Abstract
To determine whether the transient receptor potential vanilloid type 1 (TRPV1) channel provides protection against hypertension-induced renal damage, hypertension was induced by uninephrectomy and by giving deoxycorticosterone acetate (DOCA)-salt in wild type (WT) and TRPV1-null mutant (TRPV1-/-) mice. Mean arterial pressure (MAP), as determined by radiotelemetry, increased significantly and reached the peak 7 days after DOCA-salt treatment in both WT and TRPV1-/- mice. There was no difference in MAP between the two strains at the baseline or at the peak that lasted for 4 treatment weeks. DOCA-salt treatment in both WT and TRPV1-/- mice led to increased urinary excretion of albumin and 8-isoprostane, glomerulosclerosis, renal cortical tubulointerstitial injury, tubulointerstitial fibrosis, increased number of tubular proliferating cell nuclear antigen-positive cells, and renal monocyte/macrophage infiltration, all of which were much more severe in DOCA-salt-treated TRPV1-/- compared to DOCA-salt-treated WT mice. Renal TRPV1 protein expression but not the renal anandamide content was elevated in DOCA-salt-treated WT compared to vehicle-treated WT mice. Renal anandamide levels were markedly elevated in DOCA-salt-treated TRPV1-/- but not in vehicle-treated TRPV1-/- mice. Thus, our data show that ablation of TRPV1 gene exacerbates renal damage induced by DOCA-salt hypertension, indicating that TRPV1 may constitute a protective mechanism against end-organ damage induced by hypertension.
Keywords: transient receptor potential vanilloid type1 channel, hypertension, renal injury, deoxycorticosterone, mice
Introduction
It is well accepted that hypertension is a major cardiovascular risk factor and a major factor contributing to end-stage renal disease.1,2 Clinical investigation shows that patients with salt-sensitive hypertension have a greater incidence of end-stage renal disease compared to salt-resistant hypertensive individuals, suggesting that salt may be a key determinant of the development of hypertension-induced renal damage.3 Multiple factors including endothelin-1 (ET-1), angiotensin II (Ang II), and oxidative stress have been suggested to contribute to end-stage renal disease associated with salt-sensitive hypertension.4-6 However, the mechanisms underlying the progression of hypertension and end-stage renal disease in salt-sensitive hypertension are not fully elucidated.
The transient receptor potential vanilloid type 1 (TRPV1) channel is a ligand-gated cation channel.7 It functions as a molecular transducer to integrate multiple stimuli including noxious heat, low pH, and the “hot” pepper-derived vanilloid compound, capsaicin.7,8 In addition, TRPV1 can be activated by endogenous arachidonic acid derivatives, such as anandamide.9,10 TRPV1 predominantly resides in unmyelinated C-fibers and thinly myelinated Aδ-afferent nerve fibers innervating the cardiovascular system, and activation of TRPV1 expressed in these nerves causes release of a number of sensory neuropeptides including calcitonin gene-related peptide (CGRP) and substance P, which are potent vasodilators in various vascular beds.11
TRPV1-positive sensory nerves are also widely distributed in the kidney, suggesting that TRPV1-mediated action may participate in the regulation of renal function under pathophysiological conditions.11 Indeed, sodium excretion in response to sodium loading is impaired in salt-sensitive hypertension induced by surgical sensory denervation or by sensory nerve degeneration following neonatally capsaicin treatment.12,13 We also found that blockade of TRPV1 increased blood pressure in Wistar or Dahl salt-resistant rats fed a high but not normal salt diet,10,14 suggesting that high salt intake may activate TRPV1 conferring a protective effect.
The present study was designed to study the effects of TRPV1 on hypertension-induced renal damage using a gene-targeting approach. The renal function and morphological changes occurring especially in various microstructures in the renal cortex including glomeruli, tubules, and tubular interstitium were examined during the development of deoxycorticosterone acetate (DOCA)-salt hypertension in the wild type (WT) and TRPV1-null mutant (TRPV1-/-) mice.
Methods
Animals
TRPV1-/- mice were kindly provided by Dr. David Julius (University of California, San Francisco, CA) and were generated by deleting an exon encoding part of the fifth and all of the sixth putative transmembrane domains (including the interconnecting p-loop) of the channel as described by Caterina et al.7 TRPV1-/- mice were backcrossed to WT mice (C57BL/6) for at least six generation and had been shown to have impaired nociception and pain sensation.7 Ten-week-old male TRPV1-/- mice or C57BL/6 mice as the WT control (weighing ∼ 26 to 28 g) were used in this study. All of the experiments were approved by the Institutional Animals Care and Use Committee.
Telemetry Blood Pressure Assay
Mean arterial pressure (MAP) and heart rate (HR) were determined using telemetry system (Data Sciences International, St. Paul, MN) according to the manufacturer’s instruction. In brief, the mice were anesthetized with ketamine and xylazine (80 and 4 mg/kg SC, respectively), the transmitter catheter was implanted into the left carotid artery, and the transmitter body was placed subcutaneously in the lower right hand side of the abdomen. The mice were returned to their individual cages and allowed to recovery for 3 days before radiotelemetric recording was started.
