Abstract
Purpose
Performance status (PS) is a prognostic factor in patients with metastatic colorectal cancer. Clinical trials typically enroll less than 10% of patients with a PS of 2 (PS2); thus, the benefit of systemic chemotherapy in PS2 patients is uncertain.
Patients and Methods
Individual data from 6,286 patients (509 PS2 patients) from nine clinical trials were used to compare treatment efficacy by PS. Progression-free survival (PFS), grade ≥ 3 adverse events, 60-day all-cause mortality, overall survival (OS), and response rate (RR) were explored in the full set of nine trials and in the five trials comparing first-line monotherapy with combination therapy.
Results
Compared with patients with PS of 0 or 1, PS2 patients had significantly higher rates of grade ≥ 3 nausea (8.5% v 16.4%, respectively; P < .0001) and vomiting (7.6% v 11.9%, respectively; P = .006) and 60-day all-cause mortality (2.8% v 12.0%, respectively; P < .0001). PS2 was prognostic for PFS (hazard ratio [HR] = 1.52; P < .0001; median PFS, 7.6 months for PS 0 or 1 v 4.9 months for PS2), OS (HR = 2.18; P < .0001; median OS, 17.3 months for PS 0 or 1 v 8.5 months for PS2), and RR (odds ratio = 0.61; P < .0001; 43.8% for PS 0 or 1 v 32.0% for PS2). The relative benefit and toxicity of experimental versus control treatment and monotherapy versus combination therapy were not different in PS 0 or 1 patients versus PS2 patients.
Conclusion
In clinical trials, PS2 patients derive similar benefit from superior treatment as patients with PS of 0 to 1 but with an increased risk of toxicities and 12% 60-day mortality. Although current treatment provides benefit, new approaches are required to approach 1-year median survival for PS2 patients.
INTRODUCTION
Chemotherapy for advanced colorectal cancer prolongs time to progression and improves overall survival (OS) compared with best supportive care.1 Combination chemotherapy of fluorouracil (FU) with irinotecan or oxaliplatin results in improved response rate (RR) and prolonged time to progression compared with single-agent therapy.2–7 Recent trials of combination chemotherapy, many including a biologic agent, have reported median survival times near 2 years.8–10 Consequently, many clinicians consider combination therapy to be standard of care for first-line treatment of colorectal cancer. Even among practitioners who favor initial single-agent therapy, most eventually treat their patients who are candidates for additional treatment with combination therapy (usually in the form of FU/oxaliplatin, FU/irinotecan, or a chemotherapeutic with a biologic such as bevacizumab or cetuximab) after treatment failure with single-agent therapy.
However, the favorable outcomes, coupled with reasonable tolerability of combination therapy, have been demonstrated in clinical trials that have typically enrolled a fit population. There has been limited investigation as to whether the benefit of improved therapy applies equally to patients with poor performance status (PS). In most phase III trials, less than 10% of the study population is comprised of patients with an Eastern Cooperative Oncology Group PS of 2 (PS2).
The question of whether chemotherapy benefit extends to patients with poor PS is particularly relevant because PS is an established strong prognostic factor in advanced colorectal cancer, with the median survival of PS2 patients being less than half that of patients with PS of 0 at presentation.11–14 In untreated cancer patients, PS may decline either because of underlying poor health or because of cancer-related symptoms. There is some suggestion that in patients with cancer-related functional decline, FU-based chemotherapy improves patient PS and health-related quality of life.15 There is also preliminary evidence from a small study that poor PS patients may tolerate chemotherapy.16 However, there is uncertainty about the best treatment option for poor PS patients when faced with the choice between an effective but toxic therapy or a gentler alternative. Even less is known about the influence of underlying ill health on the toxicity and benefit from chemotherapy of colorectal cancer. Those with noncancer comorbidity have an increased risk of death compared with otherwise healthy colon cancer patients17; yet, at least in the adjuvant setting, these patients seem to benefit as much from chemotherapy as those without chronic comorbid illness.18
Thus, the true risks and benefits of chemotherapy in PS2 patients have not been well studied, making it difficult to counsel the poor PS patient with regard to the merits of using first-line aggressive chemotherapy. In addition, whether treatment with modern chemotherapy has the potential to overcome the poor prognosis associated with worse PS at time of presentation is unknown. Given the small numbers of PS2 patients enrolling onto clinical trials to answer these questions, we performed a pooled analysis of clinical trials using FU-based chemotherapy in conjunction with irinotecan or oxaliplatin for patients with advanced colorectal cancer. Trials including bevacizumab were not included because the pivotal trials with this agent either excluded PS2 patients entirely19 or included too few PS2 patients to provide useful analytic information.20
PATIENTS AND METHODS
Study Design
A protocol-specified retrospective analysis was conducted using individual patient data from patients with colorectal cancer enrolled onto nine clinical trials.2,4–8,21–23 Trials included were pivotal phase III clinical trials of therapy for metastatic colorectal cancer for which trial investigators agreed to provide individual patient data. Institutional review board (IRB) approval and informed consent were obtained when patients entered each individual trial. IRB approval for this pooled analysis was granted by the Mayo Clinic IRB. No attempt was made to gather individual patient data from all trials conducted within the same time period; therefore, results presented should not be interpreted as a meta-analysis of the value or relative benefit of different chemotherapy regimens. The control arms for the nine clinical trials included FU plus leucovorin (LV; five trials); infusional fluorouracil, leucovorin, and oxaliplatin (two trials); fluorouracil, leucovorin, and oxaliplatin (one trial); and irinotecan, fluorouracil, and leucovorin (one trial). Five trials specifically compared first-line monotherapy with combination therapy. Multiple different chemotherapy regimens were used; the specific treatment regimens within each trial are listed in Table 1. Progression-free survival (PFS) was prespecified as the primary end point of this analysis, with secondary end points of grade ≥ 3 adverse events, OS, and tumor RR.
