Summary
Cytokine signaling is thought to require assembly of multi-component signaling complexes at cytoplasmic segments of membrane-embedded receptors, in which receptor-proximal protein kinases are activated. Indeed, CD40, a tumor necrosis factor receptor (TNFR) family member, forms a complex containing adaptor molecules TRAF2 and TRAF3, ubiquitin conjugating enzyme Ubc13, cellular inhibitor of apoptosis proteins 1 and 2 (c-IAP1/2), IκB kinase regulatory subunit IKKγ (also called NEMO) and mitogen-activated protein kinase (MAPK) kinase kinase MEKK1 upon ligation. TRAF2, Ubc13, and IKKγ were required for complex assembly and activation of MEKK1 and MAPK cascades. However, these kinases were not activated unless the multi-component signaling complex translocated from CD40 to the cytosol upon c-IAP1/2-induced degradation of TRAF3. This two-stage signaling mechanism may apply to other innate immune receptors, accounting for spatial and temporal separation of MAPK and IKK signaling.
Cytoplasmic segments of cytokine, growth factor and antigen receptors serve as assembly sites for complexes containing adaptor proteins, protein kinases, and other signaling factors (1, 2). Ligand-induced assembly of such complexes is thought to concentrate and subsequently activate protein kinases on the receptor, thereby triggering a plethora of effector pathways that control metabolism, proliferation and survival. Amongst cytokine receptors, the tumor necrosis factor receptor (TNFR) family contains members of biomedical importance that bind trimeric ligands (3, 4). TNFR engagement results in assembly of multi-component receptor-associated signaling complexes that activate inhibitor of NF-κB (IκB) kinase (IKK) and mitogen- or stress-activated protein kinase (MAPK/SAPK) cascades (3, 5). Key players in TNFR signaling are TNF receptor-associated factors (TRAF), serving as E3 ubiquitin ligases and adaptors that recruit and activate protein kinases that act at the apex of effector pathways (3, 5–7). These protein kinases include transforming growth factor-β activated kinase 1 (TAK1) and MAP or extracellular signal-regulated kinase kinase kinase 1 (MEKK1), which in turn activate IKK (8), and the MAPK/SAPKs JNK and p38 (5, 9). Most TRAF proteins contain an N-terminal RING finger required for signal transduction and formation of K63-linked polyubiquitin chains conjugated through the C-terminal glycine of the added ubiquitin moiety and K63 of the protein-attached ubiquitin (6), and a C-terminal TRAF domain involved in self-association and binding to receptor and adaptor proteins (3).
Much of our knowledge of TNFR signaling comes from studies of type I TNF receptor (TNFR1), which interacts with TRAF2 and TRAF5 through the adaptor protein TRADD (10). Receptor recruitment of TRAF2 results in inclusion of the cellular Inhibitors of Apoptosis (c-IAP) 1 and c-IAP2 proteins in a signaling complex that also contains the protein kinase RIP1 (11). This complex, named complex I, was suggested to activate IKK, JNK, and p38, while associated with membrane-embedded TNFR1 (12, 13). IKK activation was proposed to depend on its binding to receptor-associated TRAF2 (14) and K63-mediated polyubiquitination of RIP1 that results in recruitment of TAK1, a putative IKK kinase (15, 16). IKK association with complex I may be stabilized by binding of IKKγ, its regulatory subunit (17), to K63-polyubiquitinated RIP1 (18, 19). However, it has not been formally demonstrated that kinase activity of TAK1 is actually stimulated upon receptor engagement and if so, where and when the kinase is activated.
TNFR1-induced JNK activation depends in part on the MAPK kinase kinase (MAP3K) MEKK1 (20). MEKK1 is also a major contributor to activation of JNK and p38 by two B cell TNFR family members: CD40 and BAFF receptor (BAFF-R) (9), which recruit TRAF2, TRAF3, and TRAF6 directly (21). CD40 and BAFF-R are critical for B cell maturation, survival, proliferation, and immune functions (22). Some of these functions also depend on MEKK1 (9). We find that CD40-induced activation of MEKK1 and its downstream targets requires assembly of a receptor-associated multi-component complex. But, MEKK1 is not activated unless the receptor-anchored complex is released to the cytosol via a proteolytic step. A similar mechanism accounts for activation of TAK1. These results outline an unprecedented two-stage signaling mechanism in which membrane-anchored receptors nucleate a multi-component signaling complex, but actual MAP3K activation requires complex release into the cytoplasm, where MAP3K substrates are located.