Experimental Protocol
One week after the radiotelemetric recording, the mice including WT and TRPV1-/- mice were re-anesthetized as described above, and the left kidney was removed. The DOCA pellet (24 mg/10 g body weight, Innovative Research of America, Sarasota, FL) was implanted subcutaneously in the neck area under anesthesia. Mice receiving DOCA were given 1% NaCl and 0.2% KCl to drink, and treatment with DOCA-salt continued for 4 weeks. Control groups, consisting of WT and TRPV1-/- mice, underwent uninephrectomy without receiving DOCA and saline. Each group consisted of at least 7 mice. Radiotelemetric recording continued for an additional 4 weeks.
At the end of 4-week treatment, mice were placed in metabolic cages designed for mice for 24-hour urine collection. After then, plasma, kidney, and heart were harvested, weighted, and then stored at -80°C for biochemical analysis or fixed in a 10% formaldehyde solution in phosphate buffer for histological analysis.
Plasma and Urine Analysis
Plasma and urine creatinine concentrations were assayed by the use of an improved Jaffe creatinine assay kit (BioAssay System, Hayward, CA). Plasma concentrations of K+ and Na+ were measured using flame photometry (Instrumentation, Upper Darby, PA). Urinary albumin was measured with an enzyme-linked immunosorbent assay kit (Exocell Inc, Philadelphia,PA). Urinary 8-isoprostane levels were determined using the kit from Cayman Chemical (Ann Arbor, MI).
Histological Analysis
The degree of sclerosis and mesangial matrix expansion within the glomerular tuft and tubulointerstitial injury was determined in paraffin-embedded tissue sections stained with periodic acid-Schiff (PAS) and Masson’s trichrome stain, respectively, by 2 examiners without knowledge of prior treatment of sections.
The degree of glomerulosclerosis was determined using a semiquantitative scoring method as described previously.15 Fifty glomeruli per section were selected randomly, and scored as follows: grade 0, normal glomeruli; grade 1, sclerotic area ≤ 25% of total glomerular area or distinct adhesion present between capillary tuft and Bowman’s capsule; grade 2, sclerotic area between 25% and 50%; grade 3, sclerotic area 50% to 75%; and grade 4, sclerotic area 75% to 100% of the total glomerular area. The ultimate score was obtained by multiplying the degree of changes by the percentage of glomeruli with the same degree of injury and adding up these scores.
The degree of mesangial matrix expansion was assessed using an in-house image-analysis software, which based on the total area of a glomerulus occupied by mesangial matrix stained PAS positively. The degree of the matrix expansion is expressed as the percentage of the total glomerular area.
Tubulointerstitial injury was graded according to Shih et al16 on a scale of 0 to 4: grade 0, normal; grade 0.5, small focal areas of damage; grade 1, involvement of <10% of the cortex; grade 2, involvement of 10% to 25% of the cortex; grade 3, involvement of 25% to 75% of the cortex; and grade 4, extensive damage involving >75% of the cortex.
Immunohistochemistry
Immunohistochemical staining was performed using 3-μm-thick paraffin-embedded kidney sections obtained 4 weeks after the treatment as described previously.17 Kidney sections were incubated with a primary antibody that recognizes proliferating cell nuclear antigen (PCNA) (mouse anti-human PCNA, BioGenex, San Ramon, CA) or F4/80 (rat anti-mouse macrophage monoclonal antibody, Serotec, Oxford, UK) for overnight at 4°C. Sections were then incubated with appropriate secondary antibody conjugated with horseradish peroxidase and visualized by incubating the sections with the substrate 3,3′-diaminobenzidine (DAB) or vector fast red, both of which were purchased from Vector Laboratories (Burlingame, CA). Negative control experiments were performed by omitting incubation with the primary antibody.
The quantitation of PCNA and F4/80-positive cells in the cortex was carried out in a blind fashion under ×400 magnification. For each section, 15 randomly selected cortical fields were examined. The number of positive cells was expressed as cells per square millimeter.
Hydroxyproline Assay
The collagen content of renal tissue was determined by hydroxyproline assay. Kidney samples were processed as described by Peng et al.5 Hydroxyproline content was determined with a colorimetric assay and a standard curve of 0 to 5 μg hydroxyproline (Sigma-Aldrich Co., St. Louis, MO).18 Data were expressed as μg collagen/mg dry weight, assuming that collagen contains an average of 13.5% hydroxyproline.5
Endocannabinoid Analysis
Renal anandamide extraction procedure was performed prior to separation and detection by reversed-phase high performance liquid chromatography (HPLC) with ultraviolet (UV) detector as described by Folch et al.19 Briefly, separations were carried out on Discovery BIO Wide pore ODS column (Supelco, St. Louis, MO, 5 μm, 45 × 4.6 mm i.d.) connected with Discovery BIO Wide pore ODS guard column (5 μm, 20 × 4 mm i.d.). The gradient elution of 0.1% acetic acid (A) and acetonitrile with 0.1% acetic acid (B) was used (50% B for 3 min, linear gradient from 50-70% of B in 11.2 min, 100% B for 7.5 min) at flow rate 1.3 ml/min. The HPLC-UV was performed using a commercial system (Shimadzu, Kyoto, Japan). Quantification was based on the integration of peak areas at 204 nm.
