Abstract
None of the anterograde tracers used to label and investigate vagal preganglionic neurons projecting to the viscera has proved optimal for routine and extensive labeling of autonomic terminal fields. To identify an alternative tracer protocol, the present experiment evaluated whether dextran conjugates, which have produced superior results in the CNS, might yield widespread and effective labeling of long, fine-caliber vagal efferents in the peripheral nervous system. The dextran conjugates that were evaluated proved reliable and versatile for labeling the motor neuron pool in its entirety, for single- and multiple-labeling protocols, for both conventional and confocal fluorescence microscopy, and for permanent labeling protocols for brightfield microscopy of the projections to the gastrointestinal (GI) tract. Using a standard ABC kit followed by visualization with DAB as the chromagen, Golgi-like labeling of the vagal efferent terminal fields in the GI wall was achieved with the biotinylated dextrans. The definition of individual terminal varicosities was so sharp and detailed that it was routinely practical to examine the relationship of putative vagal efferent contacts (by the criteria of high magnification light microscopy) with the dendritic and somatic architecture of counterstained neurons in the myenteric plexus. Overall, dextran conjugates provide high-definition labeling of an extensive vagal motor pool in the GI tract, and offer considerable versatility when multiple-staining protocols are needed to elucidate the complexities of the innervation of the gut.
Keywords: Aging, Alpha-Synuclein, Enteric Nervous System, Gastrointestinal, Myenteric, Nitric Oxide Synthase, Preganglionic
Introduction
Vagal motor projections to the gastrointestinal (GI) tract play critical roles in controlling GI physiology. These autonomic efferents serve both as motor limbs of vago-vagal reflexes affecting gut motility and secretion and as final common pathways by which higher brain circuitry influences GI function.
Though the physiology of the vagus has been extensively investigated, research on the morphology and organization of the preganglionic projections is in its infancy. The vagal motor outflow is comprised of long, fine-caliber axons, and, for the most part, what is known to date about the peripheral architecture of the axons has come from experiments in which the efferents were labeled by extracellular injections of neural tracers into the dorsal motor nucleus of the vagus (dmnX) in the brainstem. These early labeling experiments employed carbocyanine dyes (e.g., Berthoud et al., 1990; 1991; Berthoud, 1995, 1996; Neuhuber et al. 1998), horseradish peroxidase conjugates (HRP; e.g., Phillips and Powley, 2001; Hayakawa et al., 2006), or Phaseolus vulgaris leucoagglutinin (PHA-L; Kirchgessner and Gershon, 1989; Holst et al., 1997).
Though these early tracer studies of vagal efferents established some features of the basic organization of the preganglionic circuitry in the gut, the studies also revealed that the particular tracers used all have limitations for labeling the long, fine-caliber efferent axons that comprise the vagal motor outflow. Difficulties include, for example, complications in labeling most or all of the distributed and narrow columnar vagal motor neuron pool (e.g., PHA-L), modest morphological definition (e.g., HRP), and limited compatibility with other histochemical protocols (e.g., the carbocyanine dyes).
Thus, identification of a protocol(s) that would yield extensive, selective, high definition, and versatile labeling of the preganglionic axons would make detailed characterization of the structural details of vagal projections (and presumably other autonomic efferents) more practical. Because the dextran amines have proven to be successful in labeling visceral afferents in the periphery (Powley and Phillips, 2005; Phillips and Powley, 2007; Phillips et al., 2008) as well as axons and terminals throughout the central nervous system (Wouterlood and Jorritsma-Byham, 1993; Lanciego et al., 2000), the present experiment evaluated whether injections of dextran conjugates into the dmnX would prove as effective in labeling fine vagal motor terminals in the gut and, if so, which of several conjugates of dextran and what particular processing parameters would prove especially effective for vagal preganglionics. The results indicate that dextrans offer superior labeling of vagal efferents.
