Abstract
Elevated deoxycholic acid (DCA), mutations in the adenomatous polyposis coli (APC) gene and chronic inflammation are associated with increased risk of colorectal cancer (CRC). APC status was manipulated to determine whether DCA mediates inflammatory molecules in normal or initiated colonic mucosa. DCA increased steady state mRNA and protein levels of CXCL8 in cells which do not express wild type APC. Steady state CXCL8 mRNA and protein were suppressed when cells with conditional expression of wild type APC were exposed to DCA. Immunostaining did not detect CXCL8 in normal human colonic mucosa. CXCL8 was expressed in adenomatous polyps and adenocarcinomas. CXCL8 expression correlated with nuclear β-catenin localization in epithelial cells of adenomas, but was associated with endothelial cells and neutrophils in the adenocarcinomas. DCA-mediated CXCL8 promoter-reporter activity was elevated in a mutant APC background. Wild type APC suppressed this effect. Mutation of activator protein-1 (AP-1) or nuclear factor kappa B (NF-κB) sites suppressed the activation of the CXCL8 promoter-reporter by DCA. Chromatin immunoprecipitation (ChIP) revealed that AP-1 and NF-κB binding to the 5′-promoter of CXCL8 was induced by DCA. The β-catenin transcription factor was bound to the 5′-promoter of CXCL8 in the absence or presence of DCA. Phenotypic assays determined that DCA-mediated invasion was blocked by antibody directed against CXCL8 or wild type-APC. CXCL8 exposure lead to matrix metalloproteinase-2 (MMP-2) production and increased invasion on laminin coated filters. These data suggest that DCA-mediated CXCL8 occurs in initiated colonic epithelium and neutralizing CXCL8 could reduce the invasive potential of tumors.
Keywords: colorectal cancer, molecular mechanisms of angiogenesis, tumor microcirculation and microenvironment, APC, cytokines, CXCL8, bile acid, DCA
Introduction
Colorectal cancer (CRC) is a major cause of morbidity and mortality in the United States 1, 2. Both environmental and genetic risk factors are recognized 3. High levels of fecal deoxycholic acid (DCA), a secondary bile acid, are common in high fat, western diets 4–6. Normal diets average 100 μM DCA (range 50–200 μM) while high fat diets average closer to 200 μM DCA and may exceed 700 μM 7. The carcinogenic potential of DCA has been shown to activate intracellular signaling to increase transcription factors in the nucleus AP-1, C/EBP and NF-κB 8–10.
Patients with Familial Adenomatous Polyposis (FAP) inherit a mutated Adenomatous Polyposis Coli (APC) allele and acquire another mutation in the tumor suppressor gene 11. Two somatic, allelic, APC mutations are responsible for sporadic CRC 12. Mutations in the APC gene products facilitate Wnt signaling and increase tumorigenesis through oncogenes 13, 14. Nuclear localization of transcription factors, production of proteinases and cellular invasion are consistent markers of epithelial-mesenchymal transition (EMT) 15, 16.
A causal interaction between chronic inflammation and cancer was hypothesized in 1863 by Virchow and may contribute to the tumor microenvironment 17. Markers of inflammation include chemotactic cytokines, or chemokines which normally recruit leukocytes and aid in blood vessel remodeling 18. Pathophysiological roles for chemokines include behaving as autocrine growth factors, disruption of basement membranes, increasing motility and invasion 19–22. Patients with CRC demonstrate elevated levels of circulating chemokines 23, 24. Currently chemokines are being evaluated as targeted therapies in a variety of cancers 25–27.
DCA increases CXCL8 (previously identified as interleukin-8) production in colonic 28–30 and esophageal cell lines31. Activation of the Wnt signaling pathway increases CXCL8 expression in other models 32, 33. CXCL8 is an inducible, proinflammatory, chemokine which binds to 7-transmembrane, G-protein coupled receptors 18, 28, 34. CXCL8 over expression increased angiogenesis in endothelial models, stimulated migration, EMT and acted as an autocrine growth factor in colon cell models 35–38. Over expression of CXCL8 increased the size and vessel density of metastatic lesions in lung cancer models 39. Matrix metalloproteinase-2 (MMP-2) activity has been induced by CXCL8, and shown to increase tumorigenicity 26, 40, 41. Elevated CXCL8 is associated with increased angiogenesis in normal and transformed tissue adjacent to colon cancer in patient tumors 42, 43. Increased CXCL8 protein expression in primary CRC tumors increases risk for metastatic lesions 44.
The purpose of this study was to determine if DCA-mediated CXCL8 expression occurred in normal or initiated human colon mucosa. It was unclear whether DCA acted on normal colonic mucosa or genetically transformed epithelium. In addition, it was unresolved whether the effects of DCA were restricted to CXCL8, or contributed to features of EMT and thereby CRC. DCA and activated Wnt signaling have been shown to upregulate CXCL8 expression. Yet there is no evidence for wild type APC to suppress DCA-mediated CXCL8 expression. The data suggest that wild type APC is protective for DCA-mediated CXCL8. When cells are treated with CXCL8 and grown on laminin coated filters, there is an increase in the invasion. Neutralizing CXCL8 antibody decreased the invasive phenotype of DCA treated colon cancer cells. In addition, CXCL8 increases expression of MMP-2.
Materials and Methods
Materials
Cholic acid, deoxycholic acid, zinc chloride, crystal violet, McCoy’s 5a medium, β-actin antibody (cat #A 4700) and citric acid were purchased from Sigma (St. Louis, MO). Ursodeoxycholic acid and APC antibody (cat # OP44) were purchased from Calbiochem (San Diego, CA). Hygromycin B, penicillin, streptomycin and HEPES buffer solution were purchased from Invitrogen Corporation (Carlsbad, CA). Recombinant human CXCL8 was purchased from R&D Systems (Cat # 208-IL) (Minneapolis, MN). Rabbit antihuman CXCL8 (cat #OMA1-03351) was purchased from Affinity BioReagents (Golden, CO). β-catenin (cat #9562), c-Fos (cat #4384) and NF-κB p65 (cat #3034) antibodies were purchased from Cell Signaling (Danvers, MA). NF-κB p50 antibody (cat #sc-8414) and β-catenin (cat #sc-1496), normal rabbit IgG (cat #sc-2027), horseradish peroxidase-conjugated goat anti-rabbit IgG (cat #sc-2004) and horseradish peroxidase-conjugated goat anti-mouse (cat #sc-2005) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). MMP-2 antibody (cat #MS-804-P1ABX) was purchased from Lab Vision Corporation (Fremont, CA). Chromatin immunoprecipitation kits were purchased from Active Motif (Carlsbad, CA). Dual luciferase reporter (DLR) assay and Reverse Transcription kits were purchased from Promega (Madison, WI). RNA purification kits were purchased from Qiagen (Valencia, CA). Plasmid DNA purification kits, NucleoBond, were purchased from BD Biosciences (Palo Alto, CA). PCR primers were purchased from Invitrogen Corporation. The 8.0 μM inserts were purchased from Falcon (Franklin Lakes, NJ). Nuclear and cytoplasmic extraction kit was purchased from Pierce (Rockford, IL).
