Abstract
The corpus callosum, a major interhemispheric fiber tract, mediates communication between homotopic regions within somatosensory cortex (S1). Recently, in 1-6-day-old rats, brief bursts of oscillatory activity—called spindle-bursts (SBs)—were described in cortical somatosensory areas following sensory feedback from sleep-related myoclonic twitches or specific peripheral stimulation. To determine whether interhemispheric communication via the corpus callosum modulates the expression of SBs during this early period of development, we investigated the spontaneous expression of SBs in unanesthetized 1–6-day-old rats as well as SBs evoked by plantar surface stimulation of the forepaw. We hypothesized that surgically disrupting transcallosal communication (i.e., with callosotomy) or unilateral pharmacological manipulation of S1 activity (e.g., by blocking muscarinic receptors) would alter S1 activity in one or both hemispheres. First, callosotomy doubled the rate of spontaneous, twitch-related SBs in left and right S1s by reducing the interval between successive SBs. Second, unilateral infusion into left S1 of the muscarinic receptor antagonist, scopolamine, inhibited SBs in response to right forepaw stimulation; importantly, SBs were now disinhibited in the right S1 to right forepaw stimulation, thus “unmasking” an ipsilateral representation. Subsequent callosotomy reinstated contralateral SB responses in the left S1. Finally, tactile and proprioceptive stimulation produced dissociable neurophysiological S1 responses; specifically, SBs were produced in response to proprioceptive, but not tactile, stimulation. We conclude that the corpus callosum modulates functionally inhibitory interactions between homotopic regions in left and right S1s during the early developmental period when organized neurophysiological activity is first detected in neocortex.
Keywords: sleep, spontaneous activity, callosotomy, agenesis of the corpus callosum, inhibition, myoclonic twitch, hyperexcitability, somatosensation
The corpus callosum, which in humans consists of more than 190 million axons (Tomasch, 1954; Banich & Belder, 1995), mediates interhemispheric communication between homotopic cortical areas. Callosal fibers project to specialized cortical zones comprising maps within somatosensory cortex (Wise & Jones, 1976; Akers & Killackey, 1978; Innocenti & Price, 2005). It is not known how disruption of these projections alters activity in somatosensory areas and the development of somatotopy. Indeed, very little is known about callosal functioning early in development. Such basic developmental information might help to explain the relationship between congenital malformations of the corpus callosum (including agenesis; AgCC) and a number of neurological and psychiatric disorders, including epilepsy, schizophrenia, and autism (Paul et al., 2007). Moreover, developmental investigation may help to resolve outstanding questions concerning the excitatory and inhibitory influences of callosal projections on cortical activity (Cook, 1984; Denenberg et al., 1986; Koralek & Killackey, 1990; Reggia et al., 2001; Bloom & Hynd, 2005).
Recently, it was reported that brief bursts of spatially confined oscillatory activity—so called spindle-bursts (SBs)—occur in primary somatosensory cortex (S1) in 1–6-day-old (P1–6) rats (Khazipov et al., 2004). SBs occurred in topographic fashion as limbs exhibited fine twitch movements during sleep or high-amplitude movements during wakefulness; topographically related SBs were also evoked by tactile stimulation applied to various parts of the body. Subsequently, SBs were detected in barrel and primary visual cortex in association with whisker activity (Minlebaev et al., 2007) and retinal waves (Hanganu et al., 2006; Hanganu et al., 2007), respectively. In visual cortex, SBs were modulated by the cholinergic basal forebrain acting on cortical muscarinic receptors (Hanganu et al., 2007). It has been suggested that these topographically organized SBs reflect a self-organizational process underlying sensorimotor development (Khazipov et al., 2004; Khazipov & Luhmann, 2006).
The presence of topographically organized events in S1 offers the opportunity to assess interhemispheric communication during the early postnatal period when callosal projections are undergoing rapid developmental change. Specifically, many commissural fibers have crossed the midline by the day of birth in rats and, during the first postnatal week, callosal fibers grow into maturing cortex and topographic distribution is established (Wise & Jones, 1976; Akers & Killackey, 1978; Innocenti & Price, 2005).
Here we investigate the expression of SBs in unanesthetized 1–6.-day-old rats. We hypothesized that surgical or pharmacological manipulations that would be expected to disrupt or alter callosal communication would also alter SB activity in one or both S1s. Consistent with this hypothesis, transecting the corpus callosum (i.e., callosotomy) doubled the number of spontaneous, active sleep-related SBs in both S1s by reducing the interval between successive SBs. We also found that unilateral infusion of the muscarinic receptor antagonist, scopolamine, into the left S1 inhibited contralateral SB responses and disinhibited ipsilateral SB responses evoked by right forepaw stimulation; subsequent callosotomy reinstated contralateral SB responses. These results establish a functional inhibitory role for the corpus callosum in newborn rats and provide a foundation for further investigation of the development of callosal function, its contribution to somatotopy, and recovery of cortical function after early callosal damage.
MATERIALS AND METHODS
All experiments were performed in accordance with National Institutes of Health guidelines for the care and use of animals in research and were approved by the Institution Animal Care and Use Committee of the University of Iowa. All efforts were made to minimize the number of animals used.
Subjects
A total of 48 P1–6 rats from 48 litters were used. Males and females were equally represented among the subjects. Litters were culled to 8 pups within 3 days of birth (day of birth = P0). Mothers and their litters were housed in standard laboratory cages (48 cm × 20 cm × 26 cm) in the animal colony at the University of Iowa. Food and water were available ad libitum and all animals were maintained on a 12-hr light-dark cycle with lights on at 0700 h. All experiments took place during the lights-on period.
Surgery
Under isoflurane anesthesia, a scalp incision was made in the anterior-to-posterior direction to expose the skull. The membranes were stripped away and the uncalcified skull was cleaned and Vetbond (3M, St. Paul, Minnesota, United States) was applied to strengthen the surface. As described previously (Karlsson et al., 2005), a custom-built stainless steel apparatus, designed to attach to the earbar holders of a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, United States), was secured to the skull with cyanoacrylate adhesive. Bipolar stainless steel electrodes (50 µm diameter, California Fine Wire, Grover Beach, CA) were inserted into the right nuchal and right biceps brachii muscles. A ground wire was implanted anterior to the nuchal EMG. All electrodes were secured with flexible collodion. At the end of surgery, the pup’s trunk was lightly wrapped in gauze. These surgeries lasted approximately 10 min.