Table 1.
Trials and Regimens Included
| Trial and Regimen | No. of Patients | % PS2 Patients | Arm: E or C | Initial Therapy: M v D |
|---|---|---|---|---|
| de Gramont et al4 | ||||
| FU/LV | 210 | 10 | C | M |
| FOLFOX4 | 210 | 10 | E | D |
| Douillard et al6 | ||||
| FU/LV | 187 | 7 | C | M |
| FOLFIRI | 198 | 7 | E | D |
| Seymour et al2 | ||||
| LVFU2→CPT | 710 | 9 | C | M |
| LVFU2→FOLFIRI | 356 | 8 | E* | M |
| LVFU2→FOLFOX | 356 | 9 | E* | M |
| FOLFIRI | 356 | 8 | E | D |
| FOLFOX | 357 | 8 | E | D |
| Goldberg et al22 | ||||
| IFL | 390 | 5 | C | NA |
| FOLFOX4 | 386 | 5 | E | NA |
| IROX | 383 | 5 | E | NA |
| Köhne et al7 | ||||
| FU/LV | 216 | 4 | C | M |
| FUFIRI | 214 | 5 | E | D |
| Tournigand et al8 | ||||
| FOLFOX4 | 310 | 8 | C | NA |
| Stop-and-go FOLFOX7 | 306 | 9 | E | NA |
| Porschen et al23 | ||||
| FUFOX | 232 | 8 | C | NA |
| CAPOX | 239 | 9 | E | NA |
| Saltz et al5 | ||||
| FU/LV | 219 | 14 | C | M |
| IFL | 225 | 16 | E | D |
| Tournigand et al21 | ||||
| FOLFOX6 | 113 | 6 | C | NA |
| FOLFIRI | 113 | 17 | E | NA |
Abbreviations: PS2, performance status of 2; E, experimental; C, control; M, monotherapy; D, doublet; FU, fluorouracil; LV, leucovorin; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; FOLFIRI, infusional fluorouracil, leucovorin, and irinotecan; CPT, irinotecan; IFL, irinotecan, fluorouracil, and leucovorin; NA, not available; IROX, irinotecan and oxaliplatin; FUFIRI, fluorouracil, leucovorin, and irinotecan; FUFOX, fluorouracil, leucovorin, and oxaliplatin; CAPOX, capecitabine plus oxaliplatin.
Considered control group for progression-free survival and response rate end points.
Two methods for examining the relative benefit of treatment in Eastern Cooperative Oncology Group PS 0 to 1 patients versus PS2 patients were prespecified. The initial approach compared, for each end point, the relative difference in outcomes between the experimental arm and the control therapy arm in each trial between PS 0 to 1 patients and PS2 patients. The second prespecified approach restricted the analysis to only the five trials comparing initial monotherapy (FU/LV) versus combination therapy. The classification of each regimen within each study for these two analyses is listed in Table 1.
