Abstract
CXCR4, the specific receptor for the chemokine SDF-1α that also binds CXCR4-using HIV gp120s, affects survival of different cell types, including neurons. However, current data show that the outcome of CXCR4 activation on neuronal survival may vary depending on the ligand and/or the cellular conditions. In this study, we have systematically compared the effects of SDF-1α and gp120IIIB (with or without CD4) on several intracellular pathways involved in cell survival, including MAP kinases and Akt-dependent pathways. Our data show that gp120IIIB and SDF-1α are both potent activators of MAP kinases in neuronal and non-neuronal cells, though the kinetic of these responses is slightly different. Furthermore, unlike SDF-1α, and independently of CD4, gp120IIIB is unable to stimulate Akt and some of its antiapoptotic targets (NF-κB and MDM2)—despite its ability to activate other signaling pathways in the same conditions. Finally, the viral protein is more efficient in recruiting some effectors (e.g., JNK) than others in comparison with SDF-1α (EC50 = 0.1 vs. 0.6 nM). We conclude that the intrinsic efficacy of the two ligands is significantly different and is pathway dependent. These findings have important implications for our understanding of CXCR4-mediated responses in the CNS, as well as the role of this coreceptor in HIV neuropathogenesis.
INTRODUCTION
SINCE THEIR DISCOVERY AS HIV CORECEPTORS,1 chemokine receptors have been the subject of intense investigation beyond their role in the immune system. In particular, this family of G-protein-coupled receptors (GPCRs) has been implicated in important physiological and pathological processes in the nervous system and in other areas of the body.2-4 CXCR4, the specific receptor for the chemokine stromal-derived growth factor-1 (SDF-1α/CXCL12), regulates various neuronal and glial functions.5-7 The SDF-1α/CXCR4 pair, which is constitutively expressed in the central nervous system (CNS), is also believed to play a role in the neuronal injury associated with HIV-1 infection.8-12 Abnormal activation of CXCR4 may cause neuronal damage and death via both direct (i.e., mediated by neuronal chemokine receptors) and indirect (i.e., mediated by glial and other nonneuronal receptors) mechanisms.13,14 For instance, neuronal chemokine receptors affect cell cycle proteins involved in apoptosis,15,16 whereas glial chemokine receptors may regulate excitotoxicity.17
HIV envelope proteins are potent neurotoxins in vitro and in vivo.18-24 The CXCR4-using viral protein gp120IIIB, which binds neuronal chemokine receptors with high affinity,25 activates many signaling pathways normally stimulated by SDF-1α.26,27 Nonetheless, SDF-1α can promote survival of various cell types, including neurons, and protect them from gp120-induced apoptosis.16,28,29
Binding of gp120 to chemokine receptors is influenced by CD4, a membrane glycoprotein that binds to gp120 and induces conformational changes in the viral envelope protein promoting its interaction with chemokine receptors.30 However, HIV envelope proteins can also act in a CD4-independent manner, and CD4-independent effects of gp120 in neurons and other cells have been reported.18,25,31,32 In the CNS, CD4 is mainly expressed by microglia/macrophages and other inflammatory/immune cells, major components of HIV neuropathogenesis.33-35 Thus, we sought to (1) determine whether gp120 signaling is significantly affected by CD4 and (2) evaluate the differences between SDF-1α and gp120 in the regulation of neuronal survival pathways. We tested the effect of CXCR4-using gp120 on different effectors of survival and differentiation, as well as of gp120 neurotoxicity. Hence, the effects of the HIV protein on intracellular calcium levels ([Ca2+]i), activation of MAP kinases, the Ser/Thr kinase Akt, and some of its downstream targets have been evaluated and compared to the SDF-1α-induced responses. Our data show that despite its ability to activate mitogen-activated protein kinases (MAPKs) and mobilize intracellular calcium, gp120 was unable to stimulate Akt even in the presence of CD4, which suggests a substantial difference in the intrinsic activities of the two ligands.
MATERIALS AND METHODS
Cell cultures
Human osteosarcoma cells (HOS)
HOS cells transfected with CXCR4 (CXCR4+/CD4−) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum and 1 μg/ml puromycin. Mycophenolic acid (40 μg/ml), xanthine (250 μg/ml), and hypoxanthine (13.5 μg/ml) were included in the medium to culture HOS cells expressing CD4 (alone or with CXCR4). Cells were serum starved before experimental treatments. All HOS cells were obtained through the AIDS Research and Reference Program, Division of AIDS, NIAID, NIH (from Dr. Nathaniel Landau).36,37
Human astrocytes
Cells were purchased from ScienCell Research Laboratories (San Diego, CA) and maintained in a defined culture medium provided by the vendor for three to five passages. Expression of glial fibrillary acidic protein (GFAP) was checked during the entire culture period to verify that all cells were positive to the glial marker. Cells were serum-starved before experimental treatments.
