Abstract
Objective
This study tests the hypothesis that the transient receptor potential vanilloid subtype 1 (TRPV1)-induced neuropeptide secretion and depressor response are mediated by, at least in part, activation of endoplasmic reticulum (ER)-associated Ca2+ release receptors, leading to increased cytosolic Ca2+ in dorsal root ganglion (DRG) neurons.
Methods/Results
Bolus injection of capsaicin (CAP, 10 or 50 μg/kg), a selective TRPV1 agonist, into anesthetized male Wistar rats caused dose-dependent decreases in mean arterial pressure (MAP, P<0.05). CAP (50 μg/kg)-induced depressor effects and increases in plasma calcitonin gene-related peptide (CGRP) levels (-29±2 mmHg, 82.2±5.0 pg/ml, respectively) were abolished by a selective TRPV1 antagonist, capsazepine (3 mg/kg CAPZ, -4±1 mmHg, 41.8±4.4 pg/ml, P<0.01), and attenuated by a selective ryanodine receptor (RyR) antagonist, dantrolene (5 mg/kg, -12±1 mmHg, 57.2±2.6 pg/ml, P<0.01), but unaffected by an inhibitor of ER Ca2+-ATPase, thapsigargin (50 μg/kg TG, -30±1 mmHg, 73.8±2.3 pg/ml, P>0.05), or an antagonist of the inositol (1,4,5)-trisphosphate receptor (IP3R), 2-aminoethoxydiphenyl borate (3 mg/kg 2-APB, -34±5 mmHg, 69.0±3.7 pg/ml, P>0.05). CGRP8-37 (1 mg/kg), a selective CGRP receptor antagonist, also blocked CAP-induced depressor effects. In contrast, dantrolene had no effect on CGRP (1 μg/kg)-induced depressor effects. In vitro, CAP (0.3 μM) increased intracellular Ca2+ concentrations and CGRP release from freshly isolated sensory neurons in DRG (P<0.01), which were blocked by CAPZ (10 μM) and attenuated by dantrolene but not TG or 2-APB.
Conclusion
Our results indicate that TRPV1 activation triggers RyR- but not IP3R-dependent Ca2+ release from ER in DRG neurons leading to increased CGRP release and consequent depressor effects.
Keywords: transient receptor potential vanilloid subtype 1, ryanodine receptors, calcium, calcitonin gene-related peptide, depressor effects
Introduction
The transient receptor potential vanilloid subtype 1 (TRPV1), a ligand—gated cation channel, primarily expresses in sensory neurons and their nerve terminals innervating the cardiovascular system including hearts and blood vessels.1 TRPV1 functions as a molecular integrator of multiple chemical and physical stimuli including capsaicin (CAP), proton, and noxious heat,2-4 and when activated, leads to the release of sensory neuropeptides including calcitonin gene-related peptide (CGRP) and substance P that are potent vasodilators.5 Studies have shown that TRPV1 and TRPV1-mediated CGRP release play a significant functional role in preventing salt-induced increases in blood pressure.4, 6-8 However, the molecular mechanisms underlying functional CGRP release linked to TRPV1 are largely unknown.
Activation of TRPV1, a relatively Ca2+-selective ion channel, leads to an increase in intracellular Ca2+.9 Cytoplasmic Ca2+ in neurons regulates many vital physiological processes ranging from transmitter release, somatic secretion, excitability, synaptic plasticity, to cell survival and death.10-11 Cytoplasmic Ca2+ originates mainly from two sources, i.e., Ca2+ influx through voltage and ligand-gated Ca2+ channels expressed in the plasma membrane and Ca2+ release from the endoplasmic reticulum (ER). ER is the largest intracellular organelle and constitutes an interconnected network that extends from the soma to axons, dendrites, and dendritic spines.12 ER is not only a site of protein synthesis and maturation, but also a Ca2+ store or sink that plays a key role in determining complex patterns of local or global Ca2+ signals.
Increased intracellular Ca2+ concentrations resulting from Ca2+ influx via voltage- or ligand-gated channels expressed in the plasma membrane or from Ca2+ release from intracellular stores may trigger neurotransmitter release.13-16 Ryanodine receptors (RyRs) and inositol 1,4,5-trisphosphate receptors (IP3Rs) expressed in the membrane of ER are responsible for Ca2+ release from ER.17 It has been shown that RyRs and/or IP3Rs-dependent calcium-induced calcium release (CICR) in neurons, i.e., Ca2+ influx via voltage/ligand-operated Ca2+ channels triggers Ca2+ release from ER stores that amplifies Ca2+ influx and signaling,18-19 participates and enhances neurotransmitter release.13, 20 Regardless of the pathophysiological significance of TRPV1-mediated CGRP release, intracellular molecules contributing to this process in vitro and in vivo have not been well defined. Thus, this study sets out to determine whether activation of ER-associated Ca2+ release receptors contributes to TRPV1-induced CGRP release and depressor effects.
Methods
Animals
Male Wistar rats (8 weeks old, Charles River Laboratory, Wilmington, MA) were housed in a temperature—controlled room with a 12:12-hour light/dark cycle for 1 week before the experiment. All procedures used in this study were approved by the Institutional Animals Care and Use Committee.
Surgical Preparation
The rats were anesthetized with pentobarbital (50 mg/kg, i.p.) for implantation of catheters. The left jugular vein and carotid artery were cannulated under anesthesia for administration of drugs or monitoring of mean arterial pressure (MAP) and heart rate (HR) with a Statham 231D pressure transducer coupled to a Gould 2400s recorder (Gould Instruments, Valley View, OH).
Experimental Protocols
Protocol 1
Rats were divided into 5 groups for intravenous administration of vehicle or CAP (a selective TRPV1 agonist) alone or in combination with capsazepine (CAPZ, a selective TRPV1 receptor antagonist) or CGRP8-37 (a selective CGRP receptor antagonist), and MAP responses recorded. CAP was given at 10 μg/kg or 50 μg/kg alone or with CAPZ (3 mg/kg) that was administered 10 minutes before CAP (50 μg/kg) administration. Due to its short half life, CGRP8-37 was administered at 1 mg/kg/min for 2 minutes before CAP (50 μg/kg) injection followed by continued administration at 0.5 mg/kg/min for 4 minutes. Our preliminary studies indicated that this dose and manner of CGRP8-37 administration prevented CGRP (1 μg/kg) —induced depressor response.
Protocol 2
To determine whether RyR or IP3R is involved in CAP-induced depressor effects, dantrolene (a RyR antagonist, 5 mg/kg), thapsigargin (TG, a Ca2+-ATPase inhibitor, 50 μg/kg), or 2-aminoethoxydiphenyl borate (2-APB, an IP3R antagonist, 3 mg/kg) was injected 15 minutes, 1 hour, or 10 minutes before intravenous injection of CAP (50 μg/kg), respectively. To determine if RyR plays a role in CGRP-induced depressor effects, dantrolene (5 mg/kg) was also injected 15 minutes before intravenous injection of CGRP (1 μg/kg).