Statistical Methods
Individual patient data for all randomly assigned patients were included in the pooled analysis. Safety data on all randomly assigned patients were summarized for each study by PS group. Logistic regression models were used to explore the relationship between the incidence of selected severe adverse events (grade ≥ 3) and PS group (0 or 1 v 2), controlling for sex, age, the patient's original study, and indicator variables for whether the patient's treatment included irinotecan and/or oxaliplatin. To determine whether the relative difference in adverse event rates by arm within study (experimental v control arms) varied by PS, multivariate logistic regression was used to test for a PS-treatment interaction while adjusting for main effects, as well as age, sex, and study. Adverse events were graded using the National Cancer Institute Common Toxicity Criteria version 2 grading scale. Detailed baseline data on the underlying cause for poor PS (disease v comorbidity) were not available.
Efficacy end points analyzed were PFS, OS, and RR. For the two therapeutic comparisons (experimental v control and combination v monotherapy), the relative benefit of treatment arms for RR was explored using logistic regression. For the end points of PFS and OS, all studies were included in a Cox regression model, stratified for the patient's original study.24 PFS was defined as the time to the first event of progression or death. For all end points, the presence of a treatment-study interaction was tested for by comparing a model with a single treatment term to a model with a study-specific treatment effect term using a likelihood ratio test. All logistic and survival models were adjusted for patient age and sex. PS-treatment interactions were tested using a likelihood ratio test comparing a model with PS and treatment main effects terms with a model also including a PS-by-treatment multiplicative factor. All analyses were conducted using the SAS system version 9.0 (SAS Institute, Cary, NC); P < .05 was used to denote statistical significance.
RESULTS
A total of 6,286 patients were enrolled onto the nine studies. The trials included 509 patients (8%) with PS2. PS was relatively well balanced between treatment arms within each study, and the rate of PS2 patients by study arm ranged from 4% to 17% (Table 1). The distribution of age by PS group was similar (PS 0 to 1: median age, 63 years; range, 19 to 88 years; PS2: median age, 63 years; range, 24 to 84 years; P = .12). Patients with PS of 0 to 1 were more likely to be male than PS2 patients (64% v 58%, respectively; P = .0006).
PS was strongly prognostic for efficacy outcomes, regardless of treatment. The median PFS time for PS 0 to 1 patients was 7.6 months compared with 4.9 months for PS2 patients (hazard ratio [HR] = 1.52; 95% CI, 1.38 to 1.66; P < .0001; Fig 1A). Similarly, the median OS time for PS 0 to 1 patients was 17.3 months compared with 8.5 months for PS2 patients (HR = 2.18; 95% CI, 1.98 to 2.40; P < .0001; Fig 1B). The tumor RR was significantly lower in PS2 patients as well (32.0%) compared with PS 0 to 1 patients (43.8%; odds ratio [OR] = 0.61; 95% CI, 0.49 to 0.74; P < .0001).
Fig 1.
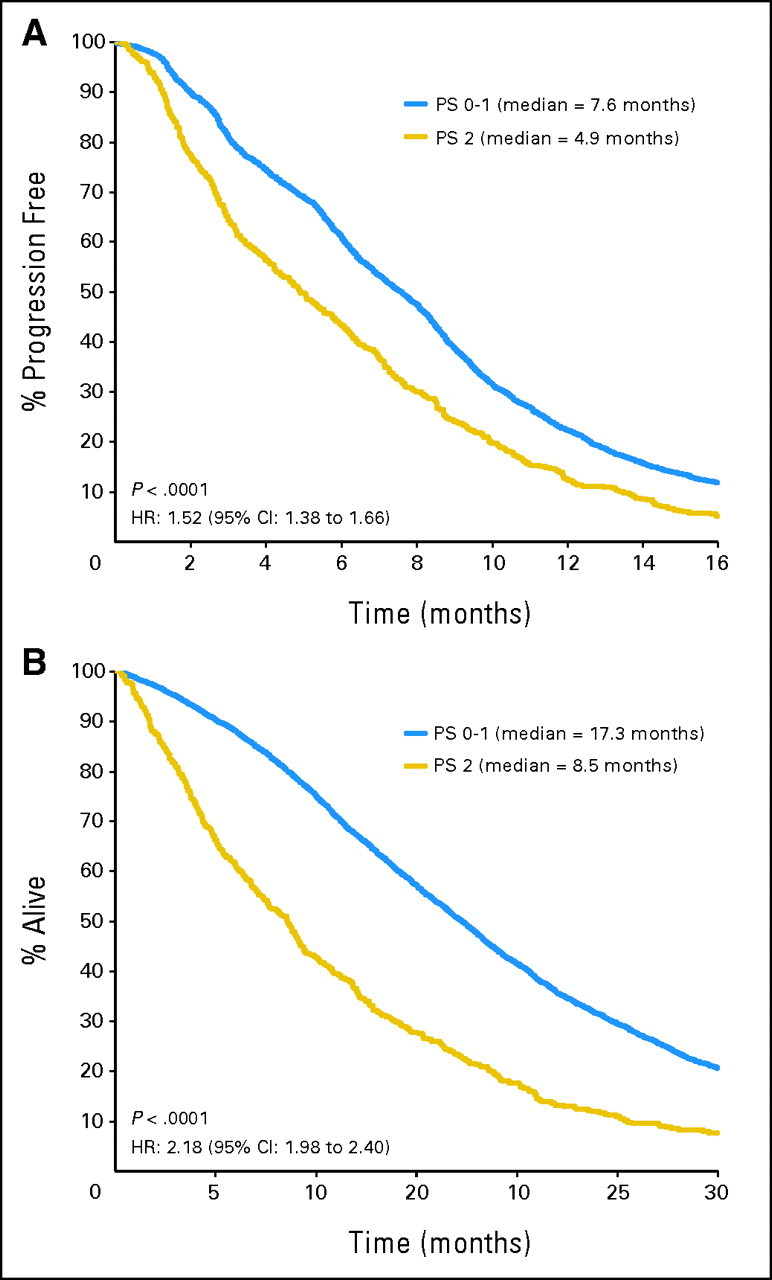
(A) Progression-free survival and (B) overall survival by performance status (PS), regardless of treatment. HR, hazard ratio.
