Abstract
Purpose
Our prior work has shown that the health behaviors of head and neck cancer patients are interrelated and are associated with quality of life; however, other than smoking, the relationship between health behaviors and survival is unclear.
Patients and Methods
A prospective cohort study was conducted to determine the relationship between five pretreatment health behaviors (smoking, alcohol, diet, physical activity, and sleep) and all-cause survival among 504 head and neck cancer patients.
Results
Smoking status was the strongest predictor of survival, with both current smokers (hazard ratio [HR] = 2.4; 95% CI, 1.3 to 4.4) and former smokers (HR = 2.0; 95% CI, 1.2 to 3.5) showing significant associations with poor survival. Problem drinking was associated with survival in the univariate analysis (HR = 1.4; 95% CI, 1.0 to 2.0) but lost significance when controlling for other factors. Low fruit intake was negatively associated with survival in the univariate analysis only (HR = 1.6; 95% CI, 1.1 to 2.1), whereas vegetable intake was not significant in either univariate or multivariate analyses. Although physical activity was associated with survival in the univariate analysis (HR = 0.95; 95% CI, 0.93 to 0.97), it was not significant in the multivariate model. Sleep was not significantly associated with survival in either univariate or multivariate analysis. Control variables that were also independently associated with survival in the multivariate analysis were age, education, tumor site, cancer stage, and surgical treatment.
Conclusion
Variation in selected pretreatment health behaviors (eg, smoking, fruit intake, and physical activity) in this population is associated with variation in survival.
INTRODUCTION
Five-year survival rates for head and neck cancer have not changed in several decades and remain at approximately 60% depending on tumor site.1,2 Although new surgical, radiation, and chemotherapy regimens hold promise, healthy lifestyle behaviors may be instrumental in improving survival among head and neck cancer patients. A clearer understanding of the pretreatment health behaviors that are associated with improved survival may provide insight into the types of behavioral interventions needed among head and neck cancer patients.
Tobacco and alcohol use are well-known primary risk factors for developing head and neck cancer and have been shown to be associated with decreased quality-of-life scores3,4 and decreased survival.5 Diets high in fruits and vegetables are protective against most cancers of the head and neck,6 can affect the occurrence of second primary cancers,7 and are associated with reduced cancer mortality.5 Malnutrition,8 cachexia,9 and weight loss10 are poor prognostic indicators for head and neck cancer patients. There is also evidence that a sedentary lifestyle may promote certain types of cancer, such as colon or breast11; however, the association with physical activity and head and neck cancer is less clear. Sleep disturbances are common in head and neck cancer patients, and although there is no evidence of causality, associations have been drawn between amount of sleep and mortality.12
In prior research, our team profiled the health behaviors of newly identified head and neck cancer patients at baseline and during the first year after diagnosis, but inadequate follow-up was available at that time to assess the associations between health behaviors and survival. Now, with longer follow-up, the present study was undertaken to determine whether five pretreatment health factors (including smoking, alcohol use, diet, exercise, and sleep) predict survival among head and neck cancer patients.
PATIENTS AND METHODS
This was a prospective cohort study of patients enrolled onto the University of Michigan Head and Neck Cancer Specialized Program of Research Excellence. The independent variables were five health factors (smoking, alcohol use, diet, exercise, and sleep). Control variables were age, sex, race, education, marital status, cancer site and stage, treatment, and comorbidities. The dependent (outcome) variable was all-cause survival.
Study Population
Research assistants approached 1,084 newly diagnosed patients with head and neck squamous cell carcinoma to participate. Exclusion criteria were as follows: age less than 18 years; pregnant; non-English speaking; psychologically or mentally unstable (eg, suicidal ideation, acute psychosis, or dementia); and non–upper aerodigestive tract cancer (eg, thyroid or skin cancer). Exclusions included 65 patients who were ineligible, 240 patients who refused, 45 patients with second primary tumors, and 110 patients who did not complete a baseline survey, which left a sample size of 625 patients. The data set was further limited for the Cox proportional hazard models analyses to 504 patients with no missing data. Similar to other studies, comparisons of those with missing data versus those without missing data are consistent with serious health problems (higher comorbidities)13 and race14 as being responsible for nonparticipation.
Human patient approval was received from the following three study sites: University of Michigan Medical Center, Ann Arbor Veterans Affairs (VA) Healthcare System, and Henry Ford Health System. Recruitment began in January 2003. Patients were censored as being dead or alive as of August 1, 2008.
Procedure
Research assistants recruited patients to the study in the waiting rooms of otolaryngology clinics by obtaining signed informed consent and providing a written survey that had questions on demographics and health behaviors. A medical record audit was also conducted. Patients were resurveyed every 3 months for 2 years and then every year thereafter.
Measures
Health behavior variables.
