Abstract
Background
This study aimed to estimate the rate of HER-2/neu (c-erbB2) immunohistochemical overexpression in different histological types of breast cancer found in the middle Euphrates region of Iraq, a region that was exposed to high levels of depleted uranium. HER-2/neu (c-erbB2) overexpression was correlated with common clinicopathological parameters such as age, grade, stage, tumor size and lymph node involvement to determine if any particular biomarker for exposure to depleted uranium could be found in the tumor samples from this region.
Materials and Methods
The present investigation was performed over a period starting from September 2007 to June 2008. Formalin-fixed, paraffin-embedded blocks from 90 patients with breast cancer were included in this study. A group of 25 patients with benign breast lesions (fibroadenoma) was included as a comparative group, and 20 breast tissue sections were used as controls. Labeled streptavidin-biotin (LSAB) complex method was employed for immunohistochemical detection of HER-2/neu.
Results
HER-2/neu immuno-expression was positive in 67.8% of breast cancer, while it was negative in all benign breast lesions (fibroadenoma) (P < 0.05). HER-2/neu immunostaining was significantly associated with histological type and recurrence of breast cancer (P < 0.05). It was positively correlated with tumor grade, but this finding was not significant (P > 0.05).
Conclusion
Based upon the findings of this study, it can be concluded that HER-2/neu overexpression plays an important role in the pathogenesis of breast cancer and is associated with a worse prognosis. The findings indicate that in regions exposed to high levels of depleted uranium, HER-2/neu overexpression is high, but its correlation with age, grade, stage, tumor size, and lymph node involvement is similar to studies that have been conducted on populations not exposed to depleted uranium.
Keywords: Breast cancer, gene overexpression, HER-2/neu receptor, immunohistochemistry
Background
Breast carcinoma is the commonest malignant tumor and the leading cause of cancer death in women, with more than one million new cases occurring worldwide annually.[1] It is well known that proto-oncogenes and tumor suppressor genes are two types of genes that play a key role in the regulation of cell growth and differentiation. Hence, any alteration in one or more of these genes appears to play an important role in the pathogenesis of most human malignancies.[2] HER-2/neu proto-oncogene amplification and/or overexpression is one of the most important alterations seen in breast cancer. The HER-2/neu proto-oncogene (also called c-erbB2) is located on chromosome 17q11[3–5] which encodes for p185, a transmembrane glycoprotein with tyrosine kinase activity that belongs to the family of epidermal growth factor receptors.[4] HER2/neu proto-oncogene is amplified and/or overexpressed in approximately 25–30% of invasive breast cancers.[5,6] An association has been found to exist between amplification and/or overexpression of HER-2/neu and a wide variety of different clinical and pathological features of breast carcinoma. From a clinical point of view, HER-2/neu receptor has become an important target for antibody-based therapy with trastuzumab (Herceptin).
In Iraq, where the population was exposed to high levels of depleted uranium following the first and second Gulf Wars, breast cancer is the most common tumor type in females.[7] Over the last ten years, there has been a three-fold increase in the incidence of breast cancer,[8] with most of this increase being attributed to a particularly aggressive type of the cancer.[9,10] This finding inspired an investigation of Her2/neu expression in a series of Iraqi women with breast cancer to see whether any differences in Her2/neu expression, in correlation with tumor characteristics, could be found in a population exposed to depleted uranium.
Materials and Methods
Ninety specimens of formalin-fixed, paraffin embedded breast cancer tissue, collected from breast cancer patients over a period from September 2007 to June 2008 were included in this study. All cases were collected from major hospitals and private laboratories in Kufa district area (located in the middle of Iraq). The age range of patients was 21 to 70 years, with a mean age of 48.8 years. A group of 25 patients with benign breast lesions (fibroadenoma) was included as a comparative group and 20 normal breast tissue sections were included as controls. Confirmation of histopathological diagnosis, grade and stage of tumor was carried out after reviewing all slides before proceeding to the immunohistochemical approach. Tissue sections with a thickness of 5-µ were taken from the formalin-fixed, paraffin embedded blocks for immunohistochemistry. Labeled streptavidin-biotin (LSAB) method was employed for immunohistochemical detection of HER-2/neu using Hercep Test Kit (K5204 Dako Co.). The intensity of HER-2/neu cell membrane stain was classified into score 0 (negative; no stain is observed or faint membrane staining presents in less than 10% of tumor cells), score 1+ (negative; a faint/barely perceptible membrane staining was detected in more than 10% of tumor cells; these cells exhibit incomplete membrane staining), score 2+ (weakly positive; a weak to moderate complete membrane staining was observed in more than 10% of tumor cells) and score 3+ (strongly positive; a strong complete membrane staining was observed in more than 10% of tumor cells).[11] All biopsies were classified according to the modified Bloom Richardson Grading System into three grades: Grade I, Grade II and Grade III. The results were statistically evaluated with a Chi-squared test (at a significant level of <0.05) and correlation-regression analysis (at significant level of R = 0.3) using SSPS software. The study received ethical approval from the Research Ethics Committee of the Middle Euphrates Centre for Cancer Research and followed the Tenets of the Declaration of Helsinki.