Experimental Versus Control Arms
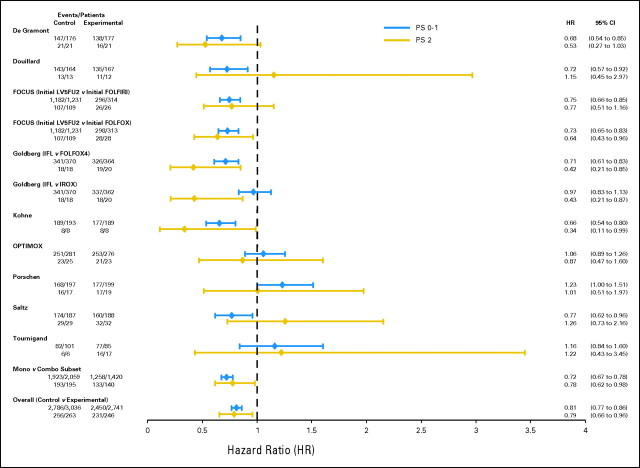
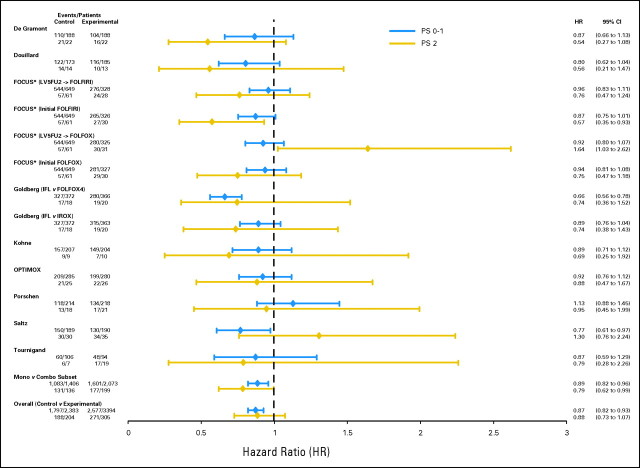
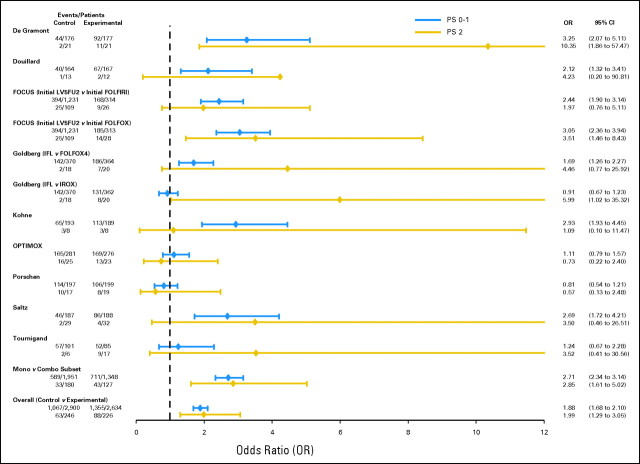
Focusing initially on the first analysis approach, where within each trial the relative benefit of experimental versus control treatment was compared between patients with PS 0 to 1 versus PS2, no difference in treatment benefit was observed by PS for any efficacy outcome. In addition, no significant between-trial heterogeneity was observed between PS and PFS or OS. For PFS, the experimental treatment provided a benefit in both PS 0 to 1 patients (HR = 0.81 for experimental v control treatment; 95% CI, 0.77 to 0.86; P < .00001; median PFS, 17.9 months for experimental treatment v 16.4 months for control) and PS2 patients (HR = 0.79 for experimental v control treatment; 95% CI, 0.66 to 0.96; P = .02; median PFS, 8.8 months for experimental treatment v 8.2 months for control). P = .68 for a PS-treatment interaction, providing no evidence of a differential treatment benefit by PS (Fig 2). Similarly, for the OS end point, the experimental treatment provided similar benefit in both PS 0 to 1 patients (HR = 0.87; 95% CI, 0.82 to 0.93; P < .00001; median OS, 8.4 months for experimental treatment v 6.7 months for control) and PS2 patients (HR = 0.88; 95% CI, 0.73 to 1.07; P = .21; median OS, 6.0 months for experimental treatment v 4.0 months for control). P = .41 for a PS-treatment interaction (Fig 3). Experimental treatment approximately doubled the likelihood of response in both PS 0 to 1 patients (OR = 1.88; 95% CI, 1.68 to 2.10; P < .0001) and PS2 patients (OR = 1.99; 95% CI, 1.29 to 3.05; P = .002), with a PS-treatment interaction P = .89 (Fig 4).
Fig 2.
Forest plot of study-specific progression-free survival (PFS) hazard ratios (HR) by performance status (PS). FU, fluorouracil; LV, leucovorin; FOLFIRI, infusional fluorouracil, leucovorin, and irinotecan; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; IFL, irinotecan, fluorouracil, and leucovorin; IROX, irinotecan and oxaliplatin.
Fig 3.
Forest plot of study-specific overall survival hazard ratios (HR) by performance status (PS). FU, fluorouracil; LV, leucovorin; FOLFIRI, infusional fluorouracil, leucovorin, and irinotecan; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; IFL, irinotecan, fluorouracil, and leucovorin; IROX, irinotecan and oxaliplatin; CPT, irinotecan.