Patients were asked to self-characterize themselves as a current smoker, former smoker (quit 1 month to > 1 year ago), or never smoker (including cigarettes, cigars, and pipe tobacco). The previously validated 10-item instrument, Alcohol Use Disorders Identification Test,15 was used to measure alcohol use; the score ranges from 0 to 40, with a score of 8 or more indicating problem drinking.16 The validated Willett food frequency questionnaire17 was used to measure average servings of fruits and vegetables in the past year and average calories per day.18 Body mass index (BMI; weight in kilograms divided by the square of height in meters) was also used to measure nutritional/physical activity status; the 1999 to 2002 population mean was 28.4 kg/m2 for adults older than 40 years in the United States.19 The validated Physical Activity Scale for the Elderly20 was used to measure activity; the population mean score for people age 65 to 100 years was 103. Given that many of our head and neck cancer patients are elderly and all of them are chronically ill, we felt that the Physical Activity Scale for the Elderly was appropriate to use because it focuses on activities of daily living versus rigorous exercise regimens, and the mean score for a population with end-stage renal disease was 90.3.21 Sleep was assessed using validated questions from the Medical Outcomes Study; scores range from 0 to 100, with a mean of 72 for adults visiting a medical clinic.22
Control variables.
Standard questions included age, sex, race (white v nonwhite), educational level (high school or less v some college or more), and marital status (married v not married). Because there were few sinus (n = 12) and nasopharynx (n = 5) cancers, tumor sites were classified into the following three groups based on proximity: larynx; oropharynx, hypopharynx, nasopharynx, or unknown primary; and oral cavity or sinus. Tumor stage (0 to IV) was classified using the American Joint Committee on Cancer staging classification system.23 Comorbidities were measured using the Adult Comorbidity Evaluation-2724 as no, mild, moderate, or severe comorbidities. Type of curative treatment received (surgery, radiation, and/or chemotherapy) was recorded by yearly chart audit or patient self-report when treated at an outside facility. By including three separate yes or no treatment variables, all possible treatment combinations are controlled for in the multivariate analysis.
Outcome variable: Survival.
By contacting patients every 3 months, patient vital status (dead or alive) was determined. For those patients who were lost to follow-up, the Social Security Administration Death Master File was used to determine whether and when the patients had died. Patients lost to follow-up and not found on the Death Master File were assumed to be alive as of August 1, 2008.
Statistical Analysis
Means and frequency distributions were examined for all variables. To assess collinearity between health behaviors and control variables, Pearson's correlation coefficients were calculated, and variance inflation factors were evaluated. To avoid confounding, hospital site was not included as a control variable because it was highly correlated with race and sex, with more males coming from the VA and more blacks coming from the VA and Henry Ford Hospital. Kaplan-Meier plots and the log-rank test were used to compare the health behavior variables with survival. Univariate and multivariate Cox proportional hazards models were used to study the relationship between health behaviors, control variables, and survival.
RESULTS
Descriptive Statistics
Descriptive characteristics of the sample are listed in Table 1. At the time of diagnosis, more than one quarter of patients were current smokers, whereas more than half were former smokers. More than one quarter of the patients screened positive for problem drinking. Approximately one third of patients ate fewer than four servings of fruit per month, and more than two-thirds ate less than one vegetable per day. The mean calorie intake was 2,351 calories per day, and the mean BMI was 26.7 kg/m2. The mean physical activity score was 115, and the mean sleep score was 67. The 2-year death rate was 24.2% (SE = 1.99%). The median follow-up time was 999 days (range, 19 to 2,010 days).
