Abstract
Obestatin is produced in the stomach from proghrelin by post-translational cleavage. The initial report claimed anorexigenic effects of obestatin in mice. Contrasting studies indicated no effect of obestatin on food intake (FI). We investigated influences of metabolic state (fed/fasted), environmental factors (dark/light phase) and brain Fos response to intraperitoneal (ip) obestatin in rats, and used the protocol from the original study assessing obestatin effects in mice. FI was determined in male rats injected ip before onset of dark or light phase, with obestatin (1 or 5 μmol/kg), CCK8S (3.5 nmol/kg) or 0.15 M NaCl, after fasting (16 h, n = 8/group) or ad libitum (n = 10-14/group) food intake. Fos expression in hypothalamic and brainstem nuclei was examined in freely fed rats 90 min after obestatin (5 μmol/kg), CCK8S (1.75 nmol/kg) or 0.15 M NaCl (n = 4/group). Additionally, fasted mice were injected ip with obestatin (1 μmol/kg) or urocortin 1 (2 nmol/kg) 15 min before food presentation. No effect on FI was observed after obestatin administration during the light and dark phase under both metabolic conditions while CCK8S reduced FI irrespectively of the conditions. The number of Fos positive neurons was not modified by obestatin while CCK8S increased Fos expression in selective brain nuclei. Obestatin did not influence the refeeding response to a fast in mice, while urocortin was effective. Therefore, peripheral obestatin has no effect on FI under various experimental conditions and did not induce Fos in relevant central neuronal circuitries modulating feeding in rodents.
Keywords: Obestatin, Food intake, CCK, Urocortin 1, Rats, Mice, Dark phase, Light phase
1. Introduction
Obestatin is a novel 23-amino acid peptide that originates from a post-translational cleavage of proghrelin located in X/A-like cells of the stomach [45]. Ghrelin, a 28-amino acid peptide [10,26] is a well-characterized hormone that stimulates food intake in rodents [1,30,43,47,49,50] and humans [48]. In contrast to ghrelin, obestatin has been initially reported to elicit anorexigenic effects in mice and rats after peripheral or intracerebroventricular injection [7,18,27,51]. Furthermore, chronic administration of obestatin reduces body weight gain in mice [27,51]. These findings suggest that obestatin may be involved in the regulation of energy homeostasis in rodents. However, several recent studies performed in rats and mice under various experimental conditions showed that obestatin injected intraperitoneally (ip) has no inhibitory effect on short-term food intake [11,14-17,21,32,41,54] and body weight gain [32,42]. The responsiveness to exogenous obestatin could be influenced by circadian rhythm and might account for the varying effects of peripheral obestatin on food intake found in recent studies [7,15,18,21,27,32,40-42,51,54].
It has been initially suggested that obestatin could inhibit food intake by stimulating key hypothalamic nuclei involved in feeding control, such as the arcuate nucleus (ARC) [35]. Moreover, it has been reported that peripheral obestatin rapidly crosses the blood brain barrier in mice, thus central pathways might play a role in the modulation of food intake by obestatin [35]. It has not yet been investigated if obestatin induces neuronal activity in relevant brain areas of the hypothalamus and brainstem involved in the regulation of feeding behavior. Over the last years, there is a consistent evidence that food intake alterations in response to peripheral administration of gut peptides (e.g. sulfated cholecystokinin octapeptide, CCK-8S, ghrelin, bombesin or amylin) are mediated at least partly by changes in neuronal activity in distinct brain nuclei [5,20,37,45]. For instance, specific brain pathways activated by intraperitoneally (ip) injected CCK-8S, as revealed by Fos-immunoreactivity (Fos-ir), include the paraventricular nucleus of the hypothalamus (PVN), the dorsomedial hypothalamic nucleus (DMH), the nucleus of the solitary tract (NTS) and the area postrema (AP) [23,25,29,33,38].
Thus, the aim of the present study was first to investigate whether obestatin injected ip influences food intake in male rats under fasting or ad libitum feeding conditions during the dark and light phases as conflicting reports may result from differencial responses depending upon the metabolic state of the animals (fed or fasted), and environmental factors (dark and light phase). The effects of obestatin on food intake were compared with those induced by CCK-8S injected ip. Second, we examined the effect on brain neuronal activity induced by the ip administration of obestatin as assessed by Fos-immunohistochemistry in different hypothalamic (PVN, VMH, DMH, ARC), and brainstem nuclei (NTS) which are important for the modulation of feeding behavior. Studies were performed under ad libitum feeding conditions at the beginning of the light phase to obtain metabolically stable conditions. Fasting itself causes metabolic stress and induces significant increase in neuronal activity in the PVN [8]. In addition, it is known that orexigenic neuropeptides (e.g. ghrelin, neuropeptide Y) induce Fos expression in hypothalamic brain nuclei namely the ARC nucleus [31,34,47]. Therefore, the activation of the orexigenic systems at the beginning of dark phase could also exert a stimulating effect on neuronal activity in the brain. We administered obestatin at the dose of 1 μmol/kg, and included an additional 5-fold higher dose based on our previous studies that obestatin injected ip in doses ranging from 0.1 to 3 mg/kg (∼0.04 to ∼1.2 μmol/kg) did not influence food intake in rats [14]. Lastly, in view of recent report by Zhang et al. [52] that a 15 min interval between peptide injection and food replacement is essential to demonstrate the inhibitory effect of obestatin on food intake in fasted mice, additional studies were carried out in mice using similar experimental protocol.
2. Methods
2.1. Animals
Male Sprague-Dawley rats (body weight: 258 ± 26 g; Harlan Winkelmann GmbH, Borchen, Germany) and lean male mice (C57BL/6, 25-30 g; Harlan, San Diego, CA) were housed in groups of 4 rats/cage under conditions of controlled illumination (12 h light:12 h dark cycle, lights on/off: 6:30 a.m./6:30 p.m.), humidity, and temperature (22 ± 2 °C). Animals were fed with a standard rodent diet and tap water ad libitum. Rats and mice were accustomed to the experimental conditions for a period of at least 7 days by handling them daily and putting them in the position to mimic the procedure of intraperitoneal (ip) injection. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the state authority for animal research conduct.