Results
HER-2/neu immuno-expression was positive in 67.8% of breast cancer cases and negative in all sections of the normal breast tissue and benign breast lesions (fibroadenomas) with significant differences among these groups (P < 0.05) [Table 1]. HER-2/neu overexpression was detected in 73.2% of invasive ductal carcinoma (IDC) cases and in 12.5% of invasive lobular carcinoma. The difference was statistically significant (P < 0.05).
Table 1.
HER-2/neu(C-ErbB-2) overexpression and histological characteristics
| Parameters | Total | c-ErbB-2 protein overexpression | P value | R test | |
|---|---|---|---|---|---|
| No. of patients | (Negative) | (Positive) | |||
| Benign fibroadenoma | 25 | 25 (100) | 0 (-) | <0.05 | |
| Normal breast tissue | 20 | 20 (100) | 0 (-) | ||
| Malignant cases | 90 | 29 (32.2) | 61(67.8) | ||
| Histological type | |||||
| Ductal carcinoma** | 82 | 22 (26.8) | 60 (73.2) | <0.05 | |
| Lobular carcinoma | 8 | 7 (87.5) | 1 (12.5) | ||
| **including | |||||
| IDC + DCIS | 18 | 1 (3.6) | 17 (94.4) | ||
| IDC + Paget's | 3 | 0 | 3 (100) | ||
| Grade | |||||
| I | 2 | 2 (100) | 0 (-) | >0.05 | 0.96 |
| II | 17 | 5 (29.4) | 12 (70.6) | ||
| III | 71 | 22 (31.0) | 49 (69.0) | ||
| Tumor size | |||||
| ≤2 cm. | 3 | 0 (-) | 3 (100) | >0.05 | |
| 2–5 cm. | 43 | 18 (41.8) | 25 (58.2) | ||
| >5 cm. | 44 | 11 (25) | 33 (75) | ||
| Tumor stage | |||||
| Stage I | 5 | 3(60) | 2(40) | >0.05 | 0.07 |
| Stage II | 23 | 5 (21.7) | 18 (78.3) | ||
| Stage III | 41 | 13 (31.7) | 28 (68.3) | ||
| Stage IV | 3 | 2 (66.7) | 1 (33.3) | ||
| Age | |||||
| <50 | 46 | 15 (32.6) | 31 (67.4) | >0.05 | |
| >/50 | 44 | 14 (31.8) | 30 (68.2) | ||
| Axillary lymph nodes | |||||
| Negative | 22 | 10 (45.5) | 12 (54.5) | >0.05 | |
| Positive | 50 | 13 (26) | 37 (74) | ||
| Tumor recurrence | |||||
| Recurrent | 29 | 4 (13.8) | 25 (86.2) | <0.05 | |
| Primary | 61 | 25 (40.9) | 36 (59.1) | ||
Figures in parentheses are in percentage
Overexpression of HER-2/neu was detected in 65.6% of those with pure invasive ductal carcinomas; all of them were of a nonspecific type. A 94.4% of invasive ductal carcinoma with an in situ comedo component (DCIS) and 100% of invasive ductal carcinoma with overlying Paget's disease were HER-2/neu positive with a significant difference (P < 0.05) in comparison with pure invasive ductal carcinomas [Table 1].