Table 1.
Pretreatment Patient Characteristics of Newly Diagnosed Head and Neck Cancer Patients
| Characteristic | No. of Patients (N = 504) | % |
|---|---|---|
| Follow-up time, days | ||
| Median | 999 | |
| Range | 19-2,010 | |
| Age, years | ||
| Mean | 58.8 | |
| SD | 10.8 | |
| Range | 25-92 | |
| Body mass index, kg/m2 (population mean ≈ 28-29 kg/m2) | ||
| Mean | 26.7 | |
| SD | 5.8 | |
| Range | 15.2-64.6 | |
| Daily calorie intake, kcal (RDA = 2,000 kcal) | ||
| Mean | 2,351 | |
| SD | 954 | |
| Range | 519-5,752 | |
| PASE physical activity score (population mean = 102) | ||
| Mean | 115.0 | |
| SD | 81.5 | |
| Range | 0-472.8 | |
| MOS sleep score (population mean = 72) | ||
| Mean | 67.1 | |
| SD | 21.1 | |
| Range | 0-100 | |
| Smoking status* | ||
| Current | 133 | 26.4 |
| Former | 278 | 55.2 |
| Never | 93 | 18.4 |
| Alcohol problem (AUDIT ≥ 8) | ||
| Yes | 132 | 26.2 |
| No | 372 | 73.8 |
| Average fruit intake servings (prior year) | ||
| None to 1-3 per month | 161 | 31.9 |
| 1 per week to 2-4 per week | 189 | 37.5 |
| 5-6 per week or more | 154 | 30.6 |
| Average vegetable intake servings (prior year) | ||
| None to 1 per week | 99 | 19.6 |
| 2-4 per week | 151 | 30.0 |
| 5-6 per week | 102 | 20.2 |
| 1 per day or more | 152 | 30.2 |
| Sex | ||
| Male | 394 | 78.2 |
| Female | 110 | 21.8 |
| Race | ||
| Non-Hispanic white | 448 | 88.9 |
| Nonwhite/Hispanic | 56 | 11.1 |
| Marital status | ||
| Married | 301 | 59.7 |
| Not married | 203 | 40.3 |
| Educational level | ||
| High school or less | 242 | 48.0 |
| Some college or more | 262 | 52.0 |
| Hospital site | ||
| University of Michigan | 377 | 74.8 |
| Ann Arbor Veterans Affairs Medical Center | 58 | 11.5 |
| Henry Ford Hospital | 69 | 13.7 |
| Tumor site | ||
| Larynx | 120 | 23.8 |
| Pharynx (oro-, hypo-, or nasopharynx, or unknown primary) | 274 | 54.4 |
| Oral cavity/sinus | 110 | 21.8 |
| Tumor stage | ||
| 0 | 9 | 1.8 |
| I | 50 | 9.9 |
| II | 45 | 8.9 |
| III | 75 | 14.9 |
| IV | 325 | 64.5 |
| ACE-27 comorbidity score | ||
| None | 146 | 29.0 |
| Mild | 194 | 38.5 |
| Moderate | 109 | 21.6 |
| Severe | 55 | 10.9 |
| Treatment | ||
| Radiation | 425 | 84.3 |
| Chemotherapy | 324 | 64.3 |
| Surgery | 253 | 50.2 |
Abbreviations: SD, standard deviation; RDA, Recommended Dietary Allowance; PASE, Physical Activity Scale for the Elderly; MOS, Medical Outcomes Study; AUDIT, Alcohol Use Disorders Identification Test; ACE-27, Adult Comorbidity Evaluation-27.
Includes cigarettes, cigars, and pipe tobacco.
Univariate and Multivariate Analyses
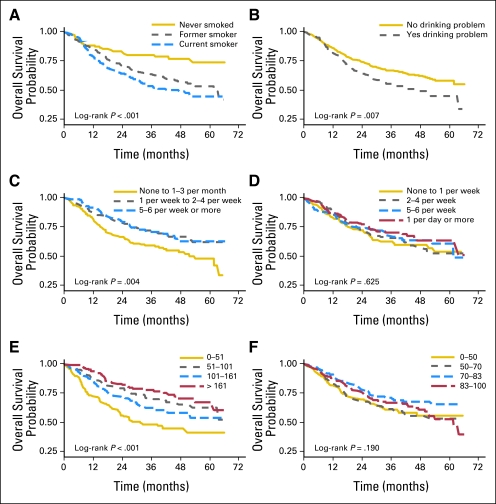
Univariate analyses showed that four of the five health behaviors (smoking status, alcohol problem, fruit intake, and physical activity, but not vegetable intake) were significantly associated with survival (Fig 1). Because there was no association between calories and BMI with survival in the univariate analyses, fruits and vegetables were chosen as the nutrition markers for the final multivariate analysis. In addition, several control variables (age, marital status, education, cancer stage, and comorbidities) were also associated with survival in the univariate analyses. The results of the univariate and multivariate Cox proportional hazards regression models are listed in Table 2.
Fig 1.
Kaplan-Meier plots of survival for health behaviors of head and neck cancer patients. (A) Smoking status (n = 712; 260 events and 452 patients censored). (B) Alcohol problem (Alcohol Use Disorders Identification Test ≥ 8; n = 689; 248 events and 441 patients censored). (C) Average prediagnosis fruit intake (n = 520; 175 events and 345 patients censored). (D) Average prediagnosis vegetable intake (n = 544; 184 events and 360 patients censored). (E) Physical Activity Scale for the Elderly score by quartile (n = 625; 223 events and 402 patients censored). (F) Medical Outcomes Study sleep scale by quartile (n = 621; 220 events and 401 patients censored).
Table 2.
