Abstract
Purpose
Vorinostat, a histone deacetylase inhibitor, represents a rational therapeutic target in glioblastoma multiforme (GBM).
Patients and Methods
Patients with recurrent GBM who had received one or fewer chemotherapy regimens for progressive disease were eligible. Vorinostat was administered at a dose of 200 mg orally twice a day for 14 days, followed by a 7-day rest period.
Results
A total of 66 patients were treated. Grade 3 or worse nonhematologic toxicity occurred in 26% of patients and consisted mainly of fatigue (17%), dehydration (6%), and hypernatremia (5%); grade 3 or worse hematologic toxicity occurred in 26% of patients and consisted mainly of thrombocytopenia (22%). Pharmacokinetic analysis showed lower vorinostat maximum concentration and area under the curve (0 to 24 hours) values in patients treated with enzyme-inducing anticonvulsants, although this did not reach statistical significance. The trial met the prospectively defined primary efficacy end point, with nine of the first 52 patients being progression-free at 6 months. Median overall survival from study entry was 5.7 months (range, 0.7 to 28+ months). Immunohistochemical analysis performed in paired baseline and post-vorinostat treatment samples in a separate surgical subgroup of five patients with recurrent GBM showed post treatment increase in acetylation of histones H2B and H4 (four of five patients) and of histone H3 (three of five patients). Microarray RNA analysis in the same samples showed changes in genes regulated by vorinostat, such as upregulation of E-cadherin (P = .02).
Conclusion
Vorinostat monotherapy is well tolerated in patients with recurrent GBM and has modest single-agent activity. Histone acetylation analysis and RNA expression profiling indicate that vorinostat in this dose and schedule affects target pathways in GBM. Additional testing of vorinostat in combination regimens is warranted.
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults and has a dismal prognosis, with a 12- to 16-month median survival despite the use of multimodality treatment.1 Treatment options are limited at recurrence. There is an urgent need for development of novel therapeutic agents.
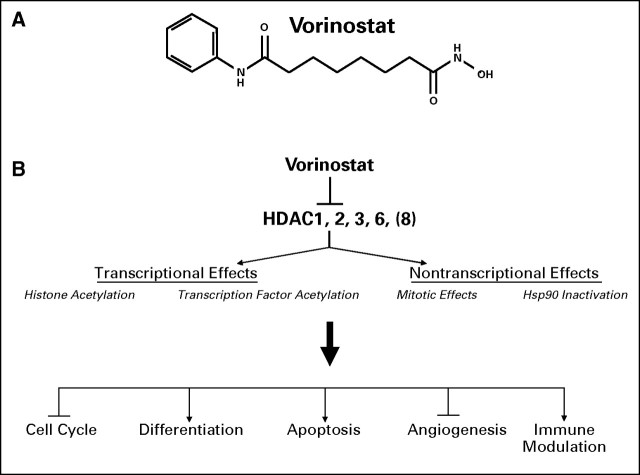
Vorinostat (suberoylanilide hydroxamic acid [SAHA]; Fig 1) is a small-molecule inhibitor of most human class I and class II histone deacetylases (HDAC) that binds directly at the enzyme's active site in the presence of zinc ion.2 The action of HDACs on nucleosomal histones leads to tight coiling of chromatin and silencing of expression of various genes, including those implicated in the regulation of cell survival, proliferation, tumor cell differentiation, cell cycle arrest, and apoptosis.3 The effects of HDACs are not limited to histone deacetylation. They also act as members of protein complexes to recruit transcription factors to the promoter region of genes, including those of tumor suppressors, and they affect the acetylation status of specific cell cycle regulatory proteins.4,5
Fig 1.
(A) Structure of vorinostat. (B) Mechanisms of vorinostat antitumor activity. HDAC, histone deacetylase.
There is preclinical evidence that vorinostat has antitumor activity against malignant glioma cell lines in vitro and orthotopic xenografts in vivo.6–8 Exposure of glioma cells to vorinostat resulted in increased expression of apoptotic and antiproliferative genes such as DR5, tumor necrosis factor α, p21Waf1, and p27Kip1 and decreased expression of antiapoptotic genes such as CDK2, CDK4, cyclin D1, and cyclin D2.8 In animal experiments, there was increased H3 and H4 acetylation in brain tissue after treatment, supporting the conclusion that vorinostat crosses the blood-brain barrier.8,9 Furthermore, suppression of tumor growth in a GL26 orthotopic glioma model and prolongation of survival8 was observed.