Protocol 3
To examine the possible effect of baroreflex on CAP-induced changes in MAP, MAP responses to CAP in baroreceptor denervated rats were studied. Baroreceptors were denervated by cutting the carotid sinus and aortic depressor nerves bilaterally.47 Each carotid bifurcation was then stripped of connective tissues and painted with 10% phenol in ethanol. Baroreceptor denervation was confirmed by the lack of change in HR when MAP was increased or decreased by the intravenous injection of phenylephrine (5 μg/kg) or nitroprusside (10 μg/kg), respectively. Both baroreceptor-denervated and neurally intact animals were allowed to recover for 2hrs after the surgical and before intravenous injection CAP (50 μg/kg).
Protocol 4
Rats were anesthetized and decapitated without subjecting to acute experiments. The cervical, thoracic, and lumbar dorsal root ganglia (DRG) were harvested and placed in the Kreb’s solution (in mM) containing of: NaCl 119, NaHCO3 25, KH2PO4 1.2, MgSO4 1.5, CaCl2 2.5, KCL 4.7, and D-glucose 11.21-22 To minimize peptide degradation, 1 μM phosphoramidon was added in the basic Krebs’s solution, which was maintained at 37°C and gassed with 95% O2 and 5% CO2. After a 60 minutes stabilization period, DRG were treated with following combinations for 20 minutes in 1ml of Kreb’s solution: vehicle, 0.08 μM or 0.3 μM CAP, 10 μM CAPZ + 0.3 μM CAP, 50 μM dantrolene + 0.3 μM CAP, 2 μM TG + 0.3 μM CAP, 100 μM 2-APB + 0.3 μM CAP, or caffeine 20 mM). For Ca2+-free solution, CaCl2 was omitted from the Kreb’s solution.23-24 At the end of the experiment, the solution was collected and added acetic acid, evaporated, and the pellets were stored at -80 °C for CGRP immunoassay.
Protocol 5
For intracellular Ca2+ imaging, the left dorsal root ganglia (T10-L2) were harvested and incubated in a Hank’s balanced salt solution containing papain (25 μl/ml) and dithioerythritol (1 mg/ml) at 37 °C in a shaking bath for 15 minutes, followed by incubation in a second Hank’s solution containing collagenase I (1 mg/ml) and trypsin inhibitor (0.75 mg/ml) at 37 °C for 10 minutes. Enzyme treated ganglia were triturated using long neck Pasteur pipettes with sequentially smaller tip sizes. After trituration, enzyme activity was blocked by adding serum containing culture medium and the cells suspension was centrifuged for 3 minutes at 500 g using a table top centrifuge. The supernatant was discarded and 5 ml of culture medium was added before centrifuging again for 3 minutes. The second supernatant was discarded and the pellet containing neurons was re-suspended using culture medium. The neurons were plated on eight poly-L-lysine (50 μg/ml) coated round coverslips (around 500 neurons/coverslip) and incubated at 37 °C in 5% CO2 overnight before calcium imaging studies.
After overnight culture, individual coverslips were placed in 1 ml of Opti-MEM (Invitrogen) in a 35 mm plastic culture dish. Neurons were incubated with Fluo-4-AM (Molecular Probes, 1 μg/ml, dissolved in DMSO) for 40 minutes at 37 °C in a 5% CO2 atmosphere. The coverslip was then placed in a perfusion chamber mounted on the stage of an upright microscope (Leica Instruments) and cells were viewed at 400 × magnification. Cells were washed with drug free HEPES buffer (pH 7.4) for ∼10 minutes to wash away any extracellular Fluo-4. Calcium induced fluorescence was imaged using a confocal microscopy (Leica) using an argon laser and a 488 nm excitation wavelength. Fluorescence signals were captured at 520- 560 nm. Images (512 × 512 pixels) were collected at 3 s intervals and for 4 minutes. Control HEPES was applied for 15 seconds before changing to drug-containing buffer using a set of solenoid controlled valves. Drugs were applied for 2 minutes before returning to drug free buffer. In pilot studies, we verified that CAP (0.3 μM) activated TRPV1 channels to cause increased Fluo-4 fluorescence by blocking CAP-induced responses with CAPZ (10 μM). In the presence of CAPZ, CAP was incapable of causing a fluorescence increase greater than 5 arbitrary fluorescence units (AFU), and only cells producing CAP responses greater than 5 AFU were considered responsive to CAP. A single coverslip was exposed to CAP only once given that subsequent responses to CAP showed significant desensitization. Moreover, each coverslip was tested with KCl (60 mM) to verify viability of the neurons at the end of the experiment. Calcium signals were analyzed offline by subtracting the baseline fluorescence (the average fluorescence in the first 5 images captured before drug application), and peak fluorescence changes in response to drugs were obtained.
Sample collection
At the end of the experiments, blood samples were collected in chilled EDTA tubes and separated by centrifugation at 1,750 g for 20 minutes at 4°C. Plasma was stored at -80°C for CGRP assay.
Radioimmunoassay
A rabbit anti-rat CGRP radioimmunoassay kit (Penisula Laboratories. Inc) was used to determine CGRP contents in plasma and DRG tissues as described previously.7 This antibody has 100% cross-reactivity with rat α-CGRP and 79% with rat β-CGRP. There is no cross-reactivity with rat amylin, calcitonin, somatostatin, or substance P.
Immunohistochemistry
Colocalization of TRPV1 and RyR in DRG was performed by confocal analysis of double-immuofluorescence staining as described previously.8 Briefly, DRG were fixed with Zamboni’s fixative solution for 4 hours at 4°C. Sections were cut in 10 μm in thickness, and incubated in 0.4% Triton-100 and 5% fetal bovine serum in phosphate-buffered saline for 1 hour at room temperature to block nonspecific binding. The sections were then incubated with polyclonal guinea pig anti-TRPV1 receptor antibody, (1:1000, Chemicon) for 8 h at 4°C, followed by overnight incubation with mouse anti-rat ryanodine antibody (1:1000, Calbiochem) at 4°C. Subsequently, the sections were incubated in Cy3-conjugated donkey anti-guinea pig IgG (1:300, Jackson ImmunoResearch) for 1 hour in room temperature, followed by incubation with FITC-conjugated donkey anti-mouse IgG (1:100, Jackson ImmunoResearch) for 1h at room temperature. The slides were viewed with a Zeiss Pascal confocal laser scanning microscope using 488-nm and 543-nm laser. Negative control for possible cross-reactivity between the fluorescent reagents was performed by incubating the sections with only one of the primary antibodies, followed by incubation with a mixture of secondary antibodies. No cross-reactivity was observed.
