Abstract
Purpose
The investigational arm of INT0116, a fluorouracil (FU) and leucovorin–containing chemoradiotherapy regimen, is a standard treatment for patients with resected gastric cancer with a 2-year disease-free survival rate (DFS) of 52%. Toxicity is also significant. More beneficial and safer regimens are needed.
Patients and Methods
We performed a randomized phase II study among 39 cancer centers to evaluate two paclitaxel and cisplatin–containing regimens, one with FU (PCF) and the other without (PC) in patients with resected gastric cancer. Patients received two cycles of postoperative chemotherapy followed by 45 Gy of radiation with either concurrent FU and paclitaxel or paclitaxel and cisplatin. The primary objective was to show an improvement in 2-year DFS to 67% as compared with INT 0116.
Results
From May 2001 to February 2004 (study closure), 78 patients entered this study, and 73 were evaluable. At the planned interim analysis of 22 patients on PCF, grade 3 or higher GI toxicity was 59%. This was significantly worse than INT0116, and this arm was closed. Accrual continued on PC. The median DFS was 14.6 months for PCF and has not been reached for PC. For PC the 2-year DFS is 52% (95% CI, 36% to 68%).
Conclusion
Though PC appears to be safe and the median DFS favorable, the DFS failed to exceed the lower bound of 52.9% for the targeted 67% DFS at 2 years and can not be recommended as the adjuvant arm for future randomized trials.
INTRODUCTION
On a global basis, cancer of the stomach is one of the most prevalent malignancies. Postoperative adjuvant chemoradiotherapy has been demonstrated to result in a significant improvement in overall and disease-free survival (DFS). In the United States, the national Intergroup trial (Intergroup 0116–Radiation Therapy Oncology Group[RTOG] 90-18) had 556 evaluable patients who underwent an R0 resection were randomly assigned to fluorouracil (FU) and leucovorin (LV) chemotherapy plus concurrent external beam radiation therapy (RT) or to expectant observation.1 A highly statistically significant improvement in 1-, 2-, and 3-year disease-free of 69%, 52%, and 48%, respectively, was reported; median DFS was 30 months versus 19 months, P = .0002; and median overall survival was 40 months versus 26 months. The results of this study indicate that chemoradiotherapy has a positive influence on increasing the overall survival for patients at high risk for recurrence after undergoing surgery alone.
However, despite the improvement in outcome, over 50% of patients will still die within 3 years of a potentially curative operation, emphasizing the need for the development of newer treatment regimens. The high recurrence rate, even in the superior chemoradiotherapy arm, clearly indicates the need for improved systemic therapies. In addition, toxicity in INT 0116 was significant, with grade 3+ overall toxicities occurring in 73% of the cases. Of 263 patients with available toxicity information, grade 3, 4, and 5 toxicities observed were observed in 39%, 32%, and 1% of patients, respectively. Grade 3 and 4 GI toxicity was observed in 29% and 3% of patients; whereas grade 3 and 4 hematologic toxicities were observed in 26% and 28% of patients, respectively. In fact, only 63% of the patients were able to complete treatment as planned.
Paclitaxel is an active agent against gastroesophageal cancers and acts as a radiation sensitizer.2–4 The combination of paclitaxel, FU, and cisplatin is also active against adenocarcinoma of the esophagus or gastroesophageal junction.5 The use of paclitaxel has been evaluated in two adjuvant and neoadjuvant trials in the treatment of gastro-esophageal cancers. In a phase I trial conducted at Memorial Sloan-Kettering, patients with locally advanced gastric cancer were treated with fixed doses of weekly cisplatin at 30 mg/m2 and RT with 5040 Gy in 180 Gy fractions 5 days per week for 6 weeks.6 For purposes of radiosensitization, paclitaxel was also given in low daily doses (15 mg/m2/day) as a 96-hour continuous infusion (CIV) each week throughout the radiotherapy. Of the 34 patients evaluable for response, 22 patients (64%) had objective clinical responses and 12 (35%) had complete pathologic responses. With a median follow-up of 47 months, 13 (35%) are alive without evidence of disease. No acute esophagitis or long-term toxicities were reported.