Western Blot Analysis
Western blot analysis was performed as described previously,14 with the use of goat anti-rat TRPV1 polyclonal IgG (1:800, Santa Cruz Biotechnology, Santa Cruz, CA) and horseradish peroxidase-conjugated bovine anti-goat IgG (1:500, Santa Cruz Biotechnology, Santa Cruz, CA). Detection was accomplished with enhanced chemiluminescence Western blot test (ECL, Amersham Biosciences, Piscataway, NJ). Band intensity was densitometrically measured. β-Actin was used to normalize protein loaded on blots.
Statistical Analysis
All values are expressed as mean ± SE. The significance of differences between groups for the blood pressure data was evaluated with an ANOVA for repeated measures followed by a Bonferroni’s test. The differences among groups were analyzed using one-way analysis of variance followed by a Bonferroni’s adjustment for multiple comparisons. An unpaired t test was applied to compare the differences between two groups. Differences were considered statistically significant at P < 0.05.
Results
As shown in Table 1, there was no significant difference in body weight and plasma levels of electrolytes between groups at the end of the experimental period. Heart-to-body weight and kidney-to-body weight ratio increased significantly in DOCA-salt hypertensive WT and TRPV1-/- mice compared to their respective control groups (P<0.05), but no difference was seen between DOCA-salt-treated WT and TRPV1-/- groups (P>0.05). Urinary output increased markedly in DOCA-salt hypertensive WT and TRPV1-/- mice compared to their respective controls (P<0.01), but there was no difference (P>0.05) between DOCA-salt-treated WT and TRPV1-/- groups albeit there was a tendency to be lower in the latter group. Urinary albumin and 8-isoprostane excretion were increased significantly in DOCA-salt treated WT and TRPV1-/- mice, and the magnitude of the increase was greater in the latter group (P<0.05). Urinary creatinine clearance was significantly decreased in DOCA-salt treated WT and TRPV1-/- mice, and the degree of the decease was greater in the latter group (P<0.05).
Table 1.
. Effect of DOCA-Salt Treatment on Body Weight, Heart Weight, Kidney Weight, Plasma and Urine Chemistries in WT and TRPV1-/- Mice
| Parameters | WT | TRPV1-/- | DOCA-WT | DOCA-TRPV1-/- |
|---|---|---|---|---|
| Body Weight (g) |
28.1±0.4 | 28.7±0.3 | 28.5±0.4 | 29.4±0.4 |
| Heart Weight (mg/10 g BW) |
43.9±1.4 | 44.6±1.7 | 58.2±2.1* | 59.9±2.4* |
| Kidney Weight (mg/10 g BW) |
78.9±1.3 | 75.8±1.2 | 111.5±3.4* | 115.2±3.1* |
| Plasma [K+] (mmol/L) |
6.3±0.4 | 6.0±0.5 | 5.9±0.3 | 5.7±0.5 |
| Plasma [Na+] (mmol/L) |
148±4 | 152±3 | 149±2 | 150±5 |
| Urinary Output (ml/24 h) |
1.6±0.2 | 1.4±0.3 | 18.1±2.3* | 12.5±1.4* |
| Urinary Albumin (μg/24 h) |
5.8±0.7 | 6.5±0.9 | 26.6±2.8* | 74.8±4.5*† |
| Urinary 8-Isoprostane (ng/24 h) |
0.52±0.11 | 0.60±0.10 | 1.27±0.19* | 2.64±0.38*† |
| Creatinine Clearance (ml/24 h) |
344±23 | 334±25 | 206±17* | 114±19*† |
Values are means ± SE; n = 7-8 mice. DOCA, deoxycorticosterone acetate; WT, wild type; TRPV1-/-, the transient receptor potential vanilloid type 1 receptor-null mutant; DOCA-WT, WT mice treated with DOCA-salt; DOCA-TRPV1-/-, TRPV1-/- mice treated with DOCA-salt; BW, body weight.
P<0.05 compared with control WT or TRPV1-/- mice
P<0.05 compared with DOCA-WT mice.