2.2. Peptide preparation
For rat experiments, rat/mouse obestatin was purchased from Bachem AG (Heidelberg, Germany). For mice studies, rat/mouse obestatin was synthesized by the Dept. of Chemistry, Québec University (Montréal, Canada). Capillary zone electrophoresis and coelution on high pressure liquid chromatography show purity >90% and mass spectrometric data were as expected (calculated 2516.32, found 2516.2) (Peptide Biology Laboratories, Salk Institute, La Jolla, CA). For rat studies, obestatin was dissolved in distilled water and stored at -20 °C. CCK-8S was dissolved in water with 1% (v/v) 1N NH4OH. Immediately before starting the experiments, peptides were diluted in sterile 0.15 M NaCl (Braun, Melsungen, Germany) to reach the final concentration of 1 or 5 μmol/kg for obestatin and 1.75 and 3.5 nmol/kg (2 and 4 μg/kg) for CCK-8S. In mice experiments, obestatin as well as urocortin 1 (Peptide Biology Laboratories) were stored at -80 °C in powder form and dissolved in sterile saline (0.15 M NaCl) before use. Peptide doses in mice were based on the study by Zhang et al. [51] and in rats from our previous studies [14,23].
2.3. Procedures
2.3.1. Effects of obestatin injected ip on food intake in rats fed ad libitum or fasted for 16 h
Groups of randomized rats either deprived of food but not water for 16 h (n = 8 per group in both experiments) or freely fed (n = 10 per group in the light phase experiment, and n = 14 per group in the dark phase experiment) were injected ip (0.5 ml) with obestatin (1 or 5 μmol/kg), CCK-8S (3.5 nmol/kg; n = 4 in all experiments) or vehicle (0.15 M NaCl) 15 min before the dark or light phase started. Two minutes before the dark or light phase started, weighed rat chow was made available to the animals. Food intake was calculated as the difference between the food weights before and after the feeding period at each time interval (30 min, 1, 2, 3, 4, 5 and 12 h) and cumulative food intake was calculated by summating the values of the different time periods.
2.3.2. Effect of obestatin injected ip on food intake in mice fasted for 16 h
Mice housed singly 7 days prior each experiment. Mice were fasted for 16 h, then randomly injected ip (0.1 ml) with either obestatin (1 μmol/kg, n = 7), urocortin 1 (2 nmol/kg, n = 5) or saline (NaCl 0.9%, n = 6). Rodent weighed chow was given ad libitum 15 min after the ip injection, accordingly to the previous experimental protocol used to assess the effects of obestatin [52]. Food intake was recorded at 1, 3, and 5 h after food presentation by weighing (±0.01 g) the food and correcting for spillage, which was collected on papers placed at the bottom of the animal cages. Food intake was calculated as the difference between the food weights before and after the feeding period at each time interval and cumulative food intake was calculated by summating the values of the different time periods.
2.3.3. Effects of obestatin injected ip on Fos-ir in the hypothalamus and brainstem in rats fed ad libitum
Freely fed rats were injected ip (0.5 ml) with vehicle (0.15 M NaCl; n = 4), obestatin (5 μmol/kg; n = 4) or CCK-8S (1.75 nmol/kg; n = 4) and 90 min later deeply anesthetized with ip injections of ketamine (100 mg/kg Ketanest®, Curamed, Karlsruhe, Germany) and xylazine (10 mg/kg, Rompun® 2%, Bayer, Leverkusen, Germany) and heparinized with 2,500 U heparin ip (Liquemin®, Hoffmann-La Roche, Grenzach-Whylen, Germany). Transcardial perfusion, brain processing, and Fos immunochemistry were performed as described before [24].
2.4. Staining for Fos-immunoreactivity (Fos-ir)
Free-floating brain sections (thickness 25 μm) were pretreated with 1% (w/v) sodium borohydride (in phosphate buffer solution, PBS) for 15 min. Subsequently, sections were incubated in a solution containing 5% (w/v) bovine serum albumin (BSA) and 0.3% (v/v) Triton X-100 in PBS for 60 min to block unspecific antibody binding. Thereafter, the diluted primary antibody (rabbit anti-rat c-Fos protein; Oncogene Research Products, Boston, USA; 1:4000 in a solution of 5%, w/v BSA and 0.1%, w/v sodium azide in PBS) was applied for 42 h at room temperature. After rinsing sections in PBS three times and incubation in a solution containing 5% (w/v) BSA for 60 min, FITC-labeled goat-anti-rabbit IgG (Sigma, St. Louis, USA) was applied for 12 h at room temperature in an appropriate dilution (1:600 in 5%, w/v BSA in PBS). Sections were rinsed in PBS three times again and stained with propidium iodide (2.5 μg/ml in PBS) for 15 min to counterstain cell chromatin. Tissue sections were finally embedded in 10 μl anti-fading solution (100 mg/ml 1,4-diazabicyclo[2.2.2]octan (Sigma) in 90%, v/v glycerin, 10%, v/v PBS, pH 7.4) and analyzed using a confocal laser scanning microscope (cLSM 510, Carl Zeiss, Germany).
2.5. Data analysis
All data are expressed as mean ± S.E.M. Food intake data in rats were analyzed by one way repeated measures ANOVA followed by the Fisher LSD post hoc test and in mice by ANOVA followed by the Fisher LSD test. p < 0.05 was considered significant. Semi-quantitative assessment of Fos-ir was achieved by counting the number of Fos-ir positive cells as described before [25]. Briefly, neurons with green nuclear staining were considered Fos-ir positive. Every third of all consecutive coronal 25 μm sections was counted bilaterally for Fos-ir positive staining in the hypothalamic (ARC, PVN, VMH, and DMH) and brainstem (NTS) nuclei throughout their rostrocaudal extent. Fos-ir positive cells were counted in 10 sections per rat of the PVN and NTS, and 15 sections per rat in the ARC, VMH and DMH. Anatomic correlations were made according to landmarks given in Paxinos and Watson’s stereotaxic atlas [29]. The investigator counting the number of Fos-ir positive cells was blinded to treatments received by the animals. Data were analyzed by ANOVA followed by Fisher LSD test. p < 0.05 was considered significant.
