 1997). For these reasons it is reasonable to hypothesize that ADHD may be associated with impaired PPI. Indirectly supporting the link between ADHD and PPI is the finding that PPI is correlated with distractibility in psychotic patients (Karper et al., 1996).
Ornitz et al. found that ADHD boys with comorbid enuresis had PPI deficits (Ornitz et al., 1992; Ornitz et al., 1999). Castellanos et al. (1996) found that boys with ADHD and Tourette’s Syndrome also had PPI deficits. However, neither group found PPI deficits in boys with ADHD alone. More recently, Hawk et al. (2003) confirmed a lack of PPI abnormalities in ADHD boys under the standard startle testing conditions, but found PPI deficits compared to non-ADHD boys when the subjects were instructed to attend to the prepulse stimuli. Thus the research to date suggests that ADHD is not associated with PPI deficits under the standard testing conditions in which PPI deficits are found in a number of neuropsychiatric disorders (e.g. passive attention to prepulse stimuli). These findings are surprising given the strong attentional abnormalities associated with ADHD, and given the large number of neuropsychiatric conditions associated with attentional deficits in which PPI deficits have been found. Therefore, these negative findings in ADHD have potentially important implications for understanding the specific role of PPI in cognition and the nature of attention abnormalities in ADHD, relative to attention abnormalities in other neuropsychiatric conditions. There are, however, several limitations in the current body of literature, which prevent a definitive conclusion that ADHD is not associated with PPI abnormalities. First, the majority of the studies to date have investigated only boys, and it is well recognized that girls with ADHD often have a different phenotypic presentation associated more strongly with inattention symptoms and less with motor hyperactivity or impulsivity as compared to boys with ADHD (Staller and Faraone, 2006). Furthermore, the studies which report PPI deficits in neuropsychiatric conditions examined adults, whereas PPI in ADHD has only been studied in children. This limits any conclusion regarding PPI functioning across neuropsychiatric syndromes.
Over the past two decades ADHD, once thought to be exclusively a disorder of childhood, has increasingly become recognized to persist into adulthood in approximately half the children who have this condition (Biederman, 2005). It is unclear why some children continue to exhibit symptoms of ADHD while others do not. Nevertheless, adult ADHD likely represents a more stable and homogeneous condition, compared to the childhood version which may either represent instances of delayed central nervous system maturation, for example in children in whom ADHD will eventually remit, or a static pathological condition that persists into adulthood. Thus, children with ADHD may actually represent two distinct subpopulations and syndromes, those in which the disorder is transitory and those in which it will persist. The phenotypic expression of adults also differs somewhat from children as motor hyperactivity seems to be less prominent and inattention difficulties are more ubiquitous (Spencer et al., 2007). In this respect, the phenotypic presentation of both adult males and females with ADHD is more similar to female than male children with the disorder. Thus ADHD in adults may represent a somewhat different, more homogenous syndrome, than the childhood version. The distinct nature of adult ADHD from its childhood version is evidenced by the fact that it took many decades after the recognition of childhood ADHD to recognize its adult version. For these reasons it is important to examine sensorimotor gating in adults with ADHD; however to date, there has been no investigation of PPI function in ADHD adults. To address this issue we tested PPI in adult males and females diagnosed with ADHD and compared them to a similar cohort of non-ADHD subjects.
2. Methods and materials
2.1. Participants
The participants in this study consisted of 20 individuals (14 M, 6 F) with a DSM-IV diagnosis of attention-deficit/hyperactivity disorder (ADHD) and 17 (9 M, 8 F) healthy comparison participants (HC). The ADHD participants were recruited from the UCSD adult ADHD program under the directorship of DF. All of the ADHD participants recruited into the study were carefully assessed using a diagnostic battery that included the Structured Clinical Interview for DSM-IV disorders (SCID) to assess for comorbid psychiatric disorders including mood and anxiety disorders, the ADHD module of the Kiddie-SADS (wording modified for adults) and a symptom rating scale (ADHD-RS). All subjects met DSM-IV diagnostic criteria for ADHD including having some symptoms of ADHD prior to the age of 7, although less than half reported being formerly diagnosed with ADHD as children. Eleven subjects were diagnosed with the inattentive subtype and nine with the combined subtype. All participants were able to verbally demonstrate that they understood the nature of the study and its associated risks and benefits.