The goal of this phase II trial was to identify any clinical efficacy of vorinostat in the treatment of recurrent GBM as measured by 6 month progression-free survival, assess the safety and toxicity of vorinostat in this patient population, assess vorinostat pharmacokinetics in patients with glioblastoma, and study its biologic effects in target tumor issues.
PATIENTS AND METHODS
Eligibility Criteria
Eligible patients were 18 years of age or older and had histologic confirmation of grade 4 astrocytoma at primary diagnosis or recurrence. They were also required to be treated with a stable dose of corticosteroids or no corticosteroids for ≥ 1 week before their baseline imaging, to have received no more than one prior chemotherapy regimen for progressive or recurrent disease, to have had their last chemotherapy treatment ≥ 4 weeks before study entry (≥ 6 weeks if nitrosourea was administered), and to be ≥ 10 weeks from completion of radiotherapy. They were also required to have an Eastern Cooperative Oncology Group performance score of 0 to 2; acceptable hematologic function, defined as absolute neutrophil count ≥ 1,500/μL, platelets ≥ 100,000/μL, and hemoglobin ≥ 9 g/dL; adequate hepatic and renal function, defined as total bilirubin ≤ 1.5 mg/dL, AST ≤ 3× upper limit of normal, and creatinine ≤ 2 mg/dL. If patients were treated with valproic acid (an HDAC inhibitor), this should have been discontinued for at least 2 weeks before study entry.
Study Treatment
Vorinostat was administered at a dose of 200 mg orally twice a day for 14 days, followed by a 7-day rest period. Patients who tolerated the first treatment cycle with toxicity ≤ grade 1 had the dose of vorinostat escalated to 300 mg twice a day (administered for 14 days, followed by a 7-day rest period) during the second treatment cycle. To assess the impact of vorinostat on target tumor pathways, the study also included a group of patients who had surgery planned as part of routine management of their recurrent GBM. Patients in the surgery arm received 200 mg of vorinostat twice a day for six doses, with the last dose administered the morning of surgery. After surgical resection and recovery from surgery, vorinostat treatment was resumed. Patients in the surgical arm, however, were not included in efficacy analysis.
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria version 3.0. Vorinostat dose was decreased by 100 mg per day for grade 3 thrombocytopenia and by 100 mg twice a day for grade 4 neutropenia, thrombocytopenia, or grade 4 nonhematologic toxicity. Both hematologic and nonhematologic toxicity had to resolve to ≤ grade 1 for patients to be allowed re-treatment.
Definition of Response
Neuroimaging with magnetic resonance imaging was performed at baseline, before the third treatment cycle, and every second cycle thereafter. For patients with measurable disease, the MacDonald criteria were used for response assessment.10
For patients with nonmeasurable but assessable disease, regression was defined as unequivocal reduction in size of contrast enhancement or decrease in mass effect as determined by primary physician and quality control physicians and no new lesion, with the patient receiving stable or decreased corticosteroid dose. Progression was defined as unequivocal increase in size of contrast enhancement or increase in mass effect as assessed by primary physician and quality control physicians or appearance of new lesions. Patients with imaging findings not meeting criteria for complete response, regression, or progression were determined to have stable disease.
Statistical Considerations and Methodology
A one-stage phase II design with interim analysis based on a modified Fleming design11 was used. The primary end point of the trial was the percentage of patients alive and progression-free at 6 months (PFS6). Secondary end points included confirmed tumor response, overall survival, and time to progression. The design tested the null hypothesis that the PFS6 rate was ≤ 10%, which is the historical PFS6 rate of North Central Cancer Treatment Group (NCCTG) patients with recurrent GBM.12–14 The trial had 90% power, with an α error of 0.10 to declare the regimen active if the true PFS6 rate was at least 25% or more.