3. Results
3.1. Effects of obestatin injected ip on cumulative food intake under ad libitum feeding or fasting conditions in rats
CCK-8S (3.5 nmol/kg body wt) injected ip induced temporary satiety signaling (Figs. 1AB and 2B; Tables 1 and 2). During the light phase in ad libitum fed rats, CCK-8S injected ip decreased food intake during the first 30, 60, and 120 min compared to the vehicle group although this did not reach statistical significance due to smaller and more variable amounts of food eaten by the vehicle group under these conditions (Fig. 2A; Tables 1 and 2). In contrast to CCK-8S, obestatin at both doses (1 and 5 μmol/kg body wt, ip) did not suppress the cumulative food intake at any time point throughout the 12 h experiment compared to the vehicle-treated rats when injected 15 min before the dark or light phase in 16-h fasted or fed rats (Figs.1AB and 2AB; Tables 1 and 2).
Fig. 1.
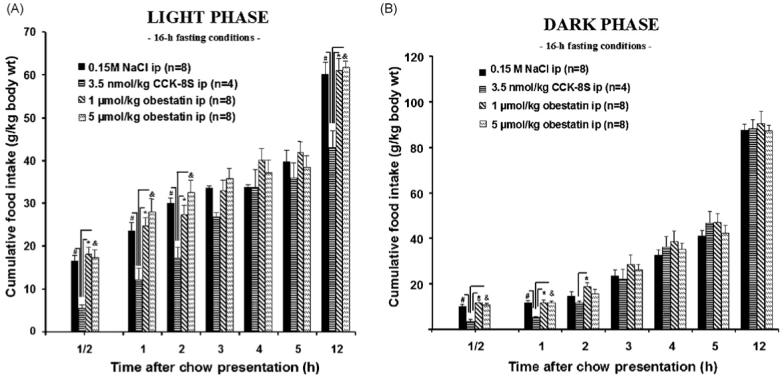
Cumulative food intake in 16-h fasted rats after intraperitoneal (ip) injection of 1 or 5 μmol/kg obestatin, 3.5 nmol/kg CCK-8S or 0.15 M NaCl 15 min before food exposure in the dark (B) or light (A) phase. While CCK-8S leads to a significant reduction of food intake, obestatin does not influence feeding behavior. The data are expressed as mean ± S.E.M. #p < 0.05 vs. vehicle, *p < 0.05 vs. 1 μmol/kg obestatin, and &p < 0.05 vs. 5 μmol/kg obestatin.
Fig. 2.
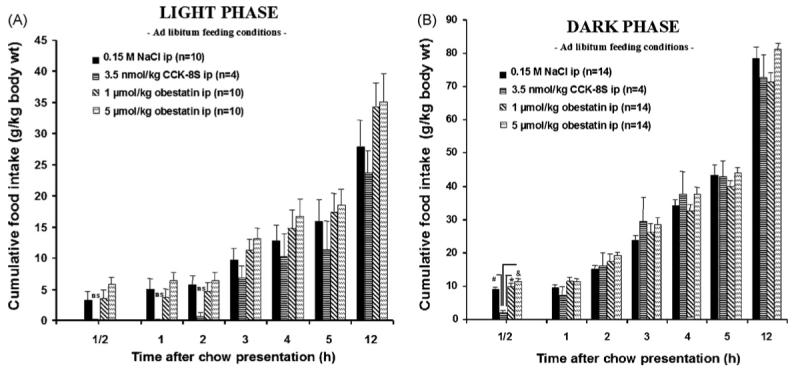
Cumulative food intake in ad libitum fed rats after intraperitoneal (ip) injection of 1 or 5 μmol/kg obestatin, 3.5 nmol/kg CCK-8S or 0.15 M NaCl 15 min before food exposure before the dark (B) or light (A) phase. CCK-8S significantly reduces food intake, while obestatin does not influence feeding behavior. The data are expressed as mean ± S.E.M. #p < 0.05 vs. vehicle, *p < 0.05 vs. 1 μmol/kg obestatin, and &p < 0.05 vs. 5 μmol/kg obestatin.
Table 1. Summary of the one way repeated measures ANOVAs on food intake experiments in rats.
| Experiment | Time (h) | Degrees of freedom | Sum of squares | Mean squares | F statistic | p-Value |
|---|---|---|---|---|---|---|
| 16 h Fasting, light phase | 1/2 | 3 | 518.432 | 172.811 | 9.883 | <0.001 |
| 1 | 3 | 844.55 | 281.517 | 6.144 | 0.005 | |
| 2 | 3 | 822.384 | 274.128 | 6.572 | 0.004 | |
| 3 | 3 | 302.995 | 100.998 | 4.427 | 0.018 | |
| 4 | 3 | 229.448 | 76.483 | 2.17 | 0.129 | |
| 5 | 3 | 90.446 | 30.149 | 0.633 | 0.604 | |
| 12 | 3 | 1079.397 | 359.799 | 7.521 | 0.002 | |
| 16 h Fasting, dark phase | 1/2 | 3 | 155.768 | 51.923 | 9.511 | 0.001 |
| 1 | 3 | 99.956 | 33.319 | 6.874 | 0.005 | |
| 2 | 3 | 95.452 | 31.817 | 5.142 | 0.015 | |
| 3 | 3 | 107.385 | 35.795 | 0.919 | 0.459 | |
| 4 | 3 | 142.622 | 47.541 | 0.766 | 0.533 | |
| 5 | 3 | 221.443 | 73.814 | 1.598 | 0.238 | |
| 12 | 3 | 54.194 | 18.065 | 0.254 | 0.857 | |
| Ad libitum feeding, light phase | 1/2 | 3 | 49.534 | 16.511 | 1.386 | 0.275 |
| 1 | 3 | 53.368 | 17.789 | 1.243 | 0.319 | |
| 2 | 3 | 37.701 | 12.567 | 0.837 | 0.489 | |
| 3 | 3 | 148.855 | 49.618 | 1.416 | 0.266 | |
| 4 | 3 | 150.595 | 50.198 | 0.646 | 0.594 | |
| 5 | 3 | 225.491 | 75.164 | 0.764 | 0.527 | |
| 12 | 3 | 436.287 | 145.429 | 0.822 | 0.496 | |
| Ad libitum feeding, dark phase | 1/2 | 3 | 150.028 | 50.009 | 4.687 | 0.009 |
| 1 | 3 | 41.504 | 13.835 | 0.814 | 0.497 | |
| 2 | 3 | 123.74 | 41.247 | 1.242 | 0.313 | |
| 3 | 3 | 315.215 | 105.072 | 1.56 | 0.22 | |
| 4 | 3 | 347.73 | 115.91 | 1.927 | 0.147 | |
| 5 | 3 | 140.395 | 46.798 | 0.717 | 0.55 | |
| 12 | 3 | 721.642 | 240.547 | 2.722 | 0.062 |
Table 2. Comparison of food intake data between rats treated with CCK-8S, obestatin or saline solution during the first 2 h after ip administration under different conditions.