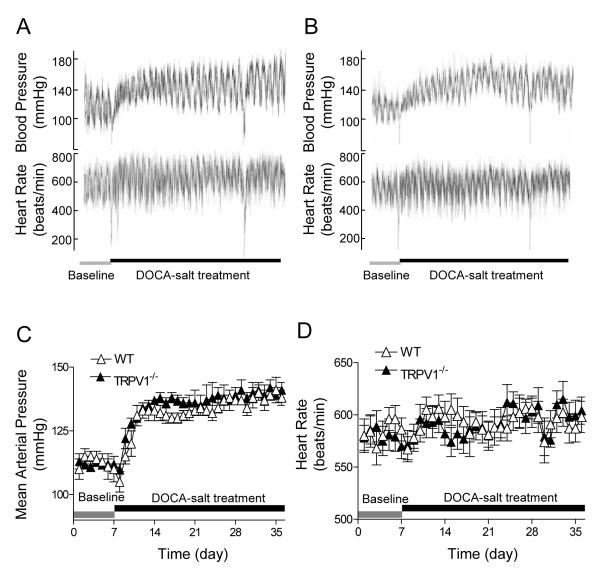
WT and TRPV1-/- mice had similar baseline MAP determined by radiotelemetry as presented in Figure 1. One week after treatment with DOCA and salt, MAP increased remarkably compared to the baseline and remained at this level for 4 weeks in both WT and TRPV1-/- mice with no difference between the two strains (P>0.05). In addition, there was no difference in HR between WT and TRPV1-/- mice during the baseline and DOCA-salt treatment period.
Figure 1.
Effect of deoxycorticosterone acetate (DOCA)-salt treatment on blood pressure and heart rate, as determined by radiotelemetry, in wild type (WT) and TRPV1-null mutant (TRPV1-/-) mice. A and B: Representative telemetric recording of blood pressure and heart rate in WT (A) and TRPV1-/- (B) mice. C and D: Graphs representing daily average 24-hour mean arterial pressure (C) and heart rate (D). Values are mean ± SE (n=6).
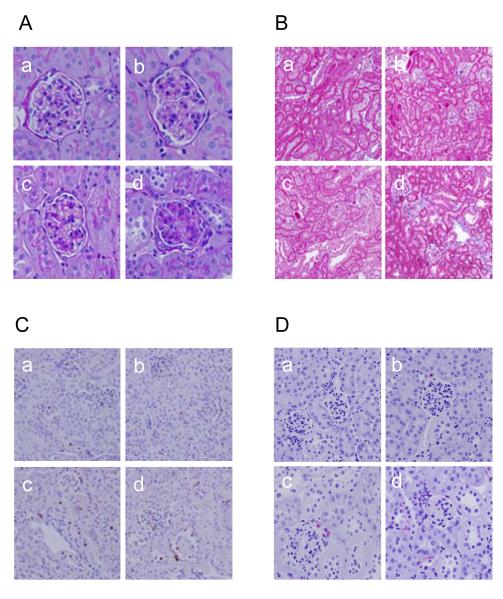
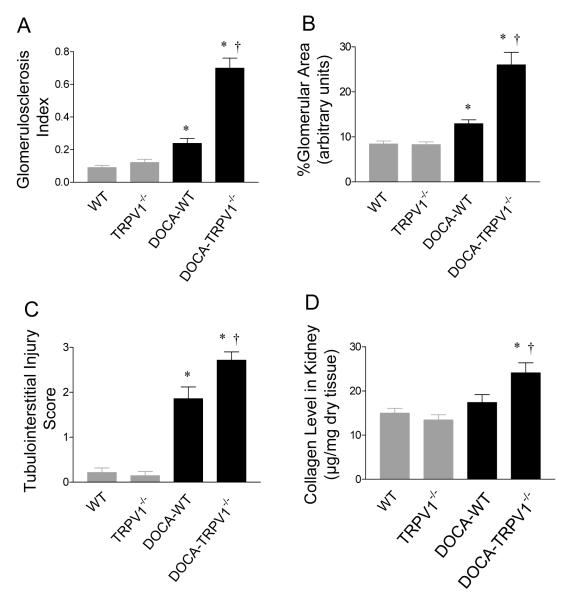
As illustrated in Figure 2, DOCA-salt treatment led to a more severe glomerulosclerosis in the kidneys of TRPV1-/- than WT mice (Figure 2A). There was no difference in the glomerulosclerosis index between control WT and TRPV1-/- mice (P>0.05) (Figure 3A). As shown in Figure 3A, glomerulosclerosis index increased significantly in DOCA-salt-treated WT and TRPV1-/- mice, and the magnitude of the increase was greater in the latter group (P<0.05). In addition, mesangial matrix increased significantly in DOCA-salt-treated WT and TRPV1-/- mice compared to the controls (P<0.05) (Figure 3B), and the degree of the increase was greater in the latter group (P<0.05).
Figure 2.
Effect of deoxycorticosterone acetate (DOCA)-salt treatment on renal morphology in wild type (WT) and TRPV1-null mutant (TRPV1-/-) mice. A and B: Periodic acid-Schiff (A) or Masson’s trichrome (B) stained kidney sections showing glomerular or cortical tubulointerstitial morphological changes in control WT and TRPV1-/- mice (a and b), and WT and TRPV1-/- mice (c and d) treated for 4 weeks with DOCA-salt. Magnification ×400 (A) or ×200 (B). C and D: Immunohistochemical stained sections from the kidney cortex demonstrating proliferating cell nuclear antigen (PCNA)-positive cells (in brown) (C) or F4/80-positive cells (monocytes/macrophages in red) (D) in the renal cortex of control WT and TRPV1-/- mice (a and b), and WT and TRPV1-/- mice (c and d) treated for 4 weeks with DOCA-salt. Magnification ×400.