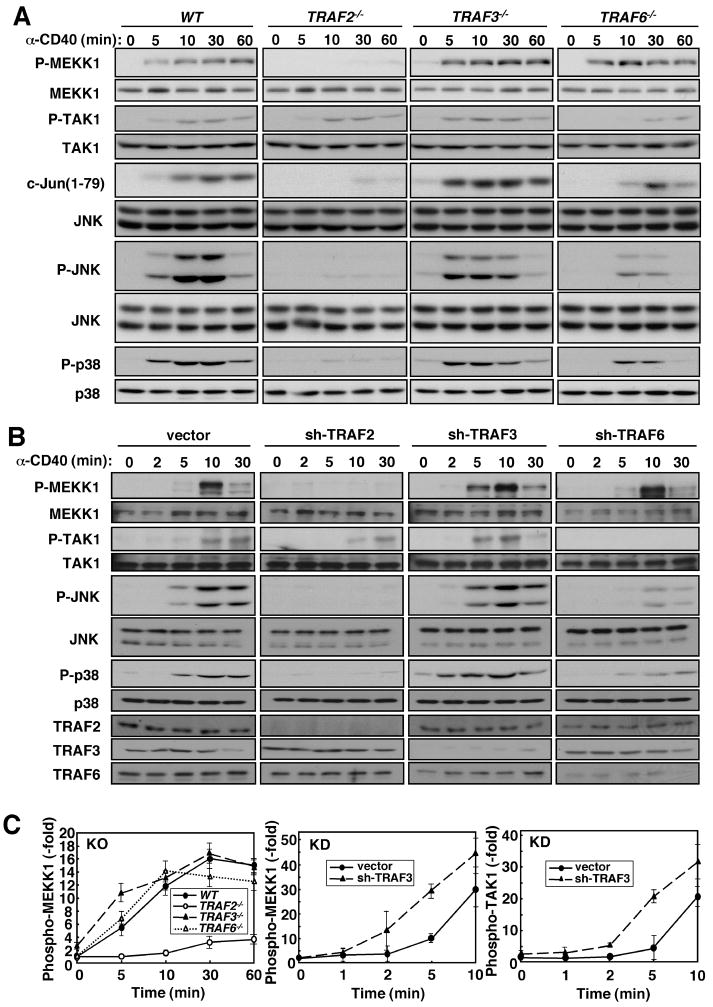
TRAF requirements for MEKK1 activation in CD40-stimulated B cells
CD40 transduces signals through TRAF proteins (22). To identify the TRAFs responsible for MEKK1 activation in CD40-stimulated B cells, we reconstituted irradiated mice with wild-type (WT), Traf2−/minus;, Traf3−/minus;, and Traf6−/minus; stem cells, and isolated splenic B cells that were stimulated with a CD40 agonistic antibody. Activation of MEKK1, measured by reactivity with a phospho-specific antibody that recognizes phospho-Thr1381 in its activation loop (9) or a kinase activity assay, was impaired in Traf2−/minus; cells, but not in Traf3−/minus; or Traf6−/minus; cells (Fig. 1A, S1A). Correspondingly, phosphorylation of JNK and p38 and JNK activity were decreased in Traf2−/minus; cells by approximately 75% (Fig. S1B). TRAF6 ablation also decreased MAPK phosphorylation and JNK activity by approximately 50%, suggesting that TRAF6 activates JNK and p38 through another MAP3K, most likely TAK1, whose activation was diminished in Traf6−/minus;, but not in Traf2−/minus; or Traf3−/minus; cells (Fig. 1A). Curiously, the absence of TRAF3 resulted in earlier MAP3K and MAPK phosphorylation and activation which were nearly maximal at the 5 min time point (Fig. 1A, S1A, B). In fact, a JNK kinase assay revealed near maximal JNK activation at 5 min in Traf3−/minus; cells, whereas in WT cells JNK activity peaked around 30 min (Fig. 1A, S1B). Depletion of TRAF2, TRAF3, and TRAF6 by lentivirus-mediated transduction of short hairpin (sh) RNAs produced similar results: CD40-induced MEKK1 activation was barely detected in TRAF2-deficient cells, whereas TRAF6 depletion prevented TAK1 activation (Fig 1B, C). Importantly, TRAF3 depletion accelerated MAP3K and MAPK activation. CD40 ligation induces MEKK1 ubiquitination (9), a modification that was dependent on TRAF2, but not on TRAF3 or TRAF6 (Fig. S2).
Fig. 1.
Requirement of TRAF2 for CD40-mediated activation of MEKK1. (A) MEKK1 activation in B cells. Splenic B cells from mice reconstituted with WT, Traf2−/minus;, Traf3−/minus;, and Traf6−/minus; fetal liver were stimulated with CD40 agonistic antibody. Cell lysates were prepared when indicated and phosphorylation of MEKK1, TAK1, JNK, and p38 was analyzed by immunoblotting. The kinase activity of immunoprecipitated JNK was measured by phosphorylation of GST-c-Jun(1–79). Immunoblotting with anti-JNK served as the loading control. (B) MEKK1 activation in a B cell line. A20 B cells transduced with lentiviruses containing no insert or shRNAs to TRAF2, 3 or 6 were stimulated with anti-CD40. MEKK1, TAK1, JNK, and p38 phosphorylation and TRAF2, 3 or 6 expression were analyzed by immunoblotting. (C) Kinetics of CD40-induced phosphorylation of MEKK1 and TAK1. Phosphorylation kinetics of MEKK1 in WT and TRAF knockout (KO) B cells were determined by densitometric analysis of two separate experiments as in (A). The early kinetics of MEKK1 and TAK1 phosphorylation in control and TRAF3 knockdown (KD) A20 B cells were determined in two separate experiments similar to the one shown in (B).