Cell cultures
Colonic adenocarcinoma cell lines HCT116 and HT29 cell lines were purchased from American Type Culture Collection (ATCC) (Rockville, MD) and maintained in McCoy’s 5a medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin. The HT29-APC and HT29-β-Gal lines were maintained as above with the addition of 0.6 mg/mL hygromycin B for selection 45. All cell cultures were kept in a humidified incubator at 37 °C with 5% CO2. The HT29 cell line has mutant APC and wild type β-catenin 46. The HCT116 cell line has wild type APC and mutant β-catenin 46. The HT29-APC cell line has an inducible wild type APC gene under control of a metallothionine promoter 47. Wild type APC was induced with 300 μM ZnCl2 48. The HT29-β-Gal cell line served as a transfection control, with the β-Galalctosidase gene inserted in place of wild type APC. Both the HT29-APC and HT29-β-Gal cell lines were pre-treated for 3 hours with 300 μM ZnCl2 and 1% FBS for optimal induction of wild type APC without significant Zn-induced toxicity 48. HCA-7 cells were maintained in DMEM media (Mediatech, Inc.) with 10% FBS, 2 mM L-glutamine, 50 I.U./mL penicillin and 50 μg/mL streptomycin 49. The HCA7 cell line has a mutated mis-match repair enzyme, wild-type β-catenin50 and a recent description of an APC-truncation mutation51. The HCT116 and HCA7 cell lines are representative of the micro satellite instability (MIN) pathway while the HT29 is representative of the chromosomal instability (CIN) pathway. The HT29, HCT116 and HCA7 cell lines all have defective WNT signaling pathways 46, 47, 50, 51.
Cells were plated at a density of 2.5 × 106/10 cm dish with media that contained 10% FBS cells and the next day were serum starved overnight. On day 3, cells were treated with the appropriate compound. Cells were harvested at the indicated time for RNA or protein analysis.
RNA analysis
Total RNA was isolated by the Qiagen RNeasy protocol. Samples were quantified then reverse transcribed using Promega Reverse Transcription System (A3500) according to manufacturer’s instructions. The product served as a template (at 100 ng/μL) for the PCR reactions that utilized puRe Taq Ready-To-Go PCR Beads (Amersham Biosciences, Piscataway, NJ). The mixture was denatured at 94°C × 5 min, then cycled 35 times 94°C × 1 min, 60°C × 1 min and 72°C × 2 min. The CXCL8 primers were designed using Vector NTI, Suite 8 (Bethesda, MD), that yielded a 246bp product: Sense -ATG ACT TCC AAG CTG GCC and Antisense - CAG ACA GAG CTC TCT TCC. GAPDH was used as a loading control with the 450 bp product: Sense-ACC ACA GTC CAT GCC ATC AC and Antisense TCC ACC ACC CTG TGA CTG TA 52. Products were visualized on agarose gels stained with ethidium bromide.
Western analysis of protein expression
Cells were lysed on ice in radio immunoprecipitation assay (RIPA) buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 10 μg/mL phenylmethylsulfonyl fluoride, 50 mM sodium orthovanadate, and 10 μg/mL apoprotinin). Protein extract was loaded onto a 15% polyacrylimide gel and electrophoresed on a sodium dodecyl sulfate polyacrylimide gel electrophretic (SDS-PAGE) apparatus. Proteins were also precipitated from the media by trichloroacetic acid (TCA) precipitation, 1 volume TCA stock (500 grams TCA in 350 mL deionized water) to 4 volumes sample. The mixture was incubated on ice, centrifuged and the pellet was washed with 200 μL cold acetone. The proteins were transferred electrophoretically to Hybond-C (Amersham Biosciences, Arlington Heights, IL) nitrocellulose membrane. The blots were blocked in Blotto A (5% w/v non-fat dry milk, 0.05% Tween-20, and Tris-buffered saline (TBS) consisting of 10 mM Tris-HCl, pH 8.0, 150 mM NaCl) for 1 hr at room temperature (RT). The primary antibodies were incubated in either Blotto A for 2 hrs at RT, or 5% BSA in TBST at 4°C overnight. The blots were washed in TBS with 0.05% Tween-20 (TBST). Secondary antibodies were incubated at RT for 1 hour and then washed with TBST. Detection was achieved with enhanced chemiluminescence (ECL) detection reagent (Amersham Biosciences, Arlington Heights, IL). Blots were stripped and reprobed with β-actin antibody as a loading control.
The protocol for the APC western blot was provided by Calbiochem and was carried out with slight modifications from the previously published protocol 48. Briefly, the cells were grown in 10 cm plates and lysed directly in cracking buffer (5% glycerol, 50 mM Tris (pH 6.8), 2 mM EDTA, 2% SDS, 144 mM 2-mercaptoethanol and 0.001% bromophenol blue). The lysate was sonicated and 100 μL of extract was loaded per lane and run on a 5% polyacrylimide gel on SDS-PAGE apparatus. Protein transfer, primary antibody incubation, washes, secondary antibody incubation and ECL detection are as described above.
Immunohistochemisty
The Human Subjects Protection Program, at the University of Arizona reviewed an approved the Immunohistochemistry (IHC) protocol. Informed consent from each participant was obtained at the time of the procedure. Patients undergoing treatment for colorectal cancer had a colonic resection. Tissue samples were obtained from the cancer, tissue adjacent to the tumor and tissue 10 cm from the anal verge that was considered normal. Patients undergoing colonoscopy had adenomatous polyps removed by polpectomy. Samples were fixed in 10% neutral buffered formalin for 24 hours, processed and then paraffin embedded. IHC was performed by the Tissue Acquisition and Cellular/Molecular Analysis Shared Service (TACMASS) Core facility at the Arizona Cancer Center. Three micrometer sections of the tissue cut from the formalin fixed, paraffin embedded (FFPE) blocks were placed on glass slides. The slides with the samples were stained on a Discovery XT Automated Immunostainer from Ventana Medical Systems, Inc (VMSI) (Tucson, AZ). Deparffinization, cell conditioning (antigen retrieval), primary antibody staining, detection with biotinylated-streptavidin-HRP and DAB, and hematoxylin counterstaining were performed on this instrument using VMSI validated reagents. The Affinity BioReagents CXCL8 antibody (cat #OMA1-03351) and the Santa Cruz β-catenin antibody (cat # sc-1496) were diluted to 1:100. Following staining on the instrument, slides were dehydrated through graded alcohols to xylene and coverslipped using Pro-Texx mounting medium. Images were captured using an Olympus BX50 and Spot camera (Model 2.3.0.). Images were standardized for light intensity.
Densitometric Quantification of Western Blots
Densitometric analysis of RT-PCR gels and Western blots was performed using Scion Imaging Software, available at www.scioncorp.com.
Transient transfection of luciferase reporter constructs
4.5 × 10 5 cells were plated in a 6-well dish and transfected with 2.5 μg plasmid DNA/well using Lipofectamine 2000 (cat #11668-027). The HCT116, HT29, HT29-APC and HT29-β-Gal cells were transfected with a plasmid that contained the full length CXCL8 promoter luciferase reporter (−1481, +44) 53. The cell lines were subsequently transfected with the promoter-reporter that had mutations in the AP-1, C/EBP or NF-κB DNA binding regions 53. A renilla luciferase reporter was used as a transfection control. HCT116 and HT29 cells were exposed to control or 300 μM deoxycholic acid. The HT29-APC and HT29-β-Gal cells were treated with control or 300 μM ZnCl2 for 3 hours and then were exposed to control or 300 μM deoxycholic acid. All cells were incubated overnight at 37°C with 5% CO2 and lysed the next day. The samples were analyzed using the dual luciferase reporter method, with the ratio of firefly to renilla luciferase reported.
Nuclear and cytoplasmic extraction
Cells were plated at a density of 2.5 × 106/10 cm dish in normal media. The next day, cells were serum starved overnight, and then treated with vehicle or DCA for 2 hours. The manufacturer’s instructions were followed for the isolation of specific fractions.