Time to progression was defined as time from study entry to disease progression; patients who died were considered to have disease progression at time of death unless there was documented evidence that no progression occurred before death. Overall survival was defined as time from study entry to death from any cause. Patients who have not died or experienced disease progression were censored at last known follow-up. Associations of categoric baseline outcome and translational data were tested using χ2 and Fisher's exact test. Comparisons of continuous baseline, outcome, and translational data were tested using Wilcoxon rank-sum test. Survival and time to progression curves were compared via the log-rank test; Cox proportional hazards models were used to assess the relationship between time-to-event end points and outcome.
Vorinostat Pharmacokinetics
Serum samples were isolated from blood (5 mL) collected from a peripheral vein into anticoagulant-free tubes before treatment and 30, 60, 120, 150, 180, 240, 360, and 480 minutes after drug administration on day 1 and day 8 of cycle 1. Samples were analyzed using an liquid chromatography with tandem mass spectometry system (Micromass, Manchester, United Kingdom). Pharmacokinetic data were analyzed by noncompartmental methods15 using the Program WINNonlin Professional, version 4.1 (Pharsight Corp, Mountain View, CA).
Immunohistochemistry for p21waf1 and p27kip1 Expression
Expression of p21waf1 and p27kip1 proteins was determined by immunohistochemistry. Formalin-fixed, paraffin-embedded samples were deparaffinized with three changes of xylene, rehydrated in a series of alcohols (100%, 95%, then 70% alcohol), placed in a preheated 1 mmol/L EDTA, pH 8.0 retrieval buffer for 30 minutes, cooled in the buffer for 5 minutes, and rinsed in running distilled water. Slides were then placed on the DAKO Autostainer (DAKO, Carpenteria, CA) for the following procedure (room temperature): incubation with 3% H2O2 in ethanol for 5 minutes, incubation with 1:35 p21 clone SX118 (M7202, DAKO Cytomation) overnight for p21waf1 detection, or with 1:100 p27 (M7203, DAKO Cytomation) for 60 minutes for p27kip1 detection, and rinsing with Tris-buffered saline TWEEN wash buffer. The secondary antibody, mEnVision+ Polymer (DAKO Cytomation) was then added for 15 minutes, and the slides were rinsed with Tris-buffered saline TWEEN wash buffer, incubated in 3,3′-diaminobenzidine (DAKO Cytomation) for 5 minutes, counterstained with modified Schmidt's hematoxylin for 5 minutes followed by a 3-minute tap water rinse, dehydrated through graded alcohols, cleared in three changes of xylene, and mounted with a permanent mounting media.
Assessment of Histone Acetylation
Histone acetylation was assessed in formalin-fixed, paraffin-embedded sections by immunohistochemical staining. Sections (5 μm) were incubated with polyclonal antibodies directed against acetylated histone H2B, lysine 5 (1:25 dilution; Cell Signaling Technology, Danvers, MA), acetylated histone H3, lysine 9 (1:25 dilution; Cell Signaling Technology) or acetylated histone H4, and lysine 8 (1:100 dilution, Cell Signaling Technology), followed by a biotinylated secondary antibody (0.5 μg/mL; Jackson Immunoresearch, West Grove, PA) and avidin-biotin complex/3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA), then hematoxylin counterstaining. Quantitative analysis was performed on an Aiol SL-50 slide scanning system (Applied Imaging, Mountain View, CA).
Gene Expression Profiling
Total RNA isolated from formalin-fixed, paraffin-embedded tissue was used to make fluorescently labeled cRNA that was hybridized to DNA oligonucleotide microarrays, as described previously.16,17 Gene expression data analysis was performed with the Rosetta Resolver gene expression analysis software (version 6.0, Rosetta Biosoftware, Seattle, WA) and MATLAB software (version 7.0.4, Mathworks, Natick, MA).
RESULTS
Patient Characteristics
Sixty-eight patients were enrolled onto the study. Two patients withdrew before treatment initiation. Table 1 lists the characteristics of the remaining 66 patients. Median number of cycles in the 66 patients receiving treatment was two (range, one to 37 cycles).
Table 1.