Primary rat neurons
Hippocampal rat neurons were cultured as previously described.18,28,38 Neurons were obtained from the brain of 17- to 18-day-old rat embryos and cultured in serum-free medium using a bilaminar cell culture system, i.e., a feeder layer of secondary astrocytes supported the growth and differentiation of the pure neuronal layer. Neurons were separated from glia immediately before the experiments, unless otherwise specified.
Fura-2 microfluorimetry
Changes of free intracellular calcium concentrations were studied by fura-2-based microfluorimetry and image analysis, as previously reported.18,28,38 Briefly, cells were loaded for 20 min with 2 μM fura-AM at room temperature in a balanced salt solution, washed, further incubated in saline for an additional 30 min, and then mounted on the stage of an inverted microscope (Olympus IX70) connected to a CCD camera (Micromax YS1300, Princeton Instruments) and a computer. The software Metamorph/Metafluor (Universal Imaging Corp.) was used for image acquisition and analysis. Calibration of the fluorescent signals was performed as described previously.28 Cells were perfused with either saline alone or saline plus experimental drugs during the whole experiment.
Western blots
After treatments, cells were washed with ice-cold balanced salt solution and scraped in lysis buffer [25 mM Tris/150 mM NaCl/5 mM NaF/1 mM ethylenediaminetetraacetic acid (EDTA)/1 mM dithiothreital (DTT)/1% Nonidet P-40/5 μg each of aprotinin, leupeptin, and pepstatin/1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride HCE (AEBSF)/1 mM vanadate]. A lower concentration of detergent (0.1%) was used for the extraction of cytosolic proteins when they had to be separated from nuclear proteins, and the pellet was further processed with a hyperosmotic buffer as previously described.28,38 Histone-1 was used as a nuclear marker and to verify the absence of nuclear proteins in the cytosolic extracts. The protein concentration in cell lysates was determined by bicinchoninic acid protein assay from Pierce. β-Actin expression was assessed to confirm equal sample loading and possible changes in constitutive proteins. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to PVDF membranes for immunoblotting. The following primary antibodies were used: anti-Akt, anti-phospho-Akt (Ser-473), and anti-posphoMDM2 (Ser-166) from Cell Signaling (1:8000, 1:4000, and 1:1000, respectively); anti-MDM2 (SMP14) and anti-NF-κB/p65 from Santa Cruz Biotechnologies (1:2000 and 1:1000, respectively); anti-ERK and phosphoERK from Transduction Laboratories and Cell Signaling (1:10,000 and 1:1000, respectively); anti-JNK and anti-phosphoJNK from Cell Signaling and Promega (1:1000 and 1:3000, respectively); anti-p38 and anti-phosphop38 from Cell Signaling (1:1,000); anti-β-actin (polyclonal antibody from Sigma-Aldrich, 1:5000); and anti-histone-1 (polyclonal antibody FL-219 from Santa Cruz Technology, 1:500). An image acquisition and analysis system from Bio-Rad (ChemiDoc System) as well as the U-Scan-IT (Silk Scientific) software were used for detection of chemiluminescent bands and densitometric analysis. Data are reported as mean ± SEM with sample size for each experiment. The intensity values from actin bands or total Akt, Erk, Jnk, and p38 band, respectively, were used to normalize phosphoprotein signals and compensate for possible variations in protein loading among samples. Data are expressed as percentage of control after normalization. Paired t test has been used to compare differences in the band densities of immunoblots.
Electromobility shift assay
Nuclear (HOS) or total (neurons) extracts (1.5 μg) were incubated with 0.25 μg/μl of 3′ biotinylated oligonucleotides containing a consensus-binding site for NF-κB (5-agtt gaggggactttcccaggc-3′). After a 20-min incubation at room temperature, the samples were resolved in a 6% nondenaturing PAGE. Streptavidin-horseradish peroxidase conjugate and the chemiluminescent substrates were provided with the LightShift EMSA kit from Pierce.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde and incubated with anti-GFAP from Santa Cruz Biotechnologies (1:200) or anti-CXCR4 (R&D System, 1:100) antibodies. Secondary antibodies conjugated to either AlexaFluor546 (Molecular Probes, 1:1000) or Cy2 (Jackson ImmunoResearch Labs, 1:500) were used. Nuclear counterstaining was obtained with Hoechst 33342 (3 μg/ml). The cells were mounted and observed under an epi-fluorescent microscope connected to a CCD camera (Micromax), and images were acquired and analyzed using the software Metamorph/Metafluor (Universal Imaging Corp.).38
Materials
Unless otherwise specified tissue culture media are from Life Technology, Rockville, MD and other general reagents are from Sigma, St. Louis, MO. SDF-1α and CD4 were purchased from R&D System, Minneapolis, MN. Proteins were reconstituted in 0.1% bovine serum albumin/phosphate-buffered saline (BSA/PBS) and aliquots stored at −20°C. Recombinant HIV-1IIIB gp120 (purchased from Intracel Corporation, Issaquah, WA) was prepared and stored as previously described.28,38 The synthetic peptides V1 and DV1 were prepared as described,39-41 while AMD3100 is from AnorMED Inc. (Langley, Canada). Custom modified oligonucleotides were obtained from Qiagen.