Chromatin immunoprecipitation
Cells were plated at a density of 2.0 × 107/15 cm plate. Cells were serum starved overnight and then treated with vehicle or DCA for 8 hours. DNA was sheared at 37 °C for 10 minutes with enzyme. The chromatin was pre-cleared for 1.5 hours. To each 150 μL aliquot of pre-cleared chromatin, 10 μL (2 μg) of antibody was added and incubated overnight on a rotator at 4°C. On day 3, tubes were incubated for 1.5 hours at 4°C with beads. Subsequent washes and elution were done according to instructions. RNA was removed with RNAse treatment and cross-links were reversed in a 65°C water bath overnight. Each of the aliquots was treated with Proteinase K for 2 hours. The purified DNA was eluted as per the manufacturer’s instructions. Amplification of the DNA was achieved with 10 pmol of each primer. CXCL8 ChIP primers were designed with Vector NTI and yielded a 313 bp product: Sense - CAC CAA ATT GTG GAG CTT CA and Antisense GGT GGT TTC TTC CTG GCT CT. For CXCL8, DNA was denatured at 94°C × 5 min, then: 94°C × 1 min, 50.5°C × 1 min, and 72°C × 2 min for 35 cycles. For GAPDH, DNA was denatured at 94°C × 3 min, then: 94°C × 20 sec, 59°C × 30 sec, and 72°C × 30 sec for 35 cycles. The PCR amplified a 166 bp product with the primers; Sense TAC TAG CGG TTT TAC GGG CG and Antisense TCG AAC AGG AGG AGC AGA GAG CGA. Products were visualized on a 2% agarose gel stained with ethidium bromide.
Invasion assays
In a 24-well plate, 500 μL of serum free media was aliquoted into each lower chamber. Cell culture inserts were pre-treated with 1μg of laminin on each side. Inserts were then placed into the cell plate. Cells were trypsinized, pelleted and the suspension was resuspended in serum free media, at a concentration of 1.0 × 106 cells/mL. Cells were dosed with the appropriate compound and 200 μL of cell suspension was plated into the upper portion of the insert. The HT29 cell line migrated for 48 hours according to previously published protocols54. The HT29-APC and HT29-β-Gal cell lines migrated for 24 hours to avoid toxicity associated with treatment48. The inserts were removed, inverted and washed 3 times with HEPES buffer. Cells in the upper portion were removed. Cells that had invaded into the lower portion were stained with 100 μL crystal violet solution (0.5% crystal violet, 20% methanol, 80% water). The stained filter was washed, dried overnight and then cut out. The crystal violet was dissolved with 200 μL 0.1 M citric acid in a 96-well plate. The dissolved dye was transferred to a new well. The optical density was read at 562 nm on BIO-TEK Instruments EL800 universal microplate reader as described by Pawar et al 55.
Results
APC status influences DCA-mediated induction of CXCL8 mRNA and protein
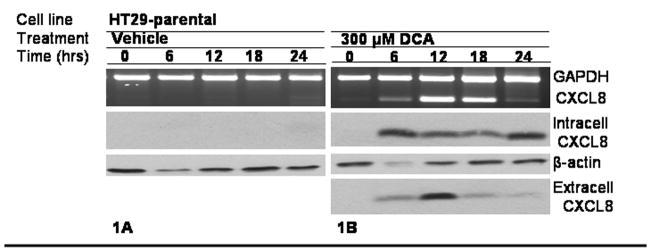
The effects of DCA on CXCL8 expression were assessed in HCT116 and HT29 cell lines. In Fig. 1A, CXCL8 was not induced by the vehicle. In Fig. 1B, HT29 mRNA and protein levels increased within 6 hours of treatment with 300 μM DCA. Similar results were found in HCT116 (Supplemental Figure 1). This effect was not seen when the cells were treated with the primary bile acid, cholic acid (CA), or with the tertiary bile acid, ursodeoxycholic acid (UDCA) (Supplemental Figure 2).
Figure 1.
To further investigate the results of intracellular induction of CXLC8, proteins in the media were precipitated by TCA after DCA exposure and then separated by SDS-PAGE. Also shown in Fig. 1B, CXCL8 antibody detected an induction of extracellular protein 6 hours after DCA exposure in the media. The extracellular CXCL8 was sustained for greater than 12 hours.
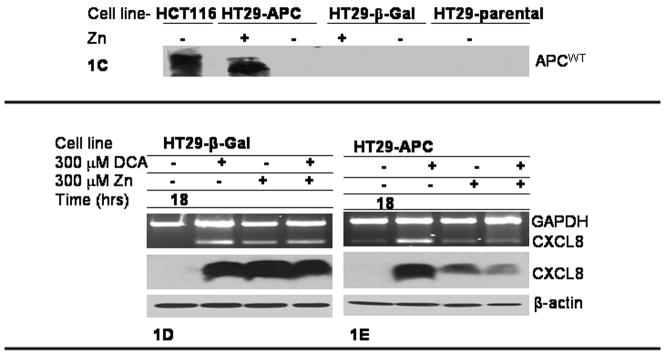
The HT29-APC and HT29-β-Gal cells were used to determine the influence of APC status on the expression of CXCL8. The HT29 cell line served as a negative control for wild type APC and the HCT116 cell line served as a positive control for wild type APC as seen in Fig 1C. HT29-APC cells treated with 300 μM ZnCl2 for 3 hours induced full length APC but not in the HT29-β-Gal cells as shown in Fig 1C. The HT29-APC and HT29-β-Gal cells were pretreated with zinc or control for 3 hours, and then treated with either 300 μM DCA or control for 18 hours. As shown in Fig. 1D, the HT29-β-Gal cells did not significantly alter the steady state CXCL8 RNA or protein in response to DCA treatment. As shown in Fig. 1E the HT29-APC cells differ in CXCL8 RNA and protein expression by their APC status. Mutant APC was permissive for the DCA-mediated CXCL8 production, but wild type APC was suppressive for the DCA-mediated CXCL8 production at both the mRNA and protein levels.
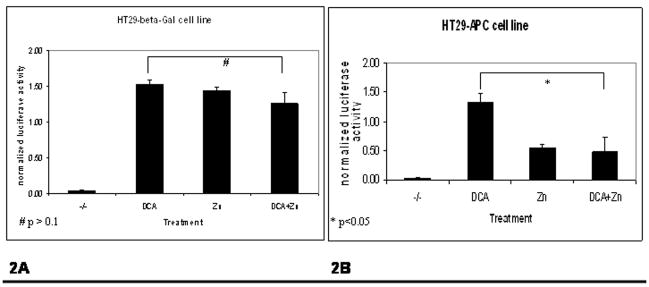
Quantification of Fig 1D and 1E is revealed in Fig 2. In Fig 2A, there is no statistically significance difference in CXCL8 induction in the HT29-β-Gal cell line. Shown in Fig 2B, there was a significant protective effect by wild type-APC status in the DCA-mediated induction of CXCL8 (p<0.05).
Figure 2.
