Abstract
Serotonin (5-hydroxytryptamine, 5-HT), a precursor for melatonin production, is produced abundantly in the pineal gland of all vertebrate animals. The synthesis of 5-HT in the pineal gland is rate limited by tryptophan hydroxylase 1 (TPH1) whose activity displays a twofold increase at night. Earlier studies from our laboratory demonstrate that pineal 5-HT secretion exhibits dynamic circadian rhythms with elevated levels during the early night, and that the increase is controlled by adrenergic signaling at night. In this study, we report that (a) 5-HT total output from the pineal gland and TPH1 protein levels both display diurnal rhythms with a twofold increase at night; (b) stimulation of cAMP signaling elevates 5-HT output in vivo; (c) 5-HT total output and TPH1 protein content in rat pineal gland are both acutely inhibited by light exposure at night. Consistent with these findings, molecular analysis of TPH1 protein revealed that (a) TPH1 is phosphorylated at the serine 58 in vitro and in the night pineal gland; and (b) phosphorylation of TPH1 at this residue is required for cAMP-enhanced TPH1 protein stability. These data support the model that increased nocturnal 5-HT synthesis in the pineal gland is mediated by the phosphorylation of TPH1 at the serine 58, which elevates the TPH1 protein content and activity at night.
Keywords: 5-hydroxytryptamine (5-HT; serotonin), cAMP, in vivo microdialysis, melatonin, phosphorylation, pineal gland, tryptophan hydroxylase (TPH)
Introduction
The pineal gland of all vertebrates produces 5-hydroxytryptamine (5-HT; serotonin) that serves as a precursor for melatonin formation [1, 2]. The synthesis of 5-HT from tryptophan in the pineal gland requires two enzymes: TPH1 [3], which limits the rate of 5-HT production, and aromatic amino acid decarboxylase, which is constitutively expressed at high levels. Unlike the aromatic amino acid decarboxylase, TPH1 enzyme activity displays a twofold increase in the night pineal gland of rats [4, 5]. Using high-resolution microdialysis [6, 7], 5-HT output was found to surge during the early night, before a precipitous decline associated with a simultaneous surge in N-acetylserotonin (NAS) and melatonin secretion [6, 8, 9]. Constitutively elevated levels of 5-HT output at night were observed when melatonin synthesis was suppressed [8]. These data demonstrate that increased TPH1 activity at night elevates 5-HT levels, which are then consumed by the nocturnal melatonin synthesis. However, the molecular regulation of the pineal TPH1 stimulation is not completely understood.
In the pineal gland of lower vertebrates including chick and frog, TPH1 mRNA levels are high at night and low during the day [10–12]. In a previous PCR-based study by Sugden, TPH1 mRNA levels were reported to be slightly higher at night in the rat pineal gland [13]. Thus, transcriptional activation of TPH1 was proposed to be the mechanism for the elevated nocturnal TPH1 activity. More recent work from our laboratory ([9] and this work); however, suggests that posttranslational regulation of TPH1 may be more important than transcriptional activation in driving the rapid rise of 5-HT output in rats.
A number of studies points to an essential role of beta-adrenergic signaling in the nocturnal stimulation of pineal TPH1 activity [4, 14] and the nocturnal surge of 5-HT synthesis [8]. Past studies also indicate that cAMP, a downstream effecter of beta-adrenergic signaling in the pineal gland, is important in regulating TPH1 activity. TPH1 is a target of cAMP-dependent protein kinase (PKA) in vitro [15–17], and PKA phosphorylates TPH1 in vitro at serine 58 (S58) [16, 17]. Phosphorylation of purified TPH1 protein by PKA increases the catalytic activity of TPH1 in vitro, and this in vitro effect of PKA depends on the intact S58 residue [16]. These studies suggest that the S58 residue is essential in mediating the stimulating effect of PKA on TPH1 catalytic activity. It remains unknown, however, whether PKA elevates TPH1 activity by increasing its protein stability and whether TPH1 is phosphorylated in vivo at the S58 site.
In this paper, we demonstrate that nocturnal 5-HT surge in Sprague–Dawley rats occurs immediately following lights off at night and persists during the entire night period. This increase in 5-HT total output is accompanied by an increase in TPH1 protein content, while TPH1 mRNA levels remain unchanged. Stimulation of cAMP signaling leads to elevated 5-HT total output in vivo and to increased TPH1 protein levels in vitro. In addition, we demonstrate that TPH1 protein is phosphorylated at the S58 residue in the night pineal gland, and that the intact S58 residue is essential for PKA induced stabilization of TPH1 protein in vitro. Consistent with our finding that termination of adrenergic signaling by light at night abolishes the nocturnal surge of 5-HT total output, TPH1 protein is reduced to the daytime levels upon light exposure of rats. Taken together, these data strongly indicate that posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland.
Materials and methods
Animals
Adult Sprague–Dawley male rats (220–250 g) from Harlan (Indianapolis, IN) were housed in a temperature-controlled room with food and water ad libitum throughout the experimental period. All rats were conditioned in the light and dark cycle of either 14:10 or 12:12 hr with defined time of lights on (see text for detail) for at least 2 wk before experiments. Illumination was supplied by white fluorescent lamps (600 lux at cage level).
Surgery, microdialysis, and HPLC analysis
We performed surgery for microdialysis probe implantation, pineal microdialysis, and automated HPLC analysis as described [6, 7]. Briefly, a CMA microdialysis guide (CMA/MD, N. Chelmsford, MA, USA) or a homemade probe (TC-4) was directly inserted into the pineal gland. Following the wound closure with dental cement, rats were allowed to recover for 24 hr. The pineal glands of the operated rats in the microdialysis chamber were then infused with artificial cerebral spinal fluid (aCSF, 2 μl/min) continuously for the remainder of the experiment. The pineal dialysate was collected via the PEEK tube into a 20-μl loop of an injector (BAS, West Lafayette, IN, USA), which was automatically injected into a HPLC column (Supelco, Bellefonte, PA, USA) at 10 min intervals using an HPLC pump (Shimadzu, Columbia, MD, USA). The injected pineal samples were separated on a reversed phase C18 column using a mobile phase consisting of 22% (v/v) acetonitrile. The detection of 5-HT, N-acetylserotonin (NAS), and melatonin was performed by a fluorescent detector (Shimadzu) on-line with the HPLC column. The automated control of the injector, the HPLC operation, and analysis of collected data were carried out with an external computer using the Shimadzu Class-VP 5.03 software. Drugs that stimulate cAMP signaling (ISO, FSK, and 8BrcAMP) were dissolved in aCSF solution before delivering into the pineal gland via microdialysis tubing.