For those subjects that were callosotomized, the following procedure was added to that above. A 2–3 mm opening was created in the skull, halfway between bregma and lambda and parallel and just lateral to midline. A thin surgical knife was inserted to a depth of approximately 5 mm. The knife was then rapidly swept in an anterior and posterior direction to transect the corpus callosum primarily in its anterior half, which contains fibers that connect left and right S1s (Wise & Jones, 1976). Sham surgeries were identical except that the surgical knife was not inserted. This additional procedure added only 30 s to the surgery. In some subjects, the opening in the skull was created under anesthesia but the callosotomy was performed later during the recording session. We note that when the callosotomy was performed in these unanesthetized subjects, they never exhibited overt signs of distress; in fact, sleeping subjects typically remained asleep during the callosotomy.
Procedure
After surgery, each pup recovered for about 1 h in a humidified incubator maintained at thermoneutrality (35°C), after which it was transferred to an electrically shielded chamber for testing. The pup’s head was fixed in the stereotaxic apparatus and its ventrum was placed on a flat support bar with its forelimbs and hindlimbs dangling freely on both sides without contacting any surface (see Figure 1A, left). Temperature-controlled water flowing through a concave double-walled glass chamber, situated beneath the pup, helped to control the thermal environment and, in conjunction with a heat lamp, maintain the pup’s brain temperature at 37°C throughout testing.
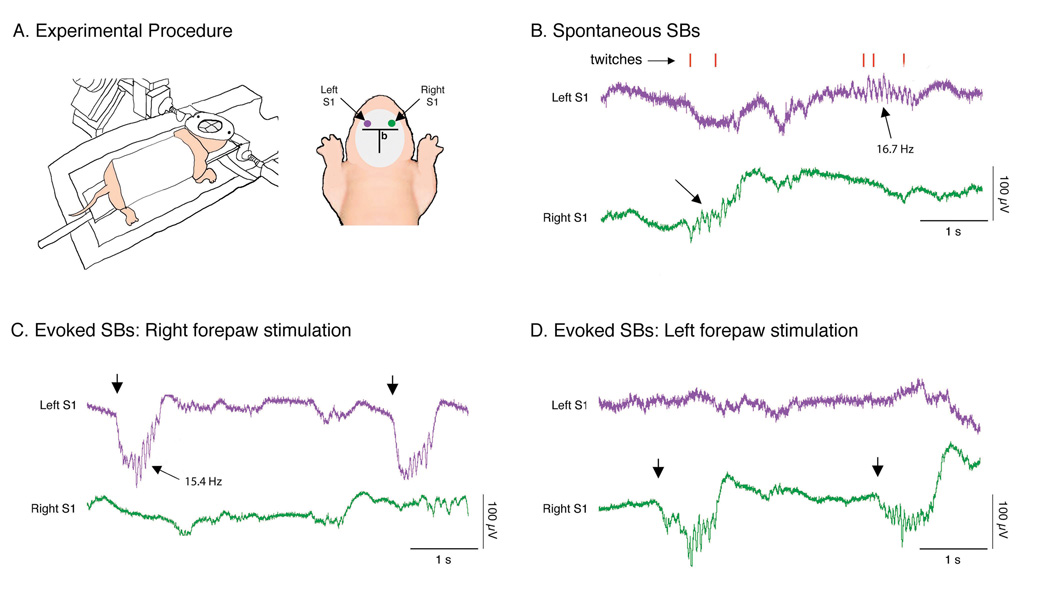
Figure 1. Spontaneous and evoked spindle-bursts (SBs) in a P5 rat.
(A) Left: Experimental procedure for recording SBs. The infant rat was head-fixed in a stereotaxic apparatus, placed on a narrow platform, lightly wrapped in gauze, and suspended over a temperature-controlled glass chamber. A heating lamp was also used to maintain brain temperature at 37°C. Right: View of skull showing approximate location of electrodes in relation to bregma (b). Pairs of Ag/AgCl electrodes were placed in left (purple dot) and right (green dot) somatosensory cortex (S1) and SB responses to contralateral forepaw plantar surface stimulation were confirmed. (B) Spontaneous SBs (denoted by arrows) in left (purple) and right (green) S1 in relation to active sleep-related myoclonic twitches of the limbs (red ticks) assessed through behavioral observation. Contralateral SBs were produced in response to right (C) and left (D) forepaw plantar surface stimulation (denoted by arrows). Note that these evoked SBs, in contrast with the spontaneous SBs in (B), were embedded within large, slow potentials. The oscillation frequencies of one spontaneous and one evoked SB are also shown.
Two recording sites were prepared for placement of electrodes over the left and right S1s (see Figure 1A, right). Each site consisted of two holes separated by approximately 2 mm and centered over the forelimb region of S1 (Khazipov et al., 2004), approximately 1 mm rostral to bregma and 2–3 mm lateral to midline. Custom-made Ag/AgCl electrodes, consisting of Teflon-insulated silver wires (0.01 in. diameter; Medwire, Mount Vernon, NY) with approximately 1 mm of each tip stripped and chlorinated, were inserted just below the cortical surface. Ground electrodes were placed in the cerebellum.
Electroencephalographic (EEG) and electromyographic (EMG) electrodes were connected to differential amplifiers (A-M Systems, Carlsborg, WA; filter setting: 0.1–3000 Hz; amplification: ×10,000). Neural and EMG signals were sampled at 12.5 kHz using a digital interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK).
After approximately 1 h of acclimation, by which time sleep-wake cycles were evident, the plantar surface of the left and right forepaws was stimulated with a fine brush (typically, these strokes also elicited dorsiflexion of the wrist). Electrode placements were judged successful when plantar surface stimulation of the forepaw resulted in a corresponding SB embedded within a slow potential in the contralateral somatosensory cortex. When SBs were not detected, the electrode placements were adjusted. Importantly, other parts of the limb and body were stimulated to assure the specificity of the SB response.
EMG and EEG data were acquired continuously throughout the test. In all subjects, visual observation of sleep-wake behaviors and EMG recording of nuchal and right biceps brachii muscles provided measures of behavioral state (Karlsson et al., 2005). When appropriate, the experimenter scored sleep-related myoclonic twitches as well as wake-related behaviors using a keyboard connected to the data acquisition system. As described elsewhere (Gramsbergen et al, 1970; Karlsson et al., 2005), myoclonic twitches, indicative of active sleep, were defined as phasic, rapid, and independent movements of the limbs and tail. High-amplitude movements, indicative of wakefulness, included locomotion, stretching, and yawning. In addition, during periods when the plantar surface of the forepaw was stimulated, the experimenter simultaneously pressed a key on the keyboard to mark this event in synchrony with the electrophysiological data.