RESULTS
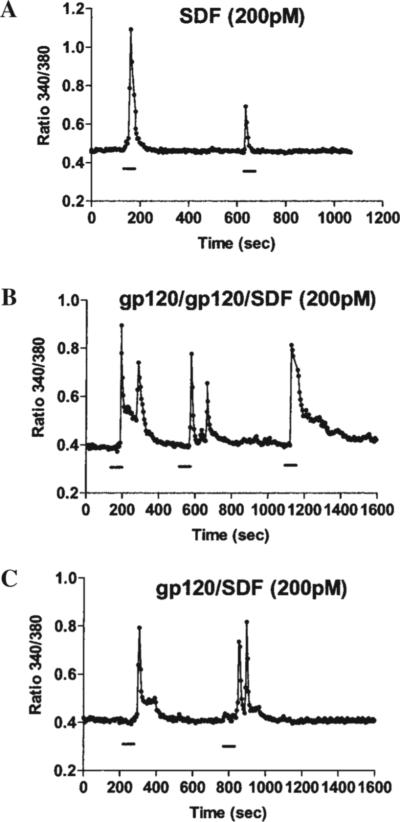
As other GPCRs, chemokine receptors are coupled to intracellular calcium ([Ca2+]i) mobilization in a variety of cell types. We have previously demonstrated that chemokines induce calcium transients in differentiated rat neurons and that CXCR4- and CCR5-using gp120s exerted a similar effect.28,38 However, it was not clear whether gp120 increases [Ca2+]i through a direct interaction with CXCR4 and what the role of CD4 in this process is. Here we used a CXCR4-transfected human cell line (HOS cells), which expresses CXCR4 with or without hCD4. Besides the advantage of an abundant and homogeneous expression of CXCR4 within the cell population, CXCR4 is also the only chemokine receptor expressed by these cells. Similar to our previous findings in primary neurons,28 both SDF-1α (200 pM–50 nM) and gp120IIIB (200 pM) evoked [Ca2+]i rises in HOS cells that expressed CXCR4 in the absence of CD4 (CXCR4+/CD4−). The [Ca2+]i rises induced by SDF-1α were blocked by pretreatment with pertussis toxin (PTx, 200 ng/ml for 18−20 hr) or anti-CXCR4 neutralizing antibodies (10 μg/ml) in these cells (Table 1). Similarly, responses to gp120IIIB were inhibited by PTx and were not observed in wild-type HOS cells (CXCR4−/CD4+) or in HOS cells transfected only with CD4 (CXCR4−/CD4+) (Table 1). These results indicate that gp120-induced calcium response is mediated by CXCR4 and is independent of CD4. Indeed, cells pre-exposed to 50 nM SDF-1α for 1 hr, which causes CXCR4 receptor desensitization,42 were unable to respond to gp120IIIB or SDF-1α (not shown). However, further experiments indicate that SDF-1α and gp120 stimulate CXCR4 in a different fashion. For instance, consecutive and relatively frequent stimulations (i.e., less than 10 min apart) of CXCR4 by SDF-1α down-regulated calcium responses in HOS cells (CXCR4+/CD4−) even when low concentrations of SDF-1α (200 pM) were used (Fig. 1A). Conversely, cells exposed to equimolar concentrations of gp120IIIB were still able to respond to a subsequent stimulation with gp120IIIB or SDF-1α (Fig. 1B and C), independently of the washout time. This suggests that receptor down-regulation/desensitization might not occur at the same rate for the two ligands. Thus, activation of CXCR4 by gp120IIIB might recruit different intracellular pathways as compared to SDF-1α. To test this hypothesis, we studied the ability of gp120IIIB and SDF-1α to stimulate MAP kinases and Akt kinase, as these pathways are primarily involved in survival mechanisms. We have systematically compared gp120- and SDF-induced responses using equimolar concentrations of both ligands. In HOS cells we found that both the chemokine and the HIV viral protein induced activation of ERK and JNK (Fig. 2). The response to SDF-1α was usually faster than that observed for gp120IIIB (not shown), but the latter produced significant activation of both kinases, even if used at low concentrations (200 pM) and in the absence of hCD4 (Fig. 2). Maximal responses induced by the two ligands were comparable. Co-incubation with soluble hCD4 (2 nM) slightly accelerated the gp120IIIB- (200 pM) induced increase in ERK phosphorylation (Fig. 2A). Equimolar concentrations of the two agonists activated JNK to a comparable extent (Fig. 2B).