| 0.15 M NaCl | 3.5 nmol/kg CCK-8S | 1 μmol/kg Obestatin | 5 μmol/kg Obestatin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Experiment | Time (h) | FI (g/kg) | FI (g/kg) | p-Value FI-reduction LSD-test to NaCl (%) vs. NaCl | FI (g/kg) | FI-reduction to NaCl (%) | FI (g/kg) | FI-reduction to NaCl (%) | |
| 16 h Fasting, light phase | 1/2 | 16.52 ± 1.30 | 5.50 ± 0.91 | <0.001 | -66.70 | 18.14 ± 1.69 | +9.80 | 17.44 ± 1.69 | +5.56 |
| 1 | 23.56 ± 2.02 | 12.23 ± 2.67 | =0.005 | -48.09 | 24.66 ± 1.98 | +4.66 | 28.08 ± 2.93 | +19.18 | |
| 2 | 30.03 ± 1.26 | 17.16 ± 2.76 | =0.007 | -42.85 | 27.33 ± 2.16 | -8.99 | 32.56 ± 2.93 | +8.42 | |
| 16 h Fasting, dark phase | 1/2 | 10.10 ± 0.87 | 3.41 ± 1.10 | =0.002 | -66.23 | 11.72 ± 1.21. | +16.03 | 10.87 ± 0.51 | +7.62 |
| 1 | 11.61 ± 1.21 | 5.28 ± 0.21 | =0.002 | -54.52 | 11.72 ± 1.21 | +0.94 | 11.68 ± 0.77 | +0.60 | |
| 2 | 14.58 ± 1.92 | 11.83 ± 0.65 | ns | -18.86 | 18.74 ± 1.84 | +28.53 | 15.66 ± 2.03 | +7.40 | |
| Ad libitum feeding, light phase | 1/2 | 3.31 ± 1.33 | 0.1 ± 0.1 | ns | -96.97 | 3.48 ± 1.41 | +5.13 | 5.77 ± 1.25 | +74.32 |
| 1 | 5.07 ± 1.59 | 0.1 ± 01 | ns | -98.02 | 3.67 ± 1.42 | -27.61 | 6,42 ± 1.32 | +26.62 | |
| 2 | 5.67 ± 1.54 | 0.67 ± 1.1 | ns | -88.18 | 4.70 ± 1.31 | -17.10 | 6.42 ± 1.32 | +13.22 | |
| Ad libitum feeding, dark phase | 1/2 | 8.99 ± 0.81 | 2.06 ± 0.67 | =0.017 | -77.08 | 10.02 ± 0.94 | +11.45 | 11.23 ± 0.93 | +24.91 |
| 1 | 9.63 ± 0.78 | 7.22 ± 2.70 | ns | -25.02 | 11.54 ± 1.24 | +19.83 | 11.41 ± 0.93 | +18.48 | |
| 2 | 15.07 ± 1.30 | 16.10 ± 3.75 | ns | +6.83 | 17.48 ± 2.27 | +15.99 | 19.17 ± 1.04 | +27.20 | |
FI = Food intake, values are mean ± S.E.M. of n = 4-14 rats/group; ns = not significant.
3.2. Effects of obestatin injected ip on cumulative food intake in fasted mice
Likewise, 16 h fasted mice injected with obestatin (1 μmol/kg body wt) 15 min before exposure to food had similar food intake as the vehicle group while ip urocortin 1 (2 nmol/kg body wt) inhibited cumulative food intake by 72, 50 and 40% at 1, 3 and 5 h, respectively, post-injection (Fig. 3).
Fig. 3.
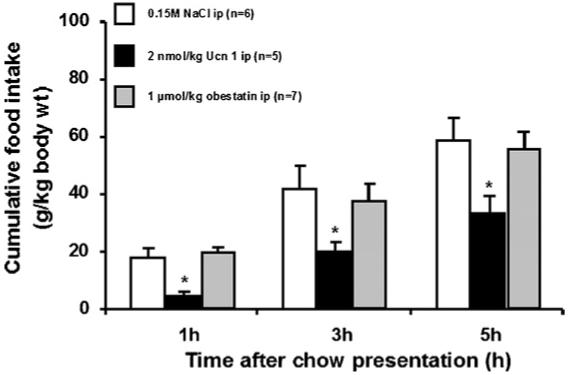
Cumulative food intake in fasted mice after intraperitoneal (ip) injection of 1 μmol/kg obestatin, 2 nmol/kg urocortin 1 or 0.15 M NaCl 15 min before exposure to food in the light phase. Obestatin and vehicle have similar food intake while urocortin 1 inhibits cumulative food intake by 72, 50 and 40% at 1, 3 and 5 h post-injection. The data are expressed as mean ± S.E.M. *p < 0.05 vs. vehicle, and *p < 0.05 vs. 1 μmol/kg obestatin.