Requirement of Ubc13 and IKKγ for MEKK1 activation
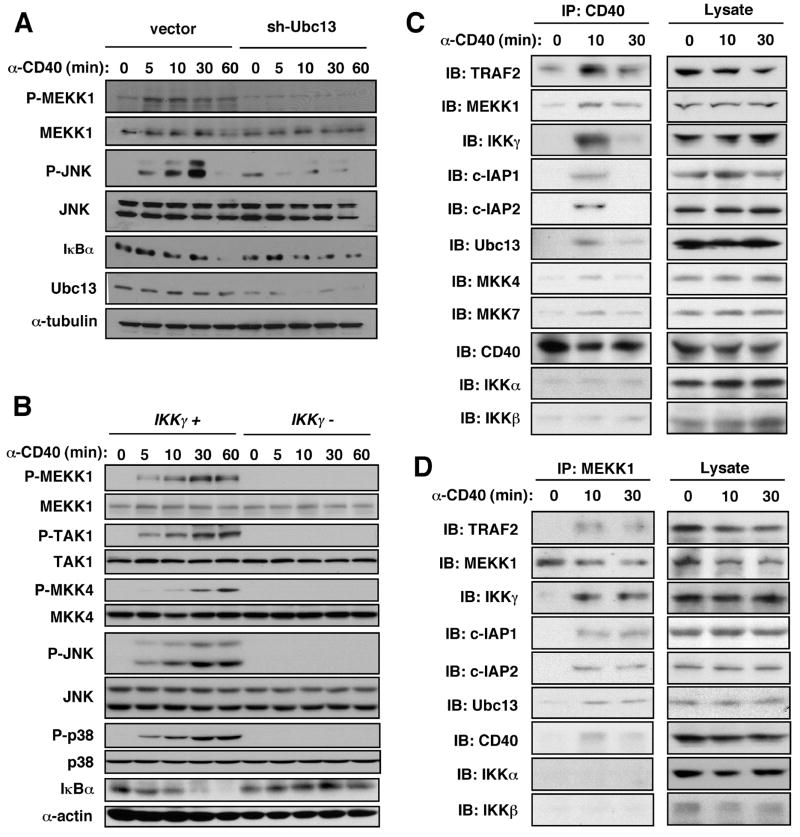
Ubc13, which together with Uev1a forms an E2 ubiquitin-conjugating enzyme that selectively catalyzes K63-polyubiquitination, is critical for CD40 signaling (23). We explored its role in MEKK1 activation by transducing A20 B cells with control or Ubc13 shRNA lentiviruses. CD40-induced phosphorylation of MEKK1 and JNK was abrogated upon depletion of Ubc13 (Fig. 2A). Ubc13 deficiency had only a minor effect on IKK activation and IκBα degradation (23). RING-dependent MEKK1 self-ubiquitination (24), was Ubc13-dependent (Fig. S3).
Fig. 2.
Dependence of MEKK1 activation on Ubc13 and IKKγ and association with formation of a multi-component signaling complex. (A) A20 B cells transduced with lentiviruses with no insert or Ubc13 shRNA were stimulated with anti-CD40. Lysates were immunoblotted to detect phosphorylation of MEKK1, JNK, and p38, degradation of IκBα, and expression of Ubc13. (B) Ikkγ-null 1.3E2 B cells and parental 70Z3 cells were stimulated with anti-CD40. At the indicated times, lysates were prepared and analyzed by immunoblotting for MAP3K and MAPK phosphorylation and IκBα degradation. (C, D) Splenic B cells were stimulated with anti-CD40. At the indicated times, cell lysates were prepared and immunoprecipitated (IP) with anti-CD40 (C) or anti-MEKK1 (D) antibodies. The gel-separated immunecomplexes were immunoblotted (IB) with the indicated antibodies.
Ubc13-dependent ubiquitination of IKKγ was proposed to function in IL-1 and TLR-mediated activation of JNK (23). Likewise, MEKK1, its substrate MKK4 and both JNK and p38 were no longer activated by CD40 in IKKγ-deficient 1.3E2 cells derived from the 70Z3 B cell lymphoma (Fig. 2B). IKKγ itself is polyubiquitinated upon CD40 engagement and this was TRAF2- and Ubc13-dependent (Fig. S4A, B). A portion of IKKγ is tightly bound to IKKα and IKKβ (17), but its involvement in MEKK1 activation suggested it might also have an IKK-independent function. We separated IKK-associated IKKγ from other forms in lysates of splenic B cells from WT or MEKK1 kinase domain-deficient (Mekk1ΔKD/ΔKD) mice by sequential immunoprecipitation. Kinase activity of MEKK1 was required for CD40-induced ubiquitination only of IKKγ that was not associated with other IKK subunits (Fig. S5).
A multi-component signaling complex is formed after CD40 engagement and undergoes membrane to cytosol translocation
CD40 immunoprecipitated from stimulated B cells was associated with TRAF2, MEKK1, IKKγ, Ubc13, c-IAP1 and c-IAP2 (Fig. 2C). This complex included trace amounts of the MEKK1 substrates MKK4 and MKK7, and at 10 min after receptor engagement did not contain detectable IKKα and IKKβ. Immunoprecipitation of MEKK1 from whole cell lysates also revealed formation of a CD40-induced multiprotein complex containing CD40, TRAF2, c-IAP1/2, Ubc13, IKKγ, and MEKK1 itself (Fig. 2D). With anti-CD40 as the immunoprecipitating antibody, the amounts of most complex components declined between 10 and 30 min after stimulation. This apparent dissociation of the complex was not seen in MEKK1 immunecomplexes.