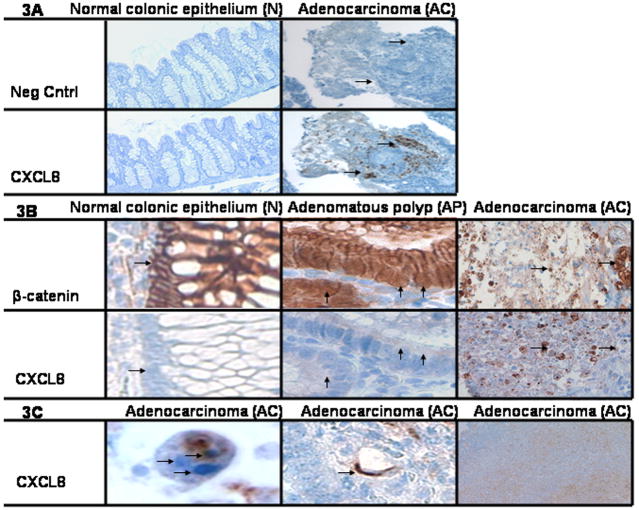
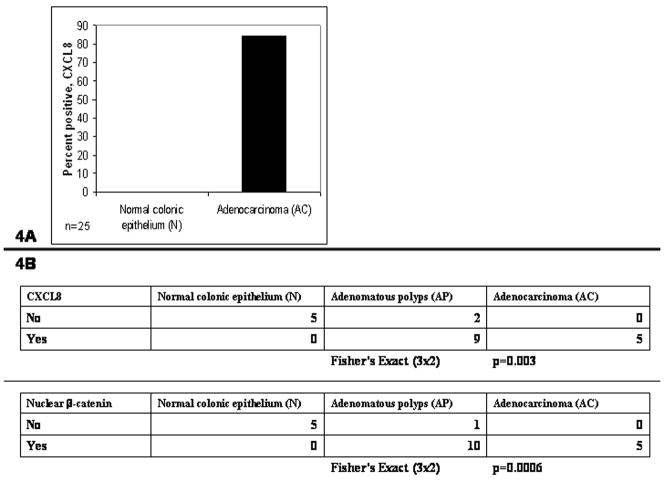
Immunohistochemistry (IHC) of patient samples was then used to determine the clinical significance of CXCL8 expression. Twenty five tissue samples of normal tissue (N) and adenomatous carcinomas (AC) from patients undergoing colonic resection were stained with CXCL8 represented in Fig. 3A. None, 0%, of the N samples were positive for CXCL8 while twenty one of twenty five, 84%, of the AC samples were positive for any CXCL8, represented in Fig. 4A. Next, CXCL8 expression in tissue from 5 normal tissue (N), 11 adenomatous polyps (AP) and 5 adenocarcinomas (AC) were then compared. N, AP, and AC tissue were also stained with β-catenin, a surrogate for Wnt signaling. Shown in Fig. 3B, the normal colonic epithelium, N, did not stain positive for nuclear β-catenin or CXCL8. By contrast, the adenomatous polyps, AP, stained positive for both nuclear β-catenin and epithelial CXCL8 protein. Adenocarcinoma, AC, demonstrated CXCL8 protein in epithelia and non-epithelial cells. In Fig. 4B, CXCL8 was associated with the adenomatous polyps (AP) and adenocarcinomas (AC), but not the normal colonic epithelium (N) (Fisher’s Exact p=0.003). Also seen In Fig. 4B, nuclear β-catenin was associated with AP and AC, but not N (Fisher’s Exact p=0.0006). As shown in Fig. 3C, CXCL8 was expressed in neutrophils, endothelial cells and in the vast necrotic regions of the tumors.
Figure 3.
Figure 4.
Transcriptional regulation of CXCL8
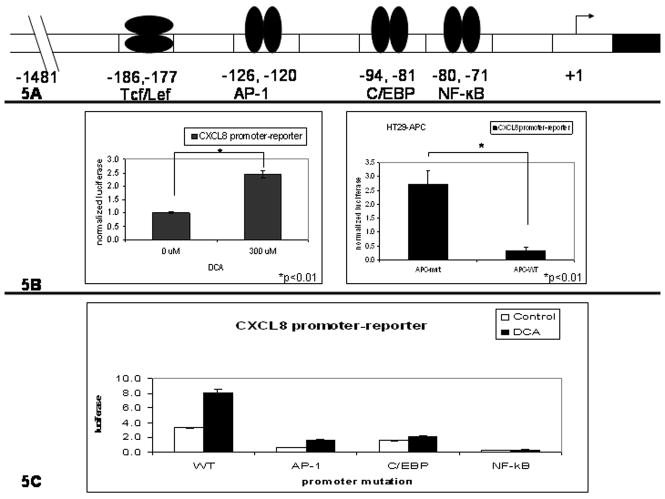
To determine the mechanism of increased CXCL8 expression, promoter-reporter assays were performed. Shown in Fig. 5A, is a representation of the 5′-promoter region of CXCL8. Shown in Fig. 5B, is the ratio of treated cells to control. “APC-mut” is the ratio of DCA treated cells to vehicle. “APC-WT” is the ratio of DCA and zinc treated cells to zinc treated cells. The first histogram of Fig 5B shows the HT29 cell line had a 2.5 fold induction of the CXCL8 promoter activity after the addition of 300 μM DCA (t-test, p<0.01). In the second histogram of Fig 5B, the HT29-APC cell line had greater than 80% reduction in DCA-mediated promoter-reporter activity by wild type APC when compared to the mutant APC controls (t-test, p<0.01).
Figure 5.
To further investigate CXCL8 induction by DCA, HT29 cells were transfected with promoter-reporter constructs containing point mutations for: AP-1, C/EBP, and NF-κB. Shown in Fig. 5C, DCA-mediated luciferase activity was decreased by 80%, 75% and 95% for AP-1, C/EBP and NF-κB mutated reporter constructs compared to the full length promoter (−1481, +44) control. Similar results were found in the HCT116 cell line (Supplemental Figure 3).
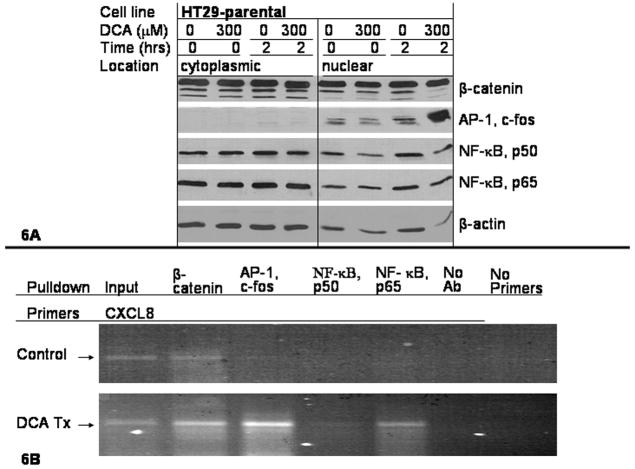
To assess how DCA affected transcription factor activation, nuclear and cytoplasmic fractions were separated after two hours treatment with 300 μM DCA and separated on an SDS-PAGE apparatus. The blot was probed with antibodies directed against the transcription factors β-catenin, AP-1, and NF-κB as seen in Fig. 6A. Downstream of mutant APC is nuclear accumulation of β-catenin. Two hours of DCA exposure did not appear to change β-catenin localization. The AP-1 transcription factor is a heterodimer of Jun and Fos subunits. The Fos family member c-Fos, increased in quantity and nuclear localization 2 hours after DCA treatment. The NF-κB transcription factor is a heterodimer of p50 and p65 subunits. Nuclear localization of the p65 subunit was not altered 2 hours after exposure to DCA. The loading control for these experiments was β-actin.
Figure 6.
