Abstract
Vascular endothelial cells respond to laminar shear stress by aligning in the direction of flow, a process which may contribute to athero-protection. Here we report that localized α4 integrin phosphorylation is a mechanism for establishing the directionality of shear stress-induced alignment in microvascular endothelial cells. Within 5 minutes of exposure to a physiological level of shear stress, endothelial α4 integrins became phosphorylated on Ser988. In wounded monolayers, phosphorylation was enhanced at the downstream edges of cells relative to the source of flow. The shear-induced α4 integrin phosphorylation was blocked by inhibitors of cAMP-dependent protein kinase A (PKA), an enzyme involved in the alignment of endothelial cells under prolonged shear. Moreover, shear-induced localized activation of the small GTPase Rac1, which specifies the directionality of endothelial alignment, was similarly blocked by PKA inhibitors. Furthermore, endothelial cells bearing a non-phosphorylatable α4(S988A) mutation failed to align in response to shear stress, thus establishing α4 as a relevant PKA substrate. We thereby show that shear-induced PKA-dependent α4 integrin phosphorylation at the downstream edge of endothelial cells promotes localized Rac1 activation, which in turn directs cytoskeletal alignment in response to shear stress.
Keywords: integrin, PKA, endothelial, Rac GTPase, alignment
INTRODUCTION
Exposure of endothelial cells to laminar shear stress elicits directional cellular behaviors. The cells assume a polarized, elongated shape parallel to the direction of flow1. When a wound perpendicular to the direction of flow is introduced into an endothelial cell monolayer, the cells at the upstream margin of the wound migrate approximately 1.5 times faster into the wound than those on the downstream margin2, 3. The aligned morphology associated with laminar flow correlates with responses associated with protection from atherosclerosis4. Thus, the capacity of endothelial cells to respond to laminar flow, to align along the flow and to migrate plays a role in vascular physiology.
In vitro studies have analyzed biochemical signaling events associated with flow-induced endothelial cell alignment. In response to the onset of flow a mechanosensory complex comprised of VE-Cadherin, VEGFR2, and PECAM-1 leads to activation of PI3-kinase, resulting in activation of integrin adhesion receptors. The activated integrins then form new attachments to the sub-endothelial extracellular matrix5. These adhesive events result in precise temporal modulation of the activity of Rho GTPases that lead to disassembly and reassembly of actin fibers. In particular, localized activation of Rac1 GTPase at the downstream cell edges is required for the alignment of the actin fibers parallel to the direction of flow6. The mechanisms that control this localized Rac1 activation are obscure.
The new integrin-mediated adhesions formed in response to shear stress contribute to Rac1 GTPase activation7. In migrating cells, α4 integrins induce highly localized Rac1 activation8-11. α4 integrins bind to paxillin at the trailing edge of migrating cells leading to suppression of adhesion-mediated Rac1 activation. Phosphorylation of the cytoplasmic tail of the α4 integrin subunit by protein kinase A (PKA) at Ser988 is localized to the leading edge of migrating cells where it blocks paxillin binding thus permitting efficient and highly localized Rac1 activation7, 12, 13. Because α4 integrins are expressed in endothelial cells14 we suspected that α4 integrin phosphorylation and its effects on localization of Rac1 activity may contribute to the endothelial cell responses to shear stress15-17. Here we show that PKA-mediated α4 integrin phosphorylation is induced by shear stress at the downstream edge of endothelial cells. This spatially restricted α4 phosphorylation is required for localized activation of Rac1 and for endothelial cell alignment in response to shear. Thus, localized α4 integrin phosphorylation informs the endothelial cell about the direction of blood flow, thereby acting as a “weather vane” of shear stress-induced endothelial cell alignment.
MATERIALS & METHODS
Complementary DNAs and other reagents
Glutathione-S-transferase fusion of the CS-1 fragment of human fibronectin was generated as described18. Collagen and α5 and α2 integrin antibodies (BD Biosciences, San Jose, CA), HP2/1 human α4 integrin antibodies (Immunotech, Westbrook, ME), and rat α-mouse CD31 antibodies (Invitrogen, Carlsbad, CA) were purchased. PSα4 monoclonal antibodies to Ser988-phospho-α4 integrin were as described19.
Generation of α4 integrin-null and S988A knock-in mice
C57/Bl6 mice harboring an α4 locus flanked by loxP sites were as described20. Generation of α4(S988A) mice is described in the online supplement.
Primary endothelial cell isolation and cell culture
Jurkat T leukemia cells and human microvascular endothelial cells were maintained as described13, 21. Primary pulmonary microvascular endothelial cells were isolated from α4(S988A) and α4 (fl/fl) mice as described21 and in the online supplement.
Shear stress assays
Cells were plated on glass slides and subjected to laminar shear in a parallel plate flow chamber as described previously22, or they were plated on coverslips which were fitted into microfluidic devices, as described elsewhere (Tkachenko et al., manuscript in preparation). Flow rates were adjusted to maintain laminar shear stress of 12 dynes/cm2. Actin staining and analysis are as described6.
Immunocytochemistry
Sterile glass coverslips or slides were coated with ECM proteins (CS-1, fibronectin or collagen) at 5 μg/ml overnight at 4°C, then blocked for an one hour with 1 mg/ml BSA. Cells were suspended with trypsin/EDTA and plated onto coated cover slips and treated as described previously12.
Fluorescence Resonance Energy Transfer
FRET assays and calculations to account for bleedthrough and background were performed as described previously23 24. The corrected 8-bit FRET images typically had a fluorescence intensity range of 0–100 and were displayed using pseudocolor, where blue was closest to 0 and red closest to 100.