Only four of the subjects had been on ADHD medication in the two months prior to testing. Each of these four had been on a stimulant. One subject discontinued the stimulant five days prior to testing and the other three had discontinued their stimulant at least three weeks before testing. In addition one of the four subjects had also been prescribed buproprion, but had discontinued it two months prior to testing.
There were no significant differences between participant groups for age [ADHD mean age = 35.3 years, HC mean age = 30.3 years, t (30) = 1.5, p = 0.15] or years of education [ADHD mean education = 14.6 years, HC mean education = 14.8 years, t (30) = 0.21, p = 0.84]. ADHD participants were excluded if they were determined to have an additional Axis I diagnosis, an unstable medical condition, history of a head injury with loss of consciousness, or history of a serious neurological disorder (e.g. frequent seizures).
The normal comparison participants underwent screening interviews to rule out Axis I and II disorders, neurological illness or head trauma, exposure to psychoactive medication, or drug abuse/dependence. Participants were excluded if they had a positive result on a urine toxicology screen.
2.2. Procedure
After a complete description of the study was given to the participants, written informed consent was obtained. The study protocol and consent forms were reviewed and approved by the UCSD Human Subjects Committee. All participants then underwent a brief hearing screening using an audiometer (Grason Stadler 17, Milford, NH) to ensure intact auditory abilities. Any participant who could not detect tones at 45 dB SPL at 500, 1000 or 6000 Hz was excluded from the study; this criterion is our laboratory’s conventional criterion for excluding subjects with impaired hearing (Minassian et al., 2007; Perry et al., 2007) and is comparable to that used by other laboratories (Kumari et al., 2008; Wynn et al., 2007). Each participant was seated comfortably in a reclining chair, in a room separated from the recording equipment by a room partition. The eyeblink component of the auditory startle reflex was measured using electromyography (EMG) of the orbicularis oculi muscle. Two miniature silver/silver chloride electrodes (In Vivo Metric, Healdsburg, CA) were positioned below and to the right of the participant’s right eye, over the orbicularis oculi muscle. Electrodes were placed to minimize potential electro-oculogram (EOG) artifact. As per our established methods (Braff, 1999; Braff et al., 1978, 1992; Perry and Braff, 1994), electrodes were fixed to the skin as close as possible to one another using adhesive collars, conforming to the location of the orbicularis oculi fibers. A ground electrode was placed behind the right ear over the mastoid. With this placement, participants could move their eye position without registering EOG activity via oscilloscope monitoring. Participants were instructed to keep their eyes open and fixed on a square on the wall. All electrode resistances were less than 10 kΩ. EMG activity was band-pass filtered (100–1000 Hz). A 60 Hz notch filter was also used to eliminate 60 Hz interference. Electro-myographic activity recorded by the electrodes was directed through a customized EMG amplifier to a computerized startle response monitoring system for digitization and analysis (SR-LAB, San Diego Instruments Inc, San Diego, CA). The system recorded 250 one-millisecond readings starting at the onset of the startle stimulus. Acoustic startle and prepulse stimuli were presented binaurally through headphones (Model TDH-39-P, Maico, Minneapolis, MN).
The startle session began with a five-minute acclimation period of 70 dB white noise, which continued throughout the session, followed by four trial blocks. Block One consisted of five pulse-alone trials. Blocks two and three each consisted of 32 trials, containing eight pulse-alone and 24 prepulse-pulse trials presented in pseudorandom order. The startle stimuli consisted of 115 dB 40 ms bursts of broadband white noise. The prepulse stimuli consisted of 20 ms 86 dB white noise that preceded the startle by either 30, 60, or 120 ms. The last block consisted of five pulse-alone trials. The inter-trial interval averaged 15 s with a range of 8–22 s; this is similar to the interval range used by other laboratories (Braff et al., 2007; Kumari et al., 2008; Roussos et al., 2008).