Univariate and Multivariate Cox Proportional Hazards Models for Survival
| Variable | Univariate Model |
Multivariate Model |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Smoking status (v never smoker) | ||||||
| Current smoker | 2.43 | 1.41 to 4.20 | .001* | 2.36 | 1.28 to 4.37 | .006* |
| Former smoker | 1.95 | 1.16 to 3.27 | .011* | 2.02 | 1.16 to 3.51 | .013* |
| Alcohol problem | 1.41 | 1.02 to 1.96 | .038* | 1.32 | 0.91 to 1.93 | .146 |
| PASE physical activity score (per 10 points) | 0.95 | 0.93 to 0.97 | < .001* | 0.98 | 0.95 to 1.00 | .085 |
| MOS sleep scale (per 10 points) | 0.94 | 0.88 to 1.02 | .118 | 0.96 | 0.89 to 1.04 | .350 |
| Low fruit intake (none to 1-3 per month) | 1.55 | 1.14 to 2.12 | .006* | 1.26 | 0.88 to 1.81 | .208 |
| Low vegetable intake (none to 2-4 per week) | 1.09 | 0.81 to 1.48 | .565 | 0.82 | 0.59 to 1.15 | .242 |
| Age (in decades) | 1.40 | 1.22 to 1.62 | < .001* | 1.50 | 1.25 to 1.79 | < .001* |
| Female | 0.72 | 0.48 to 1.07 | .101 | 0.74 | 0.47 to 1.16 | .183 |
| Nonwhite | 1.25 | 0.80 to 1.96 | .326 | 1.09 | 0.68 to 1.77 | .715 |
| Married | 0.70 | 0.51 to 0.95 | .020* | 0.87 | 0.63 to 1.21 | .413 |
| High school education or less | 1.70 | 1.25 to 2.32 | < .001* | 1.43 | 1.03 to 1.99 | .032* |
| Cancer site (v oral cavity/sinus) | ||||||
| Larynx cancer site | 0.65 | 0.42 to 1.02 | .062 | 0.41 | 0.24 to 0.69 | < .001* |
| Pharynx cancer site | 0.81 | 0.56 to 1.17 | .264 | 0.61 | 0.39 to 0.94 | .026* |
| Stage | 1.38 | 1.16 to 1.64 | < .001* | 1.52 | 1.25 to 1.85 | < .001* |
| ACE-27 comorbidity score | 1.39 | 1.18 to 1.63 | < .001* | 1.15 | 0.96 to 1.37 | .125 |
| Radiation | 1.11 | 0.71 to 1.72 | .656 | 0.75 | 0.42 to 1.32 | .318 |
| Chemotherapy | 1.04 | 0.76 to 1.43 | .801 | 0.96 | 0.62 to 1.47 | .835 |
| Surgery | 0.81 | 0.60 to 1.10 | .186 | 0.69 | 0.49 to 0.99 | .043* |
NOTE. Of 504 patients, there were 166 events and 338 patients were censored.
Abbreviations: PASE, Physical Activity Scale for the Elderly; MOS, Medical Outcomes Study; ACE-27, Adult Comorbidity Evaluation-27.
Significant at P < .05.
All control variables were included in the multivariate analyses, regardless of the univariate results. Several of the control variables were associated with each other; however, the variance inflation factor was less than 2.5 for all variables in the multivariate regression, indicating that the multicollinearity was not severe. Hence, no variables were omitted from the multivariate model as a result of concerns about collinearity.
Of the health behaviors, pretreatment smoking status was the strongest predictor of survival, with both current smokers (hazard ratio [HR] = 2.4; 95% CI, 1.3 to 4.4) and former smokers (HR = 2.0; 95% CI, 1.2 to 3.5) showing significant associations with poor survival. Pretreatment problem drinking was associated with survival in the univariate analysis but not in the multivariate model. Low fruit intake was negatively associated with survival in only the univariate analysis, whereas vegetable intake was not significant in either the univariate or multivariate analysis. Physical activity was associated with survival in univariate analysis and was approaching significance in the multivariate model (HR = 0.98; 95% CI, 0.95 to 1.00). However, sleep was not associated with survival in either the univariate or multivariate analyses. Control variables that were also independently associated with poor survival in the multivariate analysis were higher age, lower education, cancer site (oral cavity), and cancer stage. Those treated with surgery had improved survival. Sex, race, marital status, comorbidity score, and treatment with radiation or chemotherapy were not independently associated with survival in the multivariate analysis.
DISCUSSION
Because smoking is a major causative factor for head and neck cancer, it was not surprising that more than one quarter of patients smoked at diagnosis or that current smoking at diagnosis was the strongest predictor of survival among head and neck cancer patients in this population, as well as in previously reported studies.25,26 Approximately one in four head and neck cancer patients were smoking at the time of their diagnosis, and our prior work has shown that approximately half quit after diagnosis.27 Continued smoking may increase the risk of second primary cancers among head and neck cancer patients and decrease survival.28 Although it may be difficult to comprehend why a head and neck cancer patient would continue to smoke, nicotine dependence is an addiction, which is defined as “persistent compulsive use of a substance known by the user to be harmful.”29 Fortunately, efficacious cessation interventions, including medications (nicotine replacement therapy, bupropion, and varenicline), are available for head and neck cancer patients.30,31 Behavioral interventions, such as brief physician advice, nurse counseling, and 1-800-QUIT NOW telephone counseling, have been shown to increase quit rates.32
Alcohol use, in conjunction with smoking, has been found to be a causative factor for head and neck cancer,33 and continued drinking is associated with second primary tumors.28 Although approximately one quarter of patients were problem drinkers at diagnosis, unlike other studies,34,35 pretreatment alcohol problem was not a predictor of survival in the multivariate analyses in this study. Similarly, our prior work has shown that continued drinking among this population is not associated with quality of life.36 It is important to note that smoking and alcohol use are highly interrelated,27 and problem drinkers may have a harder time quitting smoking.37 Moreover, alcohol use may complicate or interfere with adherence to treatment regimens.38 For some, treatment for alcohol use must first take place before smoking cessation or other interventions can be accomplished. Unfortunately, for those who are highly alcohol dependent, inpatient detoxification programs may be needed. For others, referrals to outpatient and community-based programs such as Alcoholics Anonymous are effective strategies.