Northern blot analysis of pineal samples
RNA samples for Northern analysis were prepared using the RNeasy kit (Qiagen, Hilden, Germany). Five pineal glands were harvested and analyzed for each time point. Formaldehyde gels were loaded with 10% of the total RNA (equivalent to one-half of a pineal gland) and RNA was then blotted to a nitrocellulose membrane. The membrane was hybridized sequentially with labeled full-length rat TPH1, AANAT, and GAPDH cDNA probes.
Western blot analysis of pineal samples
For Western analysis, total protein extracts equivalent to one-fifth of a single rat pineal gland were loaded in each lane, blotted onto a nitrocellulose membrane, and probed with a TPH1 antibody (Cat# OP71L) (Calbiochem, San Diego, CA, USA). To detect TPH1 proteins phosphorylated at the S58 residue, we developed a rabbit polyclonal anti-rTPH1-pS58 antibody by immunized rabbits with a synthetic phosphopeptide, which results in antisera highly specific for the S58-phosphorylated TPH1. The antibody is now available from several commercial venders including Sigma (St. Louis, MO, USA) and AbCam (Cambridge, MA, USA). Two of the protein blots were hybridized with this antibody (1:1000 dilution). To control for sample loading, identical sets of blots were also probed with anti-14-3-3b antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Stable cell line construction
Two hundred and ninety three cells were grown in DMEM containing 10% fetal bovine serum and 1% penicillin/streptomycin in a humidified 5% CO2 incubator at 37°C. Cells at 80–90% confluency were transfected with TPH1 plasmids (rat TPH1 and TPH1-S58A coding fragments cloned in pCS2 vector; 0.9 μg per well of a 24-well plate) mixed individually with a puromycin resistance plasmid (0.1 μg) using Lipofectamine 2000 (Life Technologies). The transfected cells were split 24 hr later at a 1:10 dilution into two 10-cm plates. Puromycin (2 μg/ml) was added to the media the following day and continued for 14 additional days until colonies were detected. The clones were transferred individually into 24-well plates and screened for TPH1 protein expression by Western blot. Clones with the highest levels of TPH1 protein expression were selected for experiments shown in this paper.
Western blot analysis of transfected 293 cells
Total protein from TPH1 transfected (transient or stable) cells was extracted with RIPA buffer (50 mm Tris, 150 mm NaCl, 1 mm EDTA, 40% NP40, 1 mm Na3VO4, 20 mm NaF and protease inhibitors). Samples were then separated by electrophoresis and transferred to a membrane. The membrane was blocked with 5% non-fat dry milk in TBS-T solution (20 mm Tris–HCl, pH 7.5, 140 mm NaCl, and 0.05% Tween-20). Blots were hybridized with anti-TPH1 (Cat# OP70L from Calbiochem), anti-TPH1-pS58 (specific for S58-phosphorylated TPH1; see above for details), and anti-14-3-3b (Cat# K-19 from Santa Cruz) antibodies. The secondary antibodies used include: IRDye 700-, and IRDye 800-conjugated anti-rabbit or anti-mouse IgG antibodies (LI-COR Biosciences, Lincoln, NE). The infrared signals were detected using the Odyssey Image System, and analyzed using Odyssey software (LI-COR Biosciences, Lincoln, NE, USA).
Results
To clarify the potential mechanisms of 5-HT regulation, we analyzed the expression of TPH1 RNA isolated from the rat pineal gland. As shown in Fig. 1A, TPH1 RNA does not show detectable variation across the circadian cycle (top panel), in clear contrast to the rhythmic expression of AANAT message (middle panel). We have also analyzed TPH1 mRNA expression using quantitative RT-PCR technique and found no diurnal variation of TPH1 mRNA expression in the rat pineal gland (data not shown). When rats were exposed to continuous light for 24 hr, the diurnal expression of AANAT message was completely abolished (Fig. 1B, lane 3 in the middle panel). In contrast, no change was detected with expression levels of TPH1 following the 24 hr light treatment (Fig. 1B, lane 3 in the top panel). This experiment demonstrates the absence of diurnal regulation of TPH1 mRNA expression. Importantly, a short pulse (10 min) of light at night had no effect on mRNA expression levels for either TPH1 (top panel) or AANAT (middle panel) (Fig. 1B, lane 2).
Fig. 1.
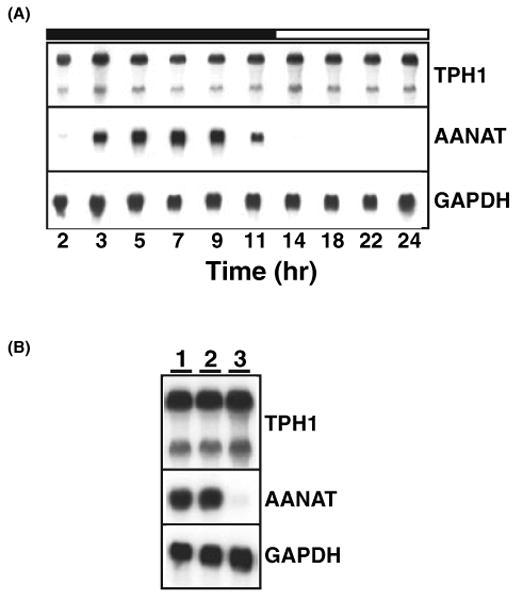
TPH1 mRNA expression is not under circadian control in rat pineal gland. (A) Circadian expression is absent for TPH1 mRNA in the rat pineal. Sprague–Dawley rats were entrained in LD 14:10 hr for more than 2 wk with lights off at 1:00 hr. Five pineal glands were isolated at each time points and analyzed for their RNA content of TPH1 (top panel), AANAT (middle panel), and GAPDH (bottom panel) sequentially. Densitometry analysis of the relative signals of TPH1 and GAPDH showed no diurnal variation of TPH1 RNA expression (data not shown). (B) Nighttime TPH1 RNA expression is insensitive to light exposure. Three cohorts of rats (five rats in each cohort) were exposed to no light (lane 1), 10 min light (8:00–8:10; lane 2), 24 hr light (from 9:00 hr the night before until 9:00 hr of the night of experiment; lane 3) and sacrificed at 9:00 hr (2 hr before light onset) under the dim red light. Three identical blots loaded with pineal total RNA were hybridized with TPH1 (top panel), AANAT (middle panel), and GAPDH (bottom panel) probes. While the 24 hr constant light completely abolished AANAT mRNA expression, it had no influence on TPH1 mRNA expression.