For the experiment in which subjects received callosotomy or sham surgery before testing, a 15-min period in which myoclonic twitching and wake-related movements were scored was followed by a 15-min period of forepaw stimulation. For the experiment in which subjects were callosotomized during testing, a 15-min period of behavioral scoring during the pre-callosotomy period was followed by a 15-min period of behavioral scoring during the post-callosotomy period (this latter period commenced with the completion of the callosotomy), followed by a final 15-min period of forepaw stimulation.
For the experiment in which subjects were infused with ACSF, bupivacaine, or scopolamine, 15 min of behavioral scoring during the pre-infusion period was followed by 15 min of behavioral scoring during the post-infusion period (this latter period commenced with the completion of the drug infusion). After the post-infusion period was complete, data were recorded during a final 15-min period of forepaw stimulation. ACSF, bupivacaine hydrochloride (Abbott Laboratories, North Chicago, IL; 0.75%), and scopolamine hydrochloride (Sigma-Aldrich, St. Louis, MO; 90 µg/µl mixed in ACSF) were infused (0.1 µl /s) in a volume of 1 µl using a Hamilton microlitre syringe with a 25 gauge needle (Model 7001, Hamilton, Reno, Nevada, United States) mounted directly above the infusion site. The syringe was lowered just below the cortical surface into a pre-drilled hole located midway between the 2 Ag/AgCl electrode sites. In a third experiment, three 15-min periods of behavioral scoring and forepaw stimulations were recorded during (i) baseline, (ii) after scopolamine infusion, and (iii) after callosotomy. Finally, in several subjects, scopolamine was again infused at the end of the experiment to assure that the observed effects of callosotomy were not due to a decrease in scopolamine’s effectiveness over time.
A final experiment was conducted to examine the specificity of S1 responses to tactile and proprioceptive stimulation. For this experiment, surgical and recording procedures were the same as described above, but now several different kinds of forepaw stimulation, each occurring during successive 15-min periods, were applied to all subjects while cortical activity was recorded. At least 20 stimulations in each category were presented to each subject and the ordering of the stimulation periods was the same in all subjects. Half of the subjects received left forepaw stimulation and the other half received right forepaw stimulation. First, using a fine brush, the plantar surface of the forepaw was stroked as in earlier experiments to produce tactile stimulation with wrist dorsiflexion; second, wrist dorsiflexion without plantar surface stimulation was produced by gently pulling a string attached to the dorsal surface of the paw (the string was attached earlier during surgery using cyanoacrylate adhesive); third, tactile stimulation of the plantar surface of the forepaw was produced while being careful to avoid wrist dorsiflexion; fourth, as a further check on the specificity of the proprioceptive response, a string was attached to the elbow joint with cyanoacrylate adhesive and the elbow was pulled repeatedly to a flexed position. Finally, tactile stimulation with wrist dorsiflexion was repeated to ensure that the recording conditions had remained stable throughout the period of testing.
Histology
Upon completion of testing, callosotomized pups were overdosed with an intraperitoneal injection of sodium pentobarbital and perfused transcardially with phosphate-buffered saline, followed by a 3% formalin solution. Brains were postfixed for at least 48 h in a formalin–sucrose solution before being sliced in the coronal plane (50 µm sections). Light microscopy was then used to assess the extent of damage to the corpus callosum.
Data Analysis
For each subject, 15-min periods of synchronized data comprising digital records of behavior, EMG activity, and EEG activity were created and analyzed using Spike2 software. For all analyses, data were averaged for each subject before statistical analysis and paired (within-subjects) or unpaired (between-subjects) t tests were performed using JMP 5.0 software (SAS, Cary, North Carolina, USA). For all tests, alpha was set at 0.05.
Spontaneous and evoked SBs were identified by referring to both the raw EEG records of the left and right S1s as well as filtered records (band-pass: 5–40 Hz). Using criteria similar to those described previously (Khazipov et al., 2004), SBs comprised at least 3 complete oscillations, were > 100 ms in duration, and contained at least one oscillation that exceeded 50 µV in amplitude (from baseline to peak). In addition, evoked SBs were embedded in large, slow potentials with amplitudes ≥ 100 µV.
SB durations and latencies were compared between intact and callosotomized subjects. For this analysis, 20 “anchor” SBs in the left S1 recording were selected at random for each subject and its duration determined. Then, for each of these SBs, the latency between it and the prior (−L) and subsequent (+L) SBs in the right S1 recording was determined.
The effects of callosotomy and drug infusion on SB oscillation frequency were also analyzed. The frequency (in Hz) of an individual SB was determined by measuring the time between two successive oscillation peaks, averaging them, and then calculating the reciprocal. Mean SB frequency was determined for each subject by averaging across 20 individual SBs. Paired or unpaired t tests were then performed.
For each type of sensory stimulation presented to each subject in the final experiment (i.e., wrist flexion plus tactile stimulation, wrist flexion only, and tactile stimulation only), we determined the percentage of contralateral S1 responses that comprised SBs alone, slow potentials alone, or SBs embedded within slow potentials. The response data were imported into Statview 5.0 (SAS Institute, Cary, NC) and paired t tests were used to test for response differences within each stimulation category. Because multiple t tests were performed, a conservative alpha of 0.001 was used.
RESULTS
S1 responses during sleep-related twitching and upon plantar surface stimulation
In all experiments, cortical activity was examined in unanesthetized newborn rats, head-fixed and suspended in a prone position (Figure 1A). Spontaneous SBs associated with sleep-related twitching of the distal limbs were reliably recorded (Figure 1B), as described previously (Khazipov et al., 2004). Also, stimulation of the plantar surface of each forepaw using a fine brush reliably evoked SBs in the contralateral S1 (i.e., > 95% of stimulations; Figure 1C, D). Under these experimental conditions, evoked SBs were distinguishable from those produced spontaneously by their being embedded within large, slow potentials with a duration typically exceeding 500 ms. Before further testing, we confirmed that SBs were specifically evoked by plantar surface stimulation of the forepaw by stimulating other parts of the body surface.