In a second trial at M. D. Anderson, patients with resectable gastric cancer first received two cycles of induction chemotherapy with infusional FU at 750 mg/m2/day by CIV on days 1 to 5, cisplatin at 15 mg/m2/day over 1 hour on days 1 to 5, and paclitaxel at a dose of 200 mg/m2 over 24 hours on day 1 of each 28-day cycle.7 This was then followed by 45 Gy with concurrent FU at 300 mg/m2/day by CIV 5 days per week and paclitaxel at 45 mg/m2 on days 1, 8, 15, 22, and 29 during the 5-week course of RT.7 In the 33 patients who underwent gastrectomy, a pathologic complete response was noted in eight patients (22%), and a pathologic partial response was noted in six patients (15%). At a median follow-up of 36 months, the median time for overall survival had not been reached. The major toxicity was GI, with grade 3 and 4 nausea, vomiting, and pain on eating observed in 44% of patients.
RTOG-0114 was therefore designed as a random assignment phase II clinical trial to determine if either of these two adjuvant treatment arms was promising enough to be pursued in a subsequent phase III study compared with the INT 0116 adjuvant arm. Both arms were designed to include cisplatin and paclitaxel, and one arm would contain FU. This decision was to be based on improvement in 2-year DFS relative to the adjuvant arm of INT 0016 and safety.
PATIENTS AND METHODS
Eligibility included patients with microscopically confirmed stages IB through IIIB adenocarcinoma of the stomach or gastroesophogeal junction, who underwent a potentially curative resection (ie, R0 resection). Additional criteria included Zubrod performance status 0 to 1 and no prior chemotherapy or prior radiation therapy to the treatment field. Exclusion criteria included any metastatic disease (M1), New York Heart Association class III or IV heart disease, history of active angina or myocardial infarction within 6 months, history of significant ventricular arrhythmia, pregnant or lactating women, nonmalignant medical illnesses that were uncontrolled or whose control may have been jeopardized by the complications of this therapy, and clinically significant hearing loss. The study was reviewed and approved by the institutional review board for each participating institution. All patients were fully informed of the investigational nature of this protocol, and all gave written informed consent before initiation of therapy.
Treatment Plan
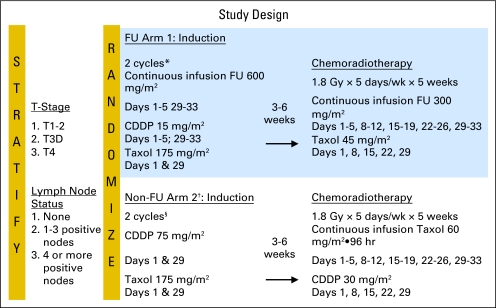
Postoperative chemotherapy was to begin within 8 weeks of surgery. Patients were randomly assigned to one of two combined-modality schedules in order to avoid any patient selection bias. As shown in Figure 1, on arm 1 containing FU (PCF), all patients received, through a double lumen Mediport (Bard Access Systems, Salt Lake City, UT), FU as CIV day 1 through 5 and 29 to 33 at a dose of 600 mg/m2/day, cisplatin on day 1 through 5 and days 29 to 33 at a dose of 15 mg/m2/day over 1 hour, and paclitaxel on days 1 and 29 at a dose of 175 mg/m2 over 3 hours. In arm 2 that did not contain FU, PC patients received on days on 1 and 29 paclitaxel at a dose of 175 mg/m2 over 3 hours and cisplatin over 1 hour at a dose of 75 mg/m2. On the basis of the National Cancer Institute Common Toxicity Criteria Version 2.0, drug doses were decreased by 25% if grade 3 nonhematologic toxicity or grade 4 hematologic toxicity occurred.
Fig 1.
Study design. FU, fluorouracil; CDDP, cisplatin, Taxol, paclitaxel.
Chemoradiotherapy was to begin 3 to 4 weeks, but no later than 6 weeks, after the postoperative chemotherapy. The intent of treatment was to deliver 45 Gy in 1.8 Gy/fraction, 5 days a week for 5 weeks, to the entire gastric bed (including anastamosis) and draining lymph nodes. Computed tomography planning was used. The protocol required films to be sent to RTOG Headquarters for rapid review.