Baseline Characteristics (N = 66)
| Characteristic | No. | % |
|---|---|---|
| Sex | ||
| Female | 29 | 44 |
| Male | 37 | 56 |
| Age, years | ||
| Median | 58 | |
| Range | 26-78 | |
| Time from radiotherapy to treatment, months | ||
| Median | 9.3 | |
| Range | 2.4-59 | |
| Performance score | ||
| 0 | 10 | 15 |
| 1 | 37 | 56 |
| 2 | 19 | 29 |
| Enzyme-inducing anticonvulsants | ||
| Yes | 19 | 29 |
| No | 47 | 71 |
| No. of prior chemotherapy regimens for recurrent disease | ||
| 0 | 30 | 45 |
| 1 | 36 | 55 |
| Prior temozolomide | ||
| Yes | 58 | 98 |
| No | 1 | 2 |
| Prior nitrosourea | ||
| Yes | 8 | 12 |
| No | 58 | 88 |
| Corticosteroid therapy at enrollment | ||
| Yes | 49 | 74 |
| No | 17 | 26 |
| Measurable disease | ||
| Measurable | 48 | 73 |
| Nonmeasurable but assessable | 18 | 27 |
Toxicity
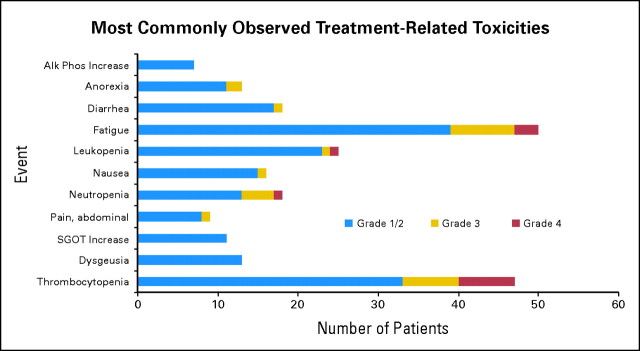
Figure 2 summarizes treated-related toxicity observed in the trial. Overall, vorinostat treatment was well tolerated. Grade 3 to 4 hematologic toxicity was observed in 26% of the patients, with most common toxicities being thrombocytopenia (11% grade 3, 11% grade 4), neutropenia (6% grade 3, 11% grade 4), and anemia (3% grade 3). The overall incidence of grade 3 to 4 nonhematologic toxicity was 26%, with most common toxicities being fatigue (12% grade 3, 5% grade 4), dehydration (6% grade 3), and hypernatremia (5% grade 3). In all, 18 (35%) of the 52 patients treated with multiple cycles required dose reduction below the 200 mg twice a day dose as a result of toxicity. In nine patients, vorinostat dose was escalated to 300 mg twice a day (2 of 3 weeks) in the second cycle as per the initial protocol design. This dose escalation resulted in increased incidence of grade 3 and 4 thrombocytopenia (44% as compared with 18% in the 200 mg twice a day dosing): the protocol was amended and intrapatient vorinostat dose escalation was aborted after the first nine patients had their dose increased to 300 mg twice a day.
Fig 2.
Most commonly observed treatment-related toxicities for patients with glioblastoma receiving vorinostat; most toxicities were grade 1 to 2. Most frequent grade 3 to 4 hematologic toxicity was thrombocytopenia (22%), and most common grade 3 to 4 nonhematologic toxicity was fatigue (17%). Alk Phos, alkaline phosphatase; SGOT, aspartate aminotransferase.
There was no significant difference in toxicity between patients receiving enzyme-inducing anticonvulsants (EIACs) and those not receiving EIACs (Appendix Table A1, online only), except for grade 3 to 4 thrombocytopenia, which was more common on patients not receiving EIACs: 28% versus 5%, (P = .05).
Response and Outcome Assessment
The trial met its primary efficacy end point, both at the interim analysis, with five of 22 patients being progression-free at 6 months, and final analysis, with nine of the first 52 patients being progression-free at 6 months. Median time to progression was 1.9 months (range, 0.3 to 28+ months). The overall percentage of patients alive and progression-free at 6 months was 15.2% (10 of 66 patients). Of note is the long duration of disease stability in patients who were progression-free at 6 months; the median was 11.2 months (range, 6.8 to 28+ months). Median time from completion of radiation therapy in the PFS6 patients was 9 months (range, 2.4 to 30.2 months). Median overall survival from study entry for all patients was 5.7 months (range, 0.7 to 28+ months). There was a slight time to progression advantage (hazard ratio = 0.54, 95% CI, 0.25 to 1.17) for patients in whom vorinostat dose was escalated to 300 mg for two or more treatment cycles, but this did not reach statistical significance (P = .09). Similarly, use of EIACs did not have an impact on outcome (hazard ratio = 1.24; P = .43).