3.3. Effects of obestatin and CCK-8S administered ip on the number of Fos-ir positive neurons in hypothalamic and brainstem nuclei in ad libitum fed rats
CCK-8S injected ip (1.75 nmol/rat) increased the number (mean ± S.E.M.) of Fos-ir positive neurons/section in the PVN (136.7 ± 7.2 vs. 39.8 ± 6.8, p < 0.001; Figs. 4 and 5), DMH (122.9 ± 5.8 vs. 67.8 ± 9.4, p = 0.03; Figs. 4 and 6), and NTS (135.1 ± 10.1 vs. 60.2 ± 6.9, p < 0.002; Figs. 4 and 6) compared to vehicle-treated animals. Obestatin (5 μmol/kg body wt, ip) had no effect on Fos expression in the PVN (44.2 ± 13.0 vs. 39.8 ± 6.8, p > 0.05; Figs. 4 and 5), ARC (15.8 ± 1.0 vs. 21.5 ± 3.7, p > 0.05; Figs. 4 and 5), VMH (38.2 ± 9.0 vs. 35.9 ± 2.8, p > 0.05; Figs. 4 and 5), DMH (67.6 ± 22.6 vs. 67.8 ± 9.4, p > 0.05; Figs. 4 and 6) and NTS (67.8 ± 16.5 vs. 60.2 ± 6.9, p > 0.05; Figs. 4 and 6) compared to vehicle treatment.
Fig. 4.
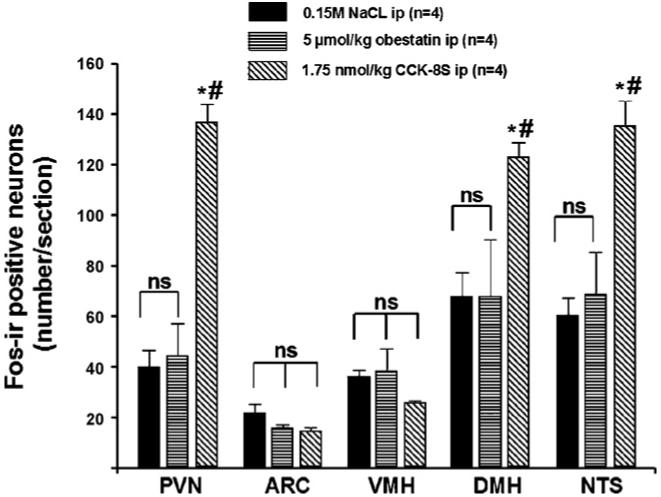
Fos expression pattern (number of Fos-ir positive neurons/section) after intraperitoneal (ip) injection of 5 μmol/kg obestatin, 1.75 nmol/kg CCK-8S or 0.15 M NaCl. Obestatin does not change neuronal activity while CCK-8S induces increased Fos expression in the PVN, DMH and NTS. The data are expressed as mean ± S.E.M. #p < 0.05 vs. vehicle, *p < 0.05 vs. 5 μmol/kg obestatin, ns = not significant.
Fig. 5.
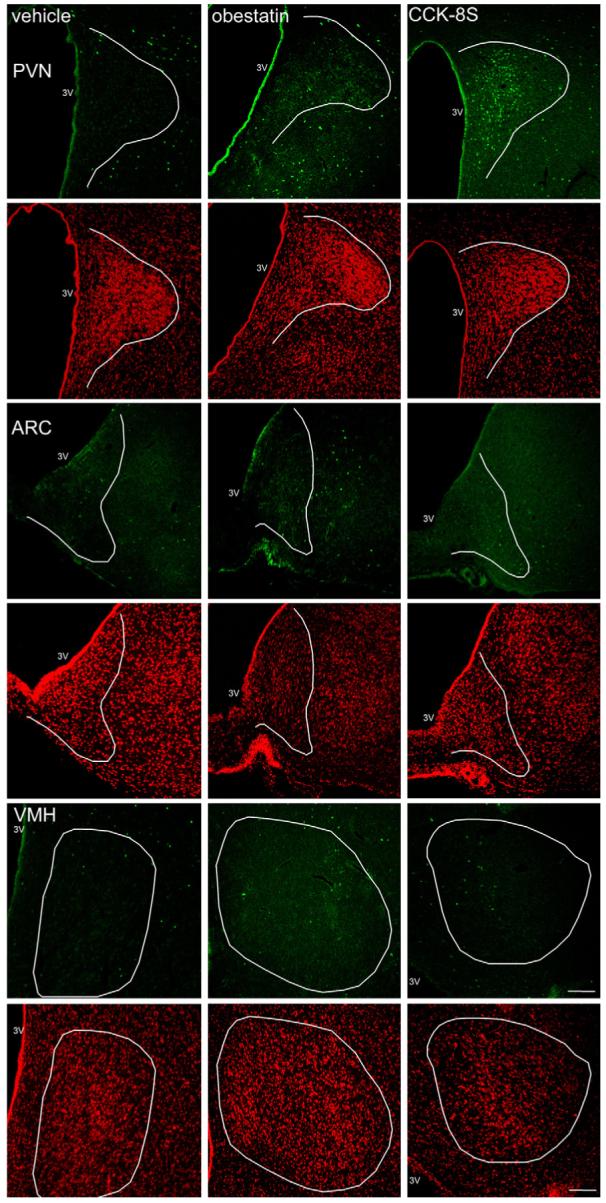
Representative images of the PVN, ARC and VMH after ip-injection of 0.15 M NaCl, 5 μmol/kg obestatin and 1.75 nmol/kg CCK-8S. Obestatin does not change neuronal activity in these hypothalamic nuclei while CCK-8S induces increased Fos expression (green staining) in the PVN. Cell nuclei are stained red as a result of the counterstaining with propidium iodide. The white outer line delineates the area of the PVN, ARC and VMH. The white scale bar represents 100 μm. 3V, third ventricle; PVN, paraventricular nucleus of the hypothalamus; ARC, arcuate nucleus of the hypothalamus; VMH, ventromedial nucleus of the hypothalamus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 6.