Table 1.
Calcium Responses in HOS Cells
| Treatment | Total number of cells | Responsive cells | Peak ratio range |
|---|---|---|---|
| CXCR4+ cells | |||
| SDF 200 pM | 176 | 30% | 0.6−0.9 |
| SDF 2 nM | 59 | 35% | 0.6−0.9 |
| SDF 20 nM | 207 | 67% | 0.6−1.3 |
| SDF 50 nM | 135 | 100% | 0.8−1.6 |
| SDF 20 nM (PTx) | 34 | 5% | — |
| SDF 20 nM (anti-X4) | 40 | 10% | — |
| gp120 200 pM | 131 | 49% | 0.6−0.9 |
| gp120 200 pM (PTx) | 74 | 1% | — |
| CXCR4− cells | |||
| gp120 200 pM (CD4−) | 30 | 0 | — |
| gp120 200 pM (CD4+) | 23 | 0 | — |
FIG. 1.
Effect of SDF-1α and gp120IIIB on intracellular calcium. SDF-1α and gp120IIIB increase intracellular calcium in CXCR4+/CD4− HOS cells. Quick and consecutive stimulation of cells with SDF-1α down-regulates calcium responses (A) whereas when stimulated with gp120IIIB, the cells generally respond to subsequent stimulations with gp120 or SDF-1α irrespective of washout time (B, C).
FIG. 2.
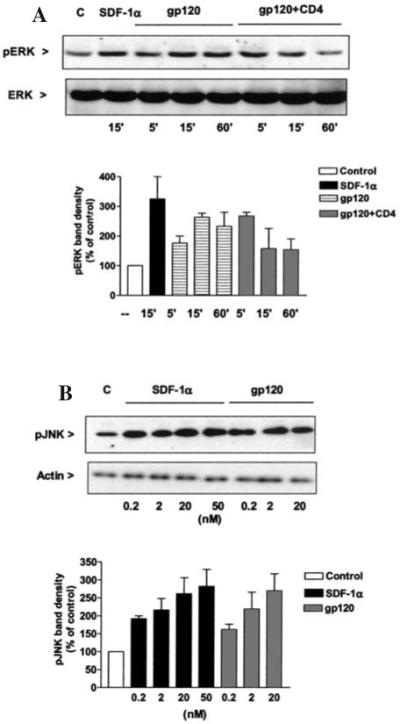
Effect of SDF-1α and gp120IIIB on ERK and JNK. (A) SDF-1α (20 nM) and gp120IIIB (200 pM) stimulate ERK phosphorylation in CXCR4+/CD4− HOS cells. Cells treated with gp120 in the presence of soluble hCD4 show a more rapid and transient phosphorylation of ERK as compared to gp120 alone. The graph indicates the densitometric analysis from three independent experiments. As in these cells the p42 ERK band is much more evident than the p44 band; it has always been used for densitometric analysis. (B) SDF-1α and gp120IIIB stimulate JNK phosphorylation in CXCR4+/CD4− HOS cells in a dose- and time-dependent manner. The graph shows the analysis of three independent experiments (15 min treatments).
Following the same experimental approach, we studied the signaling of the two CXCR4 ligands in secondary cultures of human astrocytes as these cells constitutively express functional chemokine receptors in vitro, including CXCR4.43 SDF-1α and gp120 stimulated MAP kinases in astrocytes (Fig. 3). The effects of the two ligands are blocked by various CXCR4 antagonists, such as the bicyclam AMD3100 (Fig. 3) and the synthetic peptides V1 and DV1 (not shown), confirming the involvement of this receptor in both gp120IIIB and SDF-1α action. However, gp120IIIB appears more potent than SDF-1α in activating MAP kinases, particularly JNK and p38 (EC50: 0.1 vs. 0.6 nM, for gp120IIIB and SDF-1α, respectively).
FIG. 3.