ChIP was performed to determine the binding of the transcription factors to the CXCL8 5′-UTR, with and without DCA stimulation. The HT29 cell line was treated with 300 μM DCA or control for 8 hours and then formalin fixed. Antibodies directed against β-catenin, c-Fos, p50 or p65 were incubated with sheared DNA fragments isolated from the DCA treated, or untreated control in the HT29 cell line. Isolated complexes were digested with proteinase and purified, resulting in fragments of DNA to which the transcription factors bound. Primers amplified 240 bp upstream and 73 bp downstream from the start of CXCL8 transcription that yielded a 313 bp product. This region corresponds with transcription factor binding sites, previously shown in Fig. 5A. As shown in Fig. 6B, the PCR reactions amplified a 313 bp product for the Input DNA template for both the control and DCA treated cells. β-catenin pull-down amplified a product in both the control and DCA-treated HT29 cells. The c-Fos, p50 and p65 did not amplify a product in the control group. When the HT29 cells were exposed to DCA, c-Fos and p65 amplified a significant amount of the 313 bp product.
Cellular response to CXCL8
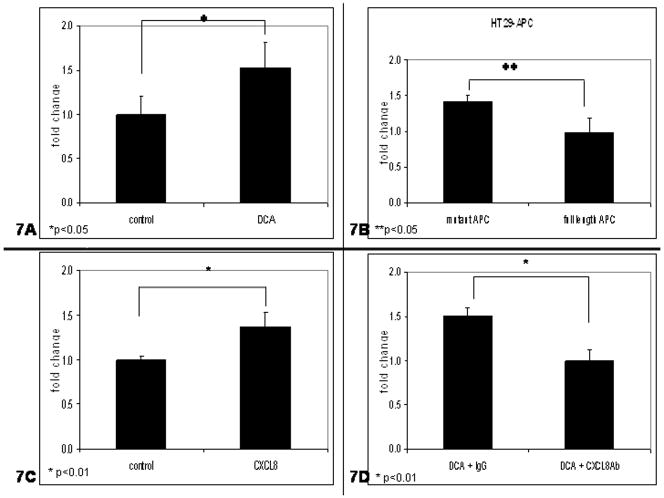
Other effects of CXCL8 were determined by invasion assays. HT29-APC and HT29 cells were exposed to 300 μM DCA, incubated on laminin coated filters and allowed to migrate for 24 48 or 48 hours 54 respectively. As shown in Fig. 7A, DCA exposure increased invasion in a mutant APC background when compared to the controls (t-test, p<0.05). Fig 7B is the ratio of treated cells to control. “APC-mut” is the ratio of DCA treated cells to vehicle. “APC-WT” is the ratio of DCA and zinc treated cells to zinc treated cells. Shown in Fig. 7B, wild type APC suppressed DCA-mediated invasion in the HT29-APC cell line when compared to controls (t-test, p <0.05). The HT29 cell line was then exposed to 2.0 μg/mL CXCL8 or control. As seen in Fig. 7C, cells treated with CXCL8 had 30% more invasion (t-test, p<0.01), when compared to the control. When cells were exposed to DCA and CXCL8 antibody, Fig. 7D, invasion was suppressed by more than 30% when compared to HT29 cell treated with DCA and IgG (t-test, p<0.01).
Figure 7.
Downstream effects of CXCL8 production
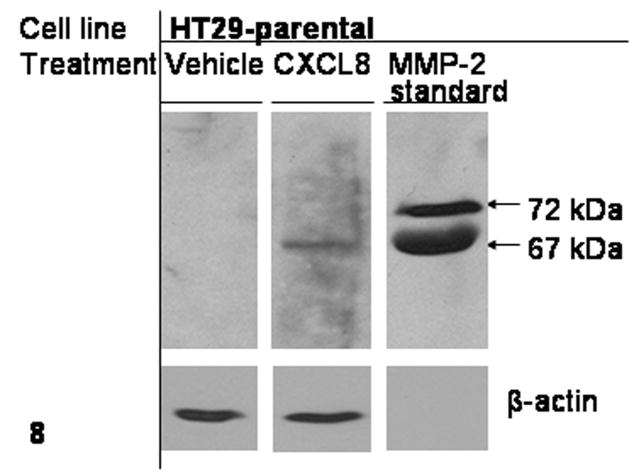
To further understand the role of CXCL8 on colonic epithelium, western analysis was performed on total cell lysate from HT29 cells treated with CXCL8. As shown in Fig. 8, matrix metalloproteinase-2 (MMP-2), a transcriptional target of CXCL8 was detected in the cell lysate 36 hours after cells were dosed with 2.0 μg/mL CXCL8.
Figure 8.
Discussion
The purpose of this study was to determine if DCA-mediated CXCL8 expression occurred in normal or initiated human colon mucosa. The data demonstrate that wild type APC suppresses, while mutant APC is permissive for, DCA-mediated CXCL8 gene expression. Further, the data demonstrate that CXCL8 induces MMP-2 production in colonic epithelium. Invasion through laminin is influenced by the APC status. The potential mechanism of DCA-mediated CXCL8 induction occurs in initiated cells and leads to a more aggressive tumor phenotype.
We originally exposed colonic epithelia to DCA asking whether multiple pro-inflammatory molecules would be induced. There was a robust induction of CXCL8 without induction of IL-1-β, TNF-α and NOS-2 at the time points we evaluated in spite of similar promoter regions for the four molecules (Supplemental Figure 4). NOS-2 did appear to respond to DCA, but not as consistently as CXCL8. Subsequent experiments were conducted in multiple colorectal cancer epithelial cells; HT29, HCT116 and HCA7 cell lines which all had defects in the Wnt signaling pathway 46, 47, 50, 51. Similar induction of CXCL8 by DCA was found across cell lines (Supplemental Figure 5). We interpreted the results to suggest a role for both DCA and Wnt signaling to induce CXCL8, but not IL-1-β, TNF-α and NOS-2.
Others had shown that DCA induced CXCL8 expression in colonic epithelial cells 28–30. However the role of APC was not evaluated. The data presented here demonstrates the important role of APC in DCA-dependent CXCL8 expression. It suggests that this induction occurs in cells with APC mutations or deletions. Our study further excludes the primary and tertiary bile acids as inducers of CXCL8 induction. Others have reported increased CXCL8 expression in cells of hepatic and endothelial lineage occurs as a result of Wnt signaling. Our findings are unique in their relevance to colon epithelial cells and the relationship to the initiation phase of colon carcinogenesis.
Many of the experiments reported here, were conducted in both HCT116 and HT29 cell lines. Similar results were found in the two cell lines and interpreted to be the result of mutations in the Wnt signaling pathway, as HCT116 have a mutant β-catenin and the HT29 have a mutant APC gene. The HCT116 also have a mutant K-ras gene and wild type p53 while the HT29 have a wild type K-ras expression and mutant p53. Therefore we show data obtained from the HT29 cell line and the derived HT29-APC and HT29-β-Gal. We express the data as conditional statements based upon the isogenic background but note that the findings may not be restricted to a single cell line.
The HT29 cell line with a mutant APC does not produce CXCL8 when treated with control alone, shown in Fig 1A, indicating that Wnt signaling may be necessary for CXCL8 production but it is not sufficient. HT29 cells produce a significant amount of CXCL8 when exposed to DCA and suggests that both mutant APC and DCA are necessary. CXCL8 is a secreted protein. While intracellular CXCL8 protein levels do not directly match RNA levels, extracellular protein levels do increase at 6 hours and are highest at 12 hours, corresponding to RNA levels. After 12 hours, extracellular protein levels drop along with RNA levels. Intracellular protein levels are a complex function of synthesis, stability and secretion and will not necessarily strictly follow RNA levels. Thus, we do not believe that there is a discrepancy in the data shown
The HT29-APC similarly does not express CXCL8 when exposed to control, Fig 1E. A robust induction of CXCL8 by DCA in HT29-APC and HT29 β-Gal is consistent with the HT29 parental strain, and serves as an internal control. In the HT29-APC, zinc does induce CXCL8 expression, Fig 1E. Fig 1B shows CXCL8 induction in HT29-parental cells over a 24 hour time period, while Fig 1D utilized the HT29-β-Gal cell line at a fixed time point of 18 hours. The protein gels in Fig. 1D were overexposed in order to detect the low level of CXCL8 protein expression in HT-29-APC cells, and are thus not comparable to the protein gels shown in Fig. 1B.