Drugs
CAP (Sigma) was dissolved in ethanol (10%, v/v), Tween-80 (10%, v/v), and normal saline. CGRP (American Peptide), CGRP8-37 (American Peptide), and caffeine (Calbiochem) were dissolved in normal saline. All other drugs were purchased from Calbiochem and dissolved in dimethylsuphoxide (DMSO 5 %, v/v) and normal saline.
Statistical Analysis
All values are expressed as mean±SE. Differences between 2 groups or before and after treatment were analyzed by using the unpaired or paired student t test. The differences among groups were analyzed using one-way ANOVA followed by a Bonferroni adjustment for multiple comparisons. Differences were considered statistically significant at P<0.05.
Results
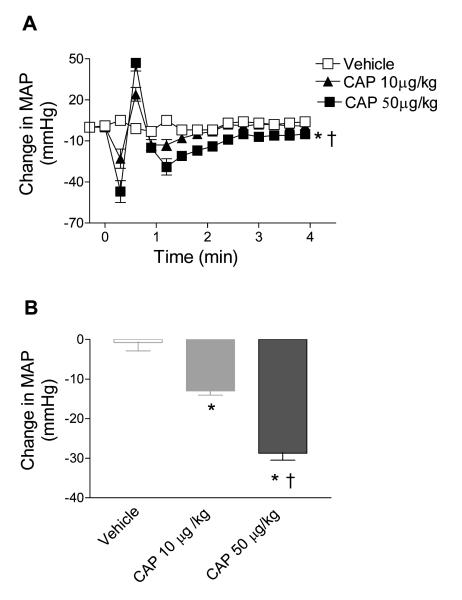
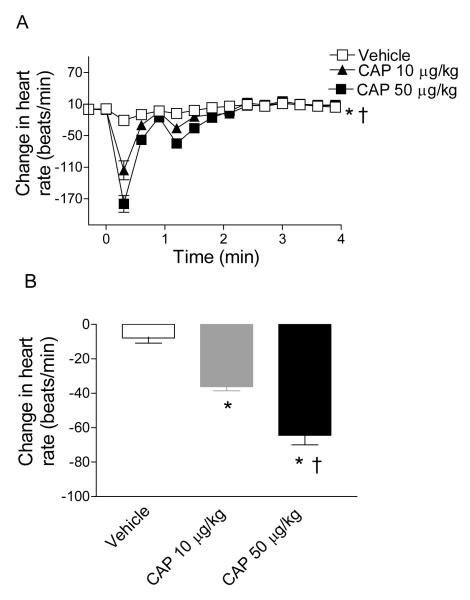
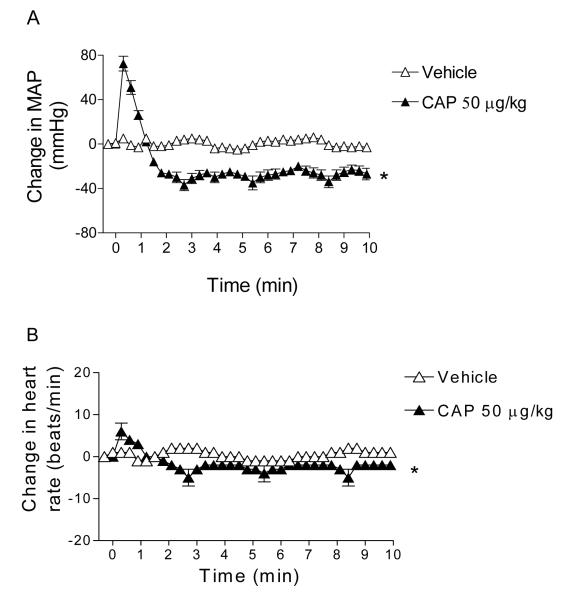
Bolus intravenous injection of CAP led to a triphasic MAP response as shown in Figure 1. The initial transient hypotension was followed by a brief pressor and a depressor phase that reached the peak at ∼1-2 minutes after CAP injection and gradually retuned to the baseline at ∼ 4 minutes. CAP (10 and 50 μg/kg) induced peak decreases in MAP were dose-dependent (13±1 mmHg and 29±2 mmHg, respectively), which corresponded with changes in heart rates (HR, Figure 2).
Figure 1.
A, Time-dependent responses of MAP to bolus injection of vehicle or CAP (10 μg/kg; 50 μg/kg). B, Changes in MAP 70 seconds after bolus injection of vehicle or CAP (10 μg/kg; 50 μg/kg). Values are mean±SE (the same for all data presented below; n = 6 to 7). *P<0.05 compared to corresponding vehicles. †P<0.05 compared to rats treated with CAP (10 μg/kg).
Figure 2.
A, Time-dependent responses of heart rate to bolus injection of vehicle or CAP (10 μg/kg; 50 μg/kg). B, Changes in heart rate 70 seconds after bolus injection of vehicle or CAP (10 μg/kg; 50 μg/kg). n = 6 to 7. *P<0.05 compared to corresponding vehicles. †P<0.05 compared to rats treated with CAP (10 μg/kg).
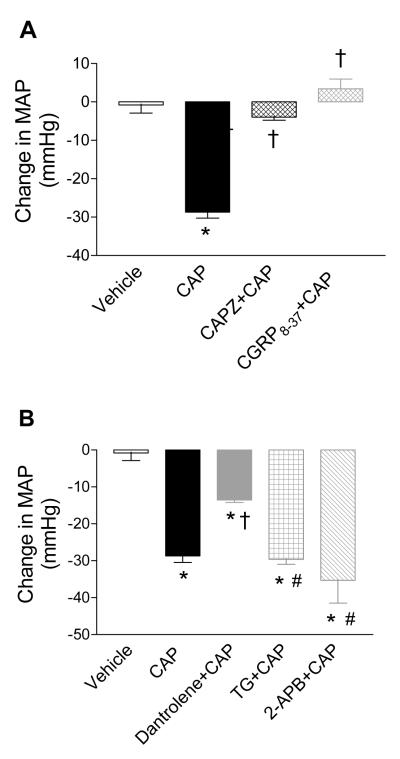
Blockade of the TRPV1 or CGRP receptors with CAPZ or CGRP8-37, respectively, abolished CAP (50 μg/kg)-induced extended depressor responses without affecting the initial two phases (4±1 mmHg or 3±1 mmHg vs. 29±2 mmHg, respectively, P<0.01) as shown in Figure 3A. The role of ER-associated Ca2+ release receptors in CAP-induced depressor responses was evaluated and shown in Figure 3B. CAP-induced depressor effects were attenuated by the RyR antagonist, dantrolene (29±2 mmHg vs. 12±1 mmHg, respectively, P<0.01), but not by the Ca2+-ATPase inhibitor, TG, or the IP3R antagonist, 2-APB (29±2 mmHg vs. 30±1 mmHg or 34±5 mmHg, respectively, P>0.05).