Figure 3.
Effect of deoxycorticosterone acetate (DOCA)-salt treatment on renal glomerular and cortical tubulointerstitial injury in wild type (WT) and TRPV1-null mutant (TRPV1-/-) mice. A: Graph presenting the average of glomerulosclerosis index. B: Graph presenting the changes in mesangial matrix of glomeruli. C: Graph presenting the average of tubulointerstitial injury score. D: Graph presenting the changes in renal collagen levels. Values are mean ± SE (n=7 to 8). *P<0.05 compared with control WT or TRPV1-/- mice; †P<0.05 compared with DOCA-salt-treated WT mice.
More severe tubular injury and interstitial fibrosis were observed in DOCA-salt-treated TRPV1-/- than in DOCA-salt-treated WT mice as demonstrated by Masson’s trichrome staining, while no observable damage was observed in control WT and TRPV1-/- kidneys (Figure 2B). Quantitative analysis showed that cortical tubular injury was significantly greater in DOCA-salt-treated TRPV1-/- than in DOCA-salt-treated WT mice (P<0.05) (Figure 3C). Renal collagen content determined using hydroxyproline assay was significantly increased in DOCA-salt-treated TRPV1-/- mice compared to DOCA-salt-treated WT mice or control TRPV1-/- or WT mice (P<0.05), while there was not significant difference between the latter three groups (P>0.05) (Figure 3D).
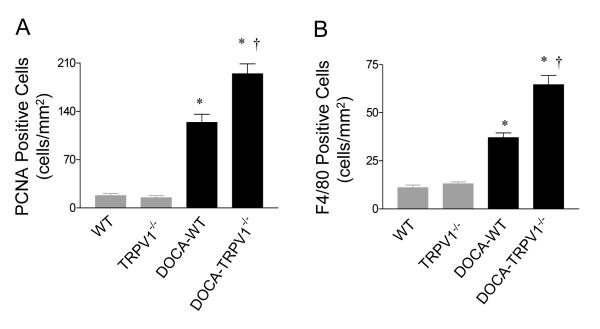
As shown in Figure 2, PCNA-positive cells were largely restricted to the renal tubular epithelium in control WT and TRPV1-/- kidneys (Figure 2C). PCNA-positive cells, particularly observed in diluted tubules, increased significantly in DOCA-salt-treated WT and TRPV1-/- mice with a greater magnitude in the latter group (P<0.05) (Figure 4A). PCNA-positive cells in interstitials were also observed in DOCA-salt-treated TRPV1-/- mice but with a lesser extent comparing to those in tubules. Monocyte/macrophage infiltration, an indicator of inflammation in the kidney, was also determined immunohistochemically (Figure 2D). Cortical F4/80-positive cells increased significantly in DOCA-salt-treated WT and TRPV1-/- mice compared to controls (P<0.05) (Figure 4B), and the magnitude of the increase was greater in the latter group (P<0.05).
Figure 4.
Effect of deoxycorticosterone acetate (DOCA)-salt treatment on renal cortex cell proliferation and monocyte/macrophage infiltration in wild type (WT) and TRPV1-null mutant (TRPV1-/-) mice. A: Graph presenting the mean value of PCNA-positive cells in the renal cortex. B: Graph presenting F4/80-positive cells (monocyte/macrophage) in the renal cortex. The results are expressed as cells per square millimeter. Values are mean ± SE (n=7 to 8). *P<0.05 compared with control WT or TRPV1-/- mice; †P<0.05 compared with DOCA-salt-treated WT mice.
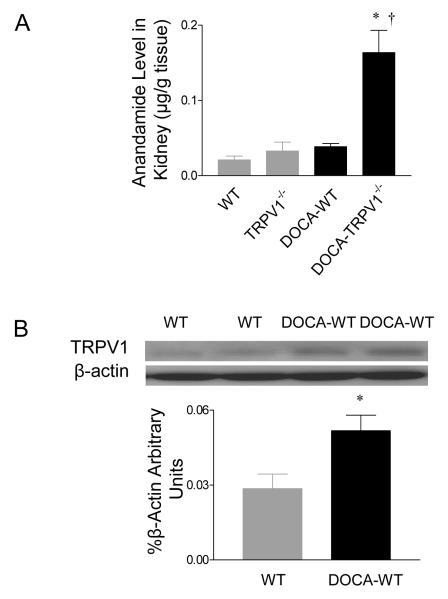
Figure 5 shows that DOCA-salt treatment increased significantly renal levels of anandamide, one of endogenous TRPV1 agonists, in TRPV1-/- mice compared to control TRPV1-/- mice (P<0.01) (Figure 5A). In contrast, DOCA-salt treatment did not change renal anandamide levels in WT mice (P>0.05). In addition, renal TRPV1 protein expression was up-regulated in DOCA-salt-treated WT mice compared to control WT mice (P<0.05) (Figure 5B). TRPV1 expression was undetectable in TRPV1-/- mice with or without DOCA-salt treatment (data not shown).