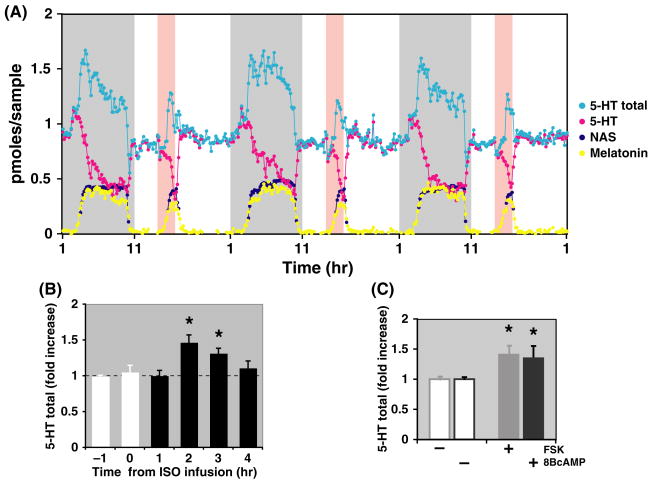
Microdialysis studies of individual rats revealed that the pineal 5-HT is constitutively high during the daytime, increases at early night, and finally decreases precipitously during the later half of night when NAS and melatonin levels are high (Fig. 2A). Because 5-HT is the precursor for synthesis of both NAS and melatonin, we calculated the total 5-HT synthesized during the night period to be (at a minimum) the sum of secreted 5-HT, NAS, and melatonin. As shown in Fig. 2A, the total 5-HT output thus calculated displays a dramatic circadian rhythm with elevated levels at night. The daily rhythms of the nightly surge of the total 5-HT output are seen even in the constant darkness (Fig. 2B), suggesting that the circadian increase of 5-HT output is driven by the circadian pacemaker in the brain. Three features are worth noting: (a) the 5-HT surge occurs soon following lights off during every night; (b) rhythmic profiles of total 5-HT output are remarkably conserved night after night in the same animals (Fig. 2A,C); and (c) these findings were consistent between all individual rats studied (Fig. 2D).
Fig. 2.
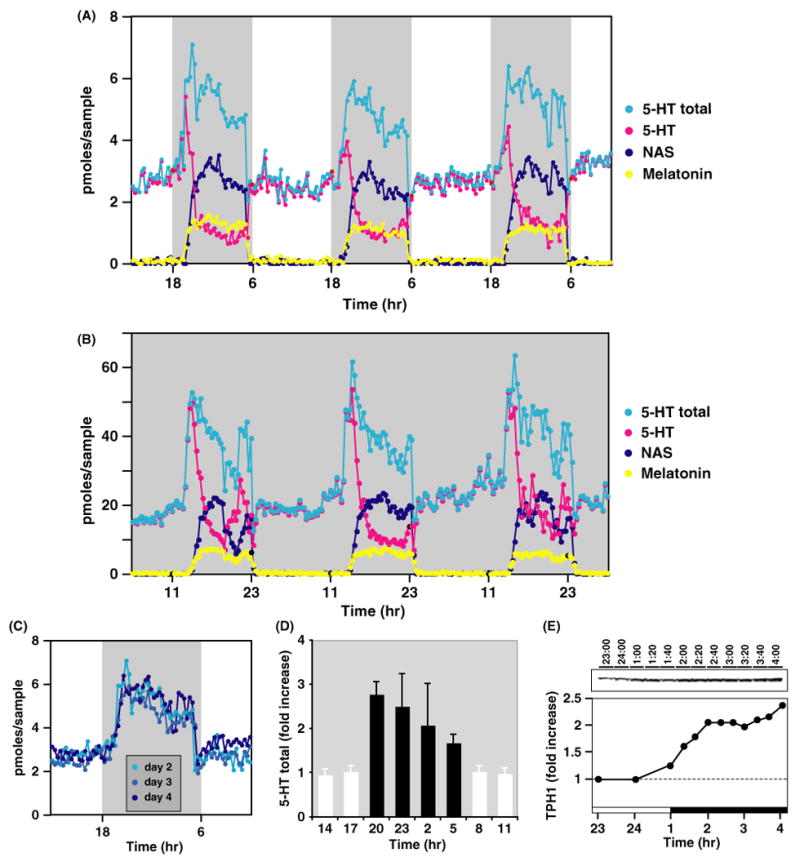
5-HT total output as well as TPH1 protein levels display dramatic circadian rhythms. (A) In vivo diurnal profiles of 5-HT (pink dots), NAS (dark blue dots), melatonin (yellow dots), and the 5-HT total output* (light blue dots) in the pineal gland of a Sprague–Dawley rat. The shaded gray area represents the dark period (18:00 to 6:00 hr). *The 5-HT total output was calculated as the sum of 5-HT, NAS, and melatonin at the circadian times indicated. Each data point represents the pineal secretory production collected over 10 min. (B) In vivo circadian profiles of 5-HT (pink dots), NAS (dark blue dots), melatonin (yellow dots), and the 5-HT total output (light blue dots) in the pineal gland of a Sprague–Dawley rat. The rat was placed in constant darkness for 1 wk prior to the sampling. (C) The comparison of the total 5-HT output from the 3-day period for the rat shown in (A). In contrast to the daytime levels of 5-HT output, the nighttime levels of the 5-HT total output display a consistent increase of more than twofolds. (D) Circadian profiles of 5-HT total output from multiple rats. The 5-HT total output was calculated from five rats and expressed as a fold of increase from their daytime average levels in each rat. (E) Pineal circadian profiles of TPH1 protein expression. The rats in this experiment experienced lights off at 1:00 hr, and lights on at 13:00 hr. The top panel shows the Western blot probed with the anti-TPH1 antibody. Each lane was loaded with total protein extracts from four rats in the amount equivalent of one-tenth of a single pineal gland. The lower panel shows quantified values of the immunoreactive bands on the top panel. The protein levels were normalized to both 14-3-3 proteins for loading control (data not shown) and to the daytime average, and graphed as the fold of the day value. The dark bar at the bottom of the graph indicates the dark period (1:00–13:00 hr). Nearly identical results were obtained in two other experiments (also see Fig. 4B).