In PCF, the chemotherapy with the RT consisted of FU at 300 mg/m2/day by continuous infusion with a portable pump 5 days each week (this treatment usually started on a Monday and ended on Friday, after the RT) plus paclitaxel at 45 mg/m2 over 3 hours each Monday for 5 weeks. In PC, the chemotherapy with the RT included paclitaxel administered through a portable pump as a 96-hour CIV at a dose of 15 mg/m2/day (60 mg/m2 total dose, starting on Monday and ending on Friday) each week, and cisplatin administered over 1 hour at the start of the paclitaxel infusion on Monday at a dose of 30 mg/m2/week. Premedication with dexamethasone was not required using the 96-hour infusion schedule of paclitaxel.
Statistical Considerations
The sample size was based on the primary end point—the 2-year DFS rate, and the study was designed to estimate this rate with a one-sided, lower-bound CI. The targeted sample size for each treatment regimen was 43 analyzable patients, in order to provide a one-sided 97.5% CI around the hypothesized 67% 2-year DFS rate with a lower-bound CI of 52.9%. This provided a 2.5% chance of observing a 2-year DFS rate of less than 52.9% if the true rate was 67%. If the lower bound of the estimated DFS rate CI was greater than or equal to 52.9%, the treatment arm would be considered for future study in a phase III trial.
Patients were stratified according to T-stage (T1/T2 v T3 v T4) and nodal status (0 v 1-3 v > 3). Patients were randomly assigned to one of the two experimental treatment arms: FU based and non–FU based (details in the Treatment Plan section), according to Zelen's permuted block randomization method,8 which ensured that the institutions had the opportunity to treat patients enrolled in different protocol arms.
Toxicity was a secondary end point. Chemotherapy and acute RT toxicity were scored using the NCI Common Toxicity Criteria version 2.0, and late RT toxicities were scored using the RTOG/ European Organisation for Research and Treatment of Cancer Late Morbidity Scoring Scheme. In INT 0116, ≥ grade 3 overall, hematologic, and GI toxicities occurred in 73%, 54% and 32% of the cases, respectively. With 43 analyzable patients and a type I error rate of 0.10, RTOG 0114 had 55% and 86% power to detect 10% (73% to 83%) and 15% (73% to 88%) absolute increases in the rate of ≥ grade 3 overall toxicity, respectively; 51% and 77% power to detect 10% (54% to 64%) and 15% (54% to 69%) absolute increases in the rate of ≥ grade 3 hematologic toxicity, respectively; and 44% and 70% power to detect 10% (32% to 42%) and 15% (32% to 47%) absolute increases in ≥ grade 3 GI toxicity, respectively. Grade 5 (fatal) toxicities occurred in 1% of patients on INT 0116. RTOG 0114 had 21% power to detect an increase of 1%, 37% power to detect a 2% increase, and 64% power to detect a 5% increase in the rate of fatal toxicities. All reported P values comparing toxicities from RTOG 0114 and INT 0116 reflect one-sided tests for increased toxicity on the RTOG 0114 treatment regimens. Early stopping for toxicity stipulated that if the rate of ≥ grade 3 GI toxicities exceeded 47% for the first 22 analyzable patients enrolled in a treatment arm, or if either treatment arm had three or more treatment-related deaths at any time, the statistician would recommend that consideration be given to closing that arm to further accrual.
Median DFS and overall survival times were estimated with the Kaplan-Meier method,9 and all analyses were performed using SAS Version 9.1 software (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Between May 2001 and February 2004, 78 patients were registered to the clinical trial from 39 different RTOG-affiliated cancer centers. Thirty patients were registered to arm 1 (PCF) and 48 patients to arm 2 (PC) (Table 1). Two patients registered to PCF were excluded—one never received therapy, and the other was ineligible for being registered more than 8 weeks after the time of surgical resection. On PC, two patients were registered and found to have stage IV disease at the time of study entry, making them ineligible, and one patient withdrew informed consent. Thus, 28 patients were considered evaluable on PCF and 45 patients on PC.
Table 1.
Evaluable Patients by Treatment Arm and Reasons for Exclusion
| Patients | FU-Based Arm 1 | Non–FU-Based Arm 2 | Total |
|---|---|---|---|
| Total entered | 30 | 48 | 78 |
| Total evaluable | 28 | 45 | 73 |
| Ineligible/no protocol | 2* | 2† | 4 |
| Withdrew consent | 0 | 1 | 1 |
Abbreviation: FU, fluorouracil.
No protocol therapy received, registered > 8 weeks from surgery.
Stage 4 disease.