Effect of callosotomy on spontaneous and evoked SBs
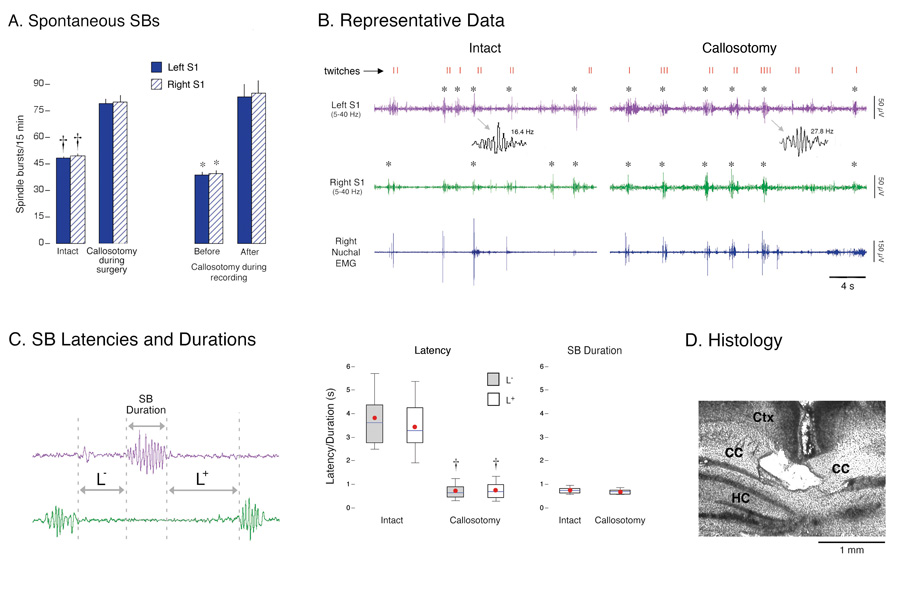
Callosotomy resulted in a significant increase in spontaneous SBs in both left and right S1s (Figure 2A, left; t10s > 7.6, Ps < 0.0001). Additionally, callosotomy resulted in a 60% increase in spontaneous SB oscillation frequency. Specifically, for intact subjects, mean SB frequency was 17.1 ± 0.3 Hz, compared with 28.9 ± 0.6 Hz for the callosotomized subjects (t10 = 18.2, P < 0.0001). In a second group of rats (n = 6), callosotomies were performed during the recording session. In these animals, callosotomy produced an immediate increase in the rate of occurrence of spontaneous SBs in both S1s in relation to the pre-callosotomy period (Figure 2A, right; t5s > 7.4, Ps < 0.0001); mean SB frequency also increased immediately and significantly after callosotomy (e.g., left S1, pre: 17.3 ± 0.4 Hz; left S1, post: 28.3 ± 0.6 Hz; t5 = 17.0, P < 0.0001).
Figure 2. Spontaneous spindle-bursts (SBs) in intact and callosotomized P1–6 rats.
(A) Left: Mean number of spontaneous SBs in left (solid) and right (hatched) S1 during 15-min recording periods. Two procedures were used: pups experienced sham surgery (intact) or callosotomy before recording (n = 6 per group), or pups were callosotomized during the recording session (n = 6). * P < 0.001 in relation to the callosotomy group or post-callosotomy period. Mean + s.e. (B) Representative periods of active sleep in an intact P6 subject and a callosotomized P5 subject. Traces depict right nuchal EMG and right (green) and left (purple) S1 EEG activity (band-pass filter: 5–40 Hz). Behaviorally scored myoclonic twitches of the limbs (red ticks) are also shown and confirmed SBs are denoted by asterisks. Note that SBs in both subjects occur reliably during periods of twitching. Two SBs are expanded to illustrate increased oscillation frequency after callosotomy. S1 activity sometimes increased during periods of twitching but was not categorized as an SB. This is because, upon closer examination, this activity did not exhibit the regular, sinusoidal features characteristic of SBs; instead, this non-SB activity was highly irregular in both frequency and amplitude. (C) Left: Illustration of the method used to measure SB durations and the latencies between SBs in different hemispheres. Right: Box plots depicting distributions of SB latencies and durations for intact and callosotomized subjects (n = 6 per group). The top, middle, and bottom horizontal lines of the box represent the 75th, 50th (median), and 25th percentiles, respectively. The thin vertical lines above and below the box represent the 90th and 10th percentiles, respectively. Red circles are means. † P < 0.0001 in relation to the intact group. (D) Coronal section showing severed corpus callosum (CC) ventral to cerebral cortex (Ctx) and dorsal to hippocampus (HC).
The callosotomy-induced increase in the occurrence of spontaneous SBs could have resulted from generalized functional disinhibition of these cortical oscillations, perhaps allowing them to occur independent of behavioral state. However, even in callosotomized pups, the close association between periods of myoclonic twitching and SBs was retained (Figure 2B). When we examined the number of limb twitches for each subject during the 15-min recording period, we found no significant difference between the intact and callosotomized subjects (intact: 343.8 ± 25.1 twitches; callosotomized: 381.0 ± 32.9 twitches; t10 = 0.9; similar results were found for subjects that were callosotomized during recording). This result was expected because, as reported previously in infant rats (Kreider & Blumberg, 2000), even complete transection of the brainstem between the cortex and mesopontine region does not alter the quantity or temporal organization of twitching.
Thus, we hypothesized that callosotomy exerted its effects on SBs by reducing mutually inhibitory interactions between homotopic areas in left and right S1s. We tested this hypothesis by examining the temporal relationship between SBs in the left S1 in relation to SBs in the right S1 (Figure 2C, left). First, the nearly 4-s mean delay between interhemispheric SBs in the intact subjects is consistent with our observation that SBs in the two hemispheres almost never overlapped. Second, callosotomy produced a significant reduction in the latencies of interhemispheric SBs (Figure 2C, right; t10s > 21.0, Ps < 0.0001); despite this reduction, SBs still rarely overlapped. SB durations did not differ significantly between intact and callosotomized subjects (t10 = 0.3, NS). It appears, then, that callosotomy reduces interhemispheric inhibition and thereby increases the probability that an SB will occur in response to twitch-related sensory feedback.
We might have expected SBs in the two disconnected hemispheres of callosotomized subjects to occasionally overlap if and when myoclonic twitches occurred simultaneously in left and right forelimbs. However, overlapping of SBs rarely occurred, which is consistent with the prior finding that myoclonic twitches are expressed in bouts comprising non-simultaneous movements of individual limbs (Robinson et al., 2000).
Finally, in callosotomized subjects, forepaw stimulation continued to evoke SB responses in the contralateral hemisphere, albeit approximately 30% less often than in intact subjects (e.g., left S1, intact: 99.3 ± 0.5%; callosotomy: 69.4 ± 6.7%; t10 = 4.4, P < 0.005). Also, as with spontaneous SBs, mean SB oscillation frequency in response to forepaw stimulation increased significantly in callosotomized subjects in relation to intact subjects (intact: 18.1 ± 0.4 Hz; callosotomy: 31.5 ± 0.9 Hz; t10 = 13.6, P < 0.0001). Again, similar results were found for subjects that were callosotomized during the test period.