There was a significant difference between the intensity of HER-2/neu overexpression and the histological type of breast cancer (P < 0.05) [Table 2]. HER-2/neu overexpression was detected in 70.6% of Grade II and 69.0% of Grade III breast cancer samples, while none of Grade I showed HER-2/neu overexpression [Table 1]. There was no significant difference in HER-2/neu overexpression between Grade II and Grade III of breast cancer (P > 0.05), but in comparison with Grade I a significant difference was noticed (P < 0.05). HER-2/neu overexpression was highly correlated with grade of tumor (R = 0.96), indicating that HER-2/neu-positive breast cancers are biologically aggressive. No statistically significant difference in the correlation between the intensity of HER-2/neu immunostaining and histological grade was found (P >0.05) [Table 2].
Table 2.
The intensity of HER-2/neu(C-ErbB-2) overexpression and histological characteristics
| Parameters | Total No. of patients | c-ErbB-2 protein overexpression intensity | P value | R test | |||
|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | ||||
| Histological type | |||||||
| IDC | 61 | 9 | 12 | 20 | 20 | <0.05 | |
| ILC | 8 | 3 | 4 | 1 | 0 | ||
| IDC+DCIS | 18 | 0 | 1 | 10 | 7 | ||
| IDC+ Paget's | 3 | 0 | 0 | 0 | 3 | ||
| Grade | |||||||
| I | 2 | 0 | 2 | 0 | 0 | <0.05 | 0.94 |
| II | 17 | 3 | 2 | 6 | 6 | ||
| III | 71 | 4 | 18 | 24 | 25 | ||
| Tumor stage | |||||||
| Stage I | 5 | 2 | 1 | 1 | 1 | >0.05 | |
| Stage II | 23 | 0 | 5 | 9 | 9 | ||
| Stage III | 41 | 3 | 10 | 16 | 12 | ||
| Stage IV | 3 | 0 | 2 | 1 | 0 | ||
| Tumor recurrence | |||||||
| Recurrent | 29 | 1 | 3 | 12 | 13 | >0.05 | |
| Primary | 61 | 11 | 14 | 19 | 17 | ||
In addition, a higher detection rate of HER-2/neu overexpression was noticed in recurrent breast cancer than primary lesions (86.2% and 59.1%, respectively) with a significant difference (P < 0.05) [Table 1]. No statistically significant differences between HER-2/neu overexpression and tumor size, tumor stage, lymph node involvement, and age of breast cancer patients were found (P > 0.05) [Table 1].
Discussion
Depleted uranium levels were estimated to be around 320–800 tons in the aftermath of the first Gulf war in 1991 with further comparable levels occurring in 2003.[8] Since the targets were always in heavily populated areas in the middle and south of Iraq, the extent of exposure on individuals was extensive but has been very hard to document accurately.[8]
This study showed that HER-2/neu overexpression was completely absent in both normal breast tissue and benign lesion (fibroadenomas) sections. The percentage of HER-2/neu overexpression in malignant breast lesions is disputed in our study as it ranges from 12.5% to 100%, but the finding is that HER-2/neu overexpression appears to be a biomarker for malignant breast tissue and does not play any role in the benign (fibroadenomas) breast lesions. This observation confirms the results of many previous studies that reached the same conclusion.[11–12]
Indeed, our study reported HER-2/neu overexpression in 67.8% out of 90 breast cancer cases [Figure 1a]. This result is significantly higher than findings reported elsewhere.[13] Furthermore, most of the ductal carcinoma cases were purely invasive ductal carcinomas of nonspecific type (74.4%) that showed significant HER-2/neu overexpression (65.6%). This provides more evidence of the hypothesis that aggressive tumors seem to show significant HER-2/neu overexpression and demonstrates the association between the nature of the biological expression of HER-2/neu by the tumor and its degree of malignancy since it has been argued that nonspecific type ductal carcinomas are the most aggressive variants of breast cancer.[14] The current study also demonstrated that 12.5% of lobular carcinoma cases exhibited HER-2/neu expression [Figure 1d].
Figure 1.