To clarify the potential mechanisms of 5-HT regulation, we performed analyses of TPH1 protein expression in rat pineal glands harvested at different times of day. As shown in Fig. 2E, pineal TPH1 protein expression displays marked diurnal rhythms with nighttime levels twice as high as the daytime average. In parallel with the rapid rise of 5-HT levels, TPH1 protein levels also increased soon after lights off (Fig. 2E). This contrasts markedly with the temporal expression pattern of TPH1 RNA, which did not show detectable variation across the circadian cycle (Fig. 1A).
Our previous work revealed that the increased nocturnal 5-HT synthesis is controlled by beta-adrenergic signaling [8], which is known to elevate intracellular cAMP levels. To further define the mechanisms of 5-HT synthesis in the living pineal gland, we infused drugs that elevate intracellular cAMP levels directly into the pineal gland during the daytime. Fig. 3A shows the secretory profiles of 5-HT, NAS, and melatonin before and after infusion of isoproterenol (ISO, 10 uM for 2.5 hr), an agonist for beta-adrenergic receptor, in a single rat over three diurnal cycles. The total 5-HT, calculated as the sum of 5-HT, NAS, and melatonin, displays a reproducible rise following each ISO infusion during daytime (the pink shaded bars in Fig. 3A). Importantly, ISO consistently elevated 5-HT output in the same animal across multiple days; this was highly reproducible between experimental animals in vivo (Fig. 3B). In addition to ISO, both forskolin (FSK), which activates adenylate cyclase, and the cAMP analogue 8-Bromo-cAMP (8BcAMP) increased pineal 5-HT total output by 50% (Fig. 3C).
Fig. 3.
Cyclic AMP signaling in the pineal gland stimulates the total output of 5-HT in vivo. (A) Effect of isoproterenol (ISO) in 5-HT total output in the same rat over a 3-day period. In vivo circadian profiles of 5-HT (pink dots), NAS (dark blue dots), melatonin (yellow dots), and the 5-HT total output (light blue circles) in the pineal gland of a Sprague–Dawley rat before and after ISO (10 μm) infusion into the pineal gland over a 3-day period. The shaded gray area represents the dark period (1–11 hr) and the shaded orange area marks the period of ISO infusion during daytime (14:30–17:00 hr). Consistent increase in 5-HT total output following ISO infusion was seen in each of the 3-day period. Each data point represents the pineal secretory production collected over 10 min. (B) Circadian profiles of 5-HT total output stimulated by ISO from multiple rats. The 5-HT total output 1 hr before and 3 hr after the ISO infusion was calculated from four rats and expressed as a fold of increase from their daytime average levels in each rat. (C) The effect of forskolin (FSK, 0.2 mg/mL) and 8-Bromo-cAMP (8BcAMP, 10 mm) in 5-HT total output. The 5-HT total output 1 hr before (−) and 2 hr after (+) the infusion of indicated drugs was calculated from four rats and expressed as a fold of increase from their daytime average levels in each rat.
To further understand the molecular mechanisms of 5-HT synthesis and TPH1 regulation, we generated a polyclonal antibody specific for the S58-phosphorylated TPH1. Cells transiently transfected with TPH1 wildtype or S58A mutant cDNA were incubated with FSK to activate PKA or phorbol-12-myristate-13-acetate (PMA) to stimulate protein kinase C (PKC). As shown in Fig. 4A, TPH1 protein is strongly phosphorylated at the S58 site in cells incubated with either FSK or PMA. No anti-TPH1-pS58 immunoreactivity was detected in cells transfected with the S58A mutant construct with or without drug exposure (top panel in Fig. 4A). The same antibody was subsequently used to probe the phosphorylation status of TPH1 protein in the night pineal gland. As shown in Fig. 4B, both TPH1 protein and TPH1-S58 phosphorylation are markedly increased in the night pineal gland.
Fig. 4.
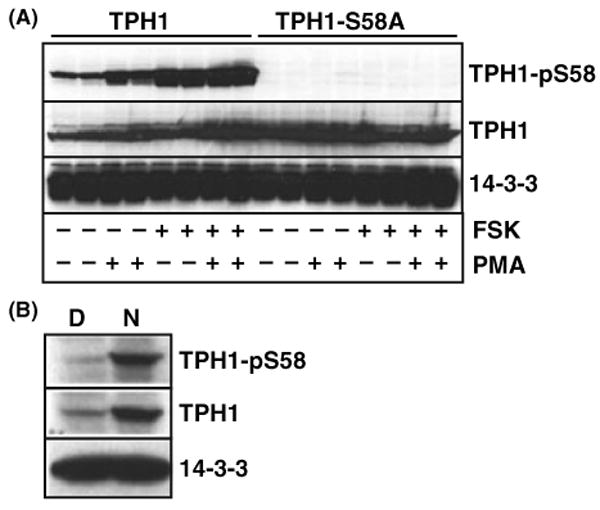
TPH1 is phosphorylated at the S58 in the night pineal gland. (A) The anti-phospho-TPH1 antibody specifically recognizes TPH1 when the serine 58 (S58) is phosphorylated. Two hundreds and ninety three cells were transfected with plasmids encoding rat TPH1 wildtype and TPH1-S58A mutant in duplicates. Cells were stimulated for 4 hr with either 10 FSK, 1 μm PMA, or both FSK and PMA 24 hr after the transfection. Identical blots were hybridized with anti-TPH1-pS58 (top panel), the anti-TPH1 (middle panel), and the anti-14-3-3 (bottom panel) antibodies. Cells expressing the S58A mutant showed no immunoreactivity following the FSK, PMA, both FSK and PMA stimulations. (B) TPH1-S58 phosphorylation occurs abundantly in the night pineal gland. Rats used in this study were housed in LD 14:10 hr with lights on at 7 am. Day (D, 2 pm) and night (N, 2 am) protein extracts from five pineal glands at each time point were prepared. The protein extracts equivalent to one-fifth of a single pineal gland was loaded in each lane. Three identical protein blots were hybridized with anti-TPH1-pS58 (top blot), anti-TPH1 (middle blot), and anti-14-3-3 (bottom blot) antibodies. Nearly identical results were obtained in at least four other experiments (data not shown).