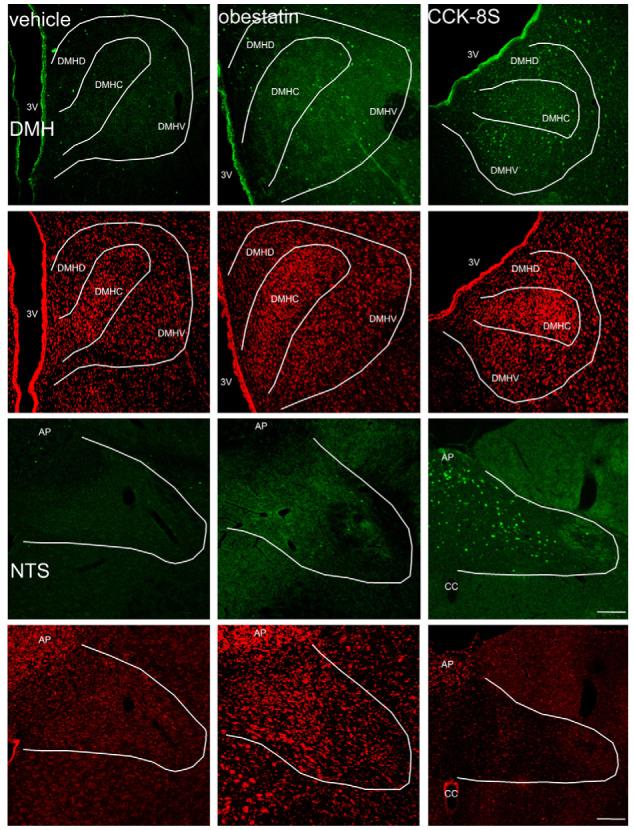
Representative images of the DMH and the NTS after ip administration of 0.15 M NaCl, 5 μmol/kg obestatin and 1.75 nmol/kg CCK-8S. Obestatin does not change neuronal activity in these nuclei while CCK-8S induces increased Fos expression (green staining) in both of them. The white outer line delineates the area of the DMH and NTS. Cell nuclei are stained red as a result of the counterstaining with propidium iodide. The white scale bar represents 100 μm. 3V, third ventricle; DMH, dorsomedial hypothalamic nucleus; DMHD, dorsomedial hypothalamic nucleus, dorsal part; DMHC, dorsomedial hypothalamic nucleus, compact part; DMHV, dorsomedial hypothalamic nucleus, ventral part; NTS, nucleus of the solitary tract; AP, area postrema; cc, canalis centralis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4. Discussion
The present experiments show that obestatin administered peripherally at 1 and 5 μmol/kg has no satiating effect in rats under ad libitum feeding or 16 h fasting/refed conditions, neither during the light phase nor the dark phase for the 12 h post-injection period. Similarly, obestatin injected ip 15 min before food exposure in 16-h fasted mice did not alter the 5-h refeeding period. In contrast, in rats, there is a significant rapid in onset decrease in food intake after peripheral injection of 3.5 nmol/kg of CCK-8S during the dark and light phase under fasted/refed conditions, and at the beginning of the dark phase in ad libitum fed rats. During the light phase under ad libitum conditions, the ∼95% reduction of food intake induced by ip CCK-8S did not reach statistical significance due to the reduced and variable amounts of food ingested in the control non-fasted group (Table 2) along with the small number of animals (n = 4) in the CCK-8S-treated group. In fasted/refed mice, we also showed that ip urocortin 1 resulted in a 70-40% suppression over the 5-h post-injection consistent with previous reports [2,46]. Moreover, after obestatin administration (5 μmol/kg, ip) to freely fed rats, no effect on Fos expression pattern was observed in the PVN, ARC, VMH, DMH, and NTS while CCK-8S (1.75 nmol/kg, ip) increased Fos expression in the PVN, DMH, and NTS which is in agreement with previous studies [23,25].
Our observation that obestatin has no inhibitory effect on 16-h fasted rats during the light phase is consistent with our previous results [14,15]. Several other research groups were also unable to demonstrate an inhibitory effect of the peptide on food intake in rodents at this dose [21,32]. All in all, data concerning obestatin’s influence on feeding behavior when injected peripherally are conflicting. There are four studies describing a significant inhibitory effect on food intake [7,18,27,51], three investigations showing a non-significant trend towards a reduction [21,40,42], and seven reports of no effect on feeding behavior after obestatin administration [11,14,15,32,41,54, and present study]. The results of these studies seem to be independent from the investigated species (mice or rats), the metabolic status (fasted or fed ad libitum) and the route of delivery (ip, intravenous, intracerebroventricular and intracisternal) [16 and present study]. Although a recent study points to the dose of obestatin administered to the animals as the decisive factor. Indeed, Lagaud et al. found that only amounts between 100 and 300 nmol/kg caused a significant decrease of food intake while higher (1 or 3 μmol/kg) or lower (10 or 30 nmol/kg) doses had no suppressive effect [27]. However, even these findings are conflicting with several studies that showed significant effects after injection of 1 μmol/kg obestatin [18,51] while in some other reports, no inhibition of food ingestion was observed with similar doses [15,21]. Recently, Zhang et al. raised the issue of the experimental conditions that are essential to detect obestatin inhibitory effects [52]. While most of the studies did not observe any effect of obestatin injected at the same time that the food was presented in fasted animals, Zhang et al. found that ip obestatin-induced inhibition of feeding when injected at 15 min, but not at 0 or 30 min before food presentation in 16-h fasted mice. In our hands, even using the critical 15-min time interval, the ip injection of obestatin did not result in any inhibition of food intake neither in rats nor in mice. Moreover, our data reveal that the effect of peripherally administered obestatin on food intake in rats depends neither on time in the circadian rhythm when the experiment is conducted, nor on the metabolic conditions of the experimental animals. The present findings are also consistent with the lack of effect of peripherally administered obestatin on gastric motor function as assessed by monitoring changes in gastric motility or emptying in both rats and mice [3,11,14,16,17] contrasting with the initial report [51].