Figure 3.
A, Effects of pretreatment with CAPZ (3 mg/kg) or CGRP8-37 (1 mg/kg/min × 2 minutes followed by 0.5 mg/kg/min × 4 minutes) on CAP (50 μg/kg)-induced changes in MAP. B, Effects of pretreatment with dantrolene (5 mg/kg), TG (50 μg/kg) or 2-APB (3 mg/kg) on CAP (50 μg/kg)-induced changes in MAP. n = 6 to 7. * P<0.05 compared to corresponding vehicles. †P<0.01 compared to rats treated with CAP alone. #P<0.01 compared to rats treated with dantrolene+CAP.
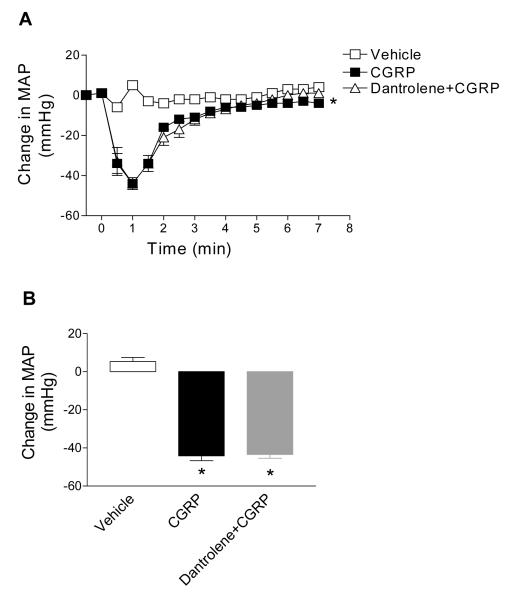
To determine whether dantrolene-induced attenuated depressor effects of CAP is due to affecting downstream CGRP effects on blood vessels, the role of dantrolene in CGRP-induced depressor effects was examined (Figure 4). Pretreatment of dantrolene had no effects on CGRP-induced depressor response (44±3 mmHg vs. 44±5 mmHg, P>0.05) (Figure 4), indicating that attenuated depressor effects of CAP induced by dantrolene was mediated, at least in part, by blockade of RyR in DRG rather than in vascular smooth muscle or endothelial cells.
Figure 4.
Effects of pretreatment with dantrolene (5 mg/kg) on CGRP (1 μg/kg)—induced changes in MAP. B, Changes in MAP 60 seconds after injection of vehicle or CGRP (1 μg/kg) with or without dantrolene. n = 6 to 7. *P<0.05 compared to corresponding vehicles.
The baseline MAP and HR were higher in barodenervated rats than in baroreceptor intact rats (117±5 mmHg vs. 106±4 mmHg; 364±9 beats/min vs. 346±9 beats/min, respectively, P<0.05). After intravenous injection of CAP, the initial fall in MAP was absent, and the intermediate pressor and the lasting depressor effects were augmented and delayed (Figure 5A). The lasting depressor phase reached as low as -40 mmHg and persisted for about 50 minutes before returning to the baseline in barodenervated rats compared to baroreceptor intact rats. Barodenervated rats showed no baroreflex changes in the heart rate (Figure 5B). These results suggest that baroreflex exerts a restraining effect on CAP-induced depressor effects.
Figure 5.
Time-dependent responses of mean arterial pressure (MAP, A) or heart rate (HR, B) to bolus injection of vehicle or CAP (50 μg/kg) in baroreceptor denervated rats. n = 6 to 7. *P<0.05 compared to corresponding vehicles.
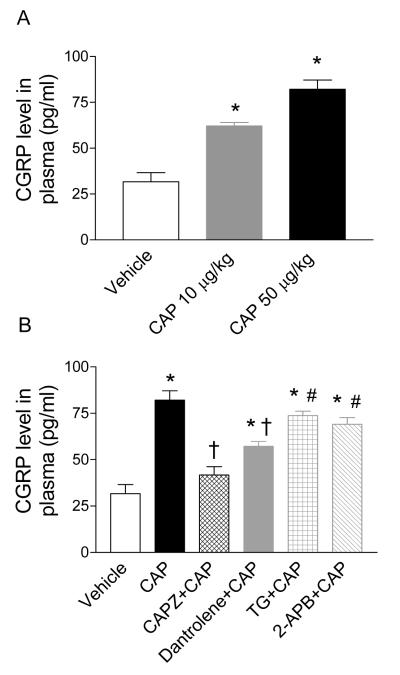
To examine the effects of TRPV1 and ER-associated Ca2+ release receptors on CAP-induced CGRP release in vivo, plasma CGRP levels at baseline and in response to CAP with or without different antagonists were determined by radioimmunoassay as shown in Figure 6. CAP (10 or 50 μg/kg) increased plasma CGRP levels compared to the vehicle (62.1±1.9 pg/ml or 82.2±5.0 pg/ml vs. vehicle 31.7±4.9 pg/ml, respectively, P<0.05) (Figure 6A). The CAP (50 μg/kg)-induced increase in plasma CGRP levels was blocked by CAPZ or attenuated by dantrolene (82.2±5.0 pg/ml vs. 41.8±4.4 pg/ml or 57.2±2.6 pg/ml, respectively, P<0.01), but not affected by TG or 2-APB (82.2±5.0 pg/ml vs. 73.8±2.3 pg/ml or 69.0±3.7 pg/ml, respectively, P>0.05) (Figure 6B). Treatment with CAPZ, dantrolene, TG, or 2-APB alone had no effect on baseline plasma CGRP levels (26.2±1.7 pg/ml; 33.0±7.4 pg/ml; 30.3±3.0 pg/ml; or 28.4±2.9 pg/ml vs. vehicle 31.7±4.9 pg/ml, respectively, P>0.05).
Figure 6.
A, Immunoactive CGRP levels in plasma of rats treated with vehicle, CAP (10 μg/kg; 50 μg/kg). B. Effects of pretreatment with CAPZ (3 mg/kg), dantrolene (5 mg/kg), TG (50 μg/kg), or 2-APB (3 mg/kg) on CAP (50 μg/kg)-induced CGRP release in plasma. n = 6 to 7. *P<0.05 compared to corresponding vehicles. †P<0.01 compared to rats treated with CAP (10 μg /kg) in panel A or CAP (50 μg/kg) alone in panel B. #P<0.05 compared to rats treated with CAPZ+CAP.