2.3. Data processing and statistical analysis
The startle measures examined were: (1) magnitude of the startle response to pulse-alone trials as measured in digital units. Startle magnitude was assessed by applying an independent samples t-test to the first block of pulse-alone startle amplitudes. (2) Percent of prepulse inhibition (PPI), calculated as the percent decrement in startle magnitude in the presence of the prepulse compared to the magnitude without the prepulse [100 − (prepulse amplitude/pulse amplitude) × 100]. PPI was calculated as an average of the two blocks that contain prepulse trials, as has been described in previous reports (Minassian et al., 2007). Data were inspected for normality and homogeneity of variance. A 3 × 2 repeated-measures analysis of variance (ANOVA) was used, with group and its three levels (ADHD Inattentive, ADHD combined, HC) as the between-subjects measure and the three interstimulus intervals (30, 60, and 120 ms) as the repeated measure. Planned comparisons among groups at each interstimulus interval were conducted. (3) Habituation of the startle response, measured by assessing the decrement in the magnitude of the startle response across all of the pulse-alone trials over the entire session. To assess group differences in habituation, mean startle magnitude for the pulse-alone trials was assessed by a 3 × 4 repeated-measures ANOVA. The significance level was set at p < 0.05 for all analyses. All statistical analyses were performed with SPSS software.
3. Results
3.1. Startle magnitude
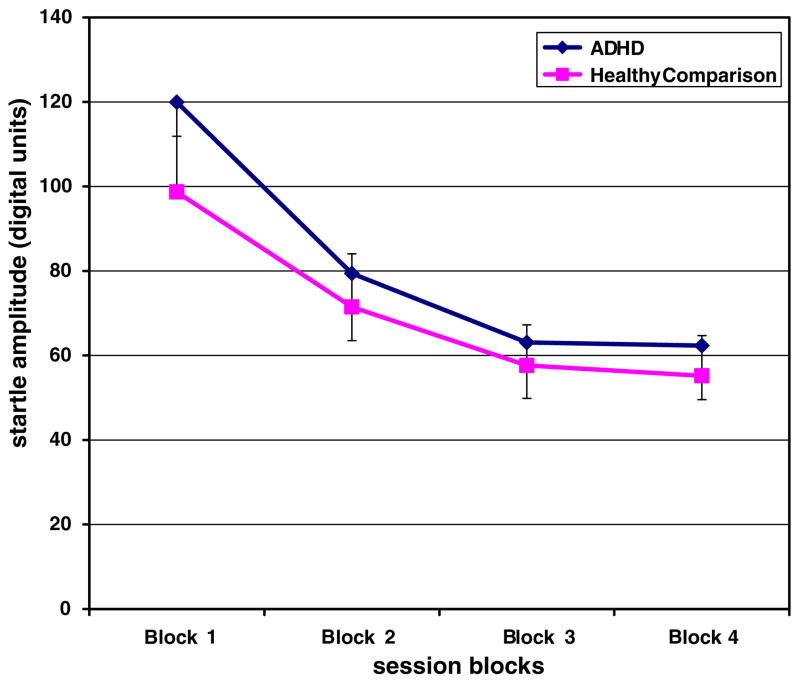
Preliminary analyses showed no gender differences in startle magnitude, PPI, or habituation, therefore gender was collapsed for the main analyses. Startle magnitude to the first block pulse-alone condition was not significantly different among the ADHD Inattentive group, the ADHD Combined group, and the HC group [F (2, 34) = 0.40, p = 0.67] (see Fig. 1).
Fig. 1.
Startle amplitude across the PPI session for ADHD subjects (n = 20) and healthy comparison subjects (n = 17). Data in the graph represent means, and error bars represent standard errors.