Here we use a zinc-inducible system to show that wild type-APC can suppress CXCL8 expression by both DCA and zinc. Zinc has been shown to induce the production of CXCL856. DCA has also been shown to induce CXCL8 but its mechanism remains unclear. In Fig 1D the induction of CXCL8 by either DCA or zinc is in the presence of a truncated APC. The interpretation is that CXCL8 is highly inducible. In Fig 1E, APC status is manipulated with varied CXCL8 induction. In Fig 1E, DCA significantly induced CXCL8 with a mutated APC shown in the 2nd column. Yet full length APC was protective for induction of CXCL8 by zinc or DCA as shown in the 3rd and 4th columns.
Quantification of Fig 1D and 1E has been provided with statistical significance, in Fig 2. Shown in Fig 2A, there is no statistically significance difference in CXCL8 induction in the HT29-β-Gal cell line. Shown in Fig 2B, there was a significant protective effect by wild type-APC status in the DCA-mediated induction of CXCL8 (p<0.05). Therefore, the interpretation is that CXCL8 is highly inducible by either DCA or zinc in the presence of truncated APC (Fig 1D and Fig 1E, column 2). Full-length APC is protective for both zinc and DCA-mediated CXCL8 expression, as shown in Fig 1E column 3 and 4. In this context, both DCA and zinc-mediated CXCL8 expression in the HT29-APC and HT29-β-Gal cells is consistent with previous findings. The novelty of data indicates that wild type APC is protective for DCA-mediated CXCL8 expression at both the steady state levels of mRNA and protein.
Elevated CXCL8 has been reported by others in human CRC 42–44. We show here that there is increased CXCL8 expression in adenocarcinoma (AC) but not in normal (N). Furthermore, we demonstrated that CXCL8 expression is associated with the nuclear localization of β-catenin in adenomatous polyps (AP). The finding is consistent with CXCL8 gene regulation by the Wnt pathway 32, 33. It extends the role of CXCL8 in CRC by placing DCA-mediated CXCL8 in the context of transformed, not normal, epithelium. CXCL8 expression was also found in neutrophils, endothelial cells and necrotic regions of the tumor consistent with their presence in CRC 43, 57. It suggests that CXCL8 may also have paracrine effects significant for recruiting infiltrating immune cells and endothelial cells. This view is consistent with tissue remodeling 17 as well as cytokine involvement in carcinogenesis 58.
The proposed mechanism of DCA-mediated CXCL8 production involves signaling mechanisms by both DCA and mutant APC. The HT29 cell line demonstrated that mutant APC was permissive for DCA-mediated CXCL8 promoter reporter activity, steady state mRNA and protein levels. This is consistent with reports showing an activated Wnt signaling pathway leading to increased CXCL8 expression 32, 33. The finding that wild type APC suppresses DCA-mediated CXCL8 is novel.
A potential mechanism of DCA-mediated increase in steady state CXCL8 mRNA has been identified through NF-κB activation 28. Yet, the AP-1, C/EBP and NF-κB transcription factor binding regions of the CXCL8 promoter have been shown to regulate gene expression in other models 34, 53, 59. Promoter reporter studies in the HT29 cells demonstrated that DCA increased the promoter activity using the CXCL8 5′-promoter. This is consistent with DCA-mediated activation of the AP-1, C/EBP, and NF-κB transcription factors 8–10. Promoter-reporter activity was significantly decreased by mutations in the AP-1 and C/EBP binding sites, and abolished by mutations in the NF-κB binding site of the CXCL8 5′-promoter. The absence of CXCL8 reporter activity suggested that NF-κB is necessary for expression and is consistent with previous studies 28, 53. AP-1 and C/EBP mutations also decreased luciferase activity and suggest the mechanism of CXCL8 regulation may involve the cooperation of AP-1, C/EBP and NF-κB.
Transcription factors found in the cytoplasm may be activated when translocated to the nucleus. The data presented here confirms the importance of DCA-mediated NF-κB activation and expands to include both β-catenin and AP-1. β-catenin bound to the 5′-promoter in the absence and presence of DCA, suggesting that it is necessary but not sufficient to regulate expression of CXCL8. DCA increased the total amount of the AP-1 family member, c-Fos as well as its translocation to the nucleus and binding to transcription factor regions. It is consistent with published data showing AP-1 activation by DCA 8. DCA treatment did not increase the total amount of NF-κB but it did increase its activation. ChIP revealed that DCA enhanced the p65 NF-κB subunit binding to the CXCL8 5′-promoter. Therefore we conclude that increased CXCL8 expression is through the combined effect of the bacterial derived luminal risk factor, DCA, and a genetic risk factor, mutant APC that drives the expression of CXCL8.
CXCL8 is an inducible cytokine that may act in autocrine and paracrine loops 38. Precipitation of CXCL8 from media of cells treated with DCA is consistent with the inducible nature of CXCL8 expression 34. HT29 cells exposed to CXCL8 increased MMP-2 production and is consistent with increased MMP-2 by CXCL8 exposure in other models 40, 41 and the role of CXCL8 in invasiveness 26, 27. The increased expression of CXCL8 in CRC cell lines is also consistent with increased expression of chemokines in patient tumor burden 19, 23, 60.
DCA has been shown to increase migration in other CRC cell lines 61. HT29 cells have alpha-3 and beta-1 integrins that suggest an ability to migrate or invade on laminin 62. We show that DCA can increase invasion on laminin, in a mutant APC background, while wild type APC suppresses this effect. HT29 cells treated with DCA and a CXCL8 antibody neutralized the invasion. Exposure of HT29 cells to CXCL8 is sufficient to increase invasion on laminin coated filters and is consistent with CXCL8-mediatied migration in other models 26, 40, 41, 63. It suggests chronic exposure of transformed cells to DCA increases the tumorigenic potential.
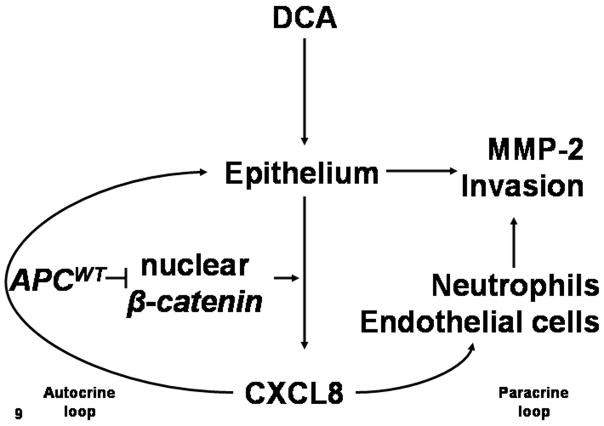
The aim of this study was to determine if DCA-mediated CXCL8 expression contributed to colon carcinogenesis. A potential mechanism for CXCL8 production involves the conversion of primary bile acid, CA, to secondary bile, DCA, by luminal bacteria. Further, the role of DCA-mediated in CXCL8 expression occurs in epithelium harboring mutant APC. A potential mechanism for DCA-mediated CXCL8 production to affect CRC is to induce MMP-2 production and increased invasion. CXCL8 may also recruit cells to remodel CRC tissue. Fig. 9 is a model of DCA and APC interaction.