Materials and Methods
Subjects
Sprague-Dawley and Fischer 344 rats (male; three- to twelve-months-old) were obtained from Harlan Industries (Indianapolis, IN; n = 18). Upon arrival, rats were single housed at 22–24°C and 40–60% humidity on a 12:12 h light:dark cycle. Solid chow (laboratory diet no. 5001; PMI Feeds, Inc., Brentwood, MO) and tap water were available ad libitum. All procedures were conducted in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), and were approved by the Purdue University Animal Care and Use Committee.
Tracer application
Rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and then mounted in a stereotaxic frame. The medulla was exposed and the obex was used as the reference point for coordinates for multiple (8 to 13) injection sites into the dorsal motor nucleus of the vagus nerve (dmnX). In separate rats, one of four different 5% solutions of lysine fixable, 10000 MW dextran conjugates in H2O was pressure injected through a glass micropipette (ID 25 μm) with a picospritzer (80 psi; General Valve Corporation, Fairfield, NJ) into the dmnX. Several 4 msec applications of the conjugate were made in each location and the pipette was left in each site for 2–3 min following the last application to allow the solution to disperse, and prevent excess leakage from the line of penetration. The four conjugates of dextran used were Texas Red (D-TR; D1863; Invitrogen, Carlsbad, CA), Rhodamine Green (D-RG; D7153; Invitrogen), tetramethylrhodamine and biotin (D-TMR-B; D3312; Invitrogen), or biotin (D-B; D1956; Invitrogen).
To evaluate the feasibility of double-labeling vagal efferents and afferents in the same rat, a subset of rats were re-anesthetized five days after dmnX injections of D-B and the left and right nodose ganglia were exposed by blunt dissection. The ganglia were then pressure injected with D-TR through a glass micropipette (ID 25 μm).
Tissue preparation
Following dmnX injections, a time course of 19 days was allowed for the dextrans to transport to the GI tract. Rats were then weighed and euthanized with a lethal dose of sodium pentobarbital (180 mg/kg, i.p.); once unresponsive to paw pinch, the abdomen and chest cavity were opened. Rats were then injected in the left ventricle of the heart with heparin (0.5 ml; 1,000 units/ml; Baxter Healthcare Corporation, Deerfield, IL) followed by transcardial perfusion with 200 ml of 0.01 M sodium phosphate-buffered saline (PBS; pH 7.4; 38°C). The stomach was distended with approximately 10 to 15 ml of PBS to provide uniformity in organ size. Tissue fixation was then achieved by intracardiac perfusion of 500 ml of 4% paraformaldehyde (PF) in 0.1 M PBS (pH 7.4; 4°C).
The stomach and small intestine from the pyloric sphincter to the ileocaecal junction were dissected out of each rat. Whole mounts of the small intestine, including the duodenum (first 8 cm anal to the pyloric sphincter), jejunum (8 cm taken from the middle of the small intestine), and ileum (first 8 cm oral to the ileocaecal junction) were designated according to the criteria of Hebel and Stromberg (1976) and saved. The stomach was separated into ventral and dorsal halves, with cuts along the greater and lesser curvatures. Intestinal segments were opened with a longitudinal cut along the mesenteric attachment and rinsed out with tap water. Whole mounts were stored for an additional 2 hours in 4 % PF prior to the removal of the mucosa, submucosa, and circular muscle by dissection with fine-tip forceps.
Fluorescent Labeling
For those rats that received dmnX injections of D-TR, D-RG, or D-TMR-B and no other labeling or staining, the removal of the mucosal, submucosal, and circular muscle layers was followed by three five-minute PBS rinses. Whole mounts were then mounted on gelatin-coated slides, air-dried overnight, dehydrated in a series of ascending alcohol rinses, cleared in xylene, and coverslipped in DPX neutral mounting medium (Aldrich Chemical Company, Inc, Milwaukee, WI).
For double-tracer-labeling observations, GI tissue from rats injected with D-B in the dmnX and D-TR in the nodose ganglia were prepared as whole mounts, rinsed in PBS, and then soaked for 3 days at room temperature in PBS containing 0.5% Triton X-100, and 0.08% Sodium Azide. Whole mounts were then rinsed in PBS and incubated for 1 h at room temperature in streptavidin ALEXA Fluor 488 (1:500; S32354; Invitrogen) diluted with PBS. Finally, labeled whole mounts were rinsed in PBS, mounted on gelatin-coated slides, air-dried overnight, dehydrated in alcohol, cleared in xylene, and cover-slipped with DePeX (13514; Electron Microscopy Sciences, Hatfield, PA).