The characteristics of these evaluable patients are listed in Table 2 for median age, sex distribution, type of surgery, the primary site of cancer within the stomach, the depth of penetration, and the TNM staging. Forty-seven men and 26 women entered the study with a median age of 57 and 54 years on arms PCF and PC, respectively. Even though PCF was closed to patient accrual early because of excessive GI toxicity, the two arms were relatively balanced by Karnofsky performance scale and TNM stage. Total gastrectomies were more common on PCF than PC (36% v 18%).
Table 2.
Patient Characteristics
| Characteristic | FU-Based Arm 1 (n = 28) |
Non–FU-Based Arm 2 (n = 45) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age | ||||
| Median | 57 | 54 | ||
| Range | 44-81 | 34-71 | ||
| Sex | ||||
| Male | 16 | 57 | 31 | 69 |
| Female | 12 | 43 | 14 | 31 |
| Zubrod performance status | ||||
| 0 | 16 | 57 | 25 | 56 |
| 1 | 12 | 43 | 20 | 44 |
| Type of surgery | ||||
| Total gastrectomy | 10 | 36 | 8 | 18 |
| Distal subtotal gastrectomy | 12 | 43 | 19 | 42 |
| Proximal subtotal gastrectomy | 6 | 21 | 18 | 40 |
| Primary site | ||||
| Cardia | 5 | 18 | 12 | 27 |
| Fundus | 0 | 3 | 7 | |
| Body | 1 | 4 | 0 | |
| Antrum | 9 | 32 | 8 | 18 |
| Pylorus | 3 | 11 | 6 | 13 |
| Lesser curvature | 6 | 21 | 8 | 18 |
| Greater curvature | 0 | 2 | 4 | |
| Stomach, NOS | 4 | 14 | 6 | 13 |
| Depth of penetration | ||||
| Submucosal | 1 | 4 | 0 | |
| Lamina propria | 0 | 1 | 2 | |
| Subserosa | 5 | 18 | 9 | 20 |
| Muscularis propria | 8 | 29 | 17 | 38 |
| Serosa | 14 | 50 | 15 | 33 |
| Spleen, transverse colon, diaphragm retroperitoneum | 0 | 2 | 4 | |
| Other | 0 | 1 | 2 | |
| T stage/pathologic | ||||
| T1 | 1 | 4 | 1 | 2 |
| T2 | 14 | 50 | 26 | 58 |
| T3 | 12 | 43 | 17 | 38 |
| T4 | 1 | 4 | 1 | 2 |
| N stage/pathologic | ||||
| N0 | 5 | 18 | 8 | 18 |
| N1 | 18 | 64 | 26 | 58 |
| N2 | 5 | 18 | 11 | 24 |
| Stage group | ||||
| IB | 4 | 14 | 3 | 7 |
| II | 10 | 36 | 18 | 40 |
| IIIA | 12 | 43 | 22 | 49 |
| IIIB | 2 | 7 | 2 | 4 |
Abbreviations: FU, fluorouracil; NOS, not otherwise specified.
Interim and Overall Toxicity Analysis
A comparison of overall chemotherapy and acute radiation toxicities (grade 1 to 4) by treatment arm is presented in Table 3. At the initial RT review, there were only two patient cases that were not in compliance with the RT fields designated by the protocol. There were no reported late RT grade 3 or 4 toxicities and no grade 5 toxicities. At the planned interim analysis of PCF of the first 22 patients, the GI grade 3 toxicity (predominantly nausea and vomiting) rate of 59% (13 of 22) was reported, with one grade 4 toxicity (anorexia) but no grade 5 (fatal) GI toxicities. This arm exceeded the boundary of 47% set in the protocol design. In contrast, on PC, at the time of the interim analysis, the GI grade 3 toxicity rate was 24% (five of 21) with no grade 4 or 5 GI toxicities reported. After discussing this data, it was decided to discontinue any further randomization to PCF because the rate of GI toxicity would be unacceptably high for an experimental arm in a subsequent phase III trial. All patients entering RTOG-0114 were then assigned to PC to complete the trial so that efficacy could be evaluated per protocol design. When PCF was closed to accrual, all institutions were notified to inform patients still receiving arm PCF treatment of the increased toxicity, and additional treatment was to be given at the discretion of their treating physician. Five patients randomly assigned to PCF chose to continue on this arm of the trial.
Table 3.