Fig 4.
Forest plot of study-specific response rate odds ratios (OR) by performance status (PS). FU, fluorouracil; LV, leucovorin; FOLFIRI, infusional fluorouracil, leucovorin, and irinotecan; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; IFL, irinotecan, fluorouracil, and leucovorin; IROX, irinotecan and oxaliplatin.
Monotherapy Versus Combination Therapy
The second analysis restricted the population to the five trials comparing initial monotherapy with FU/LV with combination therapy. In this subset of 3,814 patients (335 PS2 patients), for the PFS end point, initial combination treatment provided a benefit in both PS 0 to 1 patients (HR = 0.72 for combination v monotherapy; 95% CI, 0.67 to 0.78; P < .0001) and PS2 patients (HR = 0.78 for combination v monotherapy; 95% CI, 0.62 to 0.98; P < .0001). P = .75 for a PS-treatment interaction (Fig 2). Similarly, for the OS end point, combination therapy provided similar benefit in both PS 0 to 1 patients (HR = 0.89; 95% CI, 0.82 to 0.96; P = .003) and PS2 patients (HR = 0.79; 95% CI, 0.62 to 0.99; P = .04). P = .18 for a PS-treatment interaction (Fig 3). Treatment with initial combination therapy compared with monotherapy significantly increased the likelihood of response in both PS 0 to 1 patients (OR = 2.71; 95% CI, 2.34 to 3.14; P < .0001) and PS2 patients (OR = 2.85; 95% CI, 1.61 to 5.02; P = .0003). P = .71 for a PS-treatment interaction (Fig 4).
Toxicity
Between-study heterogeneity was observed in the rates of grade ≥ 3 adverse events; specifically, the reported adverse event rates were consistently lower in the FOCUS2 trial compared with the other included trials. For this reason, the FOCUS trial was excluded for the primary adverse event analysis. Rates of grade ≥ 3 adverse events by PS group are listed in Table 2. Nausea and vomiting of grade ≥ 3 were significantly more common in PS2 patients (nausea, 8% PS 0 to 1 v 16% PS2, P < .0001; vomiting, 8% PS 0 to 1 v 12% PS2, P = .006). The rates of stomatitis, diarrhea, and neutropenia did not differ by PS group. In a secondary analysis including the FOCUS trial, nausea and vomiting continued to be elevated in PS2 patients (P < .0001 and P = .0002, respectively). In addition, stomatitis became significantly elevated in PS2 patients (2% PS 0 to 1 v 4% PS2, P = .03).