RESULTS
Shear stress induces phosphorylation of α4 integrins at the downstream edge of endothelial cells
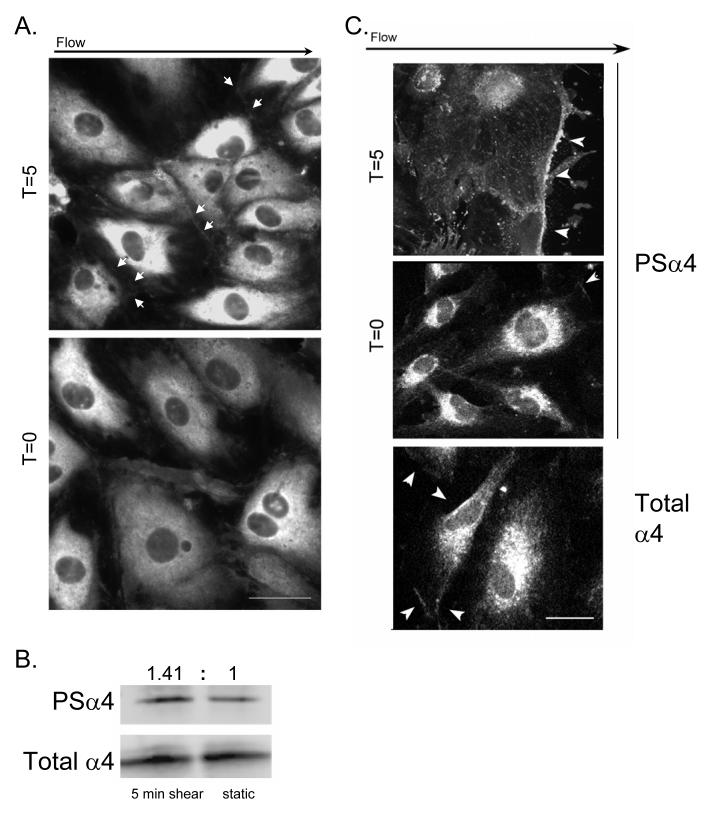
To assess a potential role for α4 integrin phosphorylation in endothelial cell alignment in response to shear stress, we first verified the expression of α4 integrins in immortalized human microvascular endothelial cells (HMECs) by fluorescence-activated cell scanning analysis (FACScan). HMECs expressed moderate levels of α4 integrin (∼20% as compared to Jurkat T cells, Supp. Fig. 1). To investigate the role for α4 integrin phosphorylation in endothelial cell responses to shear stress, we plated HMECs onto coverslips coated with the α4-binding CS-1 fragment of fibronectin and subjected the monolayers to a laminar shear stress at 12 dynes/cm2 for time intervals ranging from five to thirty minutes. After the shear exposure, cells were immediately fixed and stained with PSα4 antibody, a monoclonal antibody specific for α4 integrin phosphorylated at Ser988 12. A dramatic increase in phosphorylation of α4 in endothelial cells was observed after all times of exposure to shear, starting from the shortest tested time of 5 min (Fig. 1). We observed that α4 phosphorylation in response to shear was localized to cell boundaries, in particular at cell edges orthogonal to the direction of flow (Fig. 1A.) Labeling with secondary antibody conjugates alone showed undetectable staining (our unpublished results.) Antibodies to α4 integrin uniformly labeled the whole cell periphery (Fig. 1C), indicating that the increased concentration of phospho-α4 at the cell edges was due to increased α4 phosphorylation rather than a higher local concentration of α4. Furthermore, we detected a 1.4-fold increase in total cellular α4 phosphorylation following 5 min shear stress by western blotting of α4 immunoprecipitates (Fig. 1B), confirming that shear stress up-regulates α4 phosphorylation.
Figure 1. α4 integrin is phosphorylated at downstream cell edges in response to shear stress.
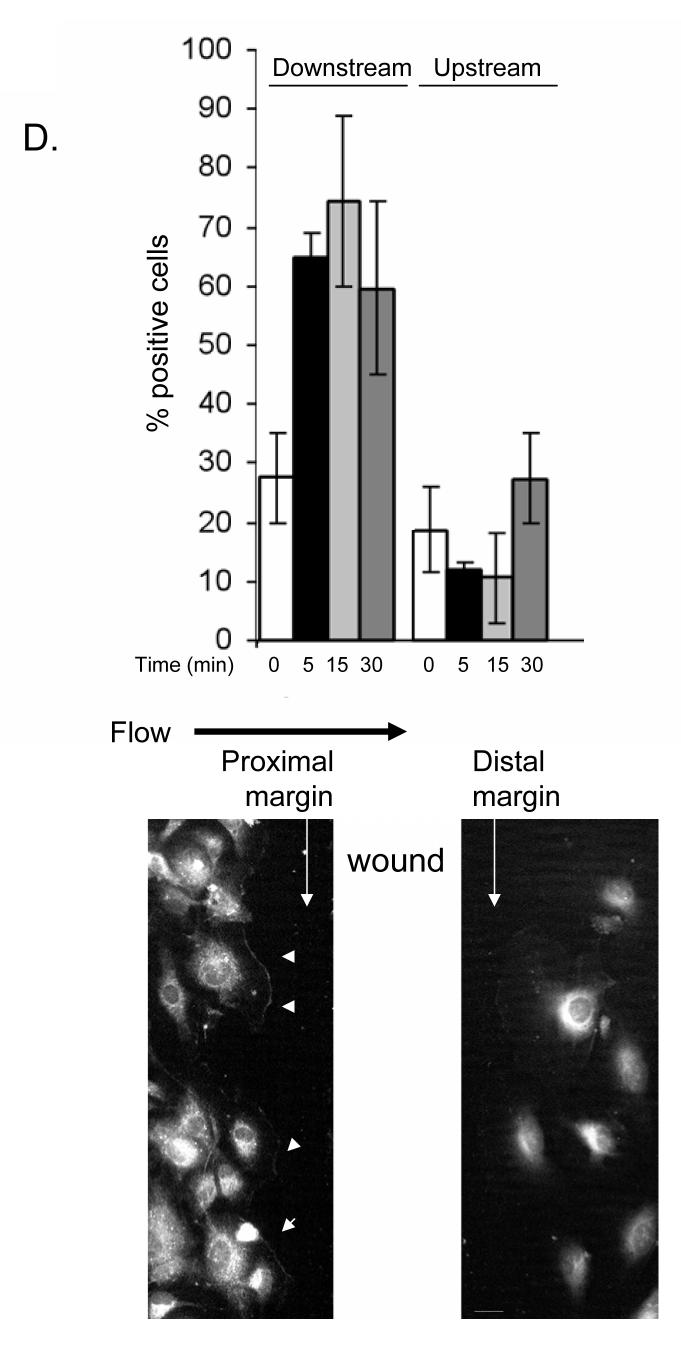
A and C. HMECs were seeded onto CS-1-coated glass slides, left confluent (A) or scratch wounded (C) and fixed either without any exposure to flow (T=0) or immediately after 5 minute exposure to flow of culture media at 12 dynes/cm2 (T=5). Fixed samples were labeled with PSα4 antibodies (A, upper panels in C) or total α4 integrin (lower panel in C). The black arrow indicates the direction of laminar flow. White arrowheads point to regions of concentrated α4 integrin phosphorylation or total α4 integrin. Bar, 10 μm. B. Western blots of α4 immunoprecipitates from HMECs subjected to shear for 0 or 5 minutes, blotted with antibodies to total α4 or PSα4. Densitometric ratios of band intensities (PSα4/total, shear:static) are indicated. D. HMECs plated on CS-1-coated slides were scratch wounded (top-to-bottom) and subjected to flow (left-to-right) for 5, 15 or 30 minutes, then stained with PSα4 antibodies. Cells at the wound margin were scored by blinded observers for phospho-α4 staining at the cell periphery. The black arrow indicates the direction of laminar flow. White arrows indicate the wound margins generated by a scratch, and small white arrowheads point of regions of concentrated α4 integrin phosphorylation.
The foregoing experiments showed that shear induced α4 phosphorylation at the cell edges perpendicular to the direction of flow, but failed to establish whether it was occurring preferentially on the proximal or distal side. To determine precisely the relationship of shear-induced α4 phosphorylation to direction of flow, we generated scratch wounds orthogonal to the flow direction in confluent monolayers of HMECs and immediately subjected the wounded monolayers to shear stress, and stained for phospho-α4. Cells at the wound margins on both sides of the scratch wound were scored for phospho-α4 staining. In the absence of shear, α4 phosphorylation was observed in ∼30% of cells at the wound margin. Application of shear induced increased α4 phosphorylation at the downstream cell edges at the wound margin proximal to the flow source but not on the upstream side of cells distal to the flow source. The α4 phosphorylation was observed in 65% (± 2.9, p<0.22) of cells at the proximal wound margin beginning five minutes after application of shear and persisting for at least thirty minutes (Fig. 1D). In contrast, α4 phosphorylation at the upstream edges of cells at the distal margin of the wound showed a trend towards inhibited phosphorylation (13% positive ± 3.1, p=0.08, Fig. 1D). Similarly, phospho-α4 was rarely observed at cell edges lateral with respect to flow direction (16% positive, unpublished results), indicating that flow increases α4 phosphorylation at the downstream edges in endothelial cells.