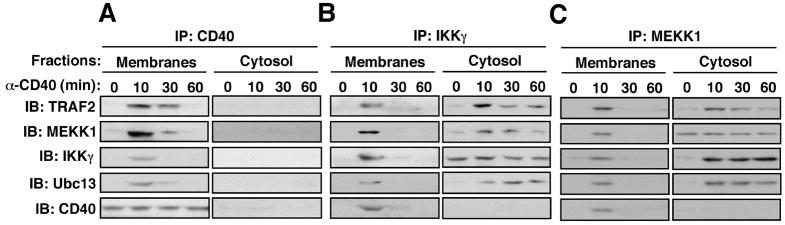
We also observed transient CD40-induced membrane association of MEKK1, TRAF2, IKKγ, and Ubc13 (Fig. S6). We therefore fractionated splenic B cells at various time points after stimulation as previously done in studies of TNFR1 signaling (12). In this protocol, nuclei and other heavy organelles are removed first and the remaining extract is divided into soluble (cytosol) and non-soluble (membrane containing) fractions. The multiprotein complex isolated with either CD40, IKKγ, or MEKK1-specific antibodies was present in the membrane-containing fraction at 10 min after stimulation but was barely detected after 30 min (Fig. 3 and S6 for loading control). By contrast, a complex, containing TRAF2, MEKK1, IKKγ and Ubc13, but not CD40, was detected in the soluble cytosol fraction within 10 min and most of its components were relatively stably associated for up to 60 min after CD40 engagement. CD40 itself remained in the membrane fraction and its amount did not decline during this time.
Fig. 3.
Release of the receptor-associated signaling complex to the cytosol. Splenic B cells were stimulated with anti-CD40 and membrane and cytosolic fractions were prepared at the indicated times. CD40 (A), IKKγ (B), or MEKK1 (C) were immunoprecipitated (IP) from lysates of each fraction, and the gel-separated immunecomplexes were immunoblotted (IB) with the indicated antibodies. The amounts of the analyzed proteins in total B cell lysates and the subcellular fractions are shown in Fig. S7.
Complex assembly occurs in two steps
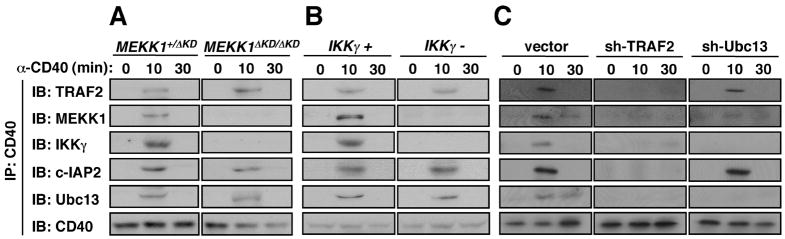
We investigated which components were critical for assembly of the CD40-anchored complex. In B cells lacking MEKK1 kinase domain, TRAF2, c-IAP2 and Ubc13 were still recruited to CD40, but IKKγ was not (Fig. 4A). Likewise, IKKγ was required for recruitment of MEKK1, but not for recruitment of TRAF2, c-IAP2 or Ubc13 (Fig. 4B). As predicted by its direct binding to CD40 (25), depletion of TRAF2 from cells abolished complex assembly (Fig. 4C). Depletion of Ubc13, however, did not interfere with recruitment of TRAF2 and c-IAP2, but prevented inclusion of MEKK1 and IKKγ (Fig. 4C). Hence, the first step in complex formation is binding of TRAF2 and c-IAP1/2 followed by recruitment of Ubc13. Once this initial complex is formed, IKKγ and MEKK1 are recruited into it inter-dependently.
Fig. 4.
Role of individual components in assembly of the CD40-associated signaling complex. (A) Role of MEKK1. Mekk1+/ΔKD or Mekk1ΔKD/ΔKD B cells were stimulated with anti-CD40 and the membrane fraction was immunoprecipitated with anti-CD40. (B) Role of IKKγ. 70Z3 and 1.3E2 B cells were stimulated and analyzed as above. (C) Roles of TRAF2 and Ubc13. A20 B cells transduced with lentiviruses containing no insert or shRNAs for TRAF2 or Ubc13 were stimulated and analyzed as above. After washing, the CD40 immunecomplexes in all 3 experiments were gel-separated and immunoblotted with the indicated antibodies.
Requirement of c-IAP and proteasome activity for cytosolic translocation of the receptor-bound complex and degradation of TRAF3
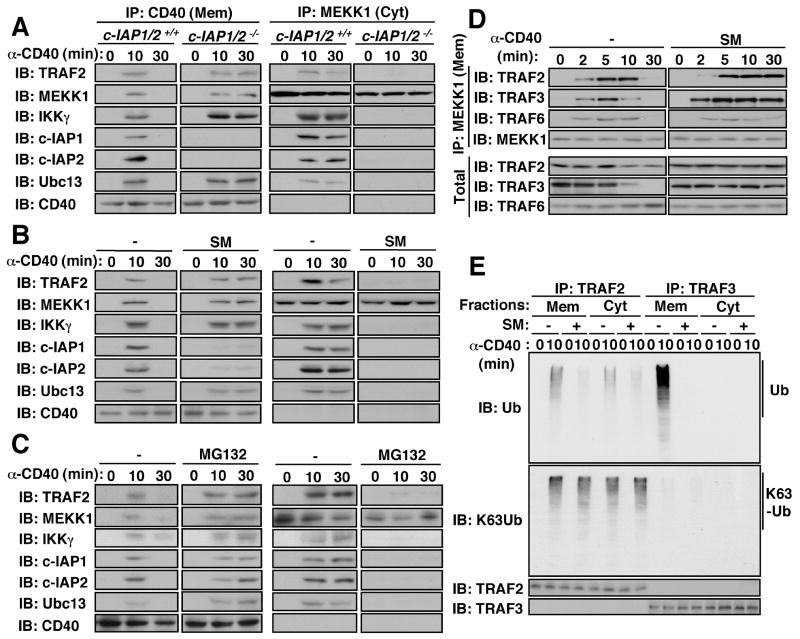
To examine the role of c-IAP1 and c-IAP2, we used multiple myeloma cells lacking these proteins as a result of an oncogenic deletion encompassing the linked c-IAP1 and c-IAP2 loci (26). Surprisingly, absence of c-IAP1/2 had no effect on complex assembly, but completely prevented its translocation to the cytosol (Fig. 5A). To further examine the role of c-IAP1/2 in complex release, we used a small molecule mimic of the pro-apoptotic protein Smac (SM), which binds to c-IAP1/2 and induces their rapid degradation (27, 28). SM also prevented cytosolic translocation of the receptor-associated signaling complex (Fig. 5B). c-IAP1/2 are K48-specific E3 ubiquitin ligases (29, 30). Proteasomal degradation of a c-IAP-targeted protein appeared to be required for cytosolic translocation of the signaling complex, because the proteasome inhibitor MG132 prevented complex release (Fig. 5C).