Objective responses were infrequent. Only two patients achieved objective responses according to the MacDonald criteria.
Pharmacokinetics
Vorinostat is metabolized via glucuronidation and β-oxidation; therefore, pharmacokinetics were investigated in a subset of patients to assess a possibility of interaction with EIACs. Pharmacokinetic analysis of vorinostat and its metabolites was performed on four patients being treated with EIACs and eight patients not being treated with EIACs after administration of the first dose on day 1 and after the morning dose on day 8. Results are summarized in Table 2. The vorinostat half-life was longer and the maximum concentration (Cmax) value was lower in patients who received EIACs. Vorinostat glucuronide half-life was longer, whereas Cmax and area under the curve (AUC0-8 hours) values were lower for patients receiving EIACs. Nevertheless, these changes did not reach statistical significance. Finally, consistent with the absence of an effect of EIACs on β-oxidation, there were no differences in half-life Cmax and AUC0-8 hours of 4-anilino-4-oxobutanoic acid.
Table 2.
Pharmacokinetics of SAHA and Its Metabolites
| Metabolite and Parameter | No EIACs (n = 8) |
EIACs (n = 4) |
||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 |
Day 8 |
Day 1 |
Day 8 |
|||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Vorinostat | ||||||||
| Half-life, hours | 1.42 | 0.88 | 2.87 | 1.85 | 2.14 | 1.08 | 1.94 | 0.27 |
| Tmax, hours | 1.01 | 0.65 | 2.19 | 2.58* | 1.76 | 0.51 | 1.75 | 1.66 |
| Cmax, ng/mL | 129 | 65 | 124 | 65 | 90 | 32 | 159 | 86 |
| AUC0-8h, ng/m·h | 278 | 84 | 369 | 175 | 271 | 120 | 353 | 165 |
| Vorinostat-glucuronide | ||||||||
| Half-life, hours | 1.89 | 1.25 | 11.3 | 19.7† | 2.12 | 0.90 | 1.97 | 0.5 |
| Tmax, hours | 1.97 | 1.00 | 2.81 | 2.40 | 1.76 | 0.51 | 1.88 | 1.55 |
| Cmax, ng/mL | 709 | 276 | 833 | 392 | 1,020 | 380 | 1,110 | 610 |
| AUC0-8h, ng/mL·h | 2,410 | 1,500 | 3,280 | 2,310 | 3,660 | 2,020 | 3,640 | 2,010 |
| 4-Anilino-4-Oxobutanoic acid | ||||||||
| Half-life, hours | 5.13 | 2.46 | 11.3 | 12.4‡ | 2.39 | 1.07 | 40.3 | 73.9 |
| Tmax, hours | 2.2 | 0.7 | 3.5 | 2.5 | 2.4 | 0.3 | 2.9 | 0.9 |
| Cmax, ng/mL | 753 | 270 | 1,060 | 340 | 748 | 316 | 1,070 | 840 |
| AUC0-8h, ng/mL·h | 3,330 | 1,080 | 5,980 | 2,350 | 3,160 | 1,240 | 5,050 | 3,240 |
Abbreviations: SAHA, suberoylanilide hydroxamic acid; EIACs, enzyme-inducing anticonvulsants; Tmax, time that peak plasma concentration is achieved; Cmax, maximum concentration; AUC0-8 hours, area under the curve 0 to 8 hours.
n = 6.
n = 7.
n = 4.