Overall Chemotherapy and Acute Radiotherapy Toxicity (Grade 1 to 4) by Treatment Arm
| Toxicity | FU-Based Arm 1 (n = 28), Grade |
Non–FU-Based Arm 2 (n = 45), Grade |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
1 |
2 |
3 |
4 |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Allergy/immunology | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | ||||||||
| Auditory/hearing | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | ||||||||
| Blood/bone marrow | 4 | 4 | 6 | 13 | 6 | 9 | 13 | 5 | ||||||||
| Cardiovascular (arrhythmia) | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | ||||||||
| Cardiovascular (general) | 0 | 3 | 4 | 2 | 3 | 0 | 4 | 1 | ||||||||
| Coagulation | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||||||||
| Constitutional symptoms | 6 | 13 | 4 | 0 | 11 | 24 | 5 | 0 | ||||||||
| Dermatology/skin | 3 | 7 | 1 | 0 | 5 | 9 | 0 | 0 | ||||||||
| Endocrine | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Gastrointestinal | 0 | 7 | 18 | 1 | 7 | 19 | 12 | 3 | ||||||||
| Hemorrhage | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | ||||||||
| Hepatic | 8 | 2 | 0 | 0 | 6 | 6 | 3 | 0 | ||||||||
| Infection/febrile neutropenia | 1 | 0 | 5 | 1 | 1 | 1 | 8 | 0 | ||||||||
| Metabolic/laboratory | 7 | 3 | 4 | 0 | 12 | 8 | 5 | 1 | ||||||||
| Musculoskeletal | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||||||
| Neurology | 5 | 5 | 0 | 1 | 13 | 4 | 6 | 0 | ||||||||
| Ocular/visual | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||||||||
| Pain | 4 | 3 | 3 | 0 | 9 | 6 | 5 | 0 | ||||||||
| Pulmonary | 2 | 1 | 1 | 0 | 5 | 8 | 1 | 0 | ||||||||
| Renal/genitourinary | 1 | 0 | 0 | 0 | 5 | 3 | 0 | 0 | ||||||||
| Worst GI | 0 | 7 | 25 | 18 | 64 | 1 | 4 | 7 | 16 | 19 | 42 | 12 | 27 | 3 | 7 | |
| Worst hematologic | 4 | 14 | 4 | 14 | 6 | 21 | 13 | 46 | 6 | 13 | 9 | 20 | 13 | 29 | 5 | 11 |
| Worst overall | 0 | 1 | 4 | 12 | 43 | 15 | 54 | 2 | 4 | 8 | 18 | 23 | 51 | 10 | 22 | |
NOTE. Scored using National Cancer Institute Common Toxicity Criteria Version 2.0.
Abbreviation: FU, fluorouracil.
Hematologic toxicity was significant on PCF. Grade 3 or greater hematologic toxicity was observed in 67% (19 of 28) of patients, with six patients with grade 3 and 13 with grade 4 toxicities. There were also six episodes of febrile neutropenia. In contrast on PC, with a total treatment of 45 patients, grade 3 and greater GI toxicity was 33% (15 of 45) and hematologic toxicity was 40% (Table 3). In comparison to the toxicity reported on the INT 0116 trial, PCF had significantly more grade 3 or higher GI toxicity (68% v 32%; P < .0001), as well as significantly more grade 3 or higher overall toxicity (97% v 73%; P < .0001). For PC, there was not a statistically significant increase in grade 3 or higher GI toxicity (34% v 32%; P = .45) nor in grade 3 or higher overall toxicity (73% v 73%; P = .50), as compared with INT 0116. In fact, the grade 3 or higher hematologic toxicity on PC was lower than that on INT 0116 (40% v 54%).