Fig. 5.
Requirement of c-IAP and proteasome activity for cytosolic translocation of the receptor-associated signaling complex and TRAF3 degradation. (A, B) Requirement of c-IAP1/2. c-IAP-deficient and control multiple myeloma cells (A), or splenic B cells treated with or without the c-IAP inhibitor Smac mimic (SM) for 4 hrs (B) were stimulated with anti-CD40. (C) Requirement of proteasome activity. Splenic B cells were treated with or without the proteasome inhibitor MG132 for 30 min prior to anti-CD40 stimulation. All cells were divided into membrane (Mem) and cytosolic (Cyt) fractions that were immunoprecipitated with anti-CD40 and anti-MEKK1, respectively. The gel-separated immunecomplexes were immunoblotted with the indicated antibodies. (D) Degradation of membrane- and MEKK1-associated TRAF3 in a c-IAP1/2-dependent manner. Splenic B cells were treated or not with SM as in (B) and stimulated with anti-CD40. At the indicated times, the cells were divided into membrane and cytosolic fractions. MEKK1 was immunoprecipitated and presence of indicated proteins in the immunecomplexes and total cell lysate was examined. (E) Requirement of c-IAP1/2 for TRAF2 and TRAF3 ubiquitination. B cells preincubated without or with SM were stimulated with anti-CD40 for 10 min. Cells were divided into membrane and cytosol fractions. TRAF2 and TRAF3 were immunoprecipitated from both fractions, extensively washed, gel-separated, and analyzed by immunoblotting with conventional or K63-specific anti-ubiquitin antibodies.
To understand the role of c-IAP1/2 in complex release, we searched for a component of the receptor-associated complex that was rapidly degraded in a c-IAP1/2-dependent manner. Our first candidate was TRAF2 as it binds directly to CD40 and its degradation may cause complex release. Although CD40 engagement resulted in reduced TRAF2 abundance that was inhibited by SM (Fig. 5D), it was not sufficiently rapid to account for complex release and ample TRAF2 remained in the cytosolic complex (Fig. S7). We therefore shifted our attention to TRAF3 as it can bind to the same site on CD40 as TRAF2, with which it may hetero-oligomerize (21, 22). Furthermore, the absence of TRAF3 accelerated MAP3K and MAPK activation (Fig. 1C). Receptor-recruited TRAF3 was rapidly degraded but was completely stabilized in cells depleted of c-IAP1/2 (Fig. 5D). Moreover, TRAF3 was only present in the membrane-associated MEKK1 complex and was not part of the cytosolic complex (Fig. S7).
Importantly, whereas polyubiquitinated TRAF2 was detected both in the membrane and cytosol fractions after CD40 engagement, polyubiquitinated TRAF3 was only seen in the membrane fraction and its appearance was completely inhibited by pretreatment with SM (Fig. 5E). Using a monoclonal antibody specific for K63-linked polyubiquitin (31), we found K63-linked polyubiquitin on TRAF2 both in the membrane and cytosol and the extent of this modification was not inhibited by pretreatment with SM, which did reduce the amount of polyubiquitinated TRAF2 detected by a conventional anti-ubiquitin antibody (Fig. 5E). No K63-linked polyubiquitinated TRAF3 could be detected. We interpret these results to suggest that TRAF3 is subjected only to degradative K48-linked polyubiquitination that is c-IAP1/2-dependent. By contrast, TRAF2 is subjected to both K63-linked and K48-linked polyubiquitination and only the K48-linked conjugates were c-IAP1/2-dependent.
Requirement of complex release for MEKK1 and MAPK activation
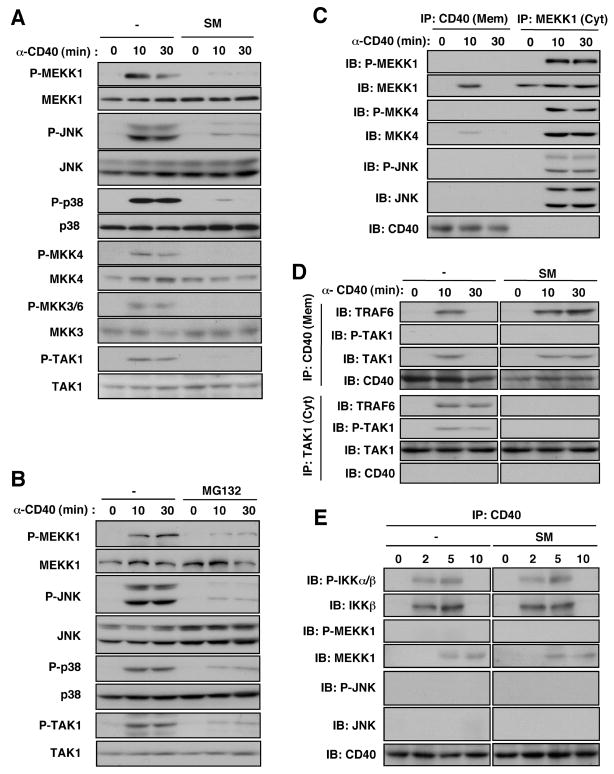
c-IAP and proteasome activity also appeared to be required for activation of MAP3Ks and MAPKs. Phosphorylation of MEKK1, TAK1, MKK4, MKK3/6, JNK, and p38 was inhibited in splenic B cells that were treated with c-IAP or proteasome inhibitors (Fig. 6A, B). Similar results were obtained in c-IAP1/2-deficient cells (Fig. S8).