Recently, it has been demonstrated that peripheral obestatin crosses the blood brain barrier in mice, indicating that central pathways may be involved in the regulation of food intake by obestatin [35]. In addition, obestatin was initially suggested to inhibit feeding through activation of brain nuclei, particularly the ARC. This was supported by the fact that obestatin was originally claimed to bind the GPR-39, which is highly expressed in the hypothalamus, as detected by Northern blot and reverse transcriptase polymerase chain reaction (RT-PCR) [51]. However, using in situ hybridization and RT-PCR, subsequent studies did not find expression of this receptor in the hypothalamus [21,22,32]. More importantly, recent studies and the retraction of initial findings by Zhang clearly established that obestatin is not the endogenous ligand that binds to GRP39 receptor [9,21,22,32,52]. In the present study, we found that ip injection of obestatin has no effect on the Fos expression pattern in the hypothalamus and brainstem. This contrast with most other gut peptides that influence feeding behavior by modulating neuronal activity in hypothalamic and brainstem nuclei. CCK-8S increases Fos expression in the PVN, DMH and NTS [23,25,29,33,38] while systemic or icv application of the long-term satiety signal leptin induces neuronal activation in PVN and DMH neurons [12,44], and peptide YY increases Fos expression in NTS, AP [6], and ARC [4]. Glucagon-like peptide 1 modulates the number of Fos-ir positive neurons in ARC [44], PVN, NTS and AP [28,39], and peripheral amylin leads to increased neuronal activation in the NTS and AP [39]. Another puzzling fact is the lack of evidence for in vivo synthesis of obestatin [13]. Using different prohormone convertases, Zhu et al. were not able to observe the formation of obestatin from proghrelin [53]. In addition, plasma obestatin levels measured by radioimmunoassay (RIA) led to varying results which might indicate that the low specificity of these tests could be due to lacking standardization and imprecise values in previous studies [19,36].
In conclusion, our data show that independently from the metabolic status (fed or fasted) and the experimental setting during dark or light phase, and in contrast to CCK-8S or urocortin 1, the ip injection of obestatin does not influence food intake in rodents and Fos expression pattern in rat hypothalamic and brainstem nuclei. Therefore, the present work does not support the assumption that obestatin influences food intake in rodents and the potential biological actions along with the receptors on which the peptide interact remain to be established.
Acknowledgements
This work was supported by grants from the German Research Foundation (DFG) to H.M. (DFG: Mö 458/4-3), and from the Charité to H.M. (Charité: UFF 2006-251), and Y.T. (Research Career Scientist Award, Department of Veterans Affairs and NIHDK R01 33061). Dr. Jean Rivier (Salk Institute, La Jolla) is acknowledged for the generous supply of urocortin 1 and purity assessment of obestatin and Mrs. Honghui Liang for technical assistance in the mice studies.
REFERENCES
- [1].Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–45. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- [2].Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology. 1999;116:1287–92. doi: 10.1016/s0016-5085(99)70491-9. [DOI] [PubMed] [Google Scholar]
- [3].Bassil AK, Haglund Y, Brown J, Rudholm T, Hellstrom PM, Naslund E, et al. Little or no ability of obestatin to interact with ghrelin or modify motility in the rat gastrointestinal tract. Br J Pharmacol. 2007;150:58–64. doi: 10.1038/sj.bjp.0706969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- [5].Bonaz B, De Giorgio R, Taché Y. Peripheral bombesin induces c-fos protein in the rat brain. Brain Res. 1993;600:353–7. doi: 10.1016/0006-8993(93)91397-b. [DOI] [PubMed] [Google Scholar]
- [6].Bonaz B, Taylor I, Taché Y. Peripheral peptide YY induces c-fos-like immunoreactivity in the rat brain. Neurosci Lett. 1993;163:77–80. doi: 10.1016/0304-3940(93)90233-b. [DOI] [PubMed] [Google Scholar]
- [7].Bresciani E, Rapetti D, Dona F, Bulgarelli I, Tamiazzo L, Locatelli V, et al. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J Endocrinol Invest. 2006;29:RC16–8. doi: 10.1007/BF03344175. [DOI] [PubMed] [Google Scholar]
- [8].Cano V, Ezquerra L, Ramos MP, Ruiz-Gayo M. Characterization of the role of endogenous cholecystokinin on the activity of the paraventricular nucleus of the hypothalamus in rats. Br J Pharmacol. 2003;140(5):964–70. doi: 10.1038/sj.bjp.0705513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chartrel N, Alvear-Perez R, Leprince J, Iturrioz X, Reaux-Le Goazigo A, Audinot V, et al. Comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake”. Science. 2007;315:766. doi: 10.1126/science.1135047. [DOI] [PubMed] [Google Scholar]
- [10].Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–61. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- [11].De Smet B, Thijs T, Peeters TL, Depoortere I. Effect of peripheral obestatin on gastric emptying and intestinal contractility in rodents. Neurogastroenterol Motil. 2007;19:211–7. doi: 10.1111/j.1365-2982.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- [12].Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138:839–42. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- [13].Garg A. The ongoing saga of obestatin: is it a hormone. J Clin Endocrinol Metab. 2007;92:3396–8. doi: 10.1210/jc.2007-0999. [DOI] [PubMed] [Google Scholar]
- [14].Gourcerol G, Coskun T, Craft LS, Mayer JP, Heiman ML, Wang L, et al. Preproghrelin-derived peptide, obestatin, fails to influence food intake in lean or obese rodents. Obesity (Silver Spring) 2007;15:2643–52. doi: 10.1038/oby.2007.316. [DOI] [PubMed] [Google Scholar]
- [15].Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, et al. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides. 2006;27(11):2811–9. doi: 10.1016/j.peptides.2006.07.012. [DOI] [PubMed] [Google Scholar]
- [16].Gourcerol G, St Pierre DH, Taché Y. Lack of obestatin effects on food intake: should obestatin be renamed ghrelin-associated peptide (GAP) Regul Pept. 2007;141(13):1–7. doi: 10.1016/j.regpep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- [17].Gourcerol G, Taché Y. Obestatin—a ghrelin-associated peptide that does not hold its promise to suppress food intake and motility. Neurogastroenterol Motil. 2007;19:161–5. doi: 10.1111/j.1365-2982.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- [18].Green BD, Irwin N, Flatt PR. Direct and indirect effects of obestatin peptides on food intake and the regulation of glucose homeostasis and insulin secretion in mice. Peptides. 2007;28(5):981–7. doi: 10.1016/j.peptides.2007.02.003. [DOI] [PubMed] [Google Scholar]