To clarify the role of S58 in TPH1 protein stability, we generated stable cell lines that express the TPH1 wildtype and S58A mutant proteins constitutively. TPH1 protein levels increased more than twofold in cell lines stimulated with FSK, compared with the control cells (Fig. 5). The FSK-induced TPH1 protein increase was completely abolished in cells expressing the S58A mutant, indicating that the phosphorylation at serine 58 is an essential step for the PKA-dependent stabilization of TPH1 protein.
Fig. 5.
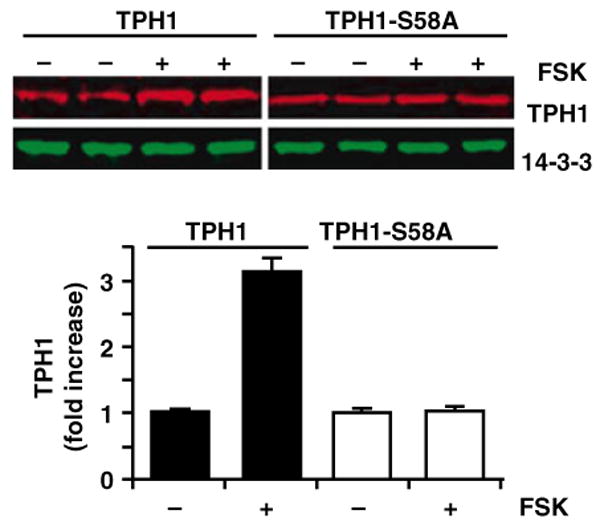
TPH1-S58 phosphorylation by PKA increases TPH1 protein content. Upper panels: 293 cells stably expressing TPH1 wildtype and the S58A mutant were stimulated with FSK (10 μm) for 4 hr. The protein extracts were blotted and probed with anti-TPH1 (top blot) and anti-14-3-3 (bottom blot) antibodies. Bottom graph: Quantitative representation of multiple experiments (n = 4) similar to those shown in the upper panels. TPH1 and TP1-S58A protein levels following the FSK treatment (+) were expressed as fold increases over those without FSK treatment (−). These studies were repeated three times and identical results were obtained (data not shown).
Nocturnal melatonin production is sensitive to light in all vertebrates [18], which is due to an abrupt and transient termination of norepinephrine release from the superior cervical ganglion [19]. We tested the effects of a 10 min pulse of light at night (22:00–22:10 hr) on total 5-HT output in freely moving rats. Immediately following the light pulse on day 2, there was an abrupt drop of NAS and melatonin output and a concomitant increase of 5-HT (Fig. 6A). This was followed by a marked reduction in total 5-HT output compared to the nights before and after (Fig. 6A). Light-induced reduction of 5-HT total output was found in all animals tested (Fig. 6B).
Fig. 6.
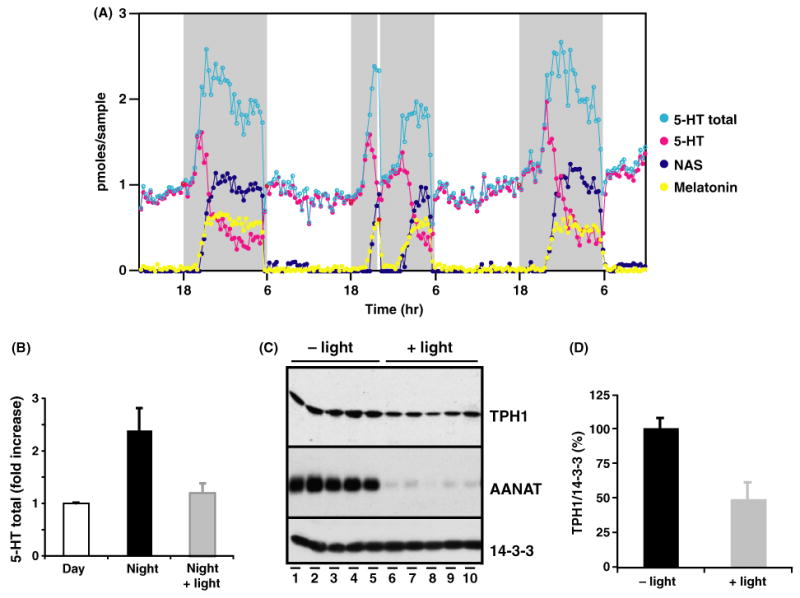
Light pulses at night suppress the nocturnal increase of both 5-HT total output and TPH1 protein levels in the living pineal gland. (A) In vivo circadian profiles of 5-HT (pink dots), NAS (dark blue dots), melatonin (yellow dots), and the 5-HT total output (light blue circles) in the pineal gland of a Sprague–Dawley rat before, during, and after a single pulse (22:00–22:10 hr) of light at night. The shaded gray area represents the dark period (18:00–18:00 hr). The 10 min light pulse, marked by a white bar, was given on day 2 of the microdialysis. (B) The effect of a single light pulse at night (4 hr following dark onset) on 5-HT total output in multiple animals in vivo. 5-HT total output was analyzed from four rats before and after a light pulse at night (22:00 hr). Light pulses suppressed the 5-HT total output to the daytime levels. (C) The effect of a single light pulse (10 min) at night (4 hr following dark onset) on TPH1 protein levels in multiple animals. Five Sprague–Dawley rats housed in LD 12:12 hr were exposed to a single light pulse for 10 min (22:00–22:10 hr, lights off at 18:00 hr), while five control rats were kept in the darkness without light exposure. Pineal protein extracts from rats sacrificed 30 min following the light pulse (lanes 1–5) or from control rats (lanes 6–10) at 22:40 hr were loaded in each lane (one-fifth of a single pineal for each lane) and analyzed for expression levels of TPH1 (top panel), AANAT (middle panel), and 14-3-3 (bottom panel). TPH1 protein content was consistently reduced following the light pulse in all rats tested (top panel). (D) Light pulse at night abolished the nighttime increase of TPH1 protein levels. TPH1 protein levels were quantified from data shown above with or without the light pulse and normalized to the nocturnal peak levels. The light pulse reduced the nighttime TPH1 protein levels by a 50%.