For triple-labeling trials, whole mounts from rats injected with D-TR in the dmnX were rinsed in PBS and then soaked for 5 to 6 days at room temperature in PBS containing 5% normal goat serum, 2% bovine serum albumin, 0.5% Triton X-100, and 0.08% Sodium Azide, followed by incubation for 24 h at room temperature in a cocktail consisting of the primary antibodies mouse alpha-synuclein (1:2,500; 610787; BD Biosciences, San Jose, CA) and rabbit NOS (1:2,500; SC-648; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted with PBS containing 2% normal goat serum, 2% bovine serum albumin, 0.3% Triton X-100, and 0.08% Na Azide. Whole mounts were then rinsed in PBS and 0.3% Triton X-100 (PBST), and incubated for 2 h at room temperature in a cocktail consisting of goat anti-mouse ALEXA Fluor 488 (1:500; A11029; Invitrogen) and biotinylated anti-rabbit IgG raised in goat (1:500; 111-065-144; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted with PBST. Next, whole mounts were further rinsed in PBS and then incubated for 1 h with streptavidin ALEXA Fluor 350 (1:500; S11249; Invitrogen). Finally, labeled whole mounts were rinsed in PBS, mounted on gelatin-coated slides, air-dried overnight, dehydrated in alcohol, cleared in xylene, and cover-slipped with DePeX (Electron Microscopy Sciences).
Permanent Labeling
Whole mounts were processed for double labeling by DAB staining of the vagal efferents following D-B injections into the dmnX and NBT staining of the subpopulation of nitrergic neurons in the myenteric plexus with nicotinamide adenine dinucleotide phosphate diaphorase (NADPHd; Phillips et al 2003) protocol. Briefly, following the post-fixation in PF, the whole mount tissue was rinsed with 0.1 M Tris–HCl (pH 7.9) and then incubated for 1 h in 0.1 M Tris–HCl (pH 7.6) containing 1.0 mg/ml b-NADPH (Sigma, St Louis, MO), 0.33 mg/ml nitroblue tetrazolium (NBT; Sigma) and 0.5% Triton X-100 at 37°C, followed by rinses in Tris–HCl (pH 7.9). Then whole mounts were rinsed in PBS followed by a 30 min soak in an endogenous peroxidase block (methanol:3% H2O2; 4:1). After additional rinses in PBS, the tissue was soaked overnight in PBS with 0.5% Triton X-100 and 0.8% Sodium Azide followed by a 60 min soak in ABC (prepared as per the directions provided with the Vectastain Elite ABC kit; Vector Laboratories, Inc., Burlingame, CA). The tissue was than rinsed in PBS, reacted with DAB for 5 min, rinsed in distilled water and mounted on slides. The slides were air-dried overnight then dehydrated in an ascending series of alcohol rinses, cleared in xylene, and coverslipped in Cytoseal mounting medium (8312; Richard-Allen Scientific, Kalamazoo, MI).
Another double labeling procedure was done by DAB staining the vagal efferents that were labeled with D-B injected into the dmnX and then counterstaining the neuronal population of the myenteric plexus with the pan-neuronal marker Cuprolinic Blue (Phillips and Powley, 2001; Phillips et al 2004). Briefly, the whole mounts were rinsed in PBS followed by a 30 min soak in an endogenous peroxidase block (methanol:3% H2O2; 4:1). After additional rinses in PBS, the tissue was rinsed in distilled water and stained for 4 h in a humidified slide warmer (38°C) with0.5% Cuprolinic blue (quinolinic phthalocyanine; Polysciences, Inc., Warrington, PA) in 0.05 M sodium acetate buffer containing 1.0 M MgCl2, pH 4.9. The whole mounts were then rinsed in distilled water, differentiated for 1 min in 0.05 M sodium acetate buffer containing 1.0 M MgCl2 (pH 4.9), and rinsed again in distilled water followed by another series of PBS rinses. The whole mounts were then soaked overnight in PBS with 0.5% Triton X-100 and 0.8% Sodium Azide followed by a 60 min soak in ABC (prepared as per the directions provided with the Vectastain Elite ABC kit; Vector Laboratories). The tissue was than rinsed in PBS, reacted with DAB for 5 min, rinsed in distilled water and mounted on slides. The tissue was allowed to air-dry overnight then dehydrated in an ascending series of alcohol rinses, cleared in xylene, and coverslipped with Cytoseal.