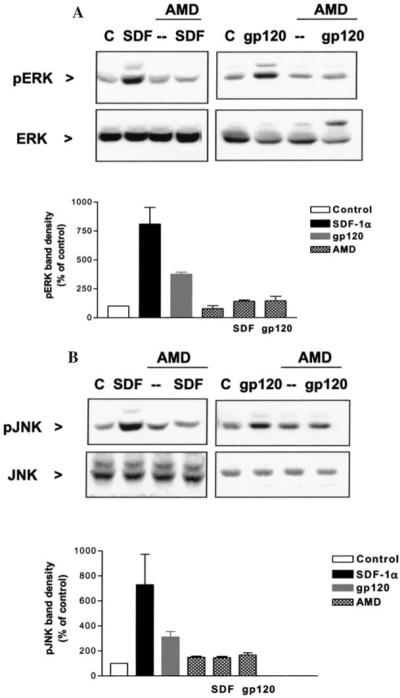
Phosphorylation of MAP kinases induced by SDF-1α or gp120IIIB is mediated by CXCR4 in human astrocytes. The CXCR4 antagonist, AMD3100 (200 ng/ml), is able to block the ERK (A) and JNK (B) activation by SDF-1α (2 nM) or gp120IIIB (200 pM). The graphs show the analysis of three independent experiments normalized with total ERK or JNK, respectively.
Next, we compared the effects of SDF-1α and gp120 on Akt, a pro-survival factor directly involved in the neuroprotective effect of chemokines.38 As reported in Figure 4, SDF-1α stimulates Akt phosphorylation in HOS-CXCR4+ cells and glia. Activation of the kinase was time and dose dependent, and maximal responses were observed within 15 min of treatment with maximal concentrations of SDF-1α, as in case of MAPKs (not shown). In contrast, gp120IIIB did not affect Akt, even after prolonged treatments (up to 60 min), at high concentrations (up to 20 nM) and/or in the presence of soluble hCD4 (Fig. 4) or in cells expressing both CXCR4 and hCD4 (not shown). SDF-1α stimulation of Akt was slightly inhibited by co-incubation with gp120IIIB (percentage inhibition of SDF-1α stimulation was 28 ± 9, n = 4) and completely blocked by treatment with PTx (not shown), as expected.
FIG. 4.
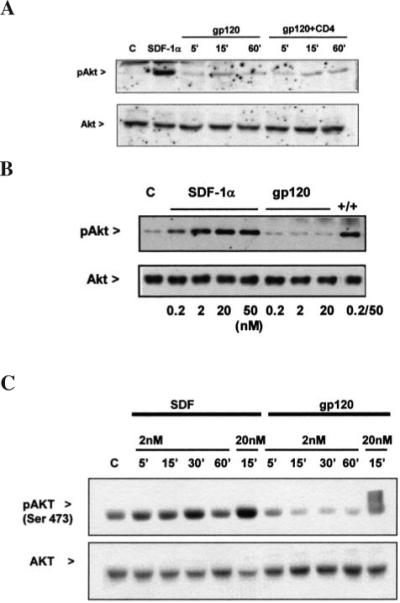
Effect of SDF-1α and gp120IIIB on Akt. SDF-1α is able to induce the phosphorylation of Akt in CXCR4+/CD4− HOS cells, whereas gp120IIIB (200 pM) is unable to do so in the presence or absence of soluble CD4 (A) as well as at high concentrations (B). Similar results were obtained in human astrocytes (C).
Parallel experiments in neurons (in the absence of soluble hCD4) also demonstrated an analogous pattern of CXCR4 activation: whereas both SDF-1α and gp120IIIB stimulated Ca fluxes and MAP kinases,28,38 SDF-1α but not gp120IIIB was able to activate Akt (Fig. 5).38 Finally, we tested the ability of the two ligands to modulate Akt targets that are implicated in cell fate, the transcription factor NF-κB, and the oncoprotein MDM2. Increased nuclear translocation of the p65NF-κB subunit was observed upon treatment of neurons with SDF-1α (Fig. 5). We did not find similar effects with gp120IIIB (Fig. 5). In addition, DNA retardation assays showed that SDF-1α increases the DNA-binding activity of p65NF-κB in HOS cells (Fig. 5). Another recently discovered target of Akt is the protein MDM2, which regulates the stability/activity of cell cycle proteins involved in apoptotic processes (i.e., p53, E2F-1 and Rb).44 MDM2 is a predominantly nuclear protein. Phosphorylation of MDM2 by Akt is critical for its nuclear localization and activation.44,45 The nuclear levels of MDM2 were significantly increased by SDF-1α in HOS (CXCR4+/CD4−) cells (Fig. 5). This effect was observed in cells treated with SDF-1α for up to 3 hr. In HOS cells, SDF-1α (but not gp120IIIB) also increased phosphorylation of MDM2 on the Ser-166, a known substrate of Akt,44,45 as determined by immunoblots with a phospho-specific antibody against MDM2 (not shown).
FIG. 5.