Fig. 6.
Requirement of c-IAP and proteasome activity for cytosolic activation of MAP3K signaling modules. (A, B) Splenic B cells were treated with or without SM (A) or MG132 (B) for 4 hrs and 30 min, respectively, prior to anti-CD40 stimulation. Kinase phosphorylation/activation was monitored by immunoblotting. (C) Subcellular location of MEKK1 module activation. Splenic B cells were stimulated with anti-CD40 and divided at the indicated times into membrane and cytosolic fractions that were immunoprecipitated with anti-CD40 or anti-MEKK1, respectively and separated on the same gel. Kinase phosphorylation/activation was monitored by immunoblotting. (D) Requirement of c-IAP1/2 for cytosolic translocation of the TRAF6-TAK1 signaling complex. Splenic B cells treated with or without SM were stimulated with anti-CD40. Cells were divided into membrane and cytosolic fractions that were immunoprecipitated with anti-CD40 and anti-TAK1, respectively. The gel-separated immunecomplexes were blotted with the indicated antibodies. (E) Splenic B cells were pretreated with or without SM and stimulated anti-CD40. At the indicated times, membranes were isolated, lysed and immunoprecipitated with anti-CD40. The gel-separated immunecomplexes were analyzed as above.
These results indicate that MEKK1 and TAK1 and their signaling modules are not activated unless the CD40-assembled signaling complex leaves the receptor and translocates to the cytosol. To investigate this point, we examined the site of kinase activation. No phosphorylation of any relevant kinase was detected in the membrane fraction, whereas the cytosolic complex contained phosphorylated (and thus, activated) MEKK1, MKK4, and JNK (Fig. 6C). CD40-associated JNK1 and JNK2 were not detected in the membrane fraction, whereas these kinases were readily detected in the cytosolic MEKK1 complex. The cytosolic complex also contained higher relative amounts of MKK4 than did the receptor-associated complex.
TAK1 and TRAF6, which also contribute to MAPK activation (Fig. 1A, B), were also recruited to CD40 upon its engagement and a complex containing TAK1 and TRAF6 was released to the cytosol (Fig. 6D). Pretreatment of cells with SM prevented the release of this complex and inhibited CD40-induced phosphorylation of TAK1, which was detected only in the cytoplasm.
IKKβ was recruited to the CD40 signaling complex as early as 2 min after receptor engagement. Maximum amounts were present at 5 min and IKKβ was no longer detected in the CD40 complex after 10 min (Fig. 6E). IKKβ was activated at the receptor and was insensitive to SM. MEKK1 recruitment was considerably slower: activated MEKK1 was first detected at 5 min after receptor engagement in the cytoplasm and its activation peaked at 10 min, the first time point at which activated JNK was detected (Fig. S9). Activation of MEKK1 and JNK as well as MEKK1 ubiquitination, which was also restricted to the cytosolic complex (Fig. S10), were inhibited by SM pretreatment. SM also inhibited TNF-induced activation of MEKK1, TAK1, and JNK, but had no effect on activation of IKK (Fig. S11). By contrast, depletion of TRAF3, which accelerated MAPK activation, also accelerated recruitment of MKK4, MKK7, and JNK to MEKK1 but had no effect on TRAF2 recruitment to CD40 (Fig. S12).