Ipsilateral “unmasking” of evoked SBs after scopolamine infusion
Previous studies have shown that network oscillations in newborn mouse neocortical slices are Na+-channel dependent (Dupont et al., 2006) and that SBs in newborn rats are modulated by cholinergic input from the basal forebrain (Hanganu et al., 2007). Therefore, to alter S1 activity unilaterally so as to observe possible callosally mediated changes in the contralateral hemisphere, we examined the effects of the local anesthetic, bupivacaine, and the muscarinic receptor antagonist, scopolamine, on spontaneous and evoked SBs. Bupivacaine’s anesthetic properties have been attributed to its ability to block voltage-gated Na+ channels as well as G protein-gated inwardly rectifying K+ channels (Zhou et al., 2001; Kindler & Yost, 2005). The general procedure was to infuse artificial cerebrospinal fluid (ACSF; n = 4), bupivacaine (n = 6), or scopolamine (n = 6), just beneath the cortical surface in the left S1 (n = 6 per group), using methods similar to those described elsewhere (Hanganu et al., 2007). After infusion, spontaneous cortical activity was recorded and, thereafter, the plantar surface of the forepaws was stimulated.
With regard to spontaneous SBs, neither bupivacaine nor scopolamine infusion into the left S1 significantly affected their rate of occurrence in either hemisphere in relation to the pre-infusion period (t5s < 1.0, NS). For example, in the left hemisphere, the average spontaneous rate of occurrence of SBs before infusion of ACSF, bupivacaine, or scopolamine, respectively, was 41.2 ± 1.6, 49.3 ± 1.4, and 48.8 ± 2.0 SBs/15 min; after infusion, the respective values were 39.75 ± 1.7, 49.8 ± 1.2, and 48.0 ± 2.6 SBs/15 min.
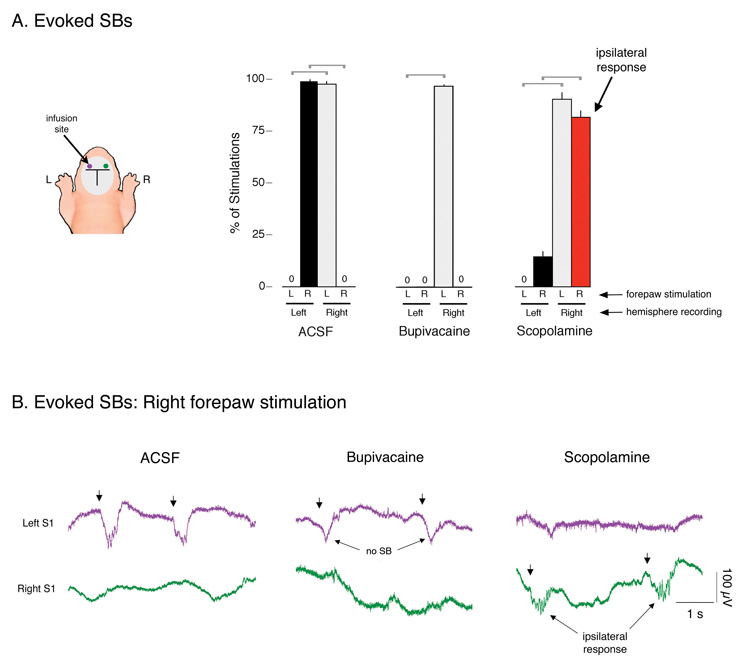
With ACSF infusion into the left S1, SB responses in the left and right S1s occurred exclusively in response to contralateral forepaw stimulation (Figure 3A; t5s > 72.5, Ps < 0.0002). For example, in the left S1, SBs occurred 98.4 ± 1.0% of the time in response to right forepaw stimulation and never in response to left forepaw stimulation. In contrast, bupivacaine infusion eliminated SBs in the left S1 to right forepaw stimulation; however, contrary to our prediction, this elimination of SBs in the left S1 was not accompanied by an effect on right S1 activity. Moreover, although SBs were eliminated by bupivacaine, the large, slow potential was still reliably evoked, thus indicating that these two components are dissociable.
Figure 3. Effects of bupivacaine and scopolamine on evoked SBs in P1–6 rats.
(A) Mean percentage of evoked SBs in left and right S1s in response to stimulation of the plantar surface of the left (L) and right (R) forepaws. Each recording period was 15 min in duration. Pups were infused with artificial cerebrospinal fluid (ACSF; n = 4), the local anesthetic, bupivacaine (n = 6), or the muscarinic receptor antagonist, scopolamine (n = 6), into the left S1. Brackets denote significant (P < 0.0001) differences between hemispheres in response to left or right forepaw stimulation. Mean + s.e. (B) Representative evoked SBs in a P4 subject in left and right S1s in response to right forepaw stimulation (denoted by arrows). ACSF infusion did not affect the normal expression of contralateral SBs in the left S1. In contrast, scopolamine infusion resulted in the expression of ipsilateral SBs in the right S1. Finally, bupivacaine eliminated SB responding in the left S1 to right forepaw stimulation, although expression of the large, slow potential was unaffected.
Scopolamine infusion into the left S1 did not interfere with the ability of left forepaw stimulation to evoke a contralateral SB response in the right S1 (contralateral: 90.3 ± 3.1%; ipsilateral: 0.0 ± 0.0%; t5 = 29.2, P < 0.0001). However, stimulation of the right forepaw now significantly reduced contralateral SBs in left S1 and “unmasked” ipsilateral SBs in right S1 (contralateral: 14.4 ± 2.5%; ipsilateral: 81.7 ± 3.4%; t5 = 11.6, P < 0.0001).
After infusion of ACSF into the left S1, mean SB oscillation frequency in the left S1 to right forepaw stimulation was 17.2 ± 0.2 Hz, compared with 17.8 + 0.5 Hz in the right S1 in response to left forepaw stimulation. After infusion of scopolamine into the left S1, the ipsilateral SBs in the right S1 (non-drug) to right forepaw stimulation had a mean frequency of 17.5 ± 0.3 Hz, which is not significantly different from the mean frequency of SBs produced in the right (non-drug) S1 to left forepaw stimulation (18.1 ± 0.3 Hz; t5 = 1.1); nor is this value significantly different from either ACSF group (t8s < 0.8). Therefore, in contrast to the effects of callosotomy, scopolamine infusion did not affect SB oscillation frequency.