Disease-Free and Overall Survival
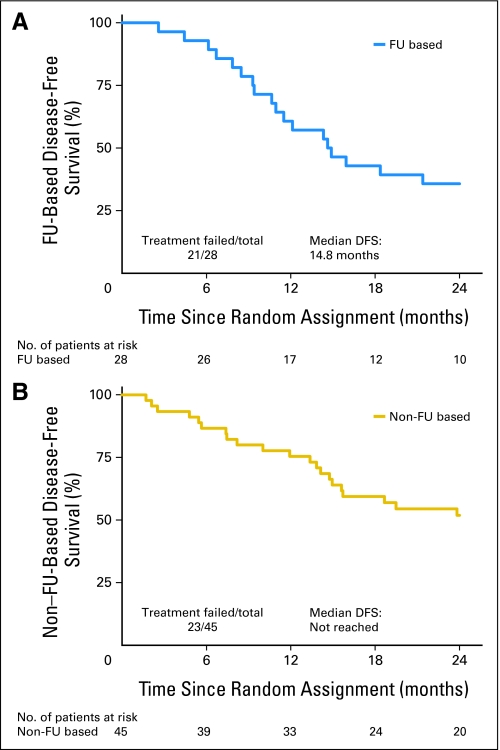
Three patients on PC were lost to follow-up for the 2-year DFS end point. With 42 evaluable patients on PC, 52% (95% CI, 36% to 68%) of patients were disease-free at 2 years. As shown on the Kaplan-Meier plots (Fig 2), median DFS on PC has not yet been reached. The median DFS is 14.8 months. As presented in Table 4, overall primary and nodal relapse was higher on PCF than PC (33% v 26%), as was the development of distant metastatic disease (25% for PCF and 17% for PC). In addition, the overall median survival is 23.8 months on PCF and has yet to be reached on PC.
Fig 2.
Disease-free survival (DFS). FU, fluorouracil.
Table 4.
Site of First Failure
| First Failure | FU Based (n = 21) |
Non–FU Based (n = 23) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Primary relapse | 3 | 11 | 8 | 18 |
| Nodal relapse | 5 | 18 | 1 | 2 |
| Primary and nodal relapse | 1 | 4 | 1 | 2 |
| Primary relapse and distant disease | 0 | 0 | 1 | 2 |
| Nodal relapse and distant disease | 0 | 0 | 1 | 2 |
| Distant disease | 7 | 25 | 6 | 13 |
| Second primary | 2 | 7 | 4 | 9 |
| Death (COD study cancer) | 1 | 4 | 0 | 0 |
| Death | 2 | 7 | 1 | 2 |
| No failure | 7 | 25 | 22 | 49 |
Abbreviations: FU, fluorouracil; COD, cause of death.
DISCUSSION
INT 0116, which used FU and LV, was the first to show that adjuvant chemoradiotherapy provides a survival benefit for patients with resected gastric cancer. However, toxicity remained significant, and the survival benefit was modest. Recent attempts have been made to combine other chemotherapy agents in both the adjuvant and neoadjuvant setting, including epirubicin and cisplatin.10,11 In addition, docetaxel, when combined with FU and cisplatin, has been show to be superior to FU and cisplatin alone in the metastatic setting.12,13
RTOG-0114 was an attempt to introduce both paclitaxel and cisplatin into two adjuvant treatment arms utilizing different doses and schedules. In addition, one arm (PC) did not include FU as part of the adjuvant treatment. As indicated, it became quite evident early on in the trial that PCF containing FU with paclitaxel and cisplatin exceeded the limits of acceptable GI toxicity for consideration as a treatment arm for a future adjuvant trial in resected gastric cancer. Even though the overall hematologic toxicity on PCF was comparable to that observed on INT 0116, the grade 3 and 4 GI toxicity of 68% observed on this arm significantly exceeded the 32% incidence of grade 3 and 4 GI toxicity observed on INT 0116. This degree of toxicity exceeded what was anticipated from this regimen based on its use as a neoadjuvant therapy for patients with resectable gastric cancer.7 This would suggest that this regimen is more difficult to manage in a postoperative rather than a preoperative setting. In addition, on the neoadjuvant trial all patients had laparoscopic placement of J-tubes.7 Therefore, the inability to initiate tube feedings, especially with the high incidence of total gastrectomies (36%) on this arm, could be a contributing factor to the increased GI toxicity observed on PCF. In contrast, with PC the incidence of grade 3 and 4 GI toxicity of 34% was essentially identical to that of INT 0116. In fact, the 40% incidence of grade 3 and 4 hematologic toxicity was less than the 54% observed on INT 0116. Thus, PC was well-tolerated, with GI and hematologic toxicity that was at least no worse than the adjuvant treatment arm of INT 0116.