3.2. Prepulse Inhibition
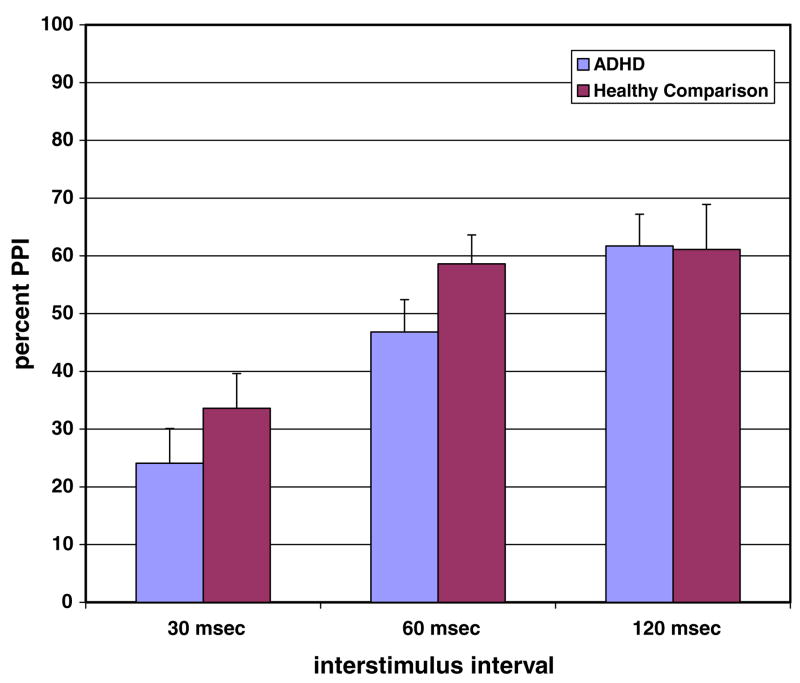
There was a significant main effect of interstimulus interval on PPI [F (2, 68) = 28.1, p < 0.001] such that PPI increased as the interstimulus interval increased (see Fig. 2). The main effect of group on overall PPI did not reach statistical significance [F (2, 34) = 0.78, p = 0.23], and there were no significant differences in PPI among ADHD Inattentive, ADHD Combined, and the HC groups. The interstimulus-by-group interaction was not significant [F (4, 68) = 1.6, p = 0.19]
Fig. 2.
Percent prepulse inhibition in ADHD subjects (n = 20) and healthy comparison subjects (n = 17). Data in the graph represent means, and error bars represent standard errors.
3.3. Habituation
Analysis of the habituation data revealed that there was an overall significant main effect for block [F (3, 102) = 49.5, p < 0.001], suggesting habituation across the startle session (see Fig. 1). There was no significant main effect of group [F (2,34) = 0.15, p = 0.86], nor was there a significant group-by-block effect [F (6, 102) = 0.81, p = 0.56].
4. Discussion
To our knowledge this is the first published report of PPI function in adults with ADHD. In this study, we found that PPI function in a sample of male and female adults with ADHD did not differ from a sample of non-ADHD adults. This result is consistent with prior reports using similar PPI measurements in young boys with ADHD (Castellanos et al., 1996; Hawk et al., 2003; Ornitz et al., 1992, 1999).
This negative finding has important implications. First, given the growing number of neuropsychiatric illnesses in which PPI deficits have been observed, the current finding indicates that PPI does have some specificity and can distinguish between neuropsychiatric illnesses with overlapping cognitive features. This finding also suggests that the deficits in attention, impulse inhibition, and hyperactivity associated with ADHD have somewhat distinct underlying neural substrates from the attention and impulse deficits associated with other conditions, such as schizophrenia, Tourette’s syndrome, Autism, and Bipolar Mania in which PPI has been observed to be deficient.