Our microdialysis data indicate that suppression of 5-HT total output, like the depletion of NAS and melatonin output, is immediate and complete following light exposure, suggesting that a posttranscriptional regulation is responsible for the suppressive effect of light on 5-HT output (Fig. 6A). Others have shown that light induced suppression of melatonin production is due to a dramatic decrease of AANAT activity, which results from the reduction of AANAT protein levels [20]. Thus, light induced AANAT degradation seems to be responsible for the rapid decline of NAS and melatonin levels in vivo. We reasoned that a posttranslational regulation of TPH1, similar to that of AANAT, might account for the light-induced reduction of 5-HT total output. A cohort of rats was exposed to a 10 min pulse of light 4 hr after darkness onset. Pineal extracts from rats exposed to light and from rats kept in darkness were analyzed for TPH1 protein content. As shown in Fig. 6C, all rats exposed to light (lanes 6–10) displayed reduced amounts of TPH1 protein compared to those housed in complete darkness (lanes 1–5). The TPH1 reduction, while not as dramatic as AANAT protein suppression (middle panel, lanes 6–10), is about 50% of the nocturnal peak levels (Fig. 6D).
Discussion
TPH is the rate-limiting enzyme in the biosynthesis of 5-HT, a key neurotransmitter in the brain and a precursor for melatonin synthesis in the pineal gland. Regulation of TPH activity has been an important area of investigation in many laboratories due to its central role in 5-HT formation. A number of novel features of the regulation of TPH1, the principle peripheral isoform of TPH, are described in the present experiments: (1) the night surge of total 5-HT output is a consistent finding across the diurnal cycles in the same animal and for all animals tested; (2) the nightly surge of 5-HT output is controlled by posttranslational regulation of TPH1, as opposed to transcriptional mechanisms; (3) cAMP signaling is responsible for elevation of total 5-HT output in vivo; (4) TPH1 phosphorylation in vivo in the night pineal gland stabilizes TPH1 protein; and (5) light at night blocks the surge of total 5-HT output by reducing TPH1 protein levels and has no effect on TPH1 mRNA levels.
Abundant evidence supports the idea that the nocturnal increase of 5-HT total output is posttranslationally regulated in the night pineal gland: Firstly, TPH1 mRNA does not show detectable circadian variations in the rat pineal gland using Northern blot analysis. Additional evidence includes the inability of a 24 hr-light exposure to affect the expression of TPH1 mRNA levels in rat pineal glands. If TPH1 mRNA levels display circadian rhythms and are upregulated by adrenergic signaling similar to AANAT [20], one would expect reduction in nighttime TPH1 RNA levels following constant light treatment. Secondly, TPH1 protein increases by twofold at night in the pineal gland. Importantly, the increase was seen immediately following lights off in Sprague–Dawley rats. Thirdly, we have demonstrated a precipitous decline of 5-HT total output and simultaneous suppression of TPH1 protein following a single pulse of light at night. Taken together, these data strongly suggest that the nocturnal stimulation of 5-HT synthesis is primarily controlled by the posttranslational regulation of TPH1 in the pineal. This data contradicts an earlier report [13] that shows that TPH1 mRNA exhibits a modest increase at night.
Previously, we demonstrated that norepinephrine secreted from the superior cervical ganglion at night elevates 5-HT synthesis in the pineal gland [8]. Beta-adrenergic receptor-mediated signaling (which elevates cAMP) has previously been shown to mediate the increase in TPH1 activity in the night pineal [4, 5]. We now provide evidence that cAMP-elevating agents are sufficient by themselves to increase in vivo levels of total 5-HT output in the daytime pineal gland. Three unrelated cAMP stimulating agents (ISO, FSK, 8BcAMP) independently led to increased output of total 5-HT in this study, demonstrating that cAMP signaling increases 5-HT synthesis in vivo. Combined with in vitro studies (Fig. 5), the robust increase in 5-HT stimulated by cAMP supports our overall model that PKA modification of TPH1 enhances 5-HT levels in vivo.
Purified TPH1 protein is phosphorylated by PKA at the S58 residue [16, 17] in vitro, suggesting that phosphorylation may play a regulatory role on TPH1 levels. Here, we present novel data showing a direct relationship between TPH1 phosphorylation, activation, and specific physiological events. Our data demonstrate that phosphorylation of TPH1 in vitro elevates TPH1 protein content, and this effect is mediated specifically by phosphorylation of the S58 residue. Furthermore, TPH1-S58 phosphorylation is markedly elevated in the night pineal gland, to a similar extent to its protein level augmentation. These data indicate that the S58 phosphorylation of TPH1 is required to stabilize the TPH1 protein, which may be a key step for the cAMP-stimulated increase of 5-HT synthesis in vivo.
PKA-mediated phosphorylation of both TPH1 and TPH2 has been well documented in vitro using purified enzyme preparations or transfected cells [16, 17, 21]. Prior to this study, it was not known whether phosphorylation of either TPH protein occurs in intact tissues under physiological conditions. Using the anti-TPH1-pS58 antibody developed in our laboratory, we were able to show for the first time that TPH1 is phosphorylated in vivo at the S58 residue, which is associated with increased TPH1 protein levels. Wide availability of the novel phosphorylation specific antibody for TPH proteins, such as the one described here for TPH1, will greatly enhance our understanding of in vivo regulation of TPH activity and stability in future studies.