Figure 5.
Effect of deoxycorticosterone acetate (DOCA)-salt treatment on renal anandamide levels (A) and TRPV1 protein expression (B) in wild type (WT) or TRPV1-null mutant (TRPV1-/-) mice. Values are mean ± SE (n=5 to 7). *P<0.05 compared with control WT or TRPV1-/- mice; †P<0.05 compared with DOCA-salt-treated WT mice.
Discussion
This is the first study to explore the influence of long-term TRPV1 deletion on renal injury induced by DOCA-salt hypertension. The results of the present study demonstrate that DOCA-salt treatment exacerbated renal damage when TRPV1 was deleted, evident by further decreased creatinine clearance, further increased albuminuria, and more deteriorate renal morphology consisting of exaggerated glomerulosclerosis, tubular dilation, tubulointerstitial proliferation, interstitial fibrosis, and increased interstitial monocyte/macrophage infiltration. These results indicate a key role of TRPV1 in protecting against the advancement of nephropathy in salt-dependent hypertension. Interestingly, mean arterial pressure determined by radiotelemetry was equally elevated by DOCA-salt treatment in TRPV1-/- and WT mice. Although systemic pressure was similar in these two strains subject to DOCA-salt treatment, we could not rule out the possibilities that the local hemodynamics were different or that the exacerbated renal injury in TRPV1-/- mice was induced by the mechanical force from hypertension because that TRPV1-/- mice were more susceptible to the pressure increase.
We found that urinary albumin excretion, an important predictor for end-stage renal disease, was ∼ 4 fold higher in DOCA-salt-treated WT mice than in control WT mice. Regardless, only moderate morphological alterations were observed in renal glomeruli of DOCA-salt-treated WT mice. The results are consistent with previous studies showing that urinary albumin was significantly increased before development of lesions detectable by light microscopy,20,21 a finding plausibly explained by ultrastructural changes preceding the light microscopic changes and leading to albuminuria. Indeed, it has been shown that ultrastructural changes, including thickening of the glomerular basement membrane and degenerative changes of podocytes, preceded light microscopic histological disturbances.17 Growing evidence suggests that podocyte injury is intimately related to proteinuria.22 Although the mechanisms underlying exacerbated urine albumin excretion in DOCA-salt-treated TRPV1-/-compared to WT mice remain to be confirmed, several possible pathways discussed below may contribute to markedly enhanced urine albumin excretion in the former and may predict higher risk of end-stage renal disease in knockout hypertensive mice.
Anandamide, one of the endogenous ligands for the cannabinoid (CB) receptor, has been shown to excite peripheral terminals of capsaicin-sensitive primary sensory neurons via TRPV1-dependent mechanisms.9,10 While no change in anandamide levels was found in the kidney of DOCA-salt-treated WT mice in the present study, renal TRPV1 expression was higher in these mice. Elevated renal TRPV1 expression in DOCA-salt-treated WT mice may constitute a compensatory mechanism contributing to kidney protection against injury. At the present time, it is unknown what leads to marked elevation of anandamide levels in DOCA-salt-treated TRPV1-/- kidneys, which deserves further investigation.
Renal cell proliferation may result from hypertension-induced renal damage, and we therefore examined PCNA expression, an indicator of cell proliferation.23 Significant proliferation of tubular epithelial cells was found in DOCA-salt-treated WT and TRPV1-/- mice, with a higher number of PCNA-positive cells in the latter. The increased cell proliferation correlated well with the degree of renal morphological damage under microscopic examination. Increased PCNA-positive cells may result from increased ET-1 and Ang II levels in the local tissues given that ET-1 and Ang II have been shown to increase renal cell proliferation via enhancing superoxide production.24,25 Alternatively, increased tubular cell proliferation may be secondary to tubular dilation induced by glomerular ischemia.
Although the precise mechanisms responsible for DOCA-salt-induced renal injury remain to be elucidated, several studies showed that enhanced activity of sympathetic nervous system might actively participate in the pathogenesis of renal damage in hypertension.26,27 Activation of TRPV1 expressed in sensory nerves leads to inhibition of sympathetic nervous activity.28,29 Thus, TRPV1 deficiency may aggravate renal injury induced by DOCA-salt treatment due to attenuated inhibition of the sympathetic nervous system. In contrast to the studies by Veelken et al showing that the arterial baroreflex is not altered in DOCA-salt rats,30 we found that DOCA-salt treatment caused similar increases in blood pressure in WT and TRPV1-/- mice without significant baroreflex bradycardia that normally accompanies such increases in blood pressure. These data, while unexpected, are consistent with the previous observations showing that DOCA-salt hypertensive rats are associated with increased sympathetic nerve activity or a predisposition to respond to environmental stress with increased sympathetic nerve activity.31,32 Given the complex interplay of several neuronhormonal systems in the central as well as peripheral sites, further studies are required to clarify the mechanisms underlying blunted baroreflex in DOCA-salt hypertension and the role of the sympathetic nervous system in DOCA-salt-induced exaggeration of renal damage in TRPV1-/- mice that showed an indistinctive baroreflex from WT mice.