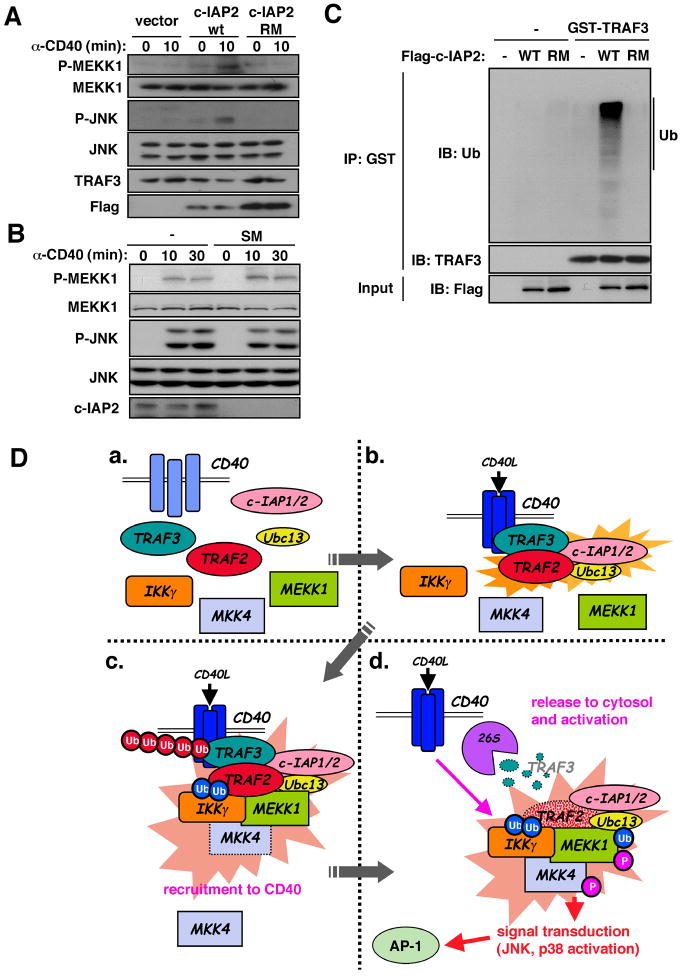
We further examined the role of c-IAP1/2 in CD40 signaling. Reconstitution of c-IAP-deficient multiple myeloma cells with c-IAP2 restored CD40-induced phosphorylation of MEKK1 (Fig. 7A). This appeared to require c-IAP2 catalytic activity because no phospho-MEKK1 was detected in cells expressing RING finger mutated c-IAP2 (32). Indeed, in vitro ubiquitination assay revealed that c-IAP2 served as a TRAF3 ubiquitin ligase and this activity depended on its RING finger (Fig. 7C). Supporting the notion that TRAF3 is the “brake” that prevents complex release and kinase activation, MAPK signaling in TRAF3-deficient cells was insensitive to SM (Fig. 7B).
Fig. 7.
MEKK1 signaling depends on c-IAP-induced TRAF3 degradation and a two-stage activation mechanism. (A) Requirement of c-IAP2 E3 ligase activity for MEKK1 activation and TRAF3 degradation. c-IAP-deficient multiple myeloma cells were transfected with vector, flag-c-IAP2(WT) and flag-c-IAP2(RM) (c-IAP2 with RING finger mutations that render the E3 ligase inactive). After G418 selection for 1 week, the cells were stimulated with anti-CD40. Lysates were analyzed by immunoblotting with the indicated antibodies. (B) TRAF3 deficiency renders MEKK1 signaling c-IAP1/2-independent. TRAF3 knockdown A20 cells were incubated with or without SM and stimulated with anti-CD40. At the indicated times, MEKK1 and JNK activation were analyzed by immunoblotting. (C) TRAF3 ubiquitination by c-IAP2. Cell lysates were prepared as well as (A). c-IAP2 WT and RM were immunoprecipitated, washed, and incubated with or without GST-TRAF3 in a reaction mixture containing ubiquitin, ATP, E1 and a mixture of E2 ubiquitin-conjugating enzymes. After 30 min, GST-TRAF3 was immunoprecipitated, gel-separated, and analyzed by immunoblotting with anti-ubiquitin and anti-TRAF3 antibodies. Amounts of c-IAP2 WT and RM were examined with anti-Flag antibody. (D) A two-stage signaling mechanism. a. In non-stimulated B cells, only CD40 is membrane-associated. b. Receptor engagement induces trimerization and recruitment of TRAF2, TRAF3, c-IAP1/2 and Ubc13. c. Next to be recruited into this complex are IKKγ and MEKK1. Interactions between IKKγ and MEKK1 and K63-linked polyubiquitin chains catalyzed by TRAF2 and Ubc13 stabilize the complex. d. c-IAP1/2 catalyze K48-linked polyubiquitination of TRAF3 whose proteasomal (26S) degradation results in translocation of the receptor-assembled signaling complex into the cytosol where MEKK1 is activated and in-turn activates downstream components of its signaling module.
Discussion
Growth factor, cytokine, and immune receptors use MAPK cascades to control many biological outcomes of receptor occupancy (33). Activation of receptor-proximal MAP3Ks that lie at the apex of MAPK cascades is thought to require assembly of multiprotein signaling complexes at receptor intracellular domains, and dissociation of receptor-associated complexes has been linked to signal termination. Our results suggest otherwise. Whereas CD40-induced MEKK1 or TAK1 activation requires assembly of a multiprotein complex at the receptor, it is the release of this complex into the cytosol that causes kinase activation. These results indicate that MAPK signaling, at least by CD40 and other TNFRs, follows a two-stage mechanism based on assembly of a multiprotein complex at the receptor that primes MAP3Ks for activation, but in which kinase activation is delayed until the complex is released to the cytoplasm (Fig. 7D).
Whereas TAK1 activation is TRAF6-dependent, activation of MEKK1 depends on TRAF2, which is directly recruited, probably as a TRAF2:3 hetero-oligomer (22), to CD40 trimers (25). TRAF2 and TRAF3 play opposing but complementary roles in MEKK1 and MAPK activation. In addition to initiating complex assembly, TRAF2 is responsible for recruitment of c-IAP1/2. Although c-IAP1/2 are dispensable for MEKK1 or TAK1 recruitment, they are critical for their activation because they are needed for degradation of TRAF3, which acts as a “brake” that prevents release of the receptor associated complexes to the cytoplasm (Fig. 7D). In this two-stage mechanism, assembly of the membrane receptor-associated signaling complex (stage I) can be separated in two steps: recruitment of TRAF2:TRAF3 (or TRAF6:TRAF3 for TAK1), c-IAP1/2 and Ubc13, followed by recruitment of IKKγ and MEKK1. Given that IKKγ can bind K63-linked polyubiquitin (18, 19), which decorates TRAF2, it is plausible that Ubc13 and TRAF2 catalyze synthesis of the polyubiquitin chains to which IKKγ binds to promote MEKK1 recruitment. MEKK1 also contains an ubiquitin interaction motif (24), which may further contribute to complex stabilization. Consistent with this notion, Ubc13 is obligatory for MAPK activation by several immune-receptors (23).