- [19].Haider DG, Schindler K, Prager G, Bohdjalian A, Luger A, Wolzt M, et al. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2007;92:1168–71. doi: 10.1210/jc.2006-1839. [DOI] [PubMed] [Google Scholar]
- [20].Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12:1047–9. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- [21].Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2006;148(1):13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- [22].Jackson VR, Nothacker HP, Civelli O. GPR39 receptor expression in the mouse brain. Neuroreport. 2006;17:813–6. doi: 10.1097/01.wnr.0000215779.76602.93. [DOI] [PubMed] [Google Scholar]
- [23].Kobelt P, Paulitsch S, Goebel M, Stengel A, Schmidtmann M, van der Voort I, et al. Peripheral injection of CCK-8S induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Res. 2006;1117(1):109–17. doi: 10.1016/j.brainres.2006.08.092. [DOI] [PubMed] [Google Scholar]
- [24].Kobelt P, Tebbe JJ, Tjandra I, Bae HG, Rüter J, Klapp BF, et al. Two immunocytochemical protocols for immunofluorescent detection of c-Fos positive neurons in the rat brain. Brain Res Brain Res Protoc. 2004;13:45–52. doi: 10.1016/j.brainresprot.2004.01.003. [DOI] [PubMed] [Google Scholar]
- [25].Kobelt P, Tebbe JJ, Tjandra I, Stengel A, Bae HG, Andresen V, et al. CCK inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol. 2005;288:R751–8. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- [26].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- [27].Lagaud GJ, Young A, Acena A, Morton MF, Barrett TD, Shankley NP. Obestatin reduces food intake and suppresses body weight gain in rodents. Biochem Biophys Res Commun. 2007;357:264–9. doi: 10.1016/j.bbrc.2007.03.138. [DOI] [PubMed] [Google Scholar]
- [28].Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–55. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- [29].Mönnikes H, Lauer G, Bauer C, Tebbe J, Zittel TT, Arnold R. Pathways of Fos expression in locus ceruleus, dorsal vagal complex, and PVN in response to intestinal lipid. Am J Physiol. 1997;273:R2059–71. doi: 10.1152/ajpregu.1997.273.6.R2059. [DOI] [PubMed] [Google Scholar]
- [30].Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- [31].Niimi M, Sato M, Taminato T. Neuropeptide Y in central control of feeding and interactions with orexin and leptin. Endocrine. 2001;14:269–73. doi: 10.1385/ENDO:14:2:269. [DOI] [PubMed] [Google Scholar]
- [32].Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, et al. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology. 2007;148:21–6. doi: 10.1210/en.2006-0915. [DOI] [PubMed] [Google Scholar]
- [33].Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res. 1992;569:238–48. doi: 10.1016/0006-8993(92)90635-m. [DOI] [PubMed] [Google Scholar]
- [34].Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- [35].Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27(4):911–6. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- [36].Qi X, Li L, Yang G, Liu J, Li K, Tang Y, et al. Circulating obestatin levels in normal subjects and in patients with impaired glucose regulation and type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2007;66:593–7. doi: 10.1111/j.1365-2265.2007.02776.x. [DOI] [PubMed] [Google Scholar]
- [37].Riediger T, Zuend D, Becskei C, Lutz TA. The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R114–22. doi: 10.1152/ajpregu.00333.2003. [DOI] [PubMed] [Google Scholar]
- [38].Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol. 1995;360:246–56. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- [39].Rowland NE, Crews EC, Gentry RM. Comparison of Fos induced in rat brain by GLP-1 and amylin. Regul Pept. 1997;71:171–4. doi: 10.1016/s0167-0115(97)01034-3. [DOI] [PubMed] [Google Scholar]
- [40].Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am J Physiol Regul Integr Comp Physiol. 2007;292:R637–43. doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]
- [41].Seoane LM, Al Massadi O, Pazos Y, Pagotto U, Casanueva FF. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest. 2006;29:RC13–5. doi: 10.1007/BF03344174. [DOI] [PubMed] [Google Scholar]
- [42].Sibilia V, Bresciani E, Lattuada N, Rapetti D, Locatelli V, De LV, et al. Intracerebroventricular acute and chronic administration of obestatin minimally affect food intake but not weight gain in the rat. J Endocrinol Invest. 2006;29:RC31–4. doi: 10.1007/BF03349204. [DOI] [PubMed] [Google Scholar]
- [43].Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- [44].Van Dijk G, Thiele TE, Donahey JC, Campfield LA, Smith FJ, Burn P, et al. Central infusions of leptin and GLP-1-(7-36) amide differentially stimulate c-FLI in the rat brain. Am J Physiol. 1996;271:R1096–100. doi: 10.1152/ajpregu.1996.271.4.R1096. [DOI] [PubMed] [Google Scholar]
- [45].Verbalis JG, Stricker EM, Robinson AG, Hoffman GE. Cholecystokinin activates c-Fos expression in hypothalamic oxytocin and corticotropin-releasing hormone neurons. J Neuroendocrinol. 1991;3:205–13. doi: 10.1111/j.1365-2826.1991.tb00264.x. [DOI] [PubMed] [Google Scholar]
- [46].Wang L, Martinez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1401–10. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- [47].Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- [48].Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- [49].Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–7. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- [50].Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–8. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- [51].Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–9. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- [52].Zhang VJ, Klein C, Ren PG, Kass S, Ver DL, Moechars D, et al. Response to comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake”. Science. 2007:766d. doi: 10.1126/science.1135047. [DOI] [PubMed] [Google Scholar]
- [53].Zhu X, Cao Y, Voogd K, Steiner DF. On the processing of proghrelin to ghrelin. J Biol Chem. 2006;281:38867–70. doi: 10.1074/jbc.M607955200. [DOI] [PubMed] [Google Scholar]
- [54].Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and GH secretion in rodents. Endocrinology. 2007;148(4):1648–53. doi: 10.1210/en.2006-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]