Surprisingly, before treatment, less than one third of patients reported eating more than five servings of fruit per week, whereas approximately one third reported eating less than four servings of fruit per month compared with an average of 1.5 servings per day for healthy adults.39 Similarly, less than one third of patients ate at least one vegetable per day, whereas one in five patients ate less than two servings of vegetables per week compared with an average of 3.7 servings per day for healthy adults.39 This is substantially less than the recommended nine servings of fruits and vegetables per day.18 Fruit intake predicted survival in the univariate analysis but did not predict survival in the multivariate analysis, whereas vegetable intake did not predict survival in either analysis. Longer follow-up may show a survival advantage for fruit and vegetable intake as shown in other studies.7,40,41
Although fruit and vegetable intake after diagnosis was not assessed in this analysis, it is possible that patients may potentially have even worse dietary intake after treatment. For example, radiation can lead to xerostomia (dry mouth), which can make it more difficult to eat particulate (rather than liquid) food items. Given poor pretreatment dietary intake, post-treatment difficulties with eating, and the potential prognostic value of fruits and vegetables, there may be a considerable and underemphasized need for nutrition counseling among head and neck cancer patients. Tailored, low-intensity nutrition interventions have been shown to increase fruit and vegetable consumption among head and neck cancer patients.42
Although pretreatment mean calorie intake was similar to the recommended levels, BMI was lower than population means, perhaps because many patients smoke and drink at problematic levels, which are both associated with lower BMI.43,44 Similar to other studies,45 neither pretreatment calorie intake nor BMI was associated with survival. Although numerous studies have shown that malnutrition, cachexia, and weight loss are poor prognostic indicators for head and neck cancer patients and are associated with higher complication rates, these conditions are likely to occur after treatment in response to radiation, chemotherapy, and surgical procedures or disease progression, all of which alter nutritional intake.
In this study, pretreatment physical activity was higher than population norms. Similar to other studies of breast,46 colorectal,47 and prostate cancer,48 physical activity predicted survival in the univariate analysis and was marginally associated with survival in the multivariate analysis. However, from these analyses, it is difficult to determine whether higher levels of pretreatment physical activity actually improved survival or whether those patients who did not survive simply displayed lower levels of physical activity as the beginning of the normal dying trajectory. While some head and neck cancer patients may be encouraged to conserve their energy, which may result in decreased muscle mass and strength, physical activity in head and neck cancer patients has been shown to be correlated with quality of life,49 fewer side effects,50 and survival.51 Yet, our prior work27 has shown that, while physical activity declines during the first 6 months after diagnosis, probably in relation to the rigors of treatment, for those who survive, physical activity levels return to pretreatment levels one year after diagnosis, even without intervention. Hence, randomized control trials are needed to determine if head and neck cancer patients should conserve energy or push themselves to engage in physical activity during the often grueling treatment period.
Similar to other studies of cancer patients,52 the sleep scores of this sample of head and neck cancer patients were lower than population means at clinically relevant levels but were not associated with poor survival. The etiology of sleep problems among cancer patients is not easy to determine and are likely to be multifactorial and, at the very least, related to pain, fatigue, and psychological distress.53 Head and neck cancer patients may also have sleep problems related to difficulty with oral secretions, dysphagia, cough, aspiration, xerostomia, pain, and sleep apnea.54 Our prior work has shown that poor sleep is correlated with nicotine use and low physical activity, which may confound the analyses. Behavioral and pharmaceutical interventions may help head and neck cancer patients improve their sleep; however, the underlying causes of insomnia should first be assessed and treated.55
Although the focus of this study was the relationship between health behaviors and survival among head and neck cancer patients, several control variables were also associated with survival. In prior studies, the impact of age has been variable56,57; however, in this study, older patients were less likely to survive than younger patients. As shown in other studies,58,59 educational level (a marker for socioeconomic status) also predicted survival. Similar to other studies,60 oral cancer patients had poorer survival than did patients with pharyngeal or laryngeal cancers. Although human papillomavirus–positive tumors have been shown to be associated with improved survival,61 unfortunately, human papillomavirus status was not available for these analyses. As shown in multiple studies,60,62,63 those with higher cancer stage had poorer survival than those with lower cancer stage. Our work64 and that of others62,63 has shown that those with increased comorbidities had poorer survival; however, in this study, comorbidity scores were significantly associated with survival only in the univariate analysis. Similar to other studies that found an association between marital status (a marker for social support) and survival,65,66 being married in the present study was significantly associated with improved survival in the univariate analysis but was not an independent predictor in the multivariate model. Surgery was the only treatment variable associated with survival in the multivariate analysis, perhaps because, in many cases, those patients who receive surgery are the ones with the best prognosis (lower cancer stage and more localized disease). The lack of effects from radiation and chemotherapy is likely a result of the fact that there was little variability in treatment in that all patients were from tertiary care centers that provided standard of care treatment for their particular cancer site and stage. Interestingly, sex and race, which have been shown to predict survival in selected other studies of cancer patients,58,67 did not predict survival in this study.