Protein kinase A activity is required for shear-induced α4 phosphorylation
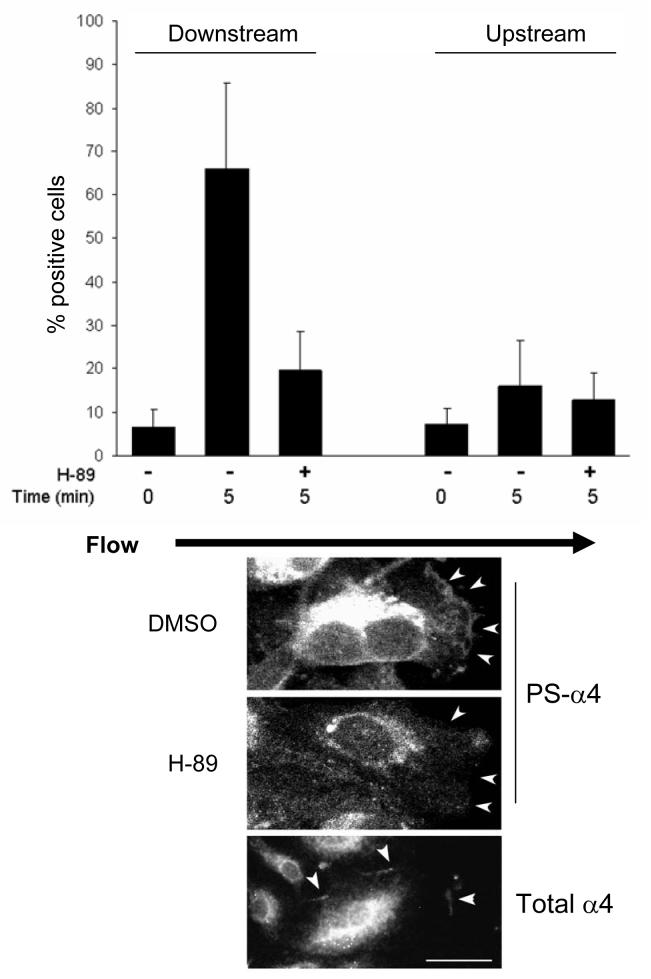
PKA phosphorylates α4 integrins at Ser988 and inhibition of PKA activity blocks α4 phosphorylation in fibroblasts and T cells12, 19. To assess the contribution of PKA to shear-induced α4 phosphorylation in endothelial cells, we pre-treated cells plated on CS-1 with the PKA inhibitor, H-89, for fifteen minutes, scratch-wounded and applied shear flow in medium containing the inhibitor. Cultures were fixed and stained for phospho-α4. A five minute exposure to shear induced α4 integrin phosphorylation at the downstream cell edges and addition of H-89 to the flow medium abrogated the α4 phosphorylation response (Fig. 2). At the upstream and lateral cell edges facing the interior of a wound, where α4 phosphorylation was low, treatment with H-89 had no apparent effect on the levels of phospho-α4 staining. As additional confirmation of this effect of PKA inhibition, we repeated these experiments using a second pharmacological PKA inhibitor, KT-5720 at 1 μM; it also blocked shear-induced phosphorylation of α4 (result not shown). Thus, shear-induced α4 phosphorylation requires PKA activity.
Figure 2. Protein kinase A activity is required for shear-induced α4 phosphorylation.
Monolayers of HMECs seeded on CS-1 were scratch wounded and subjected to flow in medium with or without 100 μM H-89 for 5 minutes. Cells were fixed and stained with PSα4 antibodies (upper panels) or antibodies to α4 (lower panel). The black arrow indicates the direction of laminar flow. White arrowheads point to areas at the cell periphery where α4 is phosphorylated. Bar, 10 μm.
Localized Rac1 activation following shear stress requires PKA activity
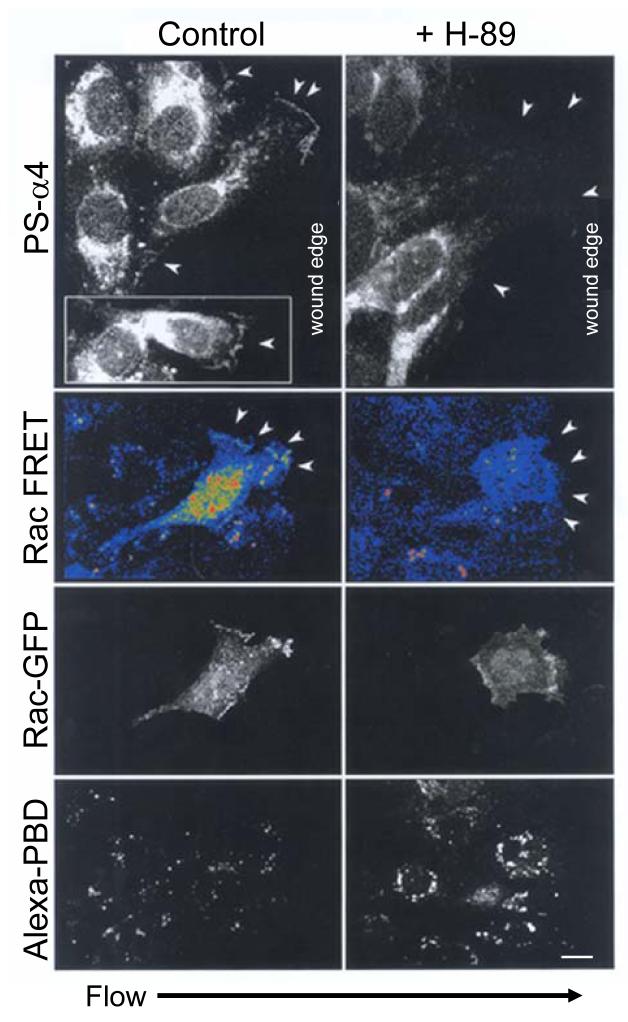
PKA-dependent phosphorylation of α4 integrin helps to localize activation of Rac1 to the leading edge of migrating cells7, 12. Furthermore, Rac1 becomes activated at the downstream edge in a sub-confluent endothelial monolayer within five minutes of exposure to laminar shear stress6. This transient, localized Rac1 activation is required for subsequent stress fiber alignment. Using a FRET-based assay, we found that shear stress induced a polarized increase of activated, GTP-bound Rac1 at the downstream edge of HMECs as previously reported6. This polarized Rac activation occurred concurrently with shear-mediated α4 integrin phosphorylation (Fig. 3, 1C). Treatment of the cells with the PKA inhibitor (H-89) that blocked shear-induced α4 phosphorylation inhibited the localized Rac1 activation, indicating that PKA activity is required for the shear induction of polarized Rac1 activation at the downstream edge of endothelial cells (Fig. 3).
Figure 3. Rac1 activation following shear stress requires PKA activity.