Table 2.
Patients Who Experienced NCI-CTC Grade 3 or Greater Adverse Events (excluding FOCUS trial)
| Grade 3+ Events | % of Patients |
P* | ||
|---|---|---|---|---|
| Overall | PS 0-1 | PS2 | ||
| Nausea | 9.1 | 8.5 | 16.4 | < .0001 |
| Vomiting | 8.0 | 7.6 | 11.9 | .006 |
| Diarrhea | 16.9 | 17.1 | 14.9 | .32 |
| Stomatitis | 2.5 | 2.3 | 5.0 | .11 |
| Neutropenia | 33.7 | 33.7 | 34.5 | .51 |
Abbreviations: NCI-CTC, National Cancer Institute Common Toxicity Criteria; PS, performance status.
P from a logistic regression for PS as a dichotomous variable (PS 0-1 v PS2) in a model controlling for study, sex, age, and indicators for whether treatment included irinotecan and/or oxaliplatin.
We conducted further analyses to determine whether the relative difference in adverse event rates by arm within study (experimental v control arms) varied by PS. Specifically, we tested for a PS-treatment interaction, which, if significant, would imply that the relative increase in adverse event rates from a more toxic versus a less toxic regimen depends on patient PS. In these models, the P value for a PS-treatment interaction was nonsignificant at the .05 level for any adverse event, indicating that any differential toxicity in experimental versus control treatments is not PS dependent. The PS-treatment interaction P values remained nonsignificant for the secondary analysis when the FOCUS trial was included.
The rate of death in the first 60 days from random assignment from any cause was significantly different by PS group, with a rate of 3% in PS 0 to 1 patients compared with 12% in PS2 patients (P < .0001). In the PS2 patients, 54% of these early deaths were preceded by disease progression, suggesting that the difference in 60-day all cause mortality is likely a result of more aggressive disease in PS2 patients, other comorbid disease beyond the patients' malignancy, and potential toxic effects of therapy.
DISCUSSION
This pooled analysis demonstrates that patients with advanced colorectal cancer who present for first-line chemotherapy on clinical trials with a PS of 2 benefit just as much from modern chemotherapy regimens as do patients with a PS of 0 or 1. In particular, the relative benefit of experimental and combination regimens was the same in PS2 patients compared with PS 0 to 1 patients. Unfortunately, despite the benefit provided to PS2 patients by newer combination therapy, the OS of PS2 patients (median OS, 8.5 months) treated with any of the regimens remained substantially shorter than the OS of PS 0 to 1 patients treated with inferior regimens. In addition, although PS2 patients receive benefit from modern treatment, severe nausea and vomiting were much more pronounced in PS2 patients than in PS 0 to 1 patients. However, there was no differential increase in toxicity in PS2 patients from the more intensive and potentially more toxic regimens, suggesting that the higher rate of toxicity may be from underlying illness rather than a more profound adverse effect profile experienced by PS2 patients from the more toxic regimens. Sixty-day mortality was also markedly higher in PS2 patients, although much of this was likely related to rapid disease progression or existing comorbidity rather than toxicity.
The benefit of the experimental and combination regimens in PS2 patients in this pooled analysis is of direct clinical relevance because these arms included the currently used, modern combinations. Although the relative benefit of modern combination therapy seems to be independent of PS, the median OS and PFS times of PS2 patients are less than half of those of PS 0 to 1 patients. Consequently, the absolute benefit afforded by these combination regimens is smaller, which is an observation that has some bearing when considering the higher incidence of severe nausea and vomiting from treatment and the high 60-day mortality experienced by PS2 patients. An important question that cannot be answered by this analysis is whether these same PS2 patients may have felt just as poorly, or worse, if treated with supportive care only. In patients with poor PS as a result of their cancer, health-related quality of life is likely poor before initiation of therapy, and nausea and/or vomiting may already be present. The lack of a treatment-PS interaction in our toxicity analysis suggests that PS 0 to 1 and PS2 patients experience a similar increase in adverse events with more toxic therapy. Thus, the higher rate of toxicity in PS2 patients is likely more influenced by the patients' pre-existing poor health than the treatment regimen chosen.