Callosotomy reinstates contralateral evoked SBs after scopolamine infusion
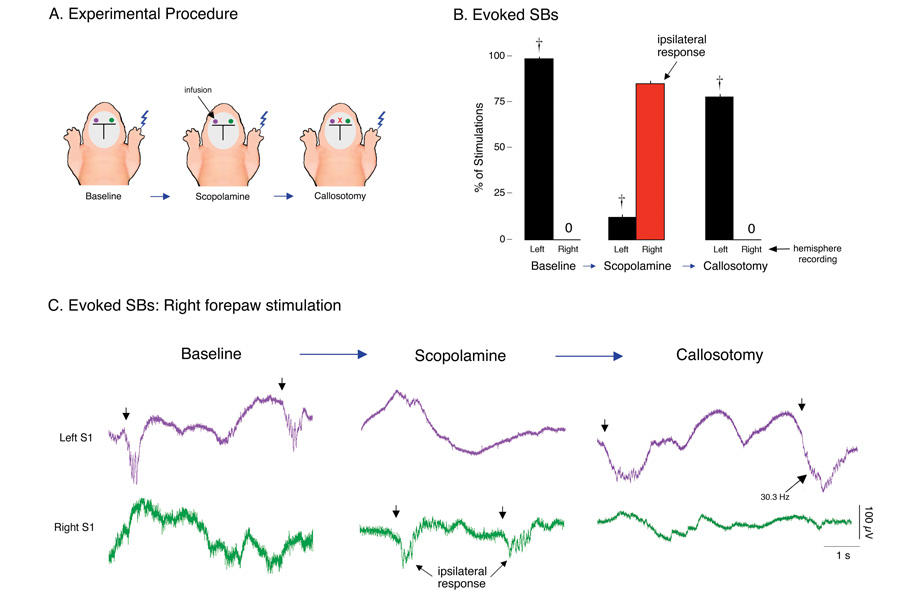
We next determined whether the ipsilateral expression of SBs after scopolamine infusion was mediated by the corpus callosum. Using 6 additional P3–6 rats, stimulations were applied to the right forepaw during 3 successive 15-min periods: (i) during baseline, (ii) after scopolamine infusion into the left S1, and (iii) after subsequent callosotomy (Figure 4A). As expected, evoked SBs during the baseline period were expressed in the contralateral (i.e., left) S1 in response to right forepaw stimulation (Figure 4B; t5 = 169.1, P < 0.0001). Next, scopolamine infusion produced the expected ipsilateral SB response (Figure 4B; t5 = 25.2, P < 0.0001). After callosotomy, ipsilateral SBs in response to right forepaw stimulations disappeared immediately and, to our surprise, contralateral responding was reinstated (t5 = 53.2, P < 0.0001; representative data are presented in Figure 4C). Finally, to confirm that the reinstatement of the contralateral SB response was due to the callosotomy and not to the loss of scopolamine’s effectiveness, several subjects received a second infusion of scopolamine at the end of the experiment; no change in SB responding was observed.
Figure 4. Callosotomy reinstates contralateral evoked spindle-bursts (SBs) after scopolamine infusion.
(A) Depiction of experimental procedure in infant rats (n = 6) showing the three 15-min recording periods: After a baseline period, scopolamine was infused into the left S1, followed by callosotomy. During each period, responses of the left and right S1s were recorded in response to plantar surface stimulation of the right forepaw. (B) Mean percentage of right forepaw plantar surface stimulations producing associated SBs. During the baseline period, only contralateral SBs were produced. After scopolamine infusion into the left S1, ipsilateral responses predominated (red bar). Subsequent callosotomy reinstated contralateral responding. † P < 0.0001 in relation to right hemisphere. Mean + s.e. (C) Representative evoked SBs in left (purple) and right (green) S1s in response to right forepaw plantar surface stimulations (denoted by arrows) in a P3 subject. Contralateral responding during the baseline period was followed by ipsilateral responding after scopolamine infusion and reinstatement of contralateral responding after subsequent callosotomy. The higher oscillation frequency of one of the post-callosotomy SBs is indicated.
Although contralateral SB responses were reinstated by callosotomy, the SBs that were reinstated were not expressed with their characteristic oscillation frequency. Specifically, during the baseline period, mean evoked SB frequency in the left S1 after right forepaw stimulation was 17.7 ± 0.3 Hz. After scopolamine infusion, mean evoked SB frequency in the right S1 after right forepaw stimulation did not change significantly (17.5 ± 0.4 Hz; t10 = 0.3, NS). In contrast, mean evoked SB frequency in the left S1 to right forepaw stimulation nearly doubled after callosotomy, 34.0 ± 0.7 Hz, which is significantly greater than both previous conditions (t10s > 28.8, Ps < 0.0001).
Dissociating the effects of tactile and proprioceptive stimulation
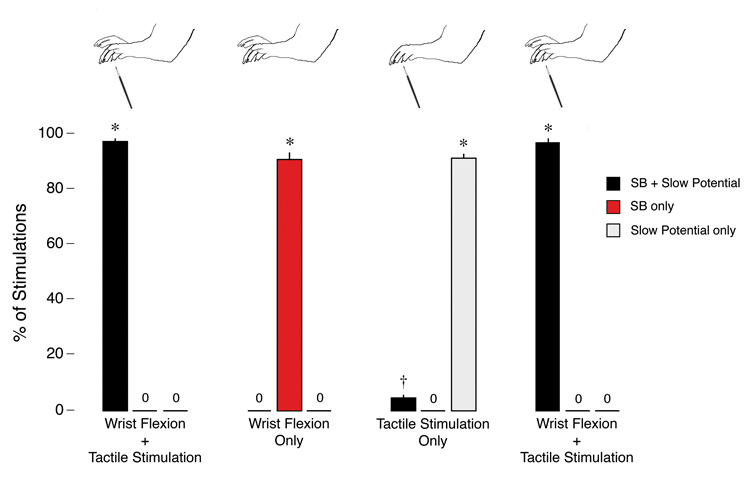
Given that bupivacaine blocked the expression of SBs without affecting expression of the slow potential, we hypothesized that the two responses could be dissociated by taking care to provide only tactile or proprioceptive stimulation to the forepaw. This hypothesis arose from our observation that the standard stimulus used here comprised tactile stimulation of the plantar surface along with dorsiflexion at the wrist. Therefore, in 8 additional P3–4 rats, subjects were prepared as in previous experiments and then presented with a succession of stimuli. As shown in Figure 5, the standard stimulus—wrist dorsiflexion with tactile stimulation—resulted in the expected SBs embedded within slow potentials (96.9 ± 1.2%). When wrist dorsiflexion alone was presented, only SBs were detected in the contralateral S1 (90.6 ± 2.5%). And when tactile stimulation alone was presented, the predominant response was a slow potential without an SB (90.9 ± 1.3%); embedded SBs were only occasionally produced (3.7 ± 0.7%), presumably due to the difficulty of completely eliminating wrist dorsiflexion. It should also be noted that a control procedure involving flexion at the elbow joint never produced activity in the contralateral S1 (data not shown). A final control period of wrist flexion with tactile stimulation again produced the expected embedded SBs in the contralateral S1 (96.7 ± 1.1%).