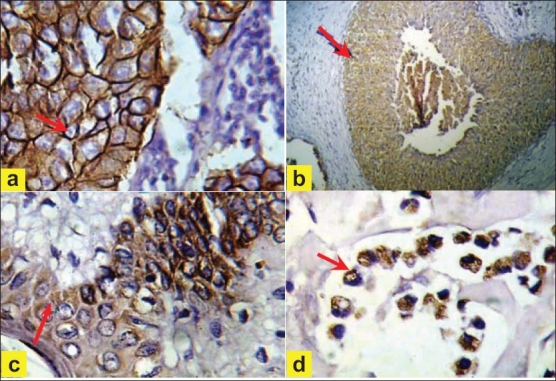
(a) Invasive ductal carcinoma, (→) showing strong complete cytoplasmic membrane staining of score 3+ (40×). (b) Insitu ductal carcinoma of comedo type, (→) showing strong complete cytoplasmic membrane staining of score 3+″positive (10×). (c) Paget's disease, (→) showing strong complete cytoplasmic membrane staining of score 3+ (40×). (d) Invasive lobular carcinoma, pleomorphic type, (→) showing moderate complete membrane staining of score 2+ (40×)
From the above results, a significant difference in HER-2/neu overexpression was observed between invasive ductal and lobular carcinoma (P < 0.05). This high discrepancy in HER-2/neu overexpression between invasive ductal and lobular carcinoma suggests that invasive lobular carcinomas are both morphologically and biologically different from invasive ductal carcinomas. However, further studies are required to provide a more definitive answer. All 18 cases of IDC with comedo-type in situ component showed HER-2/neu overexpression [Figure 1b]. The finding that HER-2/neu expression is seen more frequently in DCIS than in invasive carcinoma implicates that HER-2/neu signaling pathway is playing a critical role in the early stages of breast tumorogenesis. HER-2/neu overexpression was seen in all cases of both the invasive ductal carcinoma and Paget's disease components, when both components were seen simultaneously [Figure 1c].
HER-2/neu overexpression of low intensity (score 1+, considered as negative) was observed more frequently in ILC (50%), while weak to moderate intensity of HER-2/neu overexpression (score 2+) occurred more frequently in Invasive Ductal Carcinoma (IDC) + Ductal Carcinoma with an in situ comedo component (DCIS) (55.5%). High intensity of HER-2/neu overexpression (score 3+, considered as strong positive) was reported more frequently in Paget's disease (100%). These results suggest that the intensity of HER-2/neu overexpression is well correlated with histopathological type. Previous studies have shown faint or absent HER-2/neu overexpression in lobular carcinoma[15] and a strong positive score (score +3) in all cases of Paget's disease.[16]
The findings of this study show that a high incidence of samples of IDC with in-situ component (55.5%) were moderately stained (score +2). This compares with the findings of Lan et al.,[17] who examined the prevalence of c-erbB-2 gene amplification in moderately stained (score +2) Taiwanese specimens using IHC and found a higher prevalence of HER-2 overexpression (44.4%) than that found in similar studies from Western countries (13%-23%).[18,19]
This study found no significant association between HER-2/neu overexpression and age of the patients [Table 1], which concurs with a previous study.[16] A high proportion of HER-2/neu overexpression was seen in moderately and poorly differentiated cases of breast cancer, while a lower proportion of HER-2/neu expression was reported in cases of well differentiated breast cancer. HER-2/neu expression appears to be well correlated with tumor grade (R = 0.96). Immunohistochemical analysis showed HER-2/neu overexpression to be higher in recurrent than in primary cases of tumor. This finding was significant (P < 0.05). These results reflect the significant role of HER-2/neu overexpression in increasing the risk of local recurrence.[3]
It has been well documented that positive HER/2 breast cancer patients have good prognosis as they respond well to the blocking effect of these receptors by anti-HER/2 receptor antibody using monoclonal (Herceptin) drug that plays a role in the regression of the tumor size and prevention of recurrence.[6] All the patients in this study were advised to follow this treatment. The driving factor for the increased expression of HER-2/neu to the level of 68% compared to general expression of 30% might be due to some underlying genetic factors, although other environmental factors cannot be excluded. Further investigations are needed to ascertain the extent of the effect of depleted uranium and the types of mutations noted in the overexpressed oncogene before any final conclusions can be reached
Conclusion
Despite the three-fold increase of all types of breast cancer in Iraq due to long-term exposure to depleted uranium, this heightened exposure does not appear to be correlated with HER-2/neu overexpression. Differences may become evident with time and as mutations are passed to future generations. Further longitudinal studies and the use of techniques such as microarrays are required to investigate whether any effect of depleted uranium does manifest in the pathology of breast cancer.
Contributor Information
Esraa A. AL-Dujaily, Email: esraad@yahoo.com.
Asad A. Al-Janabi, Email: asadjanabi@yahoo.com.
Tomasz Pierscionek, Email: tomasz.pierscionek@ncl.ac.uk.