Correlative Laboratory Analysis
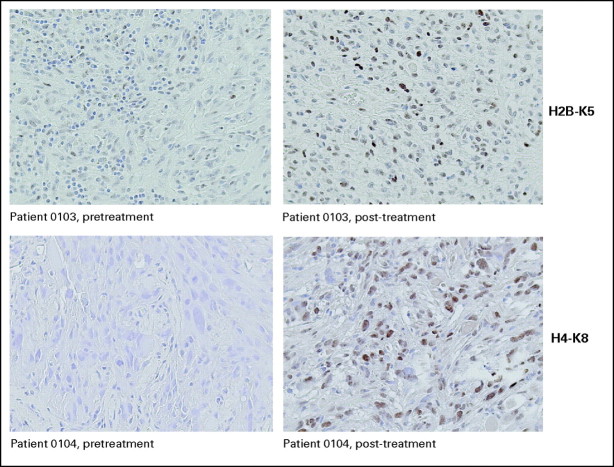
Surgical samples from five surgically treated patients who received vorinostat before surgery were analyzed by immunohistochemical staining to measure acetylation of histones H3, H2B, and H4 before and after treatment with vorinostat. Last vorinostat dose was administered the morning of surgery. Tumor cell nuclei were analyzed in nonnecrotic areas of representative sections from excised tumor tissue. Increase in acetylation of histones H2B and H4 after vorinostat treatment was observed in four of five patients and histone H3 in three of five patients (Fig 3).
Fig 3.
Increase in acetylation of histones H2B and H4 after vorinostat treatment in tumors of two study patients.
In addition, RNA from these samples was isolated and analyzed by microarrays to determine changes in genome-wide gene expression patterns induced by vorinostat therapy. Although the small number of subjects tested and the variability among subjects complicates interpretation, changes in genes known to be regulated by vorinostat were observed in posttreatment glioma samples. For example, significant increases in e-cadherin gene expression, a gene known to be upregulated in response to vorinostat, were observed in four of five patients (P = .02). These histone acetylation and gene regulation results collectively suggest that vorinostat, at doses used in our trial, reaches the glioblastoma tumor and affects vorinostat-dependent pathways.
Baseline tumor tissue for immunohistochemical analysis was available in 50 of the 66 patients of the nonsurgical group. Tumor expression levels of either p21waf1 or p27kip1, known to be upregulated by vorinostat,18 were not associated with progression-free survival (P = .92 and P = .20, respectively). Categorization of tumor staining, as well as adjustment for relevant clinical factors, also did not result in any statistically significant association between tumor staining and outcome.
DISCUSSION
HDAC inhibition is a rational therapeutic target in GBM treatment.6–8 This NCCTG phase II trial of vorinostat represents the first study of an HDAC inhibitor in patients with glioma. Vorinostat at a dose of 200 mg twice a day for 14 days every 3 weeks was well tolerated. Most common grade 3 and 4 toxicities were thrombocytopenia (11% grade 3, 11% grade 4), fatigue (12% grade 3, 5% grade 4), and dehydration (6% grade 3). The trial met its primary efficacy end point, with nine of the first 52 patients being free of progression at 6 months. PFS6 for all 66 patients was 15.2%, and median survival 5.7 months. Of note is the prolonged duration of disease stability in patients who met the trial's primary end point. Median duration of stable disease in these patients was 11.2 months, with a range of 6.8 to 28+ months. It therefore seems that there is a GBM patient subpopulation that can receive definite clinical benefit from vorinostat treatment. We are currently in the process of collecting baseline tissue for all study patients as well as pre- and posttreatment samples from a larger surgical cohort and performing RNA expression profiling in an attempt to characterize a molecular signature that can predict response to vorinostat treatment.
On the basis of the pharmacokinetic analysis performed in this trial, EIACs seem to have a small but statistically insignificant effect on vorinostat exposure. Furthermore, there was no significant difference between patients receiving and not receiving EIACs as it pertains to grade 3 and 4 treatment-related toxicity, with the exception of grade 3 to 4 thrombocytopenia (5% v 28%, P = .05). Finally, clinical outcome was not affected by EIAC use (P = .43). On the basis of these data, vorinostat dose modification is not necessary in patients receiving EIACs.