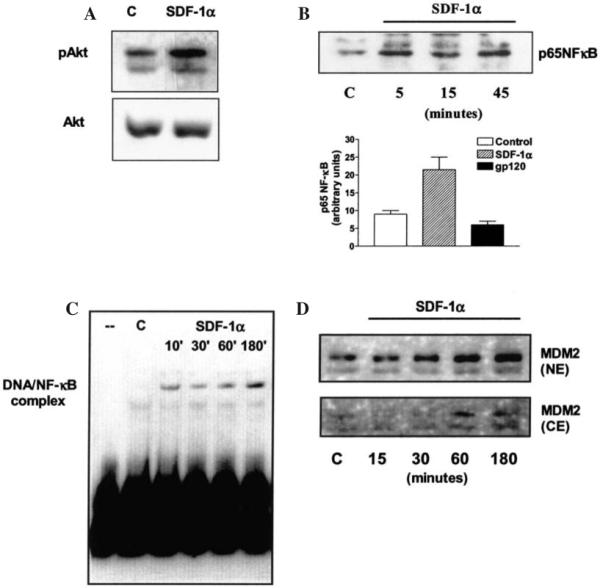
Effects of SDF-1α on Akt-mediated pathways. SDF-1α (50 nM) stimulates Akt phosphorylation in hippocampal neurons (A); the chemokine also increases p65NFκB levels in the nuclear fraction of these neurons, unlike gp120IIIB (200 pM 45 min) (B); SDF-1α (20 nM) also induces a time-dependent increase in the DNA-binding activity of p65 NFκB in CXCR4+/CD4− HOS cells. (C). In HOS cells, SDF-1α (20 nM) also induces up-regulation of MDM2 levels in the nucleus (D).
DISCUSSION
The data presented here show that although gp120 is a potent stimulator of MAP kinases, it is unable to activate pro-survival pathways such as Akt, either in the presence or in the absence of hCD4. This implies that the opposing effects of gp120 and SDF-1α on neuronal survival cannot be ascribed to the absence of hCD4, but rather to differences in the “intrinsic efficacy” of the two CXCR4 ligands to affect pro-survival pathways. Indeed, the chemokine and the viral protein differ in their ability to induce receptor internalization and desensitization in neuronal cells, as indicated by the results of our calcium imaging experiments and by studies with YFP-tagged CXCR4 showing that stimulation with SDF-1α resulted in greater endocytosis compared to gp120.31 That receptor internalization/degradation depends on the activation of specific intracellular pathways (i.e., β-arrestins, GRKs),46-48 which, in turn, are linked to other downstream effects47-49 (e.g., src activation), strengthens our conclusion that different transduction mechanisms are activated by gp120 and SDF-1α upon CXCR4 stimulation. These results are in agreement with the recent observation that gp120-induced apoptosis of dorsal root ganglia neurons is mediated by CXCR4 independently of hCD4,31 and with our previous findings regarding the involvement of Akt in the neuroprotective action of chemokines.28,38
The possibility that binding of different ligands to CXCR4 may result in pro-survival or apoptotic events is further corroborated by a very recent study showing that while full-length SDF-1α functions as a survival factor, the cleaved form of this chemokine (SDF-1α aa 5−67) is highly neurotoxic in vivo and in vitro.50 Though the N-terminal truncated molecule has a much reduced affinity for CXCR4 and is unable to induce chemotaxis of CD34+ cells, it still activates ERK (better than the full length SDF-1α) and its neurotoxicity is blocked by PTx50—suggesting that it still functions via a GPCR.
Intrinsic efficacy (i.e., the ability of a ligand to activate or inactivate a receptor) is an elusive, yet fundamental concept in molecular pharmacology, which is currently under re-evaluation.51,52 Recent evidence suggests that intrinsic efficacy might not be a single, ligand-dependent parameter but that agonists might have multiple intrinsic efficacies and that each agonist efficacy varies depending on which response (receptor behavior) is measured.51,53 Thus, intrinsic efficacy is becoming a pathway-dependent attribute and different ligands can have a range of efficacies for different receptor behaviors. Several groups have now shown that the potency and efficacy order of different drugs that act on the same receptor can, in fact, differ depending on the pathway analyzed. These concepts have been applied to a wide variety of GPCRs, including chemokine receptors.53 The idea that agonists produce only varying degrees of receptor activation is therefore becoming obsolete as it does not reconcile with the situations in which ligands induce differing patterns of responses.54 All these activities rely on the same molecular mechanism, namely the selective affinity (microaffinity) of a given ligand for a particular conformational state of the receptor.52
Therefore the neurotoxicity of gp120 (and perhaps cleaved SDF-1α) may derive from a strong affinity of the ligand for CXCR4 in a conformational state coupled to apoptotic pathways (p38, JNK) with a concomitant negligible affinity for those CXCR4 conformations that are coupled to the activation of pro-survival pathways (Akt). The idea of multiple CXCR4 conformations is also supported by previous structure–activity analyses with CXCR4 peptide antagonists, which show the remarkable flexibility of the CXCR4–ligand interface.39 The balance among the signaling cascades triggered by the various receptor conformations would ultimately determine cell survival. Akt is a major controller of these mechanisms. This kinase is not only responsible for generating pro-survival signals, but also counteracts several effectors of apoptosis, such as caspases, JNK, and the transcription factor E2F1.55-57 This is underscored by the finding that the neuroprotective activity of chemokines is lost when Akt is inhibited,38 and by the different effects of SDF-1α and gp120 on E2F1.16 In addition, a recently discovered function of Akt is the control of synaptic strength and plasticity.58 Chemokines regulate synaptic activity in neurons.28,59 Therefore, the inability of gp120 to affect Akt might have negative consequences at various levels, even those preceding apoptosis, providing a possible explanation for the discrepancy between serious neurological deficits and low degree of cell death observed in some neuroAIDS patients.