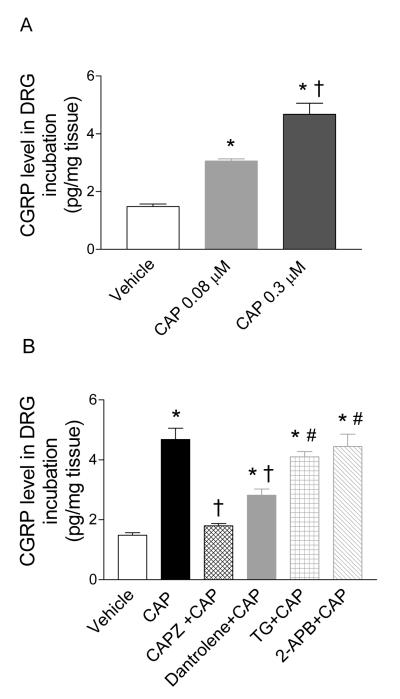
The effects of TRPV1 and ER-associated Ca2+ release receptors on CAP-induced CGRP release in isolated DRG were studied in vitro as shown in Figure 7. CAP (0.08 μM or 0.3 μM) caused a dose-dependent CGRP release in isolated DRG (3.1±0.1 pg/mg tissue vs. 4.7±0.4 pg/mg tissue or vs. vehicle 1.5±0.2 pg/mg tissue, respectively, P<0.01) (Figure 7A). The CAP (0.3 μM)-induced CGRP release was abolished by CAPZ (4.7±0.4 pg/mg tissue vs. 1.8±0.1 pg/mg tissue, respectively, P<0.01) and attenuated by dantrolene (4.7±0.4 pg/mg tissue vs. 2.8±0.2 pg/mg tissue, respectively, P<0.01), but not affected by TG or 2-APB (4.7±0.4 pg/mg tissue vs. 4.1±0.2 pg/mg tissue or 4.4±0.4 pg/mg tissue, respectively, P>0.05) (Figure 7B). The antagonists including CAPZ, dantrolene, TG, or 2-APB alone had no effect on baseline CGRP release (1.2±0.1 pg/mg tissue; 1.6±0.1 pg/mg tissue; 1.5±0.2 pg/mg tissue; or 1.3±0.1 pg/mg tissue vs. vehicle 1.5±0.2 pg/mg tissue, respectively, P>0.05).
Figure 7.
A, Immunoactive CGRP concentrations in DRG incubated with vehicle or CAP (0.08 μM; 0.3 μM). B, Effects of pretreatment with CAPZ (10 μM), dantrolene (50 μM), TG (2 μM), or 2-APB (100 μM) on CAP (0.3 μM)-induced CGRP release in DRG. n = 6. *P<0.05 compared to corresponding vehicles. †P<0.05 compared to DRG treated with CAP (0.08 μM) in panel A or CAP (0.3 μM) in panel B. # P<0.05 compared to DRG treated with CAPZ+CAP or dantrolene+CAP.
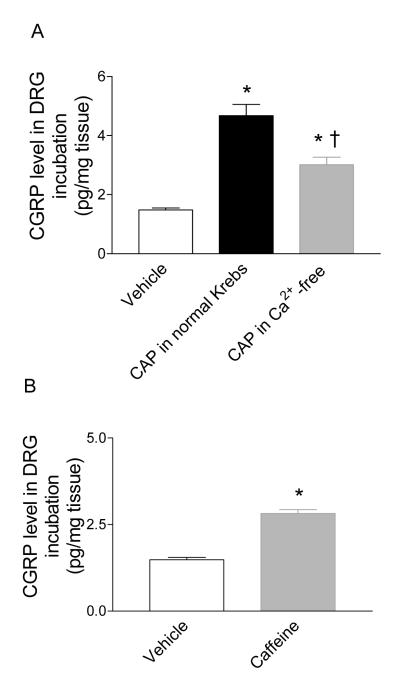
A Ca2+-free solution was used to determine the dependence of Ca2+ influx on CGRP release induced by CAP. CAP induced a smaller magnitude of CGRP release in DRG incubated with Ca2+-free solution compared to that of normal Kreb’s solution (3.0±0.3 pg/mg tissue vs. 4.7±0.4 pg/mg tissue, respectively, P<0.01) (Figure 8A). To further examine the role of RyR in CGRP release, caffeine, a selective RyR agonist, was used to treat isolated DRG (Figure 8B). Caffeine increased CGRP release (2.8±0.1 pg/mg tissue vs. 1.5±0.1 pg/mg tissue, P<0.01), which further supports a role of RyR in CAP-induced CGRP release.
Figure 8.
A. Effects of extracellular calcium (Ca2+) on CAP (0.3 μM)-induced CGRP release in DRG. B. Effects of caffeine (20 mM) on CGRP release in DRG. n = 6. *P<0.01 compared to corresponding vehicles. †P<0.01 compared to that in normal Kreb’s solution.
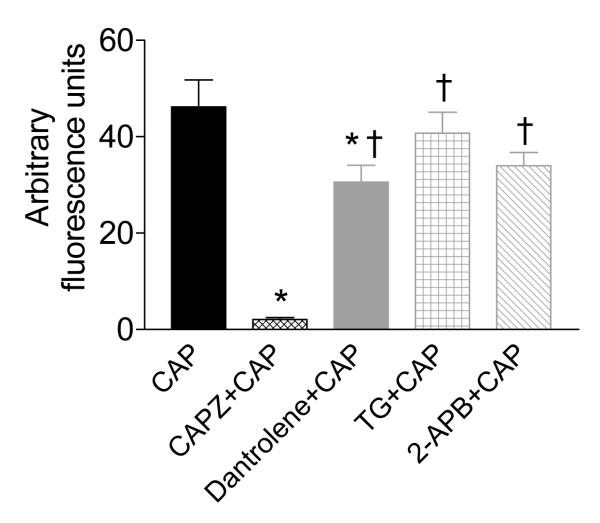
Direct intracellular Ca2+ concentrations in response to CAP with or without different ER-associated Ca2+ release receptor antagonists in isolated DRG neurons were examined and shown in Figure 8. Increased intracellular Ca2+ concentrations induced by CAP (0.3 μM) were abolished by CAPZ (10 μM) (46.2±5.6 AFU vs. 2.1±0.4 AFU, respectively, P<0.001), inhibited by dantrolene (50 μM) (46.2±5.6 AFU vs. 30.6±3.5 AFU, P<0.05), but not affected by TG (2 μM) or 2-APB (100 μM) (46.2±5.6 AFU vs. 40.7±4.4 or 34.0±2.8 AFU, respectively, P>0.05, Figure 9). These findings are consistent with that of CAP-induced CGRP release in vivo and in vitro, suggesting that the RyR- but not IP3R-dependent Ca2+ store contributes to CAP action.
Figure 9.