The major end point of this multicenter study with two complex regimens containing paclitaxel and cisplatin was to meet our hypothesized 2-year DFS of 67% with a lower bound on the 95% CI of at least 52.9% in at least one of two arms. However, our 2-year DFS for PC was only 52% (95% CI, 36% to 68%). Assuming an ideal scenario that all three patients who were not evaluable for 2-year DFS remained disease-free, this would only increase the 2-year DFS point estimate from 52% to 53% with a two-sided 95% CI of 38% to 68%. This would still fail to reach our primary study end point of a 2-year DFS of at least 67%. Therefore, based on our study end points, PCF can not be recommended because of excess toxicity, and PC can not be recommended since it failed to achieve an improvement in DFS over that what would be expected from conventional FU and LV. In addition, the local regional failure rate of 33% on PCF and 26% on PC does not represent an improvement in local control when compared with INT-0116.1 These results then indicate that use of a taxane and platinum do not represent an improvement over single agent fluoropyrimidine when combined with RT in the adjuvant treatment of resected gastric cancer. Nevertheless, this study does show that in the cooperative group setting it is possible to successfully complete large multicenter randomized clinical trials, even with complex chemotherapy regimens.
Footnotes
Supported by Radiation Therapy Oncology Group U10 Grant No. CA21661, and by the National Cancer Institute Grants No. CCOP U10 CA37422 and Stat U10 CA32115.
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, May 13-15, 2005, Orlando, Florida.
This manuscript's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Gary K. Schwartz, Kathryn Winter, Bruce D. Minsky, Christopher Willett, David Kelsen
Administrative support: Christopher Willett
Provision of study materials or patients: Gary K. Schwartz, Bruce D. Minsky, Christopher Crane, John Thomson, Howard Gross, Christopher Willett, David Kelsen
Collection and assembly of data: Gary K. Schwartz, Kathryn Winter, Pramila Anne
Data analysis and interpretation: Gary K. Schwartz, Kathryn Winter, Bruce D. Minsky, Christopher Willett, David Kelsen
Manuscript writing: Gary K. Schwartz, Kathryn Winter, Bruce D. Minsky, Christopher Willett, David Kelsen
Final approval of manuscript: Gary K. Schwartz, Kathryn Winter, Bruce D. Minsky, Christopher Crane, John Thomson, Pramila Anne, Howard Gross, Christopher Willett, David Kelsen
REFERENCES
- 1.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, Ilson DH, Daugherty K, et al. Activity of paclitaxel (Taxol) in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst. 1994;86:1086–1091. doi: 10.1093/jnci/86.14.1086. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Fairweather J, Pazdur R, et al. Phase II study of Taxol in patients with untreated metastatic gastric carcinoma. Cancer J Sci Am. 1998;4:269–274. [PubMed] [Google Scholar]
- 4.Safran H, Wanebo HJ, Hesketh PJ, et al. Paclitaxel and concurrent radiation for gastric cancer. Int J Radiat Oncol Biol Phys. 2000;46:889–894. doi: 10.1016/s0360-3016(99)00436-8. [DOI] [PubMed] [Google Scholar]
- 5.Ilson DH, Ajani JA, Bhalla K, et al. A phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol. 1998;16:1826–1834. doi: 10.1200/JCO.1998.16.5.1826. [DOI] [PubMed] [Google Scholar]
- 6.Brenner B, Ilson D, Minsky B, et al. A phase I trial of combined-modality therapy for localized esophageal cancer: Escalating doses of continuous-infusion paclitaxel with cisplatin and concurrent radiation therapy. J Clin Oncol. 2004;22:45–52. doi: 10.1200/JCO.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: Degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–1244. doi: 10.1200/JCO.2005.01.305. [DOI] [PubMed] [Google Scholar]
- 8.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Disease. 1994;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Non-parametric estimation from incomplete observation. J Am Stat. 1958;53:457–481. [Google Scholar]
- 10.Roth AD, Fazio N, Stupp R, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: A randomized phase II trial of the Swiss Group for Clinical Cancer Res. J Clin Oncol. 2007;25:3217–3223. doi: 10.1200/JCO.2006.08.0135. [DOI] [PubMed] [Google Scholar]
- 11.Leong T, Michael M, Foo K, et al. Adjuvant and neoadjuvant therapy for gastric cancer using epirubicin/cisplatin/5-fluorouracil (ECF) and alternative regimens before and after chemoradiation. Br J Cancer. 2003;89:1433–1438. doi: 10.1038/sj.bjc.6601311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Group Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: The V-325 Study Group. J Clin Oncol. 2007;25:3205–3209. doi: 10.1200/JCO.2006.10.4968. [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Moiseyenko VM, et al. Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]