One of the significant challenges in glioma trials pertains to the difficulty in assessing whether the therapeutic agent reaches the target tumor in the CNS and affects the target molecular pathways. Our analysis of tumors in five patients who received vorinostat before surgery showed increases in H2B, H4, and H3 histone acetylation and changes in gene expression profiling that are suggestive of vorinostat interaction with the target pathway. These data are consistent with data in GL26 orthotopic brain tumor xenografts8 and with data in a Huntington disease mouse model9 and indicate that orally administered vorinostat at doses used in our trial can effectively reach and block its CNS target.
On the basis of our data, incorporation of vorinostat in combination regimens as part of rationally designed combinations with other chemotherapy agents or small-molecule cell cycle inhibitors16 should be considered. The ability of HDAC inhibitors, such as vorinostat, to alter nucleosome structure and chromatin confirmation suggests that they may have the capacity to modulate sensitivity to chemotherapeutic agents targeting DNA or enzymes acting on DNA by permitting better access of DNA-targeted agents to the chromatin. Indeed, pretreatment with vorinostat increased the killing efficiency of etoposide, doxorubicin, and cisplatin against tumor lines, including the glioblastoma lines D54 and U118.9 An North American Brain Tumor Coalition phase I/II trial of vorinostat in combination with temozolomide is nearing completion (P. Wen, personal communication, December 2008),19 and a phase I/II randomized trial of vorinostat/isotretinoin versus carboplatin/isotretinoin versus vorinostat/carboplatin/isotretinoin is ongoing (V. Puduvalli, personal communication, December 2008).
Furthermore, there is evidence that vorinostat enhances radiation-induced cytotoxicity in glioblastoma cell lines18 by inducing dose-dependent inhibition of proliferation and increasing radiation-induced apoptosis. The mechanism by which vorinostat enhances radiation sensitivity is possibly related to downregulation of several oncoproteins such as epidermal growth factor receptor, Akt, and DNA damage repair proteins (DNA-Pk and RAD51) that have been implicated in mediating radiation resistance20 and decreased expression of the repair-related genes Ku70, Ku80, and Rad50.21 An NCCTG/North American Brain Tumor Coalition phase I/II trial of vorinostat in combination with temozolomide and radiation therapy followed by temozolomide/vorinostat in patients with newly diagnosed GBM is soon to be activated.
In summary, vorinostat has modest single-agent activity in patients with recurrent GBM. Nevertheless, the existence of a patient subpopulation that derived clinical benefit from this agent, its excellent tolerance, and the potential of synergy with alkylating agents, other cell cycle inhibitors, and radiation therapy support testing of vorinostat in rationally designed combinations in the treatment of patients with newly diagnosed and recurrent gliomas.
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Table A1.
Maximum Grade Treatment-Related Events by EIAC Use
| Variable | Yes (n = 19) |
No (n = 47) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Hematologic adverse event | |||||
| Grade 3 | 1 | 5 | 9 | 19 | .26 |
| Grade 4 | 1 | 5 | 6 | 13 | .67 |
| Nonhematologic adverse event | |||||
| Grade 3 | 2 | 11 | 9 | 10 | .49 |
| Grade 4 | 0 | 0 | 5 | 11 | .31 |
| Grade 3/4 thrombocytopenia | 1 | 5 | 13 | 28 | .05 |
Abbreviation: EIAC, enzyme-inducing anticonvulsants.