Figure 5. Dissociation of tactile and proprioceptive S1 responses in newborn rats.
Top: Illustrations depicting the four types of stimulation applied to the left or right forepaw in successive 15-min periods: wrist dorsiflexion with tactile stimulation; wrist dorsiflexion alone; tactile stimulation alone; and another period of wrist dorsiflexion with tactile stimulation. Bottom: percentage of forepaw stimulations that, in the contralateral S1, produced SBs embedded within slow potentials, SBs only, or slow potentials only. A fourth stimulation type, not shown here and comprising flexion of the elbow joint, never resulted in contralateral S1 activity. * significant difference from the other two response categories (p < 0.0001). † significant difference from the ‘SB only’ response category (p < 0.001). Mean + s.e.
DISCUSSION
We have revealed mutually inhibitory interactions between homotopic cortical areas mediated by the corpus callosum in unanesthetized newborn rats. This functional inhibition was immediately evident upon examination of spontaneous, active sleep-related SBs after the corpus callosum was severed: specifically, the rate and oscillation frequency of SBs increased dramatically after callosotomy, and SBs in the two hemispheres occurred in much closer temporal proximity to each other. Thus, with regard to the ongoing debate concerning the relative importance of excitatory and inhibitory functions of the corpus callosum (Cook, 1984; Denenberg et al., 1986; Koralek & Killackey, 1990; Reggia et al., 2001; Bloom & Hynd, 2005), it seems clear that in newborn rats each hemisphere functionally inhibits the other via callosal projections. Whether these projections achieve this functional inhibition through local inhibitory or excitatory postsynaptic effects remains unclear.
It may seem paradoxical that SBs were produced in closer temporal proximity between the two hemispheres in callosotomized subjects (see Figure 2). As already mentioned, it is most likely that this apparent increase in interhemispheric synchrony resulted from the loss of callosal inhibition, thereby increasing the likelihood that a limb twitch would trigger an SB. Still, it must be emphasized that the increase in temporal proximity rarely resulted in overlapping SBs in the two hemispheres, which we attribute to the dependence of SB production on peripheral sensory feedback and, in turn, to the fact that myoclonic twitches are expressed in bouts comprising contemporaneous but non-simultaneous movements of individual limbs (Robinson et al., 2000). The precise relationship among limb movements, sensory feedback, and SB production requires further study.
Unilateral infusions of the muscarinic receptor antagonist, scopolamine, provided further support for the hypothesis that the corpus callosum functionally inhibits homotopic S1s in newborn rats. Under normal conditions where SBs were evoked by stimulation of the plantar surface of a forepaw, only contralateral responses were observed. However, when scopolamine was infused unilaterally into the left S1 and the right forepaw was stimulated, contralateral SB responses were significantly reduced, thus suggesting that excitatory muscarinic activation is necessary to produce consistent contralateral SB responses to peripheral stimulation. In addition to inhibiting contralateral SBs, scopolamine infusion disinhibited ipsilateral SBs, an unexpected finding that suggests the presence of homotopic representations of each forepaw in both hemispheres. It is important to note, however, that scopolamine’s effect on ipsilateral SBs cannot merely be due to the inhibition of contralateral SBs, as bupivacaine eliminated contralateral SBs without affecting ipsilateral S1 activity.
Although it appeared to us initially that scopolamine had disrupted the ability of the left S1 to produce SBs, this was clearly not the case as subsequent callosotomy reinstated their expression (although at a higher oscillation frequency). Thus, both the inhibition of the contralateral response and the disinhibition of the ipsilateral response must rely on callosal modulation of homotopic S1 activity. Interestingly, with respect to motor behavior, alterations in interhemispheric balance in humans—as occur with asymmetric parkinsonism and other neurological disorders—can result in the disinhibition of ipsilateral pathways and the production of “mirror movements” (Li et al., 2007).
Too little is currently known about cortical circuitry and callosal connectivity, especially during the early postnatal period, to provide a realistic model of the mechanisms responsible for the cholinergically mediated disinhibition of SBs in the ipsilateral S1 or the increase in SB oscillation frequency after callosotomy. Any such model will need to take into account the neonate’s rapidly changing cortical circuitry, as well as the contributions that the cortical subplate and other transient structures might make to neurophysiological activity during the early postnatal period when the callosal fibers are establishing topographic connections (Wise & Jones, 1976; Innocenti & Price, 2005).
As shown here, SBs evoked by plantar surface stimulation were typically embedded within large, slow potentials. However, these two responses to sensory stimulation are dissociable, as the local anesthetic, bupivacaine, effectively blocked expression of SBs without affecting the slow component. We examined this issue further through specific tactile, proprioceptive, or combined stimulation of the forepaw. Whereas plantar surface stimulation with wrist dorsiflexion produced the expected embedded SB response, wrist dorsiflexion alone produced only SB responses; the predominant response upon specific tactile stimulation was the slow potential without an SB.
Spontaneous SBs were rarely embedded within slow potentials. This could reflect the fact that, in the present experiment, pups were suspended in a prone position such that the limbs did not make contact with any surface. Therefore, these test conditions allowed for proprioceptive, but not tactile, feedback from twitching limbs. Under more natural conditions where limbs can make contact with other objects, including littermates, we might expect twitching to result in both tactile and proprioceptive feedback and, consequently, embedded SBs.
The continued detection of spontaneous SBs in the left S1 after bupivacaine and scopolamine infusion was surprising. Because sleep-related twitching can produce SBs in multiple areas of S1 contemporaneously, it is possible that travelling waves from nearby cortical tissue obscured the effects of bupivacaine on local SB production; such travelling SBs have been demonstrated in newborn rats (see Supplementary Figure 3 in Khazipov et al., 2004). In contrast, SBs would not be expected to travel from nearby tissue when evoked through specific peripheral stimulation. In light of the fact that scopolamine disinhibited SB activity in the right S1 in response to ipsilateral forepaw stimulation, we might have expected a doubling of spontaneous SBs in the right S1 as a result of the combined sensory input from twitches of the contralateral and ipsilateral forepaws. That we did not observe any increase in the occurrence of spontaneous SBs after scopolamine infusion cannot be attributed to the obscuring effects of travelling waves.