HMECs were transfected with Rac1(wt)-GFP and seeded on CS-1-coated slides, shear-loaded with Alexa-PBD and scratch wounded. The cells were subjected to flow for 5 minutes in the presence or absence of H-89. Rac1 activation was assessed by FRET. White arrowheads point to the cell periphery. The black arrow indicates the direction of laminar flow for all samples. Bar, 10 μm.
Shear-induced cell alignment of the actin cytoskeleton requires PKA activity
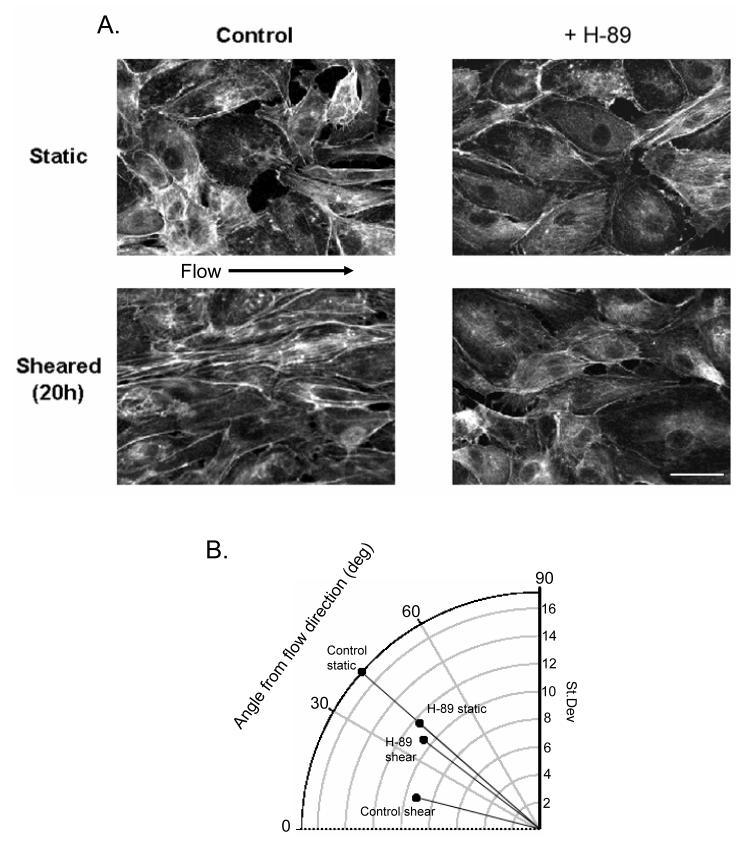
Endothelial cells respond to prolonged exposure to shear stress by remodeling their actin cytoskeleton along a dominant longitudinal cell axis, a phenomenon referred to as endothelial cell alignment25. This response requires dynamic spatio-temporal regulation of Rac1 activation, as expression of either constitutively active or dominant negative Rac1 blocks the alignment6. Because PKA activity was required for polarized Rac1 activation, we assessed whether PKA activity is necessary for alignment. HMECs were subjected to shear and actin filaments were labeled with rhodamine-phalloidin. Cells subjected to prolonged (20 hour) laminar shear stress at 12 dynes/cm2 developed an elongated, bipolar shape with actin stress fibers aligned in the direction of flow. However, the alignment and elongation did not occur if H-89 was added to the flow medium (Fig. 4A). To quantify this morphological observation, we assessed stress fiber alignment by measuring the angles of actin filament bundles relative to the direction of flow.
Figure 4. Shear-induced stress fiber alignment requires PKA activity.
A. HMECs were plated on CS-1 and subjected to flow for 20 hours in the continuous presence or absence of 100 μM H-89, then fixed and labeled with rhodamine-phalloidin. Bar, 10 μm. B. Average angles of stress fibers (mapped onto a 0-90° range) from a line parallel to the direction of flow and the S.D. of the angles are shown as the angular and radial positions of the corresponding bullets.
The average angles from the flow direction of actin filaments in control and H-89-treated cells under static conditions were 42±1.6 (17.15°=S.D.) and 41±1.1° (11.58°=S.D.), respectively, both very close to the value of 45° expected for randomly oriented fibers. Thus, actin fibers in static cells were not aligned. Actin fibers in control cells subjected to shear aligned to an average angle of 14.3±0.69° (9.23°=S.D.) from the flow direction, indicating a major bias in the alignment of actin filaments towards the direction of flow. Furthermore, the reduction in Standard Deviation (17.15→9.23) indicates a marked reduction in the variability of the orientation of the actin fibers, providing an independent measure of the alignment response. In contrast, actin filaments in sheared, H-89-treated cells aligned to a much lesser extent with an average radial displacement of 38±1° (10.61°=S.D.) (Fig. 4B and Table 1). Similar to H-89, addition of KT-5720 also dramatically reduced the alignment (Table 1). Thus, PKA activity is required for actin stress fiber re-orientation and morphological alignment of endothelial cells in response to shear stress.
Table 1. Average stress fiber angles relative to the direction of flow.
Cells were subjected to shear stress under the conditions indicated. Stress fibers were labeled with rhodamine-phalloidin and angles from lines parallel to the flow direction were measured.
| Treatment | Flow (12 dynes/cm2) | Average angle from orthogonal (°) | St. Dev. (°) |
|---|---|---|---|
| Vector control | Yes | 14.31 ± 0.69 | 9.23 |
| No | 41.48 ± 1.64 | 17.15 | |
| H-89, 30 μM | Yes | 37.63 ± 1.01 | 10.61 |
| No | 41.37 ± 1.10 | 11.58 | |
| KT-5720, 1 μM | Yes | 46.8 ± 2.62 | 22.59 |
| No | 41.6 ± 3.96 | 28.03 | |
α4 integrin is required for shear-induced alignment
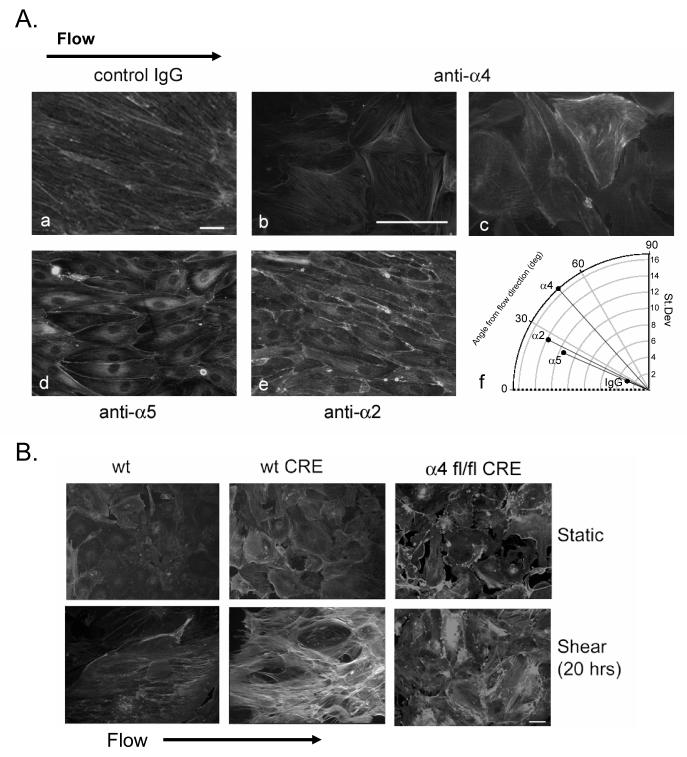
The experiments reported above showed that PKA activation was required for localized α4 integrin phosphorylation, for localized GTP loading of Rac1, and for cytoskeletal alignment in response to shear stress. These results suggested that phosphorylation of α4 integrins may provide cues for endothelial cell re-orientation and stress fiber alignment. Integrin ligation by the sub-endothelial matrix is necessary for shear-induced Rac1 activation6, and thus presumably for alignment as well. Therefore we hypothesized that blocking α4 integrin binding to ligands in the extracellular matrix would inhibit stress fiber alignment induced by shear. To test this hypothesis, we subjected confluent HMEC monolayers cultured on slides coated with fibronectin, which is a ligand for α4 and α5 integrins26, to shear stress for 20 hr in medium containing either control IgG or function-blocking antibodies to α4, α5 or α2 integrin subunits. In all cases the cells remained attached to the substratum in monolayers. After exposure to shear, cellular alignment was observed in the presence of control IgG or antibodies to α2 or α5 integrin subunits, with the mean angles relative to the flow direction of stress fibers of 20.8±0.42° (2.95°=S.D.), 23±1.6° (11.46°=S.D.), and 26±1.6° (13.84°=S.D.), respectively (Fig. 5). However, blocking antibodies to α4 integrins inhibited endothelial elongation and stress fiber re-orientation in the direction of flow, with the mean angle ± S.D. of 48±2.4° (16.68°=S.D.) (Fig. 5). In separate control experiments, these antibodies blocked the ability of endothelial cells to adhere to the relevant substrates CS-1 (anti-α4), the cell-binding domain of fibronectin (anti-α5) or collagen (anti-α2) (Supp. Fig. 2). These data indicate that α4 integrin interaction with the sub-endothelial matrix is required for shear-induced cytoskeletal alignment in microvascular endothelial cells.