Another commonly studied operational measure of information gating is the inhibition of P50, an early component of the auditory event-evoked potential. Whereas PPI is a measure of sensorimotor gating, P50 is a measure of sensory gating without a significant motor contribution. However, PPI and P50 are both considered “automatic”, or preattentive information gating processes, that require no conscious effort (Braff et al., 2007). While P50, like PPI has been shown to be deficient in schizophrenia patients, these two information gating measures appear to diverge and represent somewhat different, perhaps overlapping, aspects of attention and inhibition (Braff et al., 2007). Recently P50 inhibition was studied in adults with ADHD and was found to be comparable to normal controls and significantly higher compared to schizophrenia subjects (Olincy et al., 2000). The current PPI finding converges with this P50 finding in adults with ADHD to suggest that the attentional impairment in ADHD is distinct from that of schizophrenia and other neuropsychiatric disorders in which PPI and P50 inhibition are deficient.
The attention problems associated with ADHD are thought to more heavily involve abnormalities in the conscious or volitional ability to inhibit attention allocation toward irrelevant, distractive stimuli. In contrast, the sensory flooding often associated with schizophrenia, and thought to contribute to its symptomatic manifestation, occurs even during passive mental states and not only during attempts to volitionally control attention. Since PPI and P50 are considered measures of pre-conscious or “automatic” gating, this could account for the observed abnormalities in these measures in schizophrenia but not ADHD adults.
In this regard, Hawk et al. (2003) found that while ADHD boys showed no PPI deficits when they were instructed to ignore the prepulse stimulus, they did exhibit less enhancement of PPI than non-ADHD boys when they were instructed to attend to them. The authors interpret this as possible evidence for deficits in sensorimotor mechanisms which are invoked by volitional rather than passive allocation of attention. Future studies should investigate whether adults with ADHD display PPI deficits in a volitional attention paradigm.
A limitation of this study is its limited statistical power to detect potential differences in PPI across the groups studied, owing to the modest sample sizes and the diversity (e.g., males and females) of the subjects. There were too few females in this study to allow for an analysis of gender differences. Therefore, additional studies with larger samples of both females and males are warranted.
The ubiquity of PPI among neuropsychiatric disorders associated with attention problems has dampened enthusiasm for its utility as a meaningful endophenotype. The absence of PPI deficits in ADHD, a disorder whose core feature is attention deficiency, suggests that PPI is somewhat selectively disrupted among such disorders. Future studies should continue to elucidate the specific phenotypes associated with PPI disruption.
Acknowledgments
This work was supported in part by a grant from the National Institute of Mental Health (R01MH071916) (WP, AM). The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
The authors thank Eliza Ferguson for her technical assistance and Barbara Galangue for her administrative assistance.
Footnotes
Contributors
DF designed the study. AM tested subjects. DF, AM, and WP managed the literature searches and analyses. DF and AM conducted statistical analyses and DF wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest statement
None.
References
- Abel K, Waikar M, Pedro B, Hemsley D, Geyer M. Repeated testing of prepulse inhibition and habituation of the startle reflex: a study in healthy human controls. Journal of Psychopharmacology. 1998;12:330–7. doi: 10.1177/026988119801200402. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of startle reflex in humans. Neuroscience Letters. 2003;353:45–8. doi: 10.1016/j.neulet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychology Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biological Psychiatry. 2005;57:1215–20. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Braff D. Psychophysiological and information processing approaches to schizophrenia. In: Charney DS, Nestler E, Bunney BS, editors. Neurobiological foundation of mental illness. New York: Oxford University Press; 1999. pp. 258–71. [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49:206–15. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berlin) 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA, Swerdlow NR. Prepulse inhibition and P50 suppression are both deficient but not correlated in schizophrenia patients. Biological Psychiatry. 2007;61:1204–7. doi: 10.1016/j.biopsych.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biological Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Feifel D. Individual differences in prepulse inhibition of startle as a measure of individual dopamine function. Behaviour of Neuroscience. 1999;113:1020–9. doi: 10.1037//0735-7044.113.5.1020. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Jr, Yartz AR, Pelham WE, Jr, Lock TM. The effects of methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention-deficit hyperactivity disorder. Psychopharmacology (Berlin) 2003;165:118–27. doi: 10.1007/s00213-002-1235-7. [DOI] [PubMed] [Google Scholar]