Analysis and Photography
Tissue visualization and qualitative analysis was made using a Leica DMRE widefield microscope (Leitz, Wetzlar, Germany). Photomicrographs were acquired using a Spot RT Slider camera (Diagnostic Instruments, Sterling Heights, MI, USA). Adobe Photoshop CS3 (version 10; Adobe Systems, San Jose, CA, USA) was used to apply the text and scale bars, make minor adjustments to the color, brightness, contrast, and sharpness of the images to match as closely as possible to the appearance of the original material viewed under the microscope, and to organize the final layout of the figures.
Results
Dextran conjugates proved to be excellent, easily administered, and readily controlled compounds for labeling vagal efferents. Our observations are reviewed in terms of the four common criteria for an effective peripheral nervous system tracer, taking them in the order of (a) extent of neuronal labeling, (b) specificity of labeling, (c) definition of neuronal elements, and (d) versatility for combination with other protocols.
Extent of Labeling
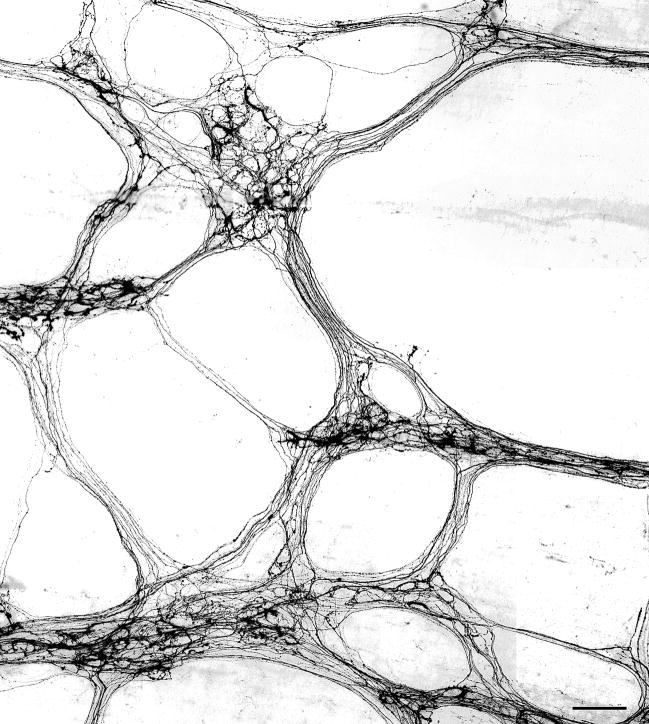
Injections of the dextran amines into the dmnX provided extensive labeling of the vagal efferent projections throughout the myenteric plexus in the stomach (Fig. 1). In cases with multiple large injections of the dmnX, it appeared that the dextran protocols generally approximated complete and total labeling of the vagal preganglionic projections, insofar as the networks of labeled axons revealed in the myenteric plexus were as extensive as those previously described (cf., for example, Holst et al., 1997). The network of vagal preganglionic projections in the proximal GI tract was so extensive, labeled fibers effectively delineated both the ganglia and the connectives of the myenteric plexus (Fig. 1). Within myenteric ganglia, many neurons were densely innervated by efferent fibers that encircled individual neurons with strings of discrete swellings, which are thought to be putative sites of transmitter release. Additionally, other labeled vagal fibers passed through the ganglia and adjacent connectives without producing any characteristic patterns suggestive of neuronal contact (i.e., varicosities).