Figure 5. α4 integrin is required for shear-induced alignment.
A. HMECs were seeded on fibronectin and subjected for 20 hours to shear flow with growth medium containing control IgG or blocking antibodies to human α2, α4 or α5 integrins. Cells were fixed and labeled with rhodamine-phalloidin. Bars, 10 μm. Average angles of stress fibers (mapped onto a 0-90° range) from a line parallel to the direction of flow and the S.D. of the angles are shown as the angular and radial positions of the corresponding bullets. B. Pulmonary lung endothelial cells isolated from wild type mice treated with vector (wt) or wild type and α4 fl/fl mice treated with Cre recombinase (wt CRE and α4 fl/fl CRE, respectively) were plated on fibronectin and subjected to flow for 20 hours, then labeled with rhodamine-phalloidin. Bar, 10 μm.
To verify separately that α4 integrins are required for shear-induced alignment, we generated endothelial cells deficient in expression of α4 integrin and compared the alignment responses of these cells with those of α4-expressing endothelial cells. To do this, we isolated pulmonary endothelial cells (MLECs) from the lungs of wild type mice and mice with a conditional α4-null allele in which exon 28 (which includes the α4 polyadenylation signal) is flanked by loxP recombination sites20). CD31-positive cell cultures derived from wild type and mutant mice were infected with adenovirus encoding Cre recombinase, leading to loss of surface α4 in the mutant cells as determined by FACS (Supp. Fig. 3); however, these cells adhered and spread on fibronectin-coated surfaces, (Fig. 5B) presumably via other fibronectin-binding integrins. Wild type and α4-null MLECs were plated onto fibronectin-coated slides and subjected to laminar shear stress for 20 hr. Wild type MLECs either infected with Cre or with empty adenovirus aligned in response to shear stress (Fig. 5B). In contrast, the α4-null (α4 fl/fl CRE) endothelial cells maintained a polygonal morphology and did not align in the direction of flow. These cells also displayed few discernable stress fibers, suggesting a disruption in the ability of actin to re-organize in α4-null cells in response to shear. Thus, α4 integrin contributes to shear stress-induced morphological alignment in primary pulmonary endothelial cells.
Phosphorylation of α4 integrins is necessary for alignment
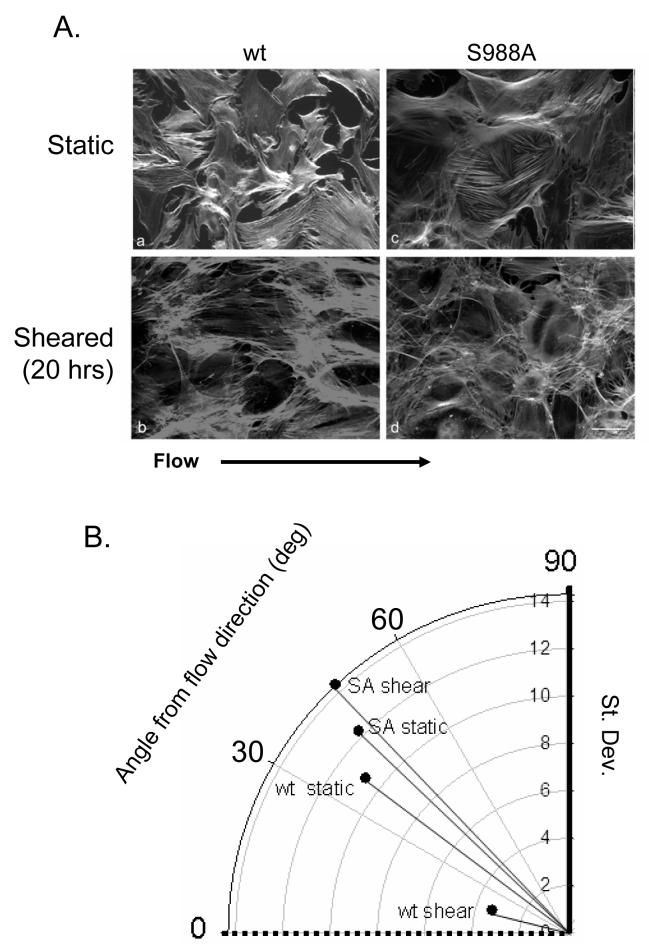
As shown above, PKA activity is required for α4 phosphorylation in response to shear in wounded monolayers and for cell alignment in prolonged shear. Therefore, we considered that α4 phosphorylation may be an early event in establishing the directionality of shear stress-induced cytoskeletal alignment. To test this possibility, we isolated endothelial cells from the lungs of mice expressing α4 integrin that harbors a Ser988→Ala mutation (S988A), which disrupts the PKA phosphorylation site in α4.
The anti-phospho-α4 PSα4 antibody did not react in western blots of lysates from endothelial cells derived from these mice, nor did these cells display any edge PSα4 staining in sheared, wounded cultures (our unpublished results and Supp. Fig. 4). Whereas wild type endothelial cells exposed to laminar shear for 20 hours aligned, α4(S988A) endothelial cells did not (Fig. 6A). As shown in Figure 6B, the average angle from the flow direction of actin filaments in wild type and mutant cells under static conditions was 37.6±0.85° (10.7°=S.D.) and 44±1.° (12.2°=S.D.), respectively (Fig. 6B). Filaments in wild type cells subjected to shear stress were orientated at an average angle of 16.9± 0.25° (3.2°=S.D.) from the flow direction, that was close to the value obtained earlier (14.3±0.69 (9.23°=S.D.), indicating that most actin filaments aligned parallel to the direction of flow. In contrast, filaments in α4(S988A) endothelial cells subjected to shear stress maintained an average angle of 47±0.92° (14.3°=S.D.), (Fig. 6B) showing that they did not align following shear. Thus, α4 integrin phosphorylation is required for stress fiber alignment induced by shear stress.