- Karper LP, Freeman GK, Grillon C, Morgan CA, 3rd, Charney DS, Krystal JH. Preliminary evidence of an association between sensorimotor gating and distractibility in psychosis. Journal of Neuropsychiatry and Clinical Neuroscience. 1996;8:60–6. doi: 10.1176/jnp.8.1.60. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Papadopoulos A, Bojang F, Poon L, Halari R, et al. A comparison of prepulse inhibition in pre- and post-menopausal women and age-matched men. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301670. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Minassian A, Feifel D, Perry W. The relationship between sensorimotor gating and clinical improvement in acutely ill schizophrenia patients. Schizophrenia Research. 2007;89:225–31. doi: 10.1016/j.schres.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Harris JG, Young DA, McAndrews MA, Cawthra E, et al. The P50 auditory event-evoked potential in adult attention-deficit disorder: comparison with schizophrenia. Biological Psychiatry. 2000;47:969–77. doi: 10.1016/s0006-3223(00)00239-0. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Hanna GL, de Traversay J. Prestimulation-induced startle modulation in attention-deficit hyperactivity disorder and nocturnal enuresis. Psychophysiology. 1992;29:437–51. doi: 10.1111/j.1469-8986.1992.tb01717.x. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Russell AT, Hanna GL, Gabikian P, Gehricke JG, Song D, et al. Prepulse inhibition of startle and the neurobiology of primary nocturnal enuresis. Biological Psychiatry. 1999;45:1455–66. doi: 10.1016/s0006-3223(98)00205-4. [DOI] [PubMed] [Google Scholar]
- Perry W, Braff DL. Information-processing deficits and thought disorder in schizophrenia. American Journal of Psychiatry. 1994;151:363–7. doi: 10.1176/ajp.151.3.363. [DOI] [PubMed] [Google Scholar]
- Perry W, Feifel D, Minassian A, Bhattacharjie I, Braff DL. Information processing deficits in acutely psychotic schizophrenia patients medicated and unmedicated at the time of admission. American Journal of Psychiatry. 2002;159:1375–81. doi: 10.1176/appi.ajp.159.8.1375. [DOI] [PubMed] [Google Scholar]
- Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Archives of General Psychiatry. 1999;56:277–81. doi: 10.1001/archpsyc.56.3.277. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biological Psychiatry. 2001;50:418–24. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biological Psychiatry. 2007;61:482–6. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Rogdaki M, Pavlakis S, Frangou S, Bitsios P. Prepulse inhibition of the startle reflex depends on the catechol O-methyltransferase Val158Met gene polymorphism. Psychological Medicine. 2008:1–8. doi: 10.1017/S0033291708002912. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60:1071–80. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. Journal of Pediatrics Psychology. 2007;32:631–42. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Staller J, Faraone SV. Attention-deficit hyperactivity disorder in girls: epidemiology and management. CNS Drugs. 2006;20:107–23. doi: 10.2165/00023210-200620020-00003. [DOI] [PubMed] [Google Scholar]
- Staller JA, Faraone SV. Targeting the dopamine system in the treatment of attention-deficit/hyperactivity disorder. Expert Review of Neurotherapy. 2007;7:351–62. doi: 10.1586/14737175.7.4.351. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biological Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: in search of an “fMRI-friendly” startle paradigm. Biological Psychiatry. 2001;50:578–85. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. Journal of Neurology and Neurosurgery Psychiatry. 1995;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Wasserman LC, Talledo JA, Casas R, Bruins P, Stephany NL. Prestimulus modification of the startle reflex: relationship to personality and physiological markers of dopamine function. Biological Psychology. 2003;62:17–26. doi: 10.1016/s0301-0511(02)00090-x. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Green MF, Sprock J, Light GA, Widmark C, Reist C, et al. Effects of olanzapine, risperidone and haloperidol on prepulse inhibition in schizophrenia patients: a double-blind, randomized controlled trial. Schizophrenia Research. 2007;95:134–42. doi: 10.1016/j.schres.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]