Figure 1.
A photomontage illustrating dense vagal efferent innervation of the myenteric plexus in the smooth muscle wall of the stomach following injection of dextran-tetramethylrhodamine-biotin into the dorsal motor nucleus of the vagus (dmnX). The fluorescent labeling was photographed and then image was inverted to better illustrate the morphology of individual fibers. Scale bar = 100 μm.
Transport time clearly can affect the amount of labeling, so it should be noted that all four of the dextran conjugates evaluated appear to have approximately the same speed of transport, because allowing 19 days for transport of all four dextrans from the dmnX to the GI tract resulted in extensive labeling of the innervation of the stomach and duodenum. Additionally, with each of the different conjugates, well labeled but sparsely distributed efferent axons and terminals were routinely observed in the whole mounts. This falloff in the density of fibers from the stomach to the distal small intestine reflects the normal distribution of vagal efferents along the GI tract (Berthoud et al., 1991); however, it is possible that different transport times following tracer injection into the dmnX might reveal alternative patterns of innervation.
Specificity of Labeling
Because (a) injections into the dmnX cannot be done routinely without risk of tracer reaching the overlying nucleus of the solitary tract and (b) dextrans can be transported both retrogradely and anterogradely, all whole mounts were screened for labeled afferent terminals that might inadvertently have been labeled by retrograde uptake from the nucleus of the solitary tract with subsequent nodose transganglionic transport of the tracer to the periphery. With transport times optimized for anterograde labeling from the dmnX (i.e. 19 days), however, no vagal afferent endings (i.e. intraganglionic laminar endings or intramuscular arrays—see Powley and Phillips, 2005) in the wall of the GI tract were labeled following injections into the dorsal vagal complex.
Because combining dmnX injections to label efferents with nodose injection to label afferents in the same animal might conceivably produce labeling interactions, the wholemounts in our efferent-afferent series were also examined for inadvertent and/or double labeling of individual processes. None of the afferent fibers and terminals (intraganglionic laminar endings; intramuscular arrays) in the periphery that were labeled by the D-TR injections in the nodose displayed additional or double labeling with D-B from the dmnX injections. On the other hand, although extremely rare, double labeled efferent fibers and their terminals in the myenteric plexus were observed in the series of cases in which injections were done in both the dmnX (D-B) and the nodose ganglion (D-TR) of the same animal. Such axonal double labeling is probably a result, however, of D-TR being taken up by motor fibers of passage that were mechanically damaged during nodose injection.
Definition or Quality of the Label
The dextran conjugates produced complete, smooth filling of the preganglionic axons and their finest terminal ramifications. This labeling equaled the definition typically ascribed to the optimal Golgi staining and PHA-L labeling.
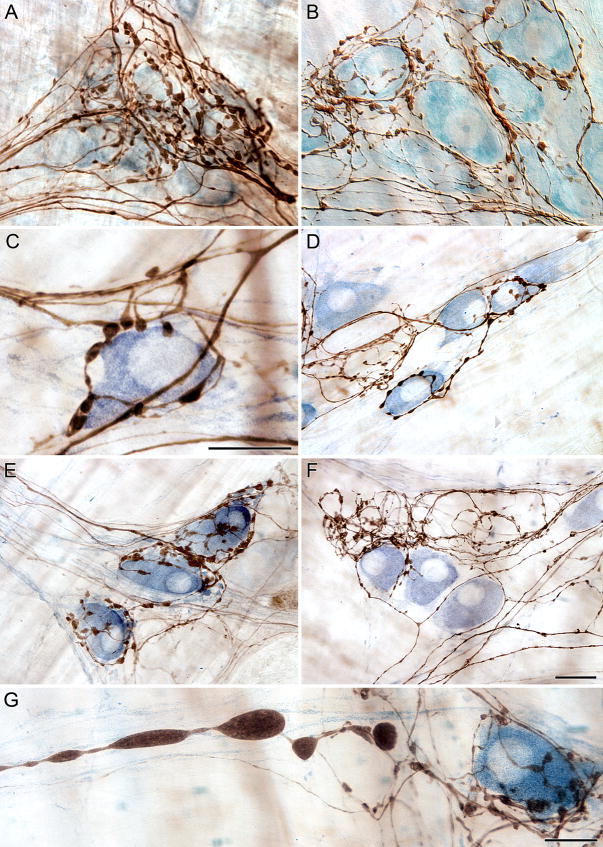
Variations of axonal diameters could be observed and evaluated, and individual fibers could be readily followed as they produced bifurcations and varicosities that surrounded myenteric neurons (Fig. 2A,B). Similarly, dextran conjugate labeling provided sufficient definition of individual fibers that it was possible to identify and examine axons with dystrophic swellings or axonopathies (e.g., Fig. 2G).