In addition to its well established role of vasodilatation, activation of TRPV1 may convey protection to tissues via other mechanisms. Although the caveat exists in the current study in which the life-long loss of the protein produced by the TRPV1 gene deleted may cause compromising changes that may impact the results, data obtained from studies using different experimental approaches show that activation of TRPV1 may increase nitric oxide release that suppresses oxidative stress via inhibition of NAD(P)H oxidase.33,34 In addition, most recent studies have demonstrated that TRPV1 attenuates endotoxin-induced proinflammatory cytokine production, inflammatory cell infiltration and migration.35,36 Indeed, the progression of hypertension-induced renal damage has been liked to oxidative stress and inflammation.6,37,38 It is likely that TRPV1 deficiency may lead to disturbed glomerular hemodynamics, enhanced oxidative stress, and/or proinflammatory responses. In the present study, we found that deletion of TRPV1 exaggerated DOCA-salt-induced renal injury which was associated with enhanced urinary 8-isoprostane excretion and renal macrophage recruitment. These data may be interpreted that an anti-oxidant and anti-inflammation effect of TRPV1 may be involved in renoprotection in DOCA-salt hypertension, or that renal injury observed in TRPV1 deficient mice may contribute to enhanced oxidative stress and inflammatory responses. To distinguish these possibilities, further studies are necessary to explore the time course of renal inflammation and injury in DOCA-salt hypertension in TRPV1 deficient and WT mice.
In summary, the data show that TRPV1 gene deletion leads to enhanced renal damage characterized by the decreased creatinine clearance, albuminuria, glomerulosclerosis, tubulointerstitial injury, and interstitial monocyte/macrophage infiltration during DOCA-salt treatment while systemic blood pressure in these mice is indistinctive from that of WT mice. These findings suggest that TRPV1 may play a protective role in preventing renal injuries possibly via inhibition of inflammatory response during hypertension.
Perspective
Impairment in sensory nerves and TRPV1 function occurs in Dahl salt-sensitive rats, a model closely mimicking human salt sensitive hypertension, suggesting that such impairment may contribute to the pathogenesis of hypertension in this model.14 The data from the present study further support the notion that development and exacerbation of salt-sensitive hypertension associated end organ damage may be attributed, at least in part, to the lack of adequate counterregulatory action of TRPV1. It follows that improvement of function of sensory nerves or TRPV1 may confer a therapeutic potential in the treatment of end-organ damage associated with salt-sensitive hypertension.
Acknowledgments
Sources of Funding This work was supported in part by National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from Michigan Economic Development Corporation.
Footnotes
Disclosures Youping Wang: None Dagmar Babánková: None Jie Huang: None Greg M. Swain: None Donna H. Wang: None
References
- 1.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 2.Atkins RC. The epidemiology of chronic kidney disease. Kidney Int. 2005;94:S14–S18. doi: 10.1111/j.1523-1755.2005.09403.x. [DOI] [PubMed] [Google Scholar]
- 3.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in incidences of treatment for end stage renal disease. N Engl J Med. 1982;306:1276–1279. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 4.Kassab S, Miller MT, Novak J, Reckelhoff J, Clower B, Grnager JP. Endothelin-A receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 1998;31:397–402. doi: 10.1161/01.hyp.31.1.397. [DOI] [PubMed] [Google Scholar]
- 5.Peng H, Carretero OA, Alfie ME, Masura JA, Rhaleb NE. Effects of angiotensin-connverting enzyme inhibitor and angiotensin type 1 receptor antagonist in deoxycorticosteron acetate-salt hypertensive mice lacking Ren-2 gene. Hypertension. 2001;37:974–980. doi: 10.1161/01.hyp.37.3.974. [DOI] [PubMed] [Google Scholar]
- 6.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45:934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 8.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 9.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, DiMarzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anandamide. Hypertension. 2005;46:986–991. doi: 10.1161/01.HYP.0000174596.95607.fd. [DOI] [PubMed] [Google Scholar]