Assembly of the receptor-associated signaling complex is insufficient for MAP3K and MAPK activation. Activation requires translocation to the cytosol, which depends on K48-linked polyubiquitination of TRAF3 catalyzed by c-IAP1/2 and subsequent proteasomal degradation (Fig. 7D). In the absence of TRAF3, which can not activate MAPK or IKK signaling upon overexpression (34), complex release and MAPK activation are accelerated and are rendered c-IAP1/2-independent. Therefore, the signaling function of TRAF3 is entirely different from that of TRAF2 or TRAF6. While the former promote kinase activation through their adaptor and ubiquitin ligase functions, TRAF3 prevents complex release and premature activation.
While it remains to be seen whether the two-stage signaling mechanism applies to activation of MAP3Ks by other cytokine and immune receptors, IKK activation depends on a different mechanism. It occurs at the receptor with considerably faster kinetics and is c-IAP1/2-independent. Spatial and temporal separation of IKK activation from MAPK activation may be related to their different cellular functions.
Supplementary Material
References and Notes
- 1.Schlessinger J. Cell. 2000;103:211. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Pawson T, Gish GD, Nash P. Trends Cell Biol. 2001;11:504. doi: 10.1016/s0962-8924(01)02154-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Goeddel DV. Science. 2002;296:1634. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 4.Bodmer JL, Schneider P, Tschopp J. Trends Biochem Sci. 2002;27:19. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 5.Baud V, Karin M. Trends Cell Biol. 2001;11:372. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 6.Xia ZP, Chen ZJ. Sci STKE. 2005;2005:pe7 . doi: 10.1126/stke.2722005pe7. [DOI] [PubMed] [Google Scholar]
- 7.Rothe M, Wong SC, Henzel WJ, Goeddel DV. Cell. 1994;78:681. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 8.Ninomiya-Tsuji J, et al. Nature. 1999;398:252. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher E, et al. Nat Immunol. 2007;8:57. doi: 10.1038/ni1421. [DOI] [PubMed] [Google Scholar]
- 10.Hsu H, Shu HB, Pan MG, Goeddel DV. Cell. 1996;84:299. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 11.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. Cell. 1995;83:1243. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 12.Micheau O, Tschopp J. Cell. 2003;114:181. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 13.Muppidi JR, Tschopp J, Siegel RM. Immunity. 2004;21:461. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Devin A, et al. Immunity. 2000;12:419. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, et al. Nature. 2001;412:346. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 16.Hacker H, Karin M. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 17.Rothwarf DM, Zandi E, Natoli G, Karin M. Nature. 1998;395:297. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 18.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Mol Cell. 2006;22:245. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Nat Cell Biol. 2006;8:398. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 20.Baud V, et al. Genes Dev. 1999;13:1297. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullen SS, Dang TT, Crute JJ, Kehry MR. J Biol Chem. 1999;274:14246. doi: 10.1074/jbc.274.20.14246. [DOI] [PubMed] [Google Scholar]
- 22.Bishop GA. Nat Rev Immunol. 2004;4:775. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, et al. Nat Immunol. 2006;7:962. doi: 10.1038/ni1367. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T. Mol Cell. 2002;9:945. doi: 10.1016/s1097-2765(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 25.Ye H, Park YC, Kreishman M, Kieff E, Wu H. Mol Cell. 1999;4:321. doi: 10.1016/s1097-2765(00)80334-2. [DOI] [PubMed] [Google Scholar]
- 26.Keats JJ, et al. Cancer Cell. 2007;12:131. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, et al. Science. 2004;305:1471. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 28.Petersen SL, et al. Cancer Cell. 2007;12:445. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaux DL, Silke J. Nat Rev Mol Cell Biol. 2005;6:287. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Yang Y, Ashwell JD. Nature. 2002;416:345. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, et al. (submitted for publication) [Google Scholar]
- 32.Huang H, et al. J Biol Chem. 2000;275:26661. doi: 10.1074/jbc.C000199200. [DOI] [PubMed] [Google Scholar]
- 33.Chang L, Karin M. Nature. 2001;410:37. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 34.Hacker H, et al. Nature. 2006;439:204. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 35.We thank Vishva M. Dixit (Genentech) for his excellent suggestions, Xiaodong Wang (University of Texas Southwestern) for SM, Joe DiDonato (Cleveland Clinic) and Gilles Courtois (INSERM U697) for IKKγ-deficient cells, Rafael Fonseca (Mayo Clinic) for c-IAP-deficient cells, John Reed and Ze’ev Ronai (Burnham) for c-IAP2 and TRAF2 expression vectors, respectively. Work was supported by NIH grant AI043477 to M.K. who is an American Cancer Society Research Professor. A.M., P.-H.T., and J.-L.L. were supported in parts by The Mochida Memorial Foundation for Medical and Pharmaceutical Research, American Lung Association of California and Life Science Foundation, respectively. H.W. and D.A.A.V were supported by the NIH (AI52199), a Cancer Center Support CORE grant (CA21765) and the American Lebanese Syrian Associated Charities (ALSAC).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.