To our knowledge, this study is the first to comprehensively assess the association of five pretreatment health behaviors and conventional prognostic factors with survival among head and neck cancer patients. Pretreatment health behaviors can identify those at risk for poor survival. Multicomponent behavioral interventions can be efficacious among head and neck cancer patients,30 and future research is needed to determine whether changes in the health behaviors after diagnosis can improve survival rates.
Footnotes
Supported by Grant No. P50 CA97248 from the National Institutes of Health through the University of Michigan's Head and Neck Specialized Program of Research Excellence.
Presented in part at the 7th International Conference on Head and Neck Cancer, American Head and Neck Society, July 19-23, 2008, San Francisco, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Sonia A. Duffy, David L. Ronis, Karen E. Fowler, Stephen B. Gruber, Gregory T. Wolf, Jeffrey E. Terrell
Financial support: Sonia A. Duffy, Gregory T. Wolf
Administrative support: Karen E. Fowler, Gregory T. Wolf, Jeffrey E. Terrell
Provision of study materials or patients: Scott McLean, Gregory T. Wolf
Collection and assembly of data: Sonia A. Duffy, Karen E. Fowler, Gregory T. Wolf
Data analysis and interpretation: Sonia A. Duffy, David L. Ronis, Scott McLean, Karen E. Fowler, Stephen B. Gruber, Gregory T. Wolf, Jeffrey E. Terrell
Manuscript writing: Sonia A. Duffy, David L. Ronis, Karen E. Fowler, Stephen B. Gruber, Jeffrey E. Terrell
Final approval of manuscript: Sonia A. Duffy, David L. Ronis, Scott McLean, Karen E. Fowler, Stephen B. Gruber, Gregory T. Wolf, Jeffrey E. Terrell
REFERENCES
- 1.Hoffman HT, Karnell LH, Funk GF, et al. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2003. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 3.Duffy SA, Terrell JE, Valenstein M, et al. Effect of smoking, alcohol, and depression on the quality of life of head and neck cancer patients. Gen Hosp Psychiatry. 2002;24:140–147. doi: 10.1016/s0163-8343(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 4.Gritz ER, Carmack CL, de Moor C, et al. First year after head and neck cancer: Quality of life. J Clin Oncol. 1999;17:352–360. doi: 10.1200/JCO.1999.17.1.352. [DOI] [PubMed] [Google Scholar]
- 5.Dikshit RP, Boffetta P, Bouchardy C, et al. Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: A multicentric European study. Int J Cancer. 2005;117:992–995. doi: 10.1002/ijc.21244. [DOI] [PubMed] [Google Scholar]
- 6.Levi F, Pasche C, La Vecchia C, et al. Food groups and risk of oral and pharyngeal cancer. Int J Cancer. 1998;77:705–709. doi: 10.1002/(sici)1097-0215(19980831)77:5<705::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Day GL, Shore RE, Blot WJ, et al. Dietary factors and second primary cancers: A follow-up of oral and pharyngeal cancer patients. Nutr Cancer. 1994;21:223–232. doi: 10.1080/01635589409514321. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(suppl 2):S51–S63. doi: 10.1016/j.ejon.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Couch M, Lai V, Cannon T, et al. Cancer cachexia syndrome in head and neck cancer patients: Part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck. 2007;29:401–411. doi: 10.1002/hed.20447. [DOI] [PubMed] [Google Scholar]
- 10.Di Fiore F, Lecleire S, Rigal O, et al. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12:4185–4190. doi: 10.3748/wjg.v12.i26.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thune I. Assessments of physical activity and cancer risk. Eur J Cancer Prev. 2000;9:387–393. doi: 10.1097/00008469-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 13.Gerber Y, Jacobsen SJ, Killian JM, et al. Participation bias assessment in a community-based study of myocardial infarction, 2002-2005. Mayo Clin Proc. 2007;82:933–938. doi: 10.4065/82.8.933. [DOI] [PubMed] [Google Scholar]
- 14.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 15.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 16.Brower KJ, Severin JD. Alcohol and other drug-related problems. In: Knesper DJ, Riba MB, Schwenk TL, editors. Primary Care Psychiatry. Philadelphia, PA: W. B. Saunders Company; 1997. [Google Scholar]
- 17.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services and US Department of Agriculture. Dietary Guidelines for Americans, 2005. Washington, DC: US Government Printing Office; 2005. [Google Scholar]