Effects of pretreatment with CAPZ (10 μM), dantrlene (50 uM), thapsigargin (20 uM), or 2-APB (100 uM) on CAP (0.3 uM)-induced intracellular calcium (Ca2+) response. n = 15 to 32. *P<0.05 compared to neurons treated with CAP alone. †P<0.05 compared to neutrons treated with CAPZ+CAP.
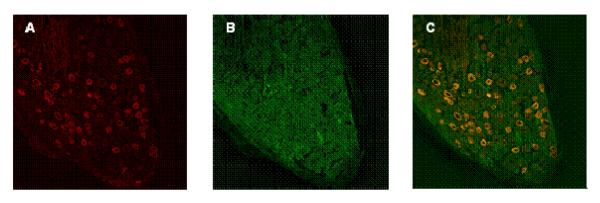
Double staining of TRPV1 and RyR revealed that both receptors were extensively distributed in DRG neurons and co-localized (Figure 10). All TRPV1-positive sensory neurons appeared to express RyR, providing anatomical basis for interaction or cross talk of these receptors.
Figure 10.
Confocal microscopic images of double-immunofluorescence staining of DRG (A—C). A, Cy3-labeled TRPV1 channels stained red. B, FITC-labeled RyR stained green. C, Colocalization of TRPV1 and RyR (yellow). A—C represent the same section of a DRG neuron.
Discussion
Despite the fact that TRPV1-mediated CGRP release may play a significant functional role under pathophysiological conditions, molecular mechanisms underlying TRPV1 action are largely unknown. This study sets out to determine whether and which ER-associated Ca2+ release receptors contribute to TRPV1-induced CGRP release in vivo and in vitro and TRPV1-mediated depressor effects. Our data show for the first time that blockade of RyR- but not IP3R-dependent Ca2+ release from ER attenuates TRPV1-mediated CGRP release in vivo and in vitro and diminishes TRPV1-mediated depressor effects. The TRPV1 effects appear to be mediated, at least in part, by RyR receptor activation in DRG neurons rather than in vascular smooth muscle or endothelial cells given that blockade of RyR had no effect on CGRP-induced depressor response. Indeed, blockade of RyR in DRG neurons diminishes TRPV1-induced increases in intracellular Ca2+ concentrations and TRPV1-mediated CGRP release, effects consistent with those obtained in vivo in which TRPV1-mediated increases in plasma CGRP levels and depressor response are attenuated by RyR blockade.
The triphasic changes in blood pressure induced by CAP administration, i.e. an initial short fall followed by a brief pressor and a prolonged depressor phase, are typical and have been documented previously.25-27 While it has been shown that the transient initial hypotensive response is mediated by the vagal reflex and the intermediate increase in blood pressure a result of a brief vasoconstriction,25-27 it is evident that the subsequent prolonged depressor effects are derived from TRPV1 activation.6, 25-27 Indeed, the initial fall in blood pressure was abolished when the baroreceptors were denervated. Also, the lasting depressor phase was markedly augmented and prolonged, suggesting that baroreflex restrains the depressor effects induced by TRPV1 action. Furthermore, blockade of TRPV1 or CGRP receptors abolishes CAP-induced prolonged depressor effects, indicating that the prolonged depressor phase induced by CAP is the result of TRPV1 activation leading to CGRP release and subsequent depressor response.
Although it is established that TRPV1 activation increases cytosolic Ca2+ levels in DRG which may underlie TRPV1-mediated CGRP release and depressor effects, it is unknown whether or which Ca2+ release receptors may contribute to TRPV1 action. Data from the present study show that blockade of RyR but not IP3R attenuates the prolonged depressor response to CAP, suggesting that Ca2+ influx resulting from TRPV1 action by CAP may trigger intracellular Ca2+ release from ER Ca2+ stores via activation of RyR. This conclusion is further supported by data obtained from in vitro studies demonstrating that CAP-induced elevations in intracellular Ca2+ concentrations and CGRP release in DRG neurons are inhibited by RyR blockade.
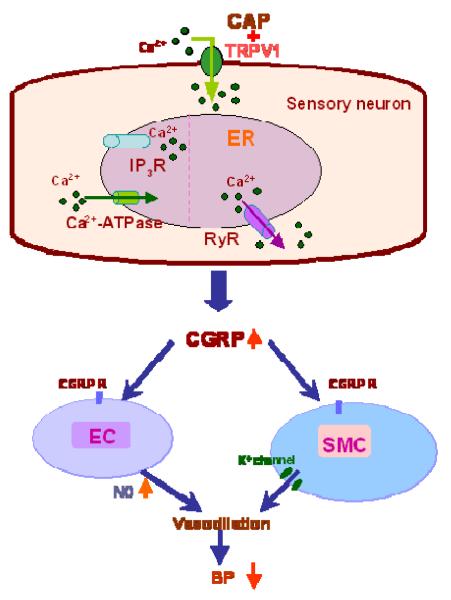
Figure 11 depicts potential molecular mechanisms involved in TRPV1-induced CGRP release in sensory neurons, i.e., CAP binds to TRPV1 on plasma membrane that leads to an increase in Ca2+ influx and activates RyR but not IP3R expressed in ER. These events result in Ca2+ release from ER and consequent CGRP release that in turn activates CGRP receptors expressed in vascular endothelial cells or smooth muscle cells to cause vasodilation. CGRP released from sensory nerves upon TRPV1 activation is one of the most powerful vasodilators, which causes vasodilation via suppression of adrenergic nerve-induced vasoconstriction or an endothelium-dependent mechanism.28-30 Altered CGRP expression in hypertensive animal models appears to be significant given that genetic deletion of CGRP receptors results in a significant increase in baseline blood pressure.31,32
Figure 11.
Schematic representation of the potential molecular mechanisms involved in TRPV1-induced CGRP release in sensory neurons. CAP, a selective TRPV1 agonist, binds to TRPV1 on plasma membrane that leads to an increase in Ca2+ influx and activates RyR but not IP3R expressed in ER, resulting in Ca2+ release from ER and consequent CGRP release that in turn activates CGRP receptors (CGRP R) expressed in vascular endothelial cells (EC) or smooth muscle cells (SMC) to cause vasodilation. NO, nitric oxide; BP, blood pressure.
Endothelium-dependent vasodilatory actions induced by CGRP have been attributed to activation of endothelial nitric oxide synthase (eNOS).33-34 It has been shown that eNOS is Ca2+/calmodulin-dependent, suggesting that eNOS activity may be modulated by cytosolic Ca2+ levels.35 Thus, it is possible that blockade of RyR leading to decreased cytosolic Ca2+ levels in endothelial cells may affect CGRP induced vasodilation and hypotension. To rule out the possibility that dantrolene-induced attenuation in depressor response induced by CAP is mediated by inhibition of downstream CGRP effects, the role of RyR in CGRP-induced hypotension was examined. Our data show that dantrolene had no effect on CGRP-induced_depressor response, suggesting that RyR may not be able to alter CGRP-induced NO production,36 and that dantrolene-induced attenuation in depressor response induced by CAP is likely the result of decreased CGRP release from DRG neurons.