Footnotes
Supported by North Central Cancer Treatment Group Grants NO. U10 CA 25224 and TRI 23XS026-T18 and Cancer Center Grant No. P30 CA 15083.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: James S. Hardwick, Merck & Co (C); John F. Reilly, Merck & Co (C); Andrey Loboda, Merck & Co (C); Michael Nebozhyn, Merck & Co (C); Valeria R. Fantin, Merck & Co (C); Victoria M. Richon, Merck & Co (C) Consultant or Advisory Role: None Stock Ownership: James S. Hardwick, Merck & Co Honoraria: None Research Funding: James S. Hardwick, Merck & Co Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Evanthia Galanis, Kurt A. Jaeckle, James Zwiebel, Jan C. Buckner
Provision of study materials or patients: Evanthia Galanis, Kurt A. Jaeckle, Patrick J. Flynn, Dennis F. Moore Jr, Jan C. Buckner
Collection and assembly of data: Evanthia Galanis, Matthew J. Maurer, Joel M. Reid, Matthew M. Ames, James S. Hardwick, John F. Reilly, Andrey Loboda, Michael Nebozhyn, Valeria R. Fantin, Victoria M. Richon, Bernd Scheithauer, Caterina Giannini
Data analysis and interpretation: Evanthia Galanis, Matthew J. Maurer, Joel M. Reid, Bernd Scheithauer, Caterina Giannini, James Zwiebel, Jan C. Buckner
Manuscript writing: Evanthia Galanis
Final approval of manuscript: Evanthia Galanis, Kurt A. Jaeckle, Matthew J. Maurer, Joel M. Reid, Matthew M. Ames, James S. Hardwick, John F. Reilly, Andrey Loboda, Michael Nebozhyn, Valeria R. Fantin, Victoria M. Richon, Bernd Scheithauer, Caterina Giannini, Patrick J. Flynn, Dennis F. Moore Jr, James Zwiebel, Jan C. Buckner
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Marks P, Rifkind RA, Richon VM, et al. Histone deacetylases and cancer: Causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 4.Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 5.Arts J, de Schepper S, Van Emelen K. Histone deacetylase inhibitors: From chromatin remodeling to experimental cancer therapeutics. Curr Med Chem. 2003;10:2343–2350. doi: 10.2174/0929867033456657. [DOI] [PubMed] [Google Scholar]
- 6.Eyupoglu IY, Hahnen E, Buslei R, et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93:992–999. doi: 10.1111/j.1471-4159.2005.03098.x. [DOI] [PubMed] [Google Scholar]
- 7.Ugur HC, Ramakrishna N, Bello L, et al. Continuous intracranial administration of suberoylanilide hydroxamic acid (SAHA) inhibits tumor growth in an orthotopic glioma model. J Neurooncol. 2007;83:267–275. doi: 10.1007/s11060-007-9337-z. [DOI] [PubMed] [Google Scholar]
- 8.Yin D, Ong JM, Hu J, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: Effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13:1045–1052. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Blake M, Baek JH, et al. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–7300. [PubMed] [Google Scholar]
- 10.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 11.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 12.Reid J, Buckner JC, Schaaf L. Pharmacokinetics of irinotecan (CPT-11) in recurrent glioma patients: Results of an NCCTG phase II trial. Proc Am Soc Clin Oncol. 1999;18:141a. abstr 540. [Google Scholar]
- 13.Galanis E, Buckner JC, Maurer MJ, et al. Phase I/II trial of pyrazoloacridine and carboplatin in patients with recurrent glioma: A North Central Cancer Treatment Group trial. Invest New Drugs. 2005;23:495–503. doi: 10.1007/s10637-005-2910-4. [DOI] [PubMed] [Google Scholar]
- 14.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 15.Gibaldi M, Perrier D. Non compartmental analysis based on statistical moment therapy. In: Gibaldi M, Perrier D, editors. Pharmacokinetics. ed 2. New York, NY: Marcel Dekker; 1982. pp. 409–417. [Google Scholar]
- 16.Hughes TR, Mao M, Jones AR, et al. Expression profiling using microarrays fabricated by an ink-jet oligonucleotide synthesizer. Nat Biotechnol. 2001;19:342–347. doi: 10.1038/86730. [DOI] [PubMed] [Google Scholar]
- 17.Marton MJ, DeRisi JL, Bennett HA, et al. Drug target validation and identification of secondary drug target effects using DNA microarrays. Nat Med. 1998;4:1293–1301. doi: 10.1038/3282. [DOI] [PubMed] [Google Scholar]
- 18.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 19.Wen PY, Puduvalli V, Kuhn J, et al. Phase I study of vorinostat (suberoylanilide hydroxamic acid) in combination with temozolomide (TMZ) in patients with malignant gliomas (NABTC 04-03) J Clin Oncol. 2007;25:2039. [Google Scholar]
- 20.Chinnaiyan P, Vallabhaneni G, Armstrong E, et al. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005;62:223–229. doi: 10.1016/j.ijrobp.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 21.Munshi A, Tanaka T, Hobbs ML, et al. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther. 2006;5:1967–1974. doi: 10.1158/1535-7163.MCT-06-0022. [DOI] [PubMed] [Google Scholar]