- 11.Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- 12.Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension. 2003;42:968–973. doi: 10.1161/01.HYP.0000097549.70134.D8. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Chen AF, Wang DH. ETA receptor blockade prevents renal dysfunction in salt-sensitive hypertension induced by sensory denervation. Am J Physiol. 2005;289:H2005–H2011. doi: 10.1152/ajpheart.00370.2005. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47:609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- 15.Saito T, Sumithran E, Glasgow EF, Atkins RC. The enhancement of aminonucleoside nephrosis by the co-administration of protamine. Kidney Int. 1987;32:691–699. doi: 10.1038/ki.1987.262. [DOI] [PubMed] [Google Scholar]
- 16.Shih W, Hines WH, Neilson EG. Effects of cyclosporine A on the development ofimmune-mediated interstitial nephritis. Kidney Int. 1988;33:1113–1118. doi: 10.1038/ki.1988.119. [DOI] [PubMed] [Google Scholar]
- 17.Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopahty of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–1093. doi: 10.1161/01.HYP.0000222003.28517.99. [DOI] [PubMed] [Google Scholar]
- 18.Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Stanley GH Sloane. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Springate JE, Feld LG, Ganten D. Renal function in hypertensive rats transgenic for mouse rennin gene. Am J Physiol. 1994;266:F731–F737. doi: 10.1152/ajprenal.1994.266.5.F731. [DOI] [PubMed] [Google Scholar]
- 21.Van Liew JB, Davis FB, Davis PJ, Noble B, Bernardis LL. Calorie restriction decreases microalbuminuria associated with aging in barrier-raised Fisher 344 rats. Am J Physiol. 1992;263:F554–F561. doi: 10.1152/ajprenal.1992.263.3.F554. [DOI] [PubMed] [Google Scholar]
- 22.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 23.Scott RJ, Hall PA, Haldane JS, van Noorden S, Price Y, Lane DP, Wright NA. A comparison of immunohistochemical markers of cell proliferation with experimentally determined growth fraction. J Pathol. 1991;165:173–178. doi: 10.1002/path.1711650213. [DOI] [PubMed] [Google Scholar]
- 24.Orth SR, Odoni G, Amann K, Strzelczyk P, Raschack M, Ritz E. The ET(A) receptor blocker LU 135252 prevents chronic transplant nephropathy in the “Fisher to Lewis” model. J Am Soc Nephrol. 1999;10:387–391. doi: 10.1681/ASN.V102387. [DOI] [PubMed] [Google Scholar]
- 25.Hannken T, Schroeder R, Zahner G, Stahl RA, Wolf G. Reactive oxygen species stimulate p44/42 mitogen-activated protein kinase and induce p27Kip1: role in angiotensin II-mediated hypertrophy of proximal tubular cells. J Am Soc Nephrol. 2000;11:1387–1397. doi: 10.1681/ASN.V1181387. [DOI] [PubMed] [Google Scholar]
- 26.Koomans HA, Blankestijn PJ, Joles JA. Sympathetic hyperactivity in chronic renal failure. J Am Soc Nephrol. 2004;15:524–537. doi: 10.1097/01.asn.0000113320.57127.b9. [DOI] [PubMed] [Google Scholar]
- 27.Wofford MR, Hall JE. Pathophysiology and treatment of obesity hypertension. Curr Pharm Des. 2004;10:3621–3637. doi: 10.2174/1381612043382855. [DOI] [PubMed] [Google Scholar]
- 28.Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, Imai Y, Kayaba Y, Nishimatsu H, Suematsu Y, Hirata Y, Yazaki Y, Nagai R, Kuwaki T, Kurihara H. Elevated sympathetic nervous activity in mice deficient in αCGRP. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- 29.Ralevic V, Karoon P, Burnstock G. Long-term sensory denervation by neonatal capsaicin treatment augments sympathetic neurotransmission in rat mesenteric arteries by increasing levels of norepinephrine and selectively enhancing postjunctional actions. J Pharmacol Exp Ther. 1995;274:64–71. [PubMed] [Google Scholar]
- 30.Veelken R, Hilgers KF, Ditting T, Leonard M, Mann JF, Geiger H, Luft FC. Impaired cardiovascular reflexs precede deoxycorticosterone acetate-salt hypertension. Hypertension. 1994;24:564–570. doi: 10.1161/01.hyp.24.5.564. [DOI] [PubMed] [Google Scholar]
- 31.O’Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension. 2006;48:658–663. doi: 10.1161/01.HYP.0000238140.06251.7a. [DOI] [PubMed] [Google Scholar]
- 32.Koepke JP, Jones S, Dibona GF. Renal nerve activity and renal function during environmental stress in DOCA-NaCl rats. Am J Physiol. 1986;251:R289–R294. doi: 10.1152/ajpregu.1986.251.2.R289. [DOI] [PubMed] [Google Scholar]
- 33.Gray DW, Marshall I. Human α-CGRP stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br J Pharmacol. 1992;107:691–696. doi: 10.1111/j.1476-5381.1992.tb14508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 2007;21:3747–3755. doi: 10.1096/fj.06-7460com. [DOI] [PubMed] [Google Scholar]
- 36.Helyes Z, Elekes K, Németh J, Pozsgai G, Sándor K, Kereskai L, Börzsei R, Pintér E, Szabó A, Szolcsányi J. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol. 2007;292:L1173–L1181. doi: 10.1152/ajplung.00406.2006. [DOI] [PubMed] [Google Scholar]
- 37.Hartner A, Porst M, Gauer S, Pröls F, Veelken R, Hilgers KF. Glomerular osteopontin expression and macrophage infiltration in glomerulosclerosis of DOCA-salt rats. Am J Kidney Dis. 2001;38:153–164. doi: 10.1053/ajkd.2001.25209. [DOI] [PubMed] [Google Scholar]
- 38.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]