Interestingly, CAP is capable of stimulating significant albeit small in magnitude of CGRP release from DRG under the Ca2+-free condition, a result consistent with a previous report showing that CAP increases intracellular Ca2+ concentrations in a Ca2+-free solution.37 Given that CAP is lipid-soluble, it is likely that CAP enters the cell and binds to TRPV1 expressed in ER which acts as a Ca2+ release receptor 38 or stimulates RyR to increase intracellular Ca2+ concentrations leading to CGRP release in DRG neurons. Indeed, our data show that stimulation of RyR by caffeine leads to CGRP release in DRG, a result consistent with that showing caffeine causes Ca2+ spark and CGRP release in vitro.39
It has been suggested that ER Ca2+ pools are compartmentalized in neuron.40-41 Spatially and functionally distinct Ca2+ stores in sarcoplasmic or endoplasmic reticulum have been shown.40 However, controversy data exist, in that neuronal ER has been shown to act as a single functional Ca2+ store shared by RyR and IP3R in rat DRG.41 Our data appear to be consistent with the former results, showing that RyR and IP3R are distinct Ca2+ stores considering that blockade of RyR but not IP3R attenuates CAP-induced elevations in intracellular Ca2+ concentrations and CGRP release. These results are in agreement with previous reports showing that IP3-PLC pathway inhibitors, U-73122 and neomycin, had no effect on CAP-induced mobilization of intracellular Ca.2, 37, 42-43 Based on these results, it is reasonable to conclude that IP3R is not involved in CAP-induced intracellular Ca2+ mobilization and subsequent CGRP release. In contrast, IP3-depedent Ca2+ release has been linked with CAP-triggered substance P release in rat DRG.44 Taken together, these data suggest that specific local and global Ca2+ signals in the nervous system may be generated and controlled in order to regulated numerous Ca2+-dependent processes simultaneously.45
In summary, our data show that TRPV1 activation induces CGRP release in vitro and in vivo and causes depressor response, all of which are likely to be mediated, at least in part, by activation of RyR expressed in ER leading to increased cytosolic Ca2+ levels in DRG neurons. The possible sequence of TRPV1 action is depicted in Figure 11, which is further explained in the figure legend.
Perspectives
Accumulating evidence indicates that defects in sensory nerves and/or TRPV1 expression/function exist in spontaneously hypertensive rats (SHR) and Dahl salt sensitive hypertensive rats.48,6 Although the mechanisms responsible for such defects remain to be elucidated, recent studies showed that RyR function in various tissues including the heart and oesophageal striated muscle is impaired in experimental hypertensive rats or SHR.49-50 Given the fact that RyR may play an important role in TRPV1-mediated CGRP release and hypotension, it is conceivable that dysfunction of RyR may contribute to impaired action mediated by TRPV1 and TRPV1-positive sensory nerves in hypertension, and that agents that modulate the RyR function may be beneficial in improving sensory nerve function and in treating hypertension. Furthermore, it has been shown that the baroreflex restraining function is diminished in hypertensive states,46-47 leading to the possibility that TRPV1 activation may constitute a stronger effect on preventing elevated blood pressure.
Acknowledgement
This work was supported in part by National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from Michigan Economic Development Corporation.
Glossary
- (TRPV1)
transient receptor potential vanilloid subtype 1
- (ER)
endoplasmic reticulum
- (DRG)
dorsal root ganglion
- (CAP)
capsaicin
- (MAP)
mean arterial pressure
- (CAPZ)
capsazepine
- (RyR)
ryanodine receptor
- (TG)
thapsigargin
- (IP3R)
inositol [1,4,5]-trisphosphate receptor
- (2-APB)
2-aminoethoxydiphenyl borate
- (CGRP)
calcitonin gene-related peptide
- (CICR)
calcium-induced calcium release
- (MAP)
mean arterial pressure
- (HR)
heart rate
- (EC)
endothelial cells
- (SMC)
smooth muscle cells
- (NO)
nitric oxide
References
- 1.Szalasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 2.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 3.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anadamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Kaminski NE, Wang DH. Anadamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- 5.Brian SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiology Review. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl saltsensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47:609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Wang DH. Prevention of endothelin-1-induced increases in blood pressure: role of endogenous CGRP. Am J Physiol. 2004;287:H1868–H1874. doi: 10.1152/ajpheart.00241.2004. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anadamide. Hypertension. 2005;46:986–991. doi: 10.1161/01.HYP.0000174596.95607.fd. [DOI] [PubMed] [Google Scholar]
- 9.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 11.David E. Calcium singnalling. Cell. 1995;80:259–268. [Google Scholar]
- 12.Verkhratsky AJ, Petersen OH. Neuronal calcium stores. Cell Calcium. 1998;24:333–343. doi: 10.1016/s0143-4160(98)90057-4. [DOI] [PubMed] [Google Scholar]
- 13.Smith AB, Cunnane TC. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea pig. J Physiol. 1996;497:657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Y. Ryanodine-sensitive component of calcium transient evoked by nerve firing at presynaptic nerve-terminals. J.Neurosci. 1996;16:6703–6712. doi: 10.1523/JNEUROSCI.16-21-06703.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-purkinje cell synapse. J Neurosci. 2003;23:11229–11234. doi: 10.1523/JNEUROSCI.23-35-11229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calciumtransients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 17.Berridge MJ. Neuronal Calcium Signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 18.Shmigol A, Verkhratsky A, Isenberg G. Calcium-induced calcium release in rat sensory neurons. J Physiol. 1995;489:627–636. doi: 10.1113/jphysiol.1995.sp021078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verkhratsky A, Shmigol A. Calcium-induced calcium release in neurons. Cell Calcium. 1996;19:1–14. doi: 10.1016/s0143-4160(96)90009-3. [DOI] [PubMed] [Google Scholar]