Akeel A. Yasseen, Email: a.yasseen@hotmail.com.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: A direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;21:3651–64. doi: 10.1200/JCO.2000.18.21.3651. [DOI] [PubMed] [Google Scholar]
- 3.Hung MC, Lau YK. Basic science of HER-2/neu: A review. Semin Oncol. 1999;26:51–9. [PubMed] [Google Scholar]
- 4.Coussens L, Yang-Feng TL, Lioa YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–9. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 5.Schecter AL, Stern DF, Vaidyanathan L, Decker S, Drebin JA, Greene MI, et al. The neu oncogene: An erbB-related gene encoding a 185,000-Mr tumor antigen. Nature. 1984;312:513–6. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Goldolphin W, Jones LA, Holt GA, Wong SG, Keith DE, et al. Studies of the HER-2/proto-oncogene in human breast cancer and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 7.Results of Iraqi cancer registry 1997-2002. Baghdad Iraq: Iraqi Cancer Board, Iraqi Cancer Registry Ministry of Health; [Google Scholar]
- 8.Al-Azzawi SN. Depleted uranium radioactive contamination In Iraq: An overview. Global Research. 2006 Aug 31;Vol. 1:4. [Google Scholar]
- 9.Choi DH, Shin DB, Lee MH, Lee DW, Dhandapani D, Carter D, et al. A comparison of five immunohistochemical biomarkers and HER-2/neu gene amplification by fluorescence in situ hybridization in white and Korean patients with early-onset breast carcinoma. Cancer. 2003;98:1587–95. doi: 10.1002/cncr.11703. [DOI] [PubMed] [Google Scholar]
- 10.Nunes RA, Harris LN. The HER2 extracellular domain as a prognostic and predictive factor in breast cancer. Clin Breast Cancer. 2002;3:125–35. doi: 10.3816/cbc.2002.n.017. [DOI] [PubMed] [Google Scholar]
- 11.Dowsett M, Bartlett JM, Ellis IO, Salter J, Hills M, Mallon E, et al. Correlation between Immunohistochemistry (Hercep Test) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centers. J Pathol. 2003;199:418–23. doi: 10.1002/path.1313. [DOI] [PubMed] [Google Scholar]
- 12.Selim AG, El-Ayat G, Wells GA. Expression of c-erbB2, p53, Bcl-2, Bax, c-myc and ki-67 in apocrine metaplasia and apocrine change within sclerosing adenosis of the breast. Virchows Arch. 2002;441:449–55. doi: 10.1007/s00428-002-0691-0. [DOI] [PubMed] [Google Scholar]
- 13.Van DE, Vijver MJ, Peterse JL, Mooi WJ, Wisman P, Lomans J, et al. Neu-protein overexpression in breast cancer: Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988;319:1239–45. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- 14.Saleh F, Abdeen S. Pathobiological features of breast tumours in the State of Kuwait: A comprehensive analysis. J Carcinog. 2007;6:12. doi: 10.1186/1477-3163-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149–56. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Moundhri M, Nirmala V, Al-Mawaly K, Ganguly S, Burney I, Rizvi A, et al. Significance of P53, Bcl2 and HER-2/neu protein expression in Omani Arab females with breast cancer. Pathol Oncol Res. 2003;9:226–31. doi: 10.1007/BF02893382. [DOI] [PubMed] [Google Scholar]
- 17.Lan C, Ming Liu J, Liu TW, Hsu DH, Shuching L, Chen JR, et al. Erb-b2 amplification by fluorescence in situ hybridization in breast cancer specimens read as 2+in immunohistochemical analysis. Am J Clin Pathol. 2005;124:97–102. doi: 10.1309/R2X4KK22QCL7PLME. [DOI] [PubMed] [Google Scholar]
- 18.Huang WY, Newman B, Millikan RC, Conway K, Hulka BS, Schell MJ, et al. Risk of breast cancer according to the status of HER-2/neu oncogene amplification. Cancer Epidemiol Biomarkers and Prev. 2000;9:65–71. [PubMed] [Google Scholar]
- 19.Czerniecki BJ, Roses RE, Kosi GK. Development of vaccines for high-risk ductal carcinoma in situ of the breast. Cancer Res. 2007;67:6531–4. doi: 10.1158/0008-5472.CAN-07-0878. [DOI] [PubMed] [Google Scholar]