Figure 9.
Based on the evidence, DCA-mediated CXCL8 occurs in initiated epithelium. The CXCL8 is pro-tumorigenic through the production of proteinases, an invasive phenotype and recruitment of other cell types. The potential therapeutic intervention for highly expressing CXCL8 tumors in CRC may be through its neutralization 25. A humanized anti-CXCL8 antibody inhibits angiogenesis, tumor growth, metastasis and MMP activity in pre-clinical models 26, 27. The expectation would be a reduction in tumor burden of CRC patients based on these parameters.
Supplementary Material
Acknowledgments
This research was supported by NIH/NCI CA023074, CA041108, CA95060, CA123065.
The authors would like to thank Drs. Bert Vogelstein and Kenneth Kinzler for the HT29-APC and HT29 β-Gal cell lines. TACMASS was supported by NCI grant #2P30CA023074. This research was supported by NIH/NCI 2PO1 CA041108, CA95060, and CA023074.
Abbreviations used
- DCA
deoxycholic acid
- APC
adenomatous polyposis coli
- FAP
Familial Adenomatous Polyposis
- CRC
colorectal cancer
- AP-1
activator protein-1
- C/EBP
CAAT enhancer binding protein
- NF-κB
nuclear factor kappa B
- IL-8
interleukin-8
- CXCL8
chemotactic cytokine ligand-8
- EMT
epithelial-mesenchymal transition
- MMP-2
matrix metalloproteinase-2
- ChIP
Chromatin immunoprecipitation
- RIPA
radio immunoprecipitation assay
- SDS-PAGE
sodium dodecyl sulfate polyacrylimide gel electrophretic
- TCA
trichloroacetic acid
- IHC
Immunohistochemistry
- MIN
multiple intestinal neoplasia
- CIN
chromosomal instability
Footnotes
Novelty and impact: This manuscript addresses the potential tumor promoting activity of CXCL8 in colorectal carcinogenesis. The novelty of the paper lies in the observation that the expression of wild type APC suppresses CXCL8 production in response to DCA treatment. The impact of the paper is this novel biochemical framework which may impact CRC tumor promotion and progression.
References
- 1.ACS. American Cancer Society. 2008. Cancer facts and figures. [Google Scholar]
- 2.Jemal A, Siegal R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. A Cancer Journal for Clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Schottenfeld D, Beebe-Dimmer J. Alleviating the burden of cancer: a perspective on advances, challenges and future directions. Cancer Epidemiol Biomarkers Prev. 2006;15:2049–55. doi: 10.1158/1055-9965.EPI-06-0603. [DOI] [PubMed] [Google Scholar]
- 4.Reddy BS, Wynder EL. Large-bowel carcinogenesis: Fecal constituents of populations with diverse incidence rates of colon cancer. Journal of the National Cancer Institute. 1973;50:1437–42. doi: 10.1093/jnci/50.6.1437. [DOI] [PubMed] [Google Scholar]
- 5.Reddy BS, Weisburger JH, Wynder EL. Effects of dietary fat level and dimethylhydrazine on fecal acid and neutral sterol excretion and colon carcinogenesis in rats. Journal of the National Cancer Institute. 1974;52:507–11. doi: 10.1093/jnci/52.2.507. [DOI] [PubMed] [Google Scholar]
- 6.Hill M, Crowther JS, Drasar BS, Hawksworth G, Aries V, Williams REO. Bacteria and etiology of cancer of the large bowel. The Lancet. 1971;1:95–100. doi: 10.1016/s0140-6736(71)90837-3. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutation Research. 2005:589. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Qiao D, Chen W, Stratagoules ED, Martinez JD. Bile acid induced activation of activator protein-1 requires both extracellular signal-regulated kinase and protein kinase C signaling. J Biol Chem. 2000;275:15090–8. doi: 10.1074/jbc.M908890199. [DOI] [PubMed] [Google Scholar]
- 9.Crowley-Weber CL, Payne CM, Gleason-Guzman M, Watts GS, Futscher B, Waltmire CN, Crowley C, Dvorakova K, Bernstein C, Craven M, Garewal H, Bernstein H. Developent and molecular characterization of HCT-116 cell lines resistance to the tumor promoter and multiple stress inducer, deoxycholate. Carcinogenesis. 2002;23:2063–80. doi: 10.1093/carcin/23.12.2063. [DOI] [PubMed] [Google Scholar]
- 10.Qiao D, Im E, Qi W, Martinez JD. Activator protein-1 and CCAAT/enhancer-binding protein mediated GADD153 expression is involved in deoxycholic acid-induced apoptosis. Biochimica et Biophysica Acta. 2002;1583:108–16. doi: 10.1016/s1388-1981(02)00190-7. [DOI] [PubMed] [Google Scholar]
- 11.Kinzler K, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 12.Fearon ER, Vogelstein B. A Genetic Model for Colorectal Tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 13.Gerner EW, Ignatenko NA, Lance P, Hurley LH. A comprehensive strategy to combat colon cancer targeting the Adenomatous Polyposis Coli tumor suppressor gene. Annals of the New York Academy of Sciences. 2005;1059:97–105. doi: 10.1196/annals.1339.033. [DOI] [PubMed] [Google Scholar]
- 14.Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111–6. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- 15.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development and disease. Journal of Cell Biology. 2006;172:973–81. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal progression. Cancer Biology & Therapy. 2005;4:365–70. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenkilde M, Schwartz T. The chemokine system - a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481–95. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- 19.Rollins B. Inflammatory chemokines in cancer growth and progression. European Journal of Cancer. 2006;42:760–7. doi: 10.1016/j.ejca.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Balkwill F. Cancer and the Chemokine Network. Nature Reviews, Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 21.Van Damme J, Struyf S, Opdenakker G. Chemokine-protease interactions in cancer. Seminars in Cancer Biology. 2004;14:201–8. doi: 10.1016/j.semcancer.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Slettenaar V, Wilson JL. The chemokine network: a target in cancer biology? Advanced Drug Delivery Reviews. 2006;58:962–74. doi: 10.1016/j.addr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Baier PK, Eggstein S, Wolff-Vorbeck G, Baumgartner U, Hopt UT. Chemokines in Human Colorectal Carcinoma. Aticancer Research. 2005;25:3581–4. [PubMed] [Google Scholar]
- 24.Aggarwal B, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochemical Pharmacology. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Ali S, Lazennec G. Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007;26:401–20. doi: 10.1007/s10555-007-9073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (IL-8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. American Journal of Pathology. 2002;161:125–34. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mian BM, Dinney CPN, Bermejo CE, Sweeny P, Tellez C, Yang XD, Gudas JM, McConkey DJ, Bar-Eli M. Fully human anti-Interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kB. Clinical Cancer Research. 2003;9:3167–75. [PubMed] [Google Scholar]
- 28.Muhlbauer M, Allard B, Bosserhoff AK, Kiessling S, Herfarth H, Rogler G, Scholmerich J, Jobin C, Hellerbrand C. Differential effects of deoxycholic acid and taurodeoxycholic acid on NF-kB signal transduction and IL-8 gene expression in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003;286:G1000–G8. doi: 10.1152/ajpgi.00338.2003. [DOI] [PubMed] [Google Scholar]
- 29.Lee D, Park SY, Baik SK, Kwon SO, Chung JM, Oh ES, Kim HS. Deoxycholic acid-induced signal transduction in HT-29 cells: role of NF-kappa B and interleukin-8. Korean J Gastroenterol. 2004;43:176–85. [PubMed] [Google Scholar]