One limitation of this study is that we were unable to distinguish between patients whose poor PS was a direct result of their cancer or a result of medical comorbidity. Given the two- to three-fold increase in the odds of response in PS2 patients, the choice of a superior regimen with a higher probability of tumor regression and associated relief of cancer symptoms has the potential to improve quality of life in patients whose PS decline is a result of cancer. In contrast, patients with poor PS as a result of comorbid medical conditions may be less likely to improve or perhaps more likely to suffer severe adverse effects and further PS decline from combination therapy. Accordingly, if one is to treat patients with poor PS as a result of their cancer, a more aggressive regimen may be justified with a goal of improving cancer-related symptoms and preventing further deterioration of PS. The converse, however, may be true of those with poor PS from other comorbid medical conditions. Unfortunately, our analysis cannot distinguish between these two groups of poor PS patients because no data were collected on the reason underlying poor PS at enrollment and current methodologies to assess PS inadequately address this issue, highlighting the need for research to better define comorbidity in future trials.
A second limitation of our study is that we cannot tell to what extent the PS2 patients selected for inclusion in these clinical trials are representative of poor PS patients in the whole population. Because investigators may be more likely to enroll patients with PS decline from cancer rather than from chronic disease who have borderline PS onto a clinical trial, we suspect that many of the PS2 patients included in this analysis had PS decline from cancer.
On the basis of these results, oncologists can feel confident that treating patients who present with poor PS as a result of their cancer with maximally effective chemotherapy is likely to provide patient benefit; however, regardless of regimen, these patients need to be managed attentively with supportive care. It is unclear from these results whether the addition of biologic agents is similarly beneficial in PS2 patients. Although the biologic agents approved for use in colorectal cancer (bevacizumab, cetuximab, and panitumumab) are generally well tolerated, the addition of biologics to chemotherapy clearly increases the incidence of severe adverse events.19,24,25 Thus, if a biologic is used in addition to chemotherapy for the treatment of PS2 patients, careful monitoring for toxicity is warranted.
The benefit of modern chemotherapy for advanced colorectal cancer applies not only to fit, young patients, but also to the elderly26,27 and, now, to patients with poor PS. However, the survival of patients with poor PS, even with recent advances, continues to be quite poor. A better understanding of host and tumor biology that leads some to present with cancer once already rather ill and to die quickly thereafter will be necessary to improve the survival of these sickest of colorectal cancer patients.
Footnotes
Supported by North Central Cancer Treatment Group Grant No. CA25224 from the National Cancer Institute.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Daniel J. Sargent, Roche (C), Genentech (C), Pfizer Inc (C), Amgen Inc (C), Sanofi-Aventis (C); Aimery de Gramont, Sanofi-Aventis (C), Roche (C), Baxter (C); Ranier Porschen, Roche (C); Leonard B. Saltz, Pfizer Inc (C), Roche (C), Genentech (C), ImClone Systems Inc (C), Bristol-Myers Squibb Co (U), YM BioSciences Inc (C), Alchemia Ltd (C), Merck & Co (C), Celgene Corp (C), Bayer Pharmaceuticals (C), Novartis (C); Christopher Tournigand, Sanofi-Aventis (C), Roche (C); Axel Grothey, Sanofi-Aventis (C), Pfizer Inc (C), Genentech (C), Bristol-Myers Squibb Co (C), Amgen Inc (C), Roche (C); Richard M. Goldberg, Sanofi-Aventis (C), Pfizer Inc (C), Genentech (C), Bristol-Myers Squibb Co (C), Amgen Inc (C), ImClone Systems Inc (C) Stock Ownership: None Honoraria: Daniel J. Sargent, Roche, Genentech, Pfizer Inc, Amgen Inc, Sanofi-Aventis; Aimery de Gramont, Sanofi-Aventis, Roche, Baxter; Ranier Porschen, Roche; Christopher Tournigand, Sanofi-Aventis, Roche; Axel Grothey, Sanofi-Aventis, Pfizer Inc, Genentech, Bristol-Myers Squibb Co, Amgen Inc, Roche; Richard M. Goldberg, Sanofi-Aventis, Pfizer Inc, Genentech, Bristol-Myers Squibb Co, Amgen Inc, ImClone Systems Inc Research Funding: Leonard B. Saltz, Pfizer Inc, Roche, Genentech, ImClone Systems Inc, Bristol-Myers Squibb Co, Merck & Co, Taiho Pharmaceutical Co Ltd, Amgen Inc; Christopher Tournigand, Sanofi-Aventis, Roche Expert Testimony: None Other Remuneration: Matthew T. Seymour, Roche, Amgen Inc
AUTHOR CONTRIBUTIONS
Conception and design: Daniel J. Sargent, Claus Henning Köhne, Richard M. Goldberg
Financial support: Daniel J. Sargent, Axel Grothey, Richard M. Goldberg