There is an alternative explanation for the differential effects of bupivacaine and scopolamine on spontaneous and evoked SBs. Specifically, it is possible that spontaneous SBs triggered by the sensory feedback from self-generated myoclonic twitches recruits different cortical mechanisms than SBs evoked by externally applied peripheral stimulation. Such a possibility is further supported by the finding that callosotomy doubled the expression of spontaneous SBs but decreased by 30% the likelihood of evoked SBs to contralateral forepaw stimulation. Confirming and elucidating this distinction between spontaneous and evoked SBs will be important for understanding the possible function of sleep-related twitching for sensorimotor development.
Sensory feedback during twitching has been hypothesized to play a role in the self-organization of spinal cord circuits that support the nociceptive withdrawal reflex (Petersson et al., 2004; Schouenborg, 2008). This reflex, which entails the integration of specific sensory fields in the paw with functionally relevant motor commands, develops over the first few postnatal weeks in rats (Holmberg & Schouenborg, 1996). In that context, it is perhaps surprising that the two separable but functionally related features of distal limb stimulation used here—wrist dorsiflexion and plantar surface stimulation—result in integrated activity in somatosensory cortex so soon after birth in rats, and that they can also be transferred together across the corpus callosum to the contralateral homotopic region. Such early expression of these phenomena suggests that spontaneous movements in fetuses, which are phenomenologically similar to those expressed in neonates (Robinson et al., 2000), also elicit sensory feedback and nervous system activation that could contribute to the development of neural circuits.
If SBs and myoclonic twitching jointly contribute to the self-organization of the sensorimotor system (Khazipov et al., 2004; Seelke et al., 2005), then it is possible that interference with callosal functioning—as occurs with callosotomy—would obscure the temporal relationship between individual movements and their associated SBs, thereby disrupting map formation. Such a situation could prevail in humans with AgCC or other disorders that entail disruption of callosal functioning (Paul et al., 2007), as well as humans who have experienced callosotomy at an early age (Lassonde et al., 1991).
ACKNOWLEDGEMENTS
We thank Bob McMurray, Ralph Adolphs, Lynn Paul, and Andrew Gall for helpful suggestions. Supported by a grant (MH50701) and an Independent Scientist Award (MH66424) from the National Institutes of Health (M.S.B.).
ABBREVIATIONS
- ACSF
Artificial cerebrospinal fluid
- Ag/AgCl
Silver/silver chloride
- AgCC
Agenesis of the corpus callosum
- CC
Corpus callosum
- Ctx
Cortex
- EEG
Electroencephalogram
- EMG
Electromyogram
- HC
Hippocampus
- P
Postnatal day
- S1
Primary somatosensory cortex
- SB
Spindle-burst
REFERENCES
- Akers RM, Killackey HP. Organization of corticocortical connections in the parietal cortex of the rat. J. Comp. Neurol. 1978;181:513–537. doi: 10.1002/cne.901810305. [DOI] [PubMed] [Google Scholar]
- Banich MT, Belder A. Interhemispheric processing: Theoretical considerations and empirical processing. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge: MIT Press; 1995. pp. 427–450. [Google Scholar]
- Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol. Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- Cook ND. Homotopic callosal inhibition. Brain Lang. 1984;23:116–125. doi: 10.1016/0093-934x(84)90010-5. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Gall JS, Berrebi A, Yutzey DA. Callosal mediation of cortical inhibition in the lateralized rat brain. Brain Res. 1986;397:327–332. doi: 10.1016/0006-8993(86)90634-7. [DOI] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Dev. Psychobiol. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J. Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Staiger JF, Ben-Ari Y, Khazipov R. Cholinergic modulation of spindle bursts in the neonatal rat visual cortex in vivo. J. Neurosci. 2007;27:5694–5705. doi: 10.1523/JNEUROSCI.5233-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg H, Schouenborg J. Postnatal development of the nociceptive withdrawal reflexes in the rat: a behavioural and electromyographic study. The Journal of physiology. 1996;493(Pt 1):239–252. doi: 10.1113/jphysiol.1996.sp021379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nature reviews. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Gall AJ, Mohns EJ, Seelke AM, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biology. 2005;3:891–901. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle-bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kindler CH, Yost CS. Two-pore domain potassium channels: new sites of local anesthetic action and toxicity. Reg. Anesth. Pain Med. 2005;30:260–274. doi: 10.1016/j.rapm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Koralek KA, Killackey HP. Callosal projections in rat somatosensory cortex are altered by early removal of afferent input. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1396–1400. doi: 10.1073/pnas.87.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider JC, Blumberg MS. Mesopontine contribution to the expression of active 'twitch' sleep in decerebrate week-old rats. Brain Res. 2000;872:149–159. doi: 10.1016/s0006-8993(00)02518-x. [DOI] [PubMed] [Google Scholar]
- Lassonde M, Sauerwein H, Chicoine AJ, Geoffroy G. Absence of disconnexion syndrome in callosal agenesis and early callosotomy: brain reorganization or lack of structural specificity during ontogeny? Neuropsychologia. 1991;29:481–495. doi: 10.1016/0028-3932(91)90006-t. [DOI] [PubMed] [Google Scholar]
- Li JY, Espay AJ, Gunraj CA, Pal PK, Cunic DI, Lang AE, Chen R. Interhemispheric and ipsilateral connections in Parkinson's disease: relation to mirror movements. Mov. Disord. 2007;22:813–821. doi: 10.1002/mds.21386. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J. Neurophysiol. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nature Reviews Neuroscience. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Petersson P, Granmo M, Schouenborg J. Properties of an adult spinal sensorimotor circuit shaped through early postnatal experience. J. Neurophysiol. 2004;92:280–288. doi: 10.1152/jn.00063.2004. [DOI] [PubMed] [Google Scholar]
- Reggia JA, Goodall S, Levitan S. Cortical map asymmetries in the context of transcallosal excitatory influences. Neuroreport. 2001;12:1609–1614. doi: 10.1097/00001756-200106130-00020. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Blumberg MS, Lane MS, Kreber LA. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behav. Neurosci. 2000;114:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- Schouenborg J. Action-based sensory encoding in spinal sensorimotor circuits. Brain Res Rev. 2008;57:111–117. doi: 10.1016/j.brainresrev.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements, and the development of active and quiet sleep. Eur. J. Neurosci. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. Anat. Rec. 1954;119:119–135. doi: 10.1002/ar.1091190109. [DOI] [PubMed] [Google Scholar]
- Wise SP, Jones EG. The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J. Comp. Neurol. 1976;168:313–343. doi: 10.1002/cne.901680302. [DOI] [PubMed] [Google Scholar]
- Zhou W, Arrabit C, Choe S, Slesinger PA. Mechanism underlying bupivacaine inhibition of G protein-gated inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6482–6487. doi: 10.1073/pnas.111447798. [DOI] [PMC free article] [PubMed] [Google Scholar]