It is well known that light at night suppresses melatonin production. The effect of light on pineal 5-HT production, however, has never been examined. Our high-resolution investigation of in vivo pineal 5-HT output before and after a short light pulse at night revealed that 5-HT total output is reduced to daytime levels immediately following a short light exposure. Importantly, this occurred despite the abrupt termination of metabolic conversion of 5-HT to NAS and melatonin by light. Moreover, we have demonstrated that light intrusions at night, either continuous illumination for 24 hr or a short 10 min light pulse, had no effects on TPH1 mRNA levels. These data strongly indicate that 5-HT production itself is posttranslationally regulated and is exquisitely sensitive to light at night. The rapidity of 5-HT decline parallels the precipitous blockade of NAS and melatonin output, suggesting that similar regulatory mechanisms govern both melatonin synthesis and 5-HT formation in the night pineal gland at posttranslational levels. Consistent with this notion, TPH1 protein content in the pineal gland was rapidly reduced to daytime levels by a short pulse of light at night, similar to AANAT levels (Fig. 6), while TPH1 and AANAT RNA levels were unchanged by the 10 min light pulse (Fig. 1B). Because norepinephrine release is rapidly blocked by light at night [19], which ultimately blocks the activation of cAMP, TPH1 phosphorylation at S58 site is predicted to decrease precipitously following the light pulse. Future studies will focus on the kinetics of TPH1 phosphorylation and dephosphorylation in vivo and in vitro.
The regulatory mechanisms discussed above for TPH1 are in many ways reminiscent of AANAT regulation [22]: (1) Pineal AANAT is extremely unstable in vivo ([22, 23], and Fig. 6C of this paper); peripheral TPH1 is similarly very unstable [3, 24, 25]. In addition, acute light pulses at night abruptly suppress protein expression of both AANAT and TPH1 in the pineal gland (see Fig. 6C); (2) AANAT activity and protein can be both stimulated by increased cAMP signaling [20]. Similarly, TPH1 activity increases in response to elevated PKA activity [16, 26]. In this work, we have demonstrated that activated cAMP signaling leads to increased 5-HT production in vivo and increases TPH1 protein content in vitro; (3) PKA modifies AANAT via phosphorylation of T29 in rat [27] and T31 in sheep [28], both of which are located in the amino-terminal regulatory domain of AANAT [22]. Similarly, for TPH1, the ability of PKA to elevate TPH1 protein levels is controlled by protein phosphorylation, which we have mapped to residue S58 in this paper.
TPH1 regulation also shares many features with that of tryptophan hydroxylase 2 (TPH2), the brain specific isoenzyme identified recently [29]. Both enzymes are composed of an amino-terminal regulatory domain and a carboxyl-terminal catalytic domain [17, 24, 30–33]. The amino-terminal regulatory domains in TPH1 and TPH2 contain key residues (S58 in TPH1 and S19 in TPH2) that can be phosphorylated by PKA [17, 21]. Phosphorylation of S19 in TPH2 leads to interaction with 14-3-3 proteins and increased activity [21, 34]. We predict that phosphorylation of S58 in TPH1 will also result in 14-3-3 interaction. Furthermore, removal of the N-terminal domain in TPH2 leads to increased stability of the enzyme [33, 35]. While not examined in this report, we predict that removal of the amino-terminal regulatory region of TPH1 will likely increase the stability of TPH1 protein.
The results described above suggest the following model of TPH1 regulation in the pineal gland. In response to the release of norepinephrine from the nerve terminals of the superior cervical ganglion following the onset of darkness, the pineal gland rapidly increases production of cAMP, leading to the activation of PKA which phosphorylates TPH1 at the S58 site. The PKA-induced phosphorylation increases the protein content and catalytic activity of TPH1, which then leads to the elevated production of 5-HT in the night pineal gland. Until now, much of our understanding of regulation of 5-HT synthesis has come from in vitro studies of TPH1 and TPH2. Ideally, however, in vitro properties of enzymes should be validated by in vivo studies. The studies reported here and in our earlier publications [6, 8] represent the first attempts to understand regulation of 5-HT synthesis in real time in freely moving animals. This study is possible because of the long term in vivo microdialysis technique uniquely developed in our laboratory, which allows high resolution sampling and analysis of 5-HT and its metabolites in the living pineal in real time for long periods of time [7]. Using this system, which is amenable to the combination of molecular, cellular, and physiological techniques, much more can still be learned from the pineal gland regarding the regulation of 5-HT synthesis and secretion in freely moving animals.
Acknowledgments
We thank the Carnegie Institution of Washington for generous support of this project during its early phase, Mr Dan Allen for help with animal care, and Ms Yaxi Chen for laboratory assistance.
References
- 1.Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–C158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 2.Borjigin J, Li X, Snyder SH. The pineal gland and melatonin: molecular and pharmacologic regulation. Annu Rev Pharmacol Toxicol. 1999;39:53–65. doi: 10.1146/annurev.pharmtox.39.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Mockus SM, Vrana KE. Advances in the molecular characterization of tryptophan hydroxylase. J Mol Neurosci. 1998;10:163–179. doi: 10.1007/BF02761772. [DOI] [PubMed] [Google Scholar]
- 4.Shibuya H, Toru M, Watanabe S. A circadian rhythm of tryptophan hydroxylase in rat pineals. Brain Res. 1977;138:364–368. doi: 10.1016/0006-8993(77)90754-5. [DOI] [PubMed] [Google Scholar]