- 19.Ogden CL, Fryar CD, Carroll MD, et al. Mean body weight, height, and body mass index, United States 1960-2002. Adv Data. 2004;347:1–17. [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59:1121–1127. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 22.Hays RD, Sherbourne CD, Mazel RM. User's Manual for the Medical Outcomes Study (MOS) Core Measures of Health-Related Quality of Life. Santa Monica, CA: RAND; 1995. [Google Scholar]
- 23.American Joint Committee on Cancer. AJCC Cancer Staging Manual. ed 6. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 24.Piccirillo JF, Costas I, Claybour P, et al. The measurement of comorbidity by cancer registries. J Registry Manage. 2003;30:8–15. [Google Scholar]
- 25.Browman GP, Mohide EA, Willan A, et al. Association between smoking during radiotherapy and prognosis in head and neck cancer: A follow-up study. Head Neck. 2002;24:1031–1037. doi: 10.1002/hed.10168. [DOI] [PubMed] [Google Scholar]
- 26.Pytynia KB, Grant JR, Etzel CJ, et al. Matched-pair analysis of survival of never smokers and ever smokers with squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22:3981–3988. doi: 10.1200/JCO.2004.02.133. [DOI] [PubMed] [Google Scholar]
- 27.Duffy SA, Khan MJ, Ronis DL, et al. Health behaviors of head and neck cancer patients the first year after diagnosis. Head Neck. 2008;30:93–102. doi: 10.1002/hed.20665. [DOI] [PubMed] [Google Scholar]
- 28.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: Role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–137. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 29.Merriam-Webster's Desk Dictionary. Springfield, MA: Merriam-Webster, Inc; 1995. [Google Scholar]
- 30.Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:2203–2208. doi: 10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 31.Doggrell SA. Which is the best primary medication for long-term smoking cessation: Nicotine replacement therapy, bupropion or varenicline? Expert Opin Pharmacother. 2007;8:2903–2915. doi: 10.1517/14656566.8.17.2903. [DOI] [PubMed] [Google Scholar]
- 32.Duncan CL, Cummings SR, Hudes ES, et al. Quitting smoking: Reasons for quitting and predictors of cessation among medical patients. J Gen Intern Med. 1992;7:398–404. doi: 10.1007/BF02599155. [DOI] [PubMed] [Google Scholar]
- 33.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 34.Do KA, Johnson MM, Doherty DA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: Joint effects of smoking and alcohol (United States) Cancer Causes Control. 2003;14:131–138. doi: 10.1023/a:1023060315781. [DOI] [PubMed] [Google Scholar]
- 35.Núñez NP, Carter PA, Meadows GG. Alcohol consumption promotes body weight loss in melanoma-bearing mice. Alcohol Clin Exp Res. 2002;26:617–626. doi: 10.1097/00000374-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Duffy SA, Ronis DL, Valenstein M, et al. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007;48:142–148. doi: 10.1176/appi.psy.48.2.142. [DOI] [PubMed] [Google Scholar]
- 37.Littleton J, Barron S, Prendergast M, et al. Smoking kills (alcoholics)! Shouldn't we do something about it? Alcohol Alcohol. 2007;42:167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed AT, Karter AJ, Liu J. Alcohol consumption is inversely associated with adherence to diabetes self-care behaviours. Diabet Med. 2006;23:795–802. doi: 10.1111/j.1464-5491.2006.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krebs-Smith SM, Kantor LS. Choose a variety of fruits and vegetables daily: Understanding the complexities. J Nutr. 2001;131(suppl):487S–501S. doi: 10.1093/jn/131.2.487S. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin JK, Gridley G, Block G, et al. Dietary factors in oral and pharyngeal cancer. J Natl Cancer Inst. 1988;80:1237–1243. doi: 10.1093/jnci/80.15.1237. [DOI] [PubMed] [Google Scholar]
- 41.Petridou E, Zavras AI, Lefatzis D, et al. The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer. 2002;94:2981–2988. doi: 10.1002/cncr.10560. [DOI] [PubMed] [Google Scholar]
- 42.Cartmel B, Bowen D, Ross D, et al. A randomized trial of an intervention to increase fruit and vegetable intake in curatively treated patients with early-stage head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2848–2854. doi: 10.1158/1055-9965.EPI-05-0191. [DOI] [PubMed] [Google Scholar]
- 43.Dallongeville J, Marecaux N, Fruchart JC, et al. Cigarette smoking is associated with unhealthy patterns of nutrient intake: A meta-analysis. J Nutr. 1998;128:1450–1457. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- 44.Halsted CH. Alcohol: Medical and nutritional effects. In: Ziegler EE, Filer LJ, editors. Present Knowledge in Nutrition. ed 7. Washington, DC: ILSI Press; 1996. pp. 547–556. [Google Scholar]