Administrative support: Daniel J. Sargent, Claus Henning Köhne, Axel Grothey, Richard M. Goldberg
Provision of study materials or patients: Daniel J. Sargent, Claus Henning Köhne, Matthew T. Seymour, Aimery de Gramont, Rainer Porschen, Leonard B. Saltz, Philippe Rougier, Christophe Tournigand, Jean-Yves Douillard, Richard J. Stephens, Richard M. Goldberg
Collection and assembly of data: Daniel J. Sargent, Claus Henning Köhne, Brian M. Bot, Matthew T. Seymour, Richard M. Goldberg
Data analysis and interpretation: Daniel J. Sargent, Claus Henning Köhne, Hanna Kelly Sanoff, Brian M. Bot, Matthew T. Seymour, Richard J. Stephens, Axel Grothey, Richard M. Goldberg
Manuscript writing: Daniel J. Sargent, Claus Henning Köhne, Hanna Kelly Sanoff, Brian M. Bot, Matthew T. Seymour, Aimery de Gramont, Rainer Porschen, Richard J. Stephens, Axel Grothey, Richard M. Goldberg
Final approval of manuscript: Daniel J. Sargent, Claus Henning Köhne, Hanna Kelly Sanoff, Brian M. Bot, Matthew T. Seymour, Aimery de Gramont, Rainer Porschen, Leonard B. Saltz, Philippe Rougier, Christophe Tournigand, Jean-Yves Douillard, Richard J. Stephens, Axel Grothey, Richard M. Goldberg
REFERENCES
- 1.Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: Systematic review and meta-analysis—Colorectal Cancer Collaborative Group. BMJ. 2000;321:531–535. doi: 10.1136/bmj.321.7260.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): A randomised controlled trial. Lancet. 2007;370:143–152. doi: 10.1016/S0140-6736(07)61087-3. [DOI] [PubMed] [Google Scholar]
- 3.Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): A phase III randomised controlled trial. Lancet. 2007;370:135–142. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer: Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 7.Köhne CH, van Cutsem E, Wils J, et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol. 2005;23:4856–4865. doi: 10.1200/JCO.2005.05.546. [DOI] [PubMed] [Google Scholar]
- 8.Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—A GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 9.Saltz L, Clarke S, Diaz-Rubio E, et al. Bevacizumab (Bev) in combination with XELOX or FOLFOX4: Updated efficacy results from XELOX-1/ NO16966, a randomized phase III trial in first-line metastatic colorectal cancer. J Clin Oncol. 2007;25(suppl):170s. abstr 4028. [Google Scholar]
- 10.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C Study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 11.Sanoff HK, Sargent DJ, Campbell ME, et al. N9741: Survival update and prognostic factor analysis of oxaliplatin and irinotecan combinations for metastatic colorectal cancer. J Clin Oncol. 2007;25(suppl):180s. doi: 10.1200/JCO.2008.17.7147. abstr 4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavin P, Mittelman A, Douglass H, Jr, et al. Survival and response to chemotherapy for advanced colorectal adenocarcinoma: An Eastern Cooperative Oncology Group report. Cancer. 1980;46:1536–1543. doi: 10.1002/1097-0142(19801001)46:7<1536::aid-cncr2820460707>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Kemeny N, Braun DW., Jr Prognostic factors in advanced colorectal carcinoma. Importance of lactic dehydrogenase level, performance status, and white blood cell count. Am J Med. 1983;74:786–794. doi: 10.1016/0002-9343(83)91066-5. [DOI] [PubMed] [Google Scholar]
- 14.Köhne CH, Cunningham D, Di Costanzo F, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: Results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308–317. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- 15.Scheithauer W, Rosen H, Kornek GV, et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752–755. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tournigand C, Andre T, Chirivella I, et al. 5-Fluorouracil, folinic acid and oxaliplatin (FOLFOX) in poor prognosis patients with metastatic colorectal cancer. J Clin Oncol. 2004;22(suppl):261s. abstr 3565. [Google Scholar]
- 17.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study. Cancer. 1998;82:2123–2134. [PubMed] [Google Scholar]
- 18.Gross CP, McAvay GJ, Guo Z, et al. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109:2410–2419. doi: 10.1002/cncr.22726. [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 20.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 21.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 23.Porschen R, Arkenau HT, Kubicka S, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: A final report of the AIO Colorectal Study Group. J Clin Oncol. 2007;25:4217–4223. doi: 10.1200/JCO.2006.09.2684. [DOI] [PubMed] [Google Scholar]
- 24.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 25.Van Cutsem E, Nowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial. J Clin Oncol. 2007;25(suppl):164s. abstr 4000. [Google Scholar]
- 26.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 27.Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: Combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]