- 5.Sitaram BR, Lees GJ. Diurnal rhythm and turnover of tryptophan hydroxylase in the pineal gland of the rat. J Neurochem. 1978;31:1021–1026. doi: 10.1111/j.1471-4159.1978.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 6.Sun X, Liu T, Deng J, et al. Long-term in vivo pineal microdialysis. J Pineal Res. 2003;35:118–124. doi: 10.1034/j.1600-079x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 7.Borjigin J, Liu T. Application of long-term microdialysis in circadian rhythm research. Pharmacol Biochem Behav. 2008;90:148–155. doi: 10.1016/j.pbb.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X, Deng J, Liu T, et al. Circadian 5-HT production regulated by adrenergic signaling. Proc Natl Acad Sci U S A. 2002;99:4686–4691. doi: 10.1073/pnas.062585499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Borjigin J. Relationship between nocturnal serotonin surge and melatonin onset in rodent pineal gland. J Circadian Rhythms. 2006;4:12. doi: 10.1186/1740-3391-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green CB, Besharse JC, Zatz M. Tryptophan hydroxylase mRNA levels are regulated by the circadian clock, temperature, and cAMP in chick pineal cells. Brain Res. 1996;738:1–7. doi: 10.1016/0006-8993(96)00743-3. [DOI] [PubMed] [Google Scholar]
- 11.Florez JC, Seidenman KJ, Barrett RK, et al. Molecular cloning of chick pineal tryptophan hydroxylase and circadian oscillation of its mRNA levels. Brain Res Mol Brain Res. 1996;42:25–30. doi: 10.1016/s0169-328x(96)00104-0. [DOI] [PubMed] [Google Scholar]
- 12.Green CB, Cahill GM, Besharse JC. Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res. 1995;677:283–290. doi: 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- 13.Sugden D. Comparison of circadian expression of tryptophan hydroxylase isoform mRNAs in the rat pineal gland using realtime PCR. J Neurochem. 2003;86:1308–1311. doi: 10.1046/j.1471-4159.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- 14.Toru M, Watanabe S, Nishikawa T, et al. Physiological and pharmacological properties of circadian rhythm of tryptophan hydroxylase in rat pineals. Adv Biosci. 1978;21:253–255. [PubMed] [Google Scholar]
- 15.Vrana KE, Rucker PJ, Kumer SC. Recombinant rabbit tryptophan hydroxylase is a substrate for cAMP-dependent protein kinase. Life Sci. 1994;55:1045–1052. doi: 10.1016/0024-3205(94)00639-3. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn DM, Arthur R, States JC. Phosphorylation and activation of brain tryptophan hydroxylase: identification of serine-58 as a substrate site for protein kinase A. J Neurochem. 1997;68:2220–2223. doi: 10.1046/j.1471-4159.1997.68052220.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumer SC, Mockus SM, Rucker PJ, Vrana KE. Amino-terminal analysis of tryptophan hydroxylase: protein kinase phosphorylation occurs at serine-58. J Neurochem. 1997;69:1738–1745. doi: 10.1046/j.1471-4159.1997.69041738.x. [DOI] [PubMed] [Google Scholar]
- 18.Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- 19.Drijfhout WJ, Van Der Linde AG, De Vries JB. Microdialysis reveals dynamics of coupling between noradrenaline release and melatonin secretion in conscious rats. Neurosci Lett. 1996;202:185–188. doi: 10.1016/0304-3940(95)12245-1. [DOI] [PubMed] [Google Scholar]
- 20.Roseboom PH, Coon SL, Baler R, et al. Melatonin synthesis: analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology. 1996;137:3033–3045. doi: 10.1210/endo.137.7.8770929. [DOI] [PubMed] [Google Scholar]
- 21.Winge I, Mckinney JA, Ying M, et al. Activation and stabilization of human tryptophan hydroxylase 2 by phosphorylation and 14-3-3 binding. Biochem J. 2008;410:195–204. doi: 10.1042/BJ20071033. [DOI] [PubMed] [Google Scholar]
- 22.Klein DC. Arylalkylamine N-acetyltransferase: “the Timezyme”. J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Deng J, Borjigin J. A novel H28Y mutation in LEC rats leads to decreased NAT protein stability in vivo and in vitro. J Pineal Res. 2005;39:84–90. doi: 10.1111/j.1600-079X.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 24.D'sa CM, Arthur RE, Jr, Kuhn DM. Expression and deletion mutagenesis of tryptophan hydroxylase fusion proteins: delineation of the enzyme catalytic core. J Neurochem. 1996;67:917–926. doi: 10.1046/j.1471-4159.1996.67030917.x. [DOI] [PubMed] [Google Scholar]
- 25.Cash CD. Why tryptophan hydroxylase is difficult to purify: a reactive oxygen-derived species-mediated phenomenon that may be implicated in human pathology. Gen Pharmacol. 1998;30:569–574. doi: 10.1016/s0306-3623(97)00308-x. [DOI] [PubMed] [Google Scholar]
- 26.Florez JC, Takahashi JS. Regulation of tryptophan hydroxylase by cyclic AMP, calcium, norepinephrine, and light in cultured chick pineal cells. J Neurochem. 1996;67:242–250. doi: 10.1046/j.1471-4159.1996.67010242.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi BH, Chae HD, Park TJ, et al. Protein kinase C regulates the activity and stability of serotonin N-acetyltransferase. J Neurochem. 2004;90:442–454. doi: 10.1111/j.1471-4159.2004.02495.x. [DOI] [PubMed] [Google Scholar]
- 28.Ganguly S, Gastel JA, Weller JL, et al. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc Natl Acad Sci USA. 2001;98:8083–8088. doi: 10.1073/pnas.141118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 30.Grenett HE, Ledley FD, Reed LL, et al. Full-length cDNA for rabbit tryptophan hydroxylase: functional domains and evolution of aromatic amino acid hydroxylases. Proc Natl Acad Sci U S A. 1987;84:5530–5534. doi: 10.1073/pnas.84.16.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang XJ, Kaufman S. High-level expression and deletion mutagenesis of human tryptophan hydroxylase. Proc Natl Acad Sci U S A. 1994;91:6659–6663. doi: 10.1073/pnas.91.14.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mockus SM, Kumer SC, Vrana KE. A chimeric tyrosine/tryptophan hydroxylase. The tyrosine hydroxylase regulatory domain serves to stabilize enzyme activity. J Mol Neurosci. 1997;9:35–48. doi: 10.1007/BF02789393. [DOI] [PubMed] [Google Scholar]
- 33.Carkaci-Salli N, Flanagan JM, Martz MK, et al. Functional domains of human tryptophan hydroxylase 2 (hTPH2) J Biol Chem. 2006;281:28105–28112. doi: 10.1074/jbc.M602817200. [DOI] [PubMed] [Google Scholar]
- 34.Banik U, Wang GA, Wagner PD, et al. Interaction of phosphorylated tryptophan hydroxylase with 14-3-3 proteins. J Biol Chem. 1997;272:26219–26225. doi: 10.1074/jbc.272.42.26219. [DOI] [PubMed] [Google Scholar]
- 35.Murphy KL, Zhang X, Gainetdinov RR, et al. A regulatory domain in the N-terminus of tryptophan hydroxylase 2 controls enzyme expression. J Biol Chem. 2008;238:13216–13224. doi: 10.1074/jbc.M706749200. [DOI] [PMC free article] [PubMed] [Google Scholar]