Figure 6. Phosphorylation of α4 integrin is necessary for alignment.
A. Pulmonary lung endothelial cells isolated from wild type and α4(S988A) mice were plated on fibronectin and subjected to flow for 20 hours, then labeled with rhodamine-phalloidin. Bar, 10 μm. B. Average angles of stress fibers (mapped onto a 0-90° range) from a line parallel to the direction of flow and the standard deviations of the angles are shown as the angular and radial positions of the corresponding bullets.
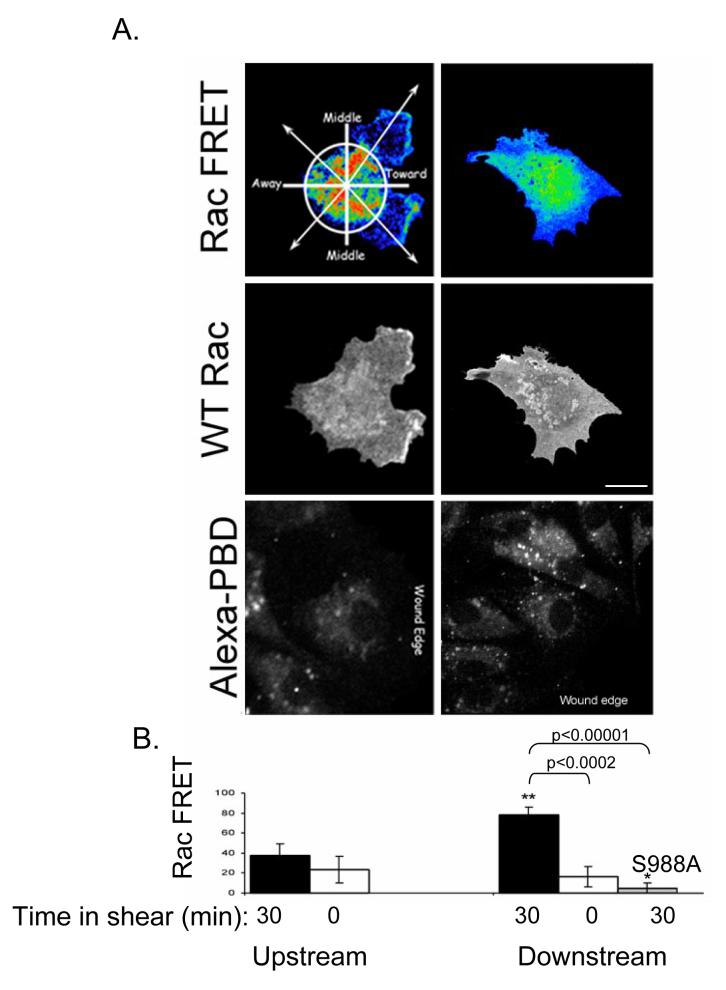
As a further test of the role of α4 integrin phosphorylation in the endothelial cell responses to shear stress, we assessed the effect of the α4(S988A) mutation on shear-induced Rac1 activation in endothelial cells. HMECs were co-transfected with a Rac FRET reporter plasmid and plasmids encoding wild type α4 or α4(S988A). These cells were plated on α4 ligands, scratch wounded and immediately subjected to 12 dynes/cm2 shear stress for five minutes, fixed, and analyzed for Rac1 activation. In cells expressing recombinant wild type α4 integrins, Rac1 activation was observed at the downstream edge of 78±8.2% of cells at the wound margins, consistent with earlier observations (Figs. 3 and 7A). In contrast, Rac1 activation was observed at the downstream edge in only 5±5% of the cells expressing recombinant α4(S988A) (Fig. 7B). Thus, ectopic expression of α4(S988A) in endothelial cells exerts a dominant inhibitory effect on the localization of shear-induced Rac1 activation. These results demonstrate that α4 integrin phosphorylation is required for shear-induced polarized Rac1 activation and consequently for endothelial cell alignment.
Figure 7. Non-phosphorylatable α4 integrin inhibits shear-induced Rac1 activation.
HMECs co-transfected with Rac1(wt)-GFP and α4(wt) or α4(S988A) were shear-loaded with recombinant PAK-1-binding domain of p21 coupled to Alexa dye (Alexa-PBD), plated on CS-1-coated slides, scratch wounded, and subjected to 12 dynes/cm2 shear stress for five minutes. Fixed cells were assayed for Rac FRET. A. Representative images of cells at the wound edge after exposure to shear stress. Bar, 25 μm. B. Quantification of polarized Rac1 activation by shear. Images of cells at the wound edges were divided into quadrants and the FRET signal at the cell periphery was assessed by visual inspection for at least 15 cells per sample. Shown are the percentages of cells in which FRET was observed in the quadrant facing the wound (“Toward” in A.) in cells over-expressing α4(wt) or α4(S988A) as indicated, +/- SEM. **, p < 0.0002; *, p < 0.00001.
DISCUSSION
Endothelial cell alignment in the direction of blood flow has been known for many years; recent work indicated that localized activation of Rac1 GTPase at the downstream side of the endothelial cell is a critical event in flow-induced alignment6. Here we report that localized α4 integrin phosphorylation leads to this localized Rac1 activation and subsequent stress fiber alignment and elongation of endothelial cells parallel to the direction of flow in response to shear stress. α4 integrins were phosphorylated within five minutes of exposure to shear stress and phosphorylation occurred predominantly at the downstream edges of the cells. Inhibition of PKA blocked α4 phosphorylation and prevented both localized Rac1 activation and alignment of stress fibers in the direction of flow. Furthermore, α4 integrins are required for endothelial cell alignment because deletion of α4 or addition of antibodies against α4 inhibited stress fiber alignment. Most importantly, PKA phosphorylation of α4 is involved in alignment because endothelial cells bearing α4(S988A), a mutation which disrupts the PKA phosphorylation site, fail to align in the direction of flow. Together these results show that shear-induced, PKA-dependent α4 phosphorylation is localized to the downstream edge of endothelial cells. The localized α4 phosphorylation leads to localized activation of Rac1 at the downstream edge, enabling re-orientation of endothelial cells in the direction of flow. Previous studies showed how a mechanosensory complex led to integrin activation that resulted in activation of Rac15, 6. The present studies elucidate the pathway whereby α4 integrin phosphorylation informs the endothelial cell about the direction of flow by localizing this Rac1 activation to the downstream edge, thereby acting as a “weather vane” of shear-induced endothelial cell re-orientation.