In conclusion, we have shown that SDF-1α and gp120 differ in their ability to activate intracellular pathways downstream of the activation of CXCR4 in neuronal and nonneuronal cells, and that gp120 may possess a different intrinsic efficacy for CXCR4 as compared to that of the natural CXCR4 ligand. A better understanding of the regulation of CXCR4-dependent pathways in normal and pathological conditions is essential, not only to improve our knowledge of HIV neuropathogenesis and foster the development of new pharmacological tools, but also to elucidate the role of chemokines in the CNS.
ACKNOWLEDGMENTS
This work was supported by grants from NIH (DA15014-01), the American Foundation of AIDS Research (amfAR 02816-30-RG), and the W.W. Smith Charitable Trust (A0302) to O.M., and by NIH GM067892 to A.F. The authors wish to thank Dr. R. Nichols for critical discussion of the manuscript and B.J. Musser for technical assistance. The continuous support from the NIH AIDS Research and Reference Reagent Program is appreciated.
REFERENCES
- 1.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 2.Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: Phvsiopathological implications. J Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 3.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, Mc-Clanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 4.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 5.Ragozzino D. CXC chemokine receptors in the central nervous system: Role in cerebellar neuromodulation and development. J Neurovirol. 2002;8:559–572. doi: 10.1080/13550280290100932. [DOI] [PubMed] [Google Scholar]
- 6.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 7.Tran PB, Miller RJ. Chemokine receptors: Signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- 8.Lavi E, Kolson DL, Ulrich AM, Fu L, Gonzalez-Scarano F. Chemokine receptors in the human brain and their relationship to HIV infection. J Neurovirol. 1998;4:301–311. doi: 10.3109/13550289809114531. [DOI] [PubMed] [Google Scholar]
- 9.Miller RJ, Meucci O. AIDS and the brain: Is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- 10.Gabuzda D, Wang J. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J Neurovirol. 2000;6(Suppl 1):S24–32. [PubMed] [Google Scholar]
- 11.Martin-Garcia J, Kolson DL, Gonzalez-Scarano F. Chemokine receptors in the brain: Their role in HIV infection and pathogenesis. AIDS. 2002;16:1709–1730. doi: 10.1097/00002030-200209060-00003. [DOI] [PubMed] [Google Scholar]
- 12.Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, Hunter DD, Nath A, Hedreen JC, Navia BA. SDF-1 alpha is expressed in astrocytes and neurons in the AIDS dementia complex: An in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62:617–626. doi: 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- 13.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 14.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: An evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 15.Jordan-Sciutto KL, Murray Fenner BA, Wiley CA, Achim CL. Response of cell cycle proteins to neurotrophic factor and chemokine stimulation in human neuroglia. Exp Neurol. 2001;167:205–214. doi: 10.1006/exnr.2000.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MZ, Brandimarti R, Musser BJ, Resue DM, Fatatis A, Meucci O. The chemokine receptor CXCR4 regulates cell-cycle proteins in neurons. J Neurovirol. 2003;9:300–314. doi: 10.1080/13550280390201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: Amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 18.Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: Protective action of TGF-beta 1. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toggas SM, Masliah E, Rockenstein EM, Rail GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, Rana F, Silva C, Ethier J, Wehrly K, Chesebro B, Power C. Human immunodeficiency virus type 1 envelope-mediated neuronal death: uncoupling of viral replication and neurotoxicity. J Virol. 2003;77:6899–6912. doi: 10.1128/JVI.77.12.6899-6912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krucker T, Toggas SM, Mucke L, Siggins GR. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- 22.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- 24.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 25.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: Functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 26.Davis CB, Dikic I, Unutmaz D, Hill CM, Arthos J, Siani MA, Thompson DA, Schlessinger J, Littman DR. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C, Liu QH, Tomkowicz B, Yi Y, Freedman BD, Collman RG. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J Leukoc Biol. 2003;74:676–682. doi: 10.1189/jlb.0503206. [DOI] [PubMed] [Google Scholar]