- 20.Mothet JP, Fossier P, Meunier FM, Stinnakre J, Tauc L, Baux G. Cyclic ADP-ribose and calcium-induced calcium release regulate neurotransmitter release at a cholinergic synapse of Aplysia. J Physiol. 1998;507:405–414. doi: 10.1111/j.1469-7793.1998.405bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tognetto M, Amadesi S, Harrison S, Creminon C, Trevisani M, Carreras M, Matera M, Geppetti P, Bianchi A. Ananamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. J. Neurosci. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peter MZ, Jesper P, David AA, Huai-hu C, Morten S, Vincenzo DM, David J, Edward DH. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 23.Shen E, Wu SY, Dun NJ. Spontaneous and transmitter-induced rhythmic activity in neonatal rat sympathetic preganglionic neurons in vitro. J Neurophysiol. 1994;71:1197–1205. doi: 10.1152/jn.1994.71.3.1197. [DOI] [PubMed] [Google Scholar]
- 24.Kurebayashi N, Yamashita H, Nakazato Y, Daida H, Oqawa Y. Behavior of Ca2+ waves in multicellular preparations from guineapig ventricle. Am J Physiol Cell Physiol. 2004;287:1646–1656. doi: 10.1152/ajpcell.00200.2004. [DOI] [PubMed] [Google Scholar]
- 25.Donnerer J, Lembeck F. Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:54–57. doi: 10.1007/BF00499072. [DOI] [PubMed] [Google Scholar]
- 26.Yeh JL, Lo YC, Wang Y, Chen IJ. Cardiovscular interactions of nonivamide, capsaicin analogures, and substance P antagonist in rats. Brian Res.Bull. 1993;30:641–648. doi: 10.1016/0361-9230(93)90095-s. [DOI] [PubMed] [Google Scholar]
- 27.Lo YC, Yeh JL, Wu JR, Yang JM, Chen SJ, Chen IJ. Autonomic and sensory cardiovascular activities of nonivamide; Intrathecal administration of clonidine. Brain Res.Bull. 1994;35:15–22. doi: 10.1016/0361-9230(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 28.Crossman DC, Dashwood MR, Brain SD, McEwan J, Pearson JD. Action of calcitonin gene-related peptide upon bovine vascular endothelial and smooth muscle cells grown in isolation and co-culture. Br J Pharmacol. 1990;99:71–76. doi: 10.1111/j.1476-5381.1990.tb14656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirata Y, Takagi Y, Takata S, Fukuda Y, Yoshimi H, Fujita T. Calcitonin gene-related peptide receptor in cultured vascular smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1988;151:1113–1121. doi: 10.1016/s0006-291x(88)80481-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Wang DH. Prevention of endothelin-1-induced increases in blood pressure: role of endogenous CGRP. Am J Physiol Heart Circ Physiol. 2004;287:H1868–H1874. doi: 10.1152/ajpheart.00241.2004. [DOI] [PubMed] [Google Scholar]
- 31.Zaidi M, Bevis PJ. Enhanced circulating levels of neurally derived calcitonin gene related peptide in spontaneously hypertensive rats. Cardiovasc Res. 1991;25:125–8. doi: 10.1093/cvr/25.2.125. [DOI] [PubMed] [Google Scholar]
- 32.Gangula PR, Zhao H, Supowit SC, Wimalawansa SJ, Dipette DJ, Westlund KN, Gagel RF, Yallampalli C. Increased blood pressure in alpha-calcitonin gene-related peptide/calcitonin gene knockout mice. Hypertension. 2000;35:470–5. doi: 10.1161/01.hyp.35.1.470. [DOI] [PubMed] [Google Scholar]
- 33.Brian SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 34.Gray DW, Marshall I. Human alpha-calcitonin gene-related peptide stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br J Pharmacol. 1992;107:691–696. doi: 10.1111/j.1476-5381.1992.tb14508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 36.Hutcheson IR, Griffith TM. Central role of intracellular calcium stores in acute flow-and agonist-evokd endothelial nitric oxide release. Br J Pharmacol. 1997;122:117–125. doi: 10.1038/sj.bjp.0701340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu M, Liu MC, Magoulas C, Priestley JV, Willmott NJ. Vesatile regulation of cytosolic Ca2+ by vanilloid receptor in rat dorsal root ganglion neurons. J Biol Chem. 2003;278:5462–5472. doi: 10.1074/jbc.M209111200. [DOI] [PubMed] [Google Scholar]
- 38.Eun SY, Jung SJ, Park YK, Kwak J, Kim SJ, Kim J. Effects of capsaincin on Ca2+ storesin the dorsal root ganglion cells of adult rats. Biochemical and biophysical research communications. 2001;285:114–1120. doi: 10.1006/bbrc.2001.5272. [DOI] [PubMed] [Google Scholar]
- 39.Ouyang K, Zheng H, Qing X, Zhang C, Yang D, Wang X, Wu C, Zhou Z, Cheng H. Ca2+ sparks and secretion in dorsal root ganglion neurons. Proc Natl Acad Sci. 2005;102:12259–12264. doi: 10.1073/pnas.0408494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- 41.Solovyova N, Verkhratsky A. Neuronal endoplasmic reticulum acts as a single functional Ca2+ stores shared by ryanodine and inositol-1,4,5-trisphosphate receptors as revealed by intra-ER [Ca2+] recordings in single rat sensory neurons. Pfluqers Arch. 2003;446:447–454. doi: 10.1007/s00424-003-1094-z. [DOI] [PubMed] [Google Scholar]
- 42.Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-Aminoethoxydipheny1 borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 43.Wisnoskey BJ, Sinkins WG, Schilling WP. Activation of vanilloid receptor type 1 in the endoplasmic reticulum fails to activate store-operated Ca2+ entry. Biochem. J. 2003;372:517–528. doi: 10.1042/BJ20021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang HB, Inoue A, Iwasa M, Hide I, Nakata Y. Substance P release evoked by capsaicin or potassium from rat cultured dorsal root ganglion neurons if conversely modulated with bradykinin. J Neurochem. 2006;97:1412–1418. doi: 10.1111/j.1471-4159.2006.03830.x. [DOI] [PubMed] [Google Scholar]
- 45.Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- 46.Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension. 2005;46:1194–1200. doi: 10.1161/01.HYP.0000187011.44201.2e. [DOI] [PubMed] [Google Scholar]
- 47.Gao SA, Johansson M, Rundqvist B, Lambert G, Jensen G, Friberg P. Reduced spontaneous baroreceptor sensitivity in patients with renovascular hypertension. J Hypertens. 2002;20(1):111–116. doi: 10.1097/00004872-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Supowit SC, Ramana CV, Westlund KN, DiPette DJ. Calcitonin gene-related peptide gene expression in the spontaneously hypertensive rats. Hypertension. 1993;21:1010–1014. doi: 10.1161/01.hyp.21.6.1010. [DOI] [PubMed] [Google Scholar]
- 49.Naudin V, Oliviero P, Rannou F, Beuve C Sainte, Charlemagne D. The density of ryanodine receptors decreases with pressure overload-induced rat cardiac hypertrophy. FEBS Lett. 1991;285:135–138. doi: 10.1016/0014-5793(91)80743-m. [DOI] [PubMed] [Google Scholar]
- 50.Sekiguchi F, Kawata K, Yamazoe M, Sunano S. Abnormal response to ryanodine in oesophageal striated muscle of spontaneously hypertensive rats. Eur J Pharmacol. 2004;486:91–98. doi: 10.1016/j.ejphar.2003.11.081. [DOI] [PubMed] [Google Scholar]