The tangential drag forces imposed by laminar shear stress induce phosphorylation of α4 integrin at Ser988 in endothelial cells. Using a phospho-specific anti-α4 antibody, we observed phosphorylation as early as five minutes after the application of 12 dynes/cm2 shear stress, which is within the ranges of shear stresses in medium-sized arteries27. Previous studies have suggested that other key signaling events occur within similar time frames, including c-Src activation (1 min, peak at 10 min)28, VEGFR2 phosphorylation (1 min, peak at 5 min)29, 30, and activation of Ras31 and Rac1 (5 min)6. Furthermore, α4 is phosphorylated at Ser988, a known PKA phosphorylation site19 and blocking PKA abolishes phosphorylation. The dependence of the α4 phosphorylation on the application of shear and on PKA activity suggests that shear stress may activate PKA; however, we cannot exclude the possibility that there is tonic PKA activity and that shear stress acts to suppress phosphatase activity. Nevertheless, we favor the former possibility because PKA is known to be activated in endothelial cells by shear stress, which also induces phosphorylation of several PKA substrates such as VASP32 and endothelial nitric oxide synthase33. Shear stress exerts force on endothelial cell attachments to the substrate, attachments mediated by integrins. Mechanical strain on integrins can result in enhancement of intracellular cAMP concentration leading to PKA activation34. The role of integrin attachments in initiating α4 phosphorylation warrants future study. In sum, we conclude that fluid shear stress results in PKA-dependent α4 integrin phosphorylation in microvascular endothelial cells.
Localized phosphorylation of α4 integrins induced by shear stress is an important cue for directionality of alignment. After five minutes in shear, phosphorylation was observed only at the downstream cell edge. Furthermore, stress fiber alignment required both the presence of the α4 integrin and its phosphorylation by PKA. α4 integrins strongly promote cell migration. The α4 cytoplasmic tail is sufficient for a pro-migratory response8, and we have shown that phosphorylation at Ser988 at the leading edge of the cell is the key determinant of this function; Ser988 is also the only identified site of α4 phosphorylation in vivo12, 13. Shear stress accelerates endothelial wound closure35. When a wound is orthogonal to the flow, cells on the wound margin proximal to the source of flow (in which α4 is phosphorylated) migrate into the wound space faster than cells on the distal margin (in which α4 phosphorylation does not occur)2, 3. Those latter cells have to move against the flow. Indeed, reendothelialization occurs fastest parallel to the flow direction following endothelial wounds in vivo, indicating a flow-induced enhancement of cell migration36, 37. Shear stress can promote migration of endothelial cells from the upstream edge of wounds; our studies now suggest that localized α4 phosphorylation can contribute to this enhanced directional migration.
The restriction of α4 phosphorylation to the downstream side of endothelial cells under shear serves to localize other signaling responses required for proper cytoskeletal alignment. We have now found that blocking α4 phosphorylation disrupts localized Rac1 activation to the downstream edge that is essential for endothelial cytoskeletal alignment6. One clue to the mechanism for this effect on Rac1 comes from our previous studies in migrating cells. In particular, phosphorylation of the α4 cytoplasmic tail at Ser988 by PKA enables that integrin to activate Rac1 because it prevents the binding of a protein complex that blocks Rac1 activation7, 12. There are other signaling events that are induced or enhanced by shear and play a role in cell migration. For example, PI3-kinase activity is increased by shear38. PI3-K is also typically localized to the leading edge of migrating cells39 and can promote Rac1 activation, but it is not required for the alignment response40. Both cell migration and morphological alignment under shear are driven by cytoskeletal rearrangements25, 41 in response to Rho GTPases. α4 phosphorylation by shear stress coordinates localized Rho GTPase signaling to favor cytoskeletal alignment along the flow and migration in the direction of flow.
α4 integrins and PKA play important roles in endothelial functions such as neovascularization. Endothelial cells express α4 integrins in vitro and in vivo, and fibronectin, a ligand for α4 integrins, is highly expressed in the vasculature15, 42, 43. α4 integrins mediate adhesion, spreading, proliferation and migration of endothelial cells in vitro 42, 44. In vivo, α4 expression is required for homing of hematopoietic and endothelial progenitor cells, and for efficient angiogenesis in developing embryos and in tumors. α4 antagonists also block angiogenesis in a chick chorioallantoic membrane model confirming this function of α4 integrins, although the requirement for α4 in angiogenesis may result from combined contributions in vascular endothelial and smooth muscle cells, as well as from paracrine effects in macrophages44-46. Similarly, PKA regulates adhesion, migration and survival of vascular endothelial cells47, 48 and angiogenesis49, 50. New blood vessel formation is stimulated by shear stress and requires flow conditions which promote cytoskeletal parallel alignment, indicating the importance of endothelial alignment in angiogenesis27, 51. Therefore shear-induced PKA-dependent α4 phosphorylation may be an important regulatory step in endothelial functions during vascular development and remodeling.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by American Heart Association SDG 0435295 (LEG) and 0635228N (ET), National Science Foundation NIRT 0608863 (AG), and NIH grants HL088632 (ET), GM 68524 (EG), HL085159 (SC), and AR27214, HL078784 and HL31950 (MHG). ET is an Ellison Medical Foundation New Scholar in Aging.
Footnotes
DISCLOSURES: The authors declare that they have no competing financial interests.
REFERENCES
- (1).Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999 September 17;85(6):504–14. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- (2).Gojova A, Barakat AI. Vascular endothelial wound closure under shear stress: role of membrane fluidity and flow-sensitive ion channels. J Appl Physiol. 2005 June;98(6):2355–62. doi: 10.1152/japplphysiol.01136.2004. [DOI] [PubMed] [Google Scholar]
- (3).Hsu PP, Li S, Li YS, Usami S, Ratcliffe A, Wang X, Chien S. Effects of flow patterns on endothelial cell migration into a zone of mechanical denudation. Biochem Biophys Res Commun. 2001 July 20;285(3):751–9. doi: 10.1006/bbrc.2001.5221. [DOI] [PubMed] [Google Scholar]
- (4).Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005 January;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- (5).Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001 September 3;20(17):4639–47. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Tzima E, del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002 December 16;21(24):6791–800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol. 2005 April;7(4):343–52. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- (8).Kassner PD, Kawaguchi S, Hemler ME. Minimum α chain cytoplasmic tail sequence needed to support integrin-mediated adhesion. J Biol Chem. 1994;269:19859–67. [PubMed] [Google Scholar]
- (9).Yang JT, Rayburn H, Hynes RO. Cell adhesion events by alpha4 integrins are essential in placental and cardiac development. Development. 1995;121:549–60. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- (10).Sengbusch JK, He W, Pinco KA, Yang JT. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol. 2002 May 27;157(5):873–82. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999 November;11(5):555–66. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- (12).Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of {alpha}4 integrin phosphorylation regulates lamellipodial stability and {alpha}4{beta}1-dependent cell migration. J Cell Biol. 2003 August 18;162(4):731–41. doi: 10.1083/jcb.200304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Han J, Rose DM, Woodside DG, Goldfinger LE, Ginsberg MH. Integrin alpha4 beta1-dependent cell migration requires both phosphorylation and de-phosphorylation of the alpha4 cytoplasmic domain. J Biol Chem. 2003 June 30; doi: 10.1074/jbc.M304691200. [DOI] [PubMed] [Google Scholar]