- 28.Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lataillade JJ, Clay D, Bourin P, Herodin F, Dupuy C, Jasmin C, Bousse-Kerdiles MC. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G(0)/G(1) transition in CD34(+) cells: Evidence for an autocrine/paracrine mechanism. Blood. 2002;99:1117–1129. doi: 10.1182/blood.v99.4.1117. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-Induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: Consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodner A, Toth PT, Oh SB, Lu M, Tran PB, Chin RK, Ren D, Miller RJ. CD4 dependence of gp120IIIB-CXCR4 interaction is cell-type specific. J Neuroimmunol. 2003;140:1–12. doi: 10.1016/s0165-5728(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 32.Roggero R, Robert-Hebmann V, Harrington S, Roland J, Vergne L, Jaleco S, Devaux C, Biard-Piechaczyk M. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J Virol. 2001;75:7637–7650. doi: 10.1128/JVI.75.16.7637-7650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40:240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- 34.Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 35.Kusdra L, McGuire D, Pulliam L. Changes in monocyte/macrophage neurotoxicity in the era of HAART: Implications for HIV-associated dementia. AIDS. 2002;16:31–38. doi: 10.1097/00002030-200201040-00005. [DOI] [PubMed] [Google Scholar]
- 36.Landau NR, Littman DR. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 38.Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou N, Luo Z, Luo J, Fan X, Cayabyab M, Hiraoka M, Liu D, Han X, Pesavento J, Dong CZ, Wang Y, An J, Kaji H, Sodroski JG, Huang Z. Exploring the stereochemistry of CXCR4-peptide recognition and inhibiting HIV-1 entry with D-peptides derived from chemokines. J Biol Chem. 2002;277:17476–17485. doi: 10.1074/jbc.M202063200. [DOI] [PubMed] [Google Scholar]
- 40.Luo Z, et al. Structure-function study and anti-HIV activity of synthetic peptide analogues derived from viral chemokine vMIP-II. Biochemistry. 2000;39:13545–13550. doi: 10.1021/bi000633q. [DOI] [PubMed] [Google Scholar]
- 41.Zhou N, Luo Z, Luo J, Hall JW, Huang Z. A novel peptide antagonist of CXCR4 derived from the N-terminus of viral chemokine vMIP-II. Biochemistry. 2000;39:3782–3787. doi: 10.1021/bi992750v. [DOI] [PubMed] [Google Scholar]
- 42.Fernandis AZ, Cherla RP, Chernock RD, Ganju RK. CXCR4/CCR5 down-modulation and chemotaxis are regulated by the proteasome pathway. J Biol Chem. 2002;277:18111–18117. doi: 10.1074/jbc.M200750200. [DOI] [PubMed] [Google Scholar]
- 43.Boutet A, et al. Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4. Glia. 2001;34:165–177. [PubMed] [Google Scholar]
- 44.Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27:462–467. doi: 10.1016/s0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- 45.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 47.Imamura T, Huang J, Dalle S, Ugi S, Usui I, Luttrell LM, Miller WE, Lefkowitz RJ, Olefsky JM. Beta-Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J Biol Chem. 2001;276:43663–43667. doi: 10.1074/jbc.M105364200. [DOI] [PubMed] [Google Scholar]
- 48.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 49.Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM, Power C. HIV-induced metal-loproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- 51.Clarke WP, Bond RA. The elusive nature of intrinsic efficacy. Trends Pharmacol Sci. 1998;19:270–276. doi: 10.1016/s0165-6147(97)01138-3. [DOI] [PubMed] [Google Scholar]
- 52.Kenakin T, Onaran O. The ligand paradox between affinity and efficacy: Can you be there and not make a difference? Trends Pharmacol Sci. 2002;23:275–280. doi: 10.1016/s0165-6147(02)02036-9. [DOI] [PubMed] [Google Scholar]
- 53.Kenakin T. Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- 54.Kenakin T. Inverse, protean, and ligand-selective agonism: Matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- 55.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stan-bridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 56.Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum MJ, Chao MV. Aktl regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 57.Hallstrom TC, Nevins JR. Specificity in the activation and control of transcription factor E2F-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:10848–10853. doi: 10.1073/pnas.1831408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 59.Limatola C, Giovannelli A, Maggi L, Ragozzino D, Castellani L, Ciotti MT, Vacca F, Mercanti D, Santoni A, Eusebi F. SDF-1alpha-mediated modulation of synaptic transmission in rat cerebellum. Eur J Neurosci. 2000;12:2497–2504. doi: 10.1046/j.1460-9568.2000.00139.x. [DOI] [PubMed] [Google Scholar]