- 30.Araki Y, Fujiyama Y, Andoh A, Nakamura F, Shimada M, Takaya H, Bamba T. Hydrophilic and hydrophobic bile acids exhibit different cytotoxicities through cytolysis, interleukin-8 synthesis and apoptosis in the intestinal epithelial cell lines IEC-6 and Caco-2 cells. Scand J Gastroenterol. 2001;36:533–9. doi: 10.1080/003655201750153430. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins GJS, Harries K, Doak SH, Wilmes A, Griffiths AP, Baxter JN, Parry JM. The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis. 2004;25:317–23. doi: 10.1093/carcin/bgh032. [DOI] [PubMed] [Google Scholar]
- 32.Levy L, Neuveu C, Renard CA, Charneau P, Branchereau S, Gauthier F, Van Nhieu JT, Cherqui D, Petit-Berton AF, Mathieu D, Buendia MA. Transcriptional Activation of Interleukin-8 by B-Catenin-Tcf4. The Journal of Biological Chemistry. 2002;277:42386–2393. doi: 10.1074/jbc.M207418200. [DOI] [PubMed] [Google Scholar]
- 33.Nestor T, Masckauchan H, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/B-catenin signaling induces proliferation, survival and Interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 34.Mukaida N, Shiroo M, Matasushima K. Genomic Structure of the Human Monocyte-Derived Neutrophil Chemotactic Factor IL-8. The Journal of Immunology. 1989;143:1366–71. [PubMed] [Google Scholar]
- 35.Heidemann J, Ogawa H, Dwindell MB, Rafiee P, Maaser C, Gogkel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of Interleukin 8 (CXCL8) in Human Intestinal Microvascular Endothelial Cells Are Mediated by CXCR2. The Journal of Biological Chemistry. 2003;278:8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 36.Wilson AJ, Byron K, Gibson PR. Interleukin-8 stimulates the migration of colonic epithelial cells in vitro. Clinical Science. 1999;97:385–90. [PubMed] [Google Scholar]
- 37.Bates RC, DeLeo MJ, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1 mediated chemotaxis. Experimental Cell Research. 2004;299:315–24. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Brew R, Erickson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 39.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97:2792–802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M. Expression of Interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. American Journal of Pathology. 1997;151:1105–13. [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue K, Slaton JW, Kim SJ, Perrotte P, Eve BY, Bar-Eli M, Radinsky R, Dinney CPN. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000;60:2290–9. [PubMed] [Google Scholar]
- 42.Fox SH, Whalen GF, Sanders MM, Burleson JA, Jennings K, Kurtzman S, Kreutzer D. Angiogenesis in normal tissue adjacent to colon cancer. Journal of Surgical Oncology. 1998;69:230–4. doi: 10.1002/(sici)1096-9098(199812)69:4<230::aid-jso7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Kuniyasu H, Yasui W, Shinohara H, Yano S, Ellis LM, Wilson MR, Bucana CD, Rikita T, Tahara E, Fidler I. Induction of angiogenesis by hyperplastic colonic mucosa adjacent to colon cancer. American Journal of Pathology. 2000;157:1523–35. doi: 10.1016/S0002-9440(10)64790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haraguchi M, Komuta K, Akashi A, Matsuzzaki S, Furui J, Kanematsu T. Elevated IL-8 levels in the drainage vein of resectable Duke’s C colorectal cancer indicate high risk for developing hepatic metastasis. Oncology Reports. 2002;9:159–65. [PubMed] [Google Scholar]
- 45.Morin PJ, Vogelstein B, Kinzler KW. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci. 1996;93:7950–4. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shih IMYJ, He TC, Vogelstein B, Kinzler KW. The b-Catenin binding domain of Adenomatous Polyposis Coli is sufficient for tumor suppression. Cancer Research. 2000;60:1671–6. [PubMed] [Google Scholar]
- 47.He TCSA, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 48.Fultz KE, Gerner EW. APC-Dependent regulation of ornithine decarboxylase in human colon tumor cells. Molecular Carcinogenesis. 2002;34:10–8. doi: 10.1002/mc.10043. [DOI] [PubMed] [Google Scholar]
- 49.Baines AT-PM, Goulet AC, Renaud C, Gerner EW, Nelson MA. Selenomethionine inhibits growth and suppresses cyclooxygenase-2 (COX-2) protein expression in human colon cancer cell lines. Cancer Biol Ther. 2002;1:370–4. [PubMed] [Google Scholar]
- 50.Chang WCEL, Pfeiffer GR, Cooper HS, Barusevicius A, Clapper ML. Sulindac sulfone is most effective in modulating beta-catenin-mediated transcription in cells with mutant APC. Ann NY Acad Sci. 2005;1059:41–55. doi: 10.1196/annals.1339.020. [DOI] [PubMed] [Google Scholar]
- 51.Abel-Rahman WMKJ, Shoman S, Eissa S, Ollikainen M, Elomaa O, Eliseenkova AV, Butzow R, Mohammadi M, Peltomaki P. Somatic FGF9 mutations in colorectal and endometrial carcinomas associated with membranous Beta-catenin. Human Mutation. 2008;29:390–7. doi: 10.1002/humu.20653. [DOI] [PubMed] [Google Scholar]
- 52.Tesfai Y, Brereton HM, Barritt GJ. A diacylglycerol-activated Ca2+ channel in PC12 cells (an adrenal chromaffin cell line) correlates with expression of the TRP-6 (transient receptor potential) protein. Biochem J. 2001;358:717–26. doi: 10.1042/0264-6021:3580717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freund A, Jolivel V, Durand S, Kersual N, Chalbos D, Chavey C, Vignon F, Lazennec G. Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene. 2004;23:6105–14. doi: 10.1038/sj.onc.1207815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HSLY, Kim JW, Baik SK, Kwon SO, Jang HI. Modulation of colon cancer invasiveness induced by deoxycholic acid. Korean J Gastroenterol. 2006;48:9–18. [PubMed] [Google Scholar]
- 55.Pawar SCDM, Nagle RB, Bowden GT, Cress AE. Integrin alpha6 cleavage: a novel modification to modulate cell migration. Exp Cell Res. 2007;313:1080–9. doi: 10.1016/j.yexcr.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim Y, Reed W, Wu W, Bromberg PA, Graves LM, Samet JM. Zn 2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1028–35. doi: 10.1152/ajplung.00479.2005. [DOI] [PubMed] [Google Scholar]
- 57.Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Dano K. 92 kDa type IV collegenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer. 1996;65:57–62. doi: 10.1002/(SICI)1097-0215(19960103)65:1<57::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 58.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 59.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanisms of interleukin-8 gene expression. Journal of Leukocyte Biology. 1994;56:554–8. [PubMed] [Google Scholar]
- 60.Baier PK, Vorbeck GW, Eggstein S, Baumgartner U, Hopt UT. Cytokine Expression in Colon Carcinoma. Anticancer Research. 2005;25:2135–40. [PubMed] [Google Scholar]
- 61.Milovic V, Teller IC, Murphy GM, Caspary WF, Stein J. Deoxycholic acid stimulates migration in colon cancer cells. European Journal of Gastroenterology & Hepatology. 2001;13:945–9. doi: 10.1097/00042737-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Stutzmann J, Bellissent-Waydelich A, Fontao L, Launay JF, Simon-Assman P. Adhesion complexes implicated in intestinal epithelial cell-matrix interactions. Microscopy Research and Technique. 2000;51:179–90. doi: 10.1002/1097-0029(20001015)51:2<179::AID-JEMT9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 63.Xie K. Interleukin-8 and human cancer biology. Cytokine and Growth Factor Reviews. 2001;12:375–91. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.