- (14).Priestley GV, Ulyanova T, Papayannopoulou T. Sustained alterations in biodistribution of stem/progenitor cells in Tie2Cre+ alpha4(f/f) mice are hematopoietic cell autonomous. Blood. 2007 January 1;109(1):109–11. doi: 10.1182/blood-2006-06-026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Sheppard AM, Onken MD, Rosen GD, Noakes PG, Dean DC. Expanding roles for alpha 4 integrin and its ligands in development. Cell Adhes Commun. 1994 April;2(1):27–43. doi: 10.3109/15419069409014200. [DOI] [PubMed] [Google Scholar]
- (16).Kil SH, Krull CE, Cann G, Clegg D, Bronner-Fraser M. The alpha4 subunit of integrin is important for neural crest cell migration. Dev Biol. 1998 October 1;202(1):29–42. doi: 10.1006/dbio.1998.8985. [DOI] [PubMed] [Google Scholar]
- (17).Pinco KA, Liu S, Yang JT. alpha4 integrin is expressed in a subset of cranial neural crest cells and in epicardial progenitor cells during early mouse development. Mech Dev. 2001 January;100(1):99–103. doi: 10.1016/s0925-4773(00)00503-7. [DOI] [PubMed] [Google Scholar]
- (18).Jongewaard IN, Tsai PM, Smith JW. The type III connecting segment of fibronectin contains an aspartic acid residue that regulates the rate of binding to integrin alpha 4 beta 1. Cell Adhes Commun. 1996 April;3(6):487–95. doi: 10.3109/15419069609081025. [DOI] [PubMed] [Google Scholar]
- (19).Han J, Liu S, Rose DM, Schlaepfer DD, McDonald H, Ginsberg MH. Phosphorylation of the integrin α4 cytoplasmic domain regulates paxillin binding. J Biol Chem. 2001 August 30;276:40903–9. doi: 10.1074/jbc.M102665200. [DOI] [PubMed] [Google Scholar]
- (20).Feral CC, Rose DM, Han J, Fox N, Silverman GJ, Kaushansky K, Ginsberg MH. Blocking the alpha 4 integrin-paxillin interaction selectively impairs mononuclear leukocyte recruitment to an inflammatory site. J Clin Invest. 2006 March;116(3):715–23. doi: 10.1172/JCI26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Reutershan J, Stockton R, Zarbock A, Sullivan GW, Chang D, Scott D, Schwartz MA, Ley K. Blocking p21-activated kinase reduces lipopolysaccharide-induced acute lung injury by preventing polymorphonuclear leukocyte infiltration. Am J Respir Crit Care Med. 2007 May 15;175(10):1027–35. doi: 10.1164/rccm.200612-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 March 22;227(4693):1477–9. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- (23).del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002 March;4(3):232–9. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- (24).Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000 October 13;290(5490):333–7. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- (25).McCue S, Noria S, Langille BL. Shear-induced reorganization of endothelial cell cytoskeleton and adhesion complexes. Trends Cardiovasc Med. 2004 May;14(4):143–51. doi: 10.1016/j.tcm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- (26).Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002 September 20;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- (27).Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol. 2003 April;81(3):177–99. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- (28).Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, Shyy JY. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998 February;18(2):227–34. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- (29).Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999 June 25;274(26):18393–400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- (30).Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligandindependent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003 August 22;93(4):354–63. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- (31).Li YS, Shyy JY, Li S, Lee J, Su B, Karin M, Chien S. The Ras-JNK pathway is involved in shear-induced gene expression. Mol Cell Biol. 1996 November;16(11):5947–54. doi: 10.1128/mcb.16.11.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Wei L, Muller S, Ouyang J, Stoltz JF, Wang X. Changes of vasodilator-stimulated phosphoprotein (VASP) and its phosphorylation in endothelial cells exposed to laminar flow. Clin Hemorheol Microcirc. 2003;28(2):113–20. [PubMed] [Google Scholar]
- (33).Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002 November;283(5):H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- (34).Meyer CJ, Alenghat FJ, Rim P, Fong JHJ, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nature Cell Biol. 2000;2:666–8. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- (35).Albuquerque ML, Waters CM, Savla U, Schnaper HW, Flozak AS. Shear stress enhances human endothelial cell wound closure in vitro. Am J Physiol Heart Circ Physiol. 2000 July;279(1):H293–H302. doi: 10.1152/ajpheart.2000.279.1.H293. [DOI] [PubMed] [Google Scholar]
- (36).Schwartz SM, Gajdusek CM, Reidy MA, Selden SC, III, Haudenschild CC. Maintenance of integrity in aortic endothelium. Fed Proc. 1980 July;39(9):2618–25. [PubMed] [Google Scholar]
- (37).Haudenschild CC, Schwartz SM. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 November;41(5):407–18. [PubMed] [Google Scholar]
- (38).Decave E, Rieu D, Dalous J, Fache S, Brechet Y, Fourcade B, Satre M, Bruckert F. Shear flow-induced motility of Dictyostelium discoideum cells on solid substrate. J Cell Sci. 2003 November 1;116(Pt 21):4331–43. doi: 10.1242/jcs.00726. [DOI] [PubMed] [Google Scholar]
- (39).Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002 May 31;109(5):611–23. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- (40).Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003 April 28;161(2):429–39. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003 December 5;302(5651):1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- (42).Massia SP, Hubbell JA. Vascular endothelial cell adhesion and spreading promoted by the peptide REDV of the IIICS region of plasma fibronectin is mediated by integrin alpha 4 beta 1. J Biol Chem. 1992;267:14019–26. [PubMed] [Google Scholar]
- (43).Brezinschek RI, Brezinschek HP, Lazarovits AI, Lipsky PE, Oppenheimer-Marks N. Expression of the beta 7 integrin by human endothelial cells. Am J Pathol. 1996 November;149(5):1651–60. [PMC free article] [PubMed] [Google Scholar]
- (44).Calzada MJ, Zhou L, Sipes JM, Zhang J, Krutzsch HC, Iruela-Arispe ML, Annis DS, Mosher DF, Roberts DD. Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo. Circ Res. 2004 March 5;94(4):462–70. doi: 10.1161/01.RES.0000115555.05668.93. [DOI] [PubMed] [Google Scholar]
- (45).Grazioli A, Alves CS, Konstantopoulos K, Yang JT. Defective blood vessel development and pericyte/pvSMC distribution in alpha 4 integrin-deficient mouse embryos. Dev Biol. 2006 May 1;293(1):165–77. doi: 10.1016/j.ydbio.2006.01.026. [DOI] [PubMed] [Google Scholar]
- (46).Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006 February 15;66(4):2146–52. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- (47).Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res. 2007 October 12;101(8):768–76. doi: 10.1161/CIRCRESAHA.106.146159. [DOI] [PubMed] [Google Scholar]
- (48).Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006 March 17;98(5):617–25. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest. 2002 October;110(7):933–41. doi: 10.1172/JCI14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Bakre MM, Zhu Y, Yin H, Burton DW, Terkeltaub R, Deftos LJ, Varner JA. Parathyroid hormone-related peptide is a naturally occurring, protein kinase A-dependent angiogenesis inhibitor. Nat Med. 2002 September;8(9):995–1003. doi: 10.1038/nm753. [DOI] [PubMed] [Google Scholar]
- (51).Vyalov S, Langille BL, Gotlieb AI. Decreased blood flow rate disrupts endothelial repair in vivo. Am J Pathol. 1996 December;149(6):2107–18. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.