- 45.Doerr TD, Marks SC, Shamsa FH, et al. Effects of zinc and nutritional status on clinical outcomes in head and neck cancer. Nutrition. 1998;14:489–495. doi: 10.1016/s0899-9007(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 46.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 47.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 48.Giovannucci EL, Liu Y, Leitzmann MF, et al. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165:1005–1010. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- 49.Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer. 2006;14:1012–1019. doi: 10.1007/s00520-006-0044-7. [DOI] [PubMed] [Google Scholar]
- 50.Mustian KM, Griggs JJ, Morrow GR, et al. Exercise and side effects among 749 patients during and after treatment for cancer: A University of Rochester Cancer Center Community Clinical Oncology Program Study. Support Care Cancer. 2006;14:732–741. doi: 10.1007/s00520-005-0912-6. [DOI] [PubMed] [Google Scholar]
- 51.Jones AS, Fenton JE, Husband DJ. Performance data and survival in head and neck cancer. Clin Otolaryngol Allied Sci. 2000;25:396–403. doi: 10.1046/j.1365-2273.2000.00387.x. [DOI] [PubMed] [Google Scholar]
- 52.Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: A review. Eur J Cancer Care (Engl) 2001;10:245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mercadante S, Girelli D, Casuccio A. Sleep disorders in advanced cancer patients: Prevalence and factors associated. Support Care Cancer. 2004;12:355–359. doi: 10.1007/s00520-004-0623-4. [DOI] [PubMed] [Google Scholar]
- 54.Friedman M, Landsberg R, Pryor S, et al. The occurrence of sleep-disordered breathing among patients with head and neck cancer. Laryngoscope. 2001;111:1917–1919. doi: 10.1097/00005537-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 55.O'Donnell JF. Insomnia in cancer patients. Clin Cornerstone. 2004;6(suppl 1D):S6–S14. doi: 10.1016/s1098-3597(05)80002-x. [DOI] [PubMed] [Google Scholar]
- 56.Brenner H, Arndt V. Recent increase in cancer survival according to age: Higher survival in all age groups, but widening age gradient. Cancer Causes Control. 2004;15:903–910. doi: 10.1007/s10552-004-1484-3. [DOI] [PubMed] [Google Scholar]
- 57.Pytynia KB, Grant JR, Etzel CJ, et al. Matched analysis of survival in patients with squamous cell carcinoma of the head and neck diagnosed before and after 40 years of age. Arch Otolaryngol Head Neck Surg. 2004;130:869–873. doi: 10.1001/archotol.130.7.869. [DOI] [PubMed] [Google Scholar]
- 58.Arbes SJ, Jr, Olshan AF, Caplan DJ, et al. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States) Cancer Causes Control. 1999;10:513–523. doi: 10.1023/a:1008911300100. [DOI] [PubMed] [Google Scholar]
- 59.Paterson IC, John G, Adams Jones D. Effect of deprivation on survival of patients with head and neck cancer: A study of 20,131 cases. Clin Oncol (R Coll Radiol) 2002;14:455–458. doi: 10.1053/clon.2002.0159. [DOI] [PubMed] [Google Scholar]
- 60.Baatenburg de Jong RJ, Hermans J, Molenaar J, et al. Prediction of survival in patients with head and neck cancer. Head Neck. 2001;23:718–724. doi: 10.1002/hed.1102. [DOI] [PubMed] [Google Scholar]
- 61.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 62.Pugliano FA, Piccirillo JF, Zequeira MR, et al. Clinical-severity staging system for oral cavity cancer: Five-year survival rates. Otolaryngol Head Neck Surg. 1999;120:38–45. doi: 10.1016/S0194-5998(99)70367-0. [DOI] [PubMed] [Google Scholar]
- 63.Woodard TD, Oplatek A, Petruzzelli GJ. Life after total laryngectomy: A measure of long-term survival, function, and quality of life. Arch Otolaryngol Head Neck Surg. 2007;133:526–532. doi: 10.1001/archotol.133.6.526. [DOI] [PubMed] [Google Scholar]
- 64.Duffy SA, Taylor JM, Terrell JE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 65.Vercelli M, Lillini R, Capocaccia R, et al. Cancer survival in the elderly: Effects of socio-economic factors and health care system features (ELDCARE project) Eur J Cancer. 2006;42:234–242. doi: 10.1016/j.ejca.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 66.de Graeff A, de Leeuw JR, Hordijk GJ, et al. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 67.Miller CS, Henry RG, Rayens MK. Disparities in risk of and survival from oropharyngeal squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:570–575. doi: 10.1067/moe.2003.108. [DOI] [PubMed] [Google Scholar]