Figure 2.
Counterstaining of the myenteric plexus to label either the total population of neurons (A,B) or the nitrergic subpopulation of neurons (C–D) provided excellent contrast to efferent axons and terminals labeled with the anterograde tracer dextran-biotin visualized using a routine DAB protocol. Fibers of passage, distinguishable by their smooth appearance, were routinely observed passing through and within close proximity to ganglia (A,C,F). Vagal efferents formed distinct “basket-like” endings around both nitrergic (C,E) and presumed cholinergic (F) neurons. Dextrans were just as effective in aging rats (G) as they were in younger rats. Scale bar = 16 μm in F for A, B; 25 μm for D-F; Scale bars in C and G = 16 μm.
Illustrating the types of assessments that the dextran conjugates facilitated, Individual efferent fibers appeared to preferentially innervate one or another neuronal subpopulation, as some axons were observed in close registration with NADPHd neurons (Fig. 2C–E) and other fibers were observed to target sets of NADPHd-negative neurons. The clarity of the label was so remarkable that putative terminals were observed branching off and forming the characteristic features of terminal boutons along the side of nitergic neurons (Fig. 2C). In contrast to the preganglionics that seemed to select out NADPHd neurons, other vagal axons passed by NADPHd neurons forming few, if any, varicosities and surrounded neighboring neurons with characteristic baskets (Fig. 2F). Fibers of passage without any apparent contact with neurons of a given ganglion could also be followed through the ganglion and surrounding connectives (Fig. 2A–C,F). At higher magnification axons could be followed through their course from one neuron to another, even changing direction within a ganglion to make contact with another specific neuron (Fig. 2C,D).
In the tissue specimens that were studied, all four of the injected dextran conjugates provided wide spread labeling of axonal projections, but with varying degrees of resolution (see below).
Versatility
The dextran conjugates readily combined with double-label protocols for permanent labeling as well as with double- and triple-label fluorochrome preparations, offering a number of means of exploring the organization of the preganglionic terminals. Consistent with the fact that the four dextran conjugates utilized are all lysine-fixable, none of the labels diffused or leaked from the labeled axons, even with prolonged storage. While the dextrans prove to be versatile there are some observed differences, as discussed below.
As a fluorescent marker, D-TMR-B provided fiber definition similar to that of the conjugate D-TR, but the intensity of the D-TMR-B was not as distinguishable from the background as D-TR. Additionally, reliable double-immunofluorescence labeling requires that the fluorochromes used exhibit good spectral separation, and the overlap between the emission spectra of green dyes (e.g., FITC or Alexa Fluor 488) and TMR renders simultaneous excitation of probes labeled with these dyes unsatisfactory for conventional fluorescent microscopy and confocal laser scanning microscopy. The emission spectrum of the fluorochrome Texas red is better separated from spectra of the green dyes, making D-TR a better choice than D-TMR-B for multiple labeling protocols.
The final conjugate that was investigated, D-RG, extensively labeled the peripheral axons, but its appearance was very faint and granular. The observer’s ability to resolve fine varicosities and terminals in the myenteric ganglia was hampered by the quality of D-RG labeling. Although D-RG may be useful in some instances, our initial observations suggest that it would be impractical to use D-RG when detailed high-resolution labeling is desired.
We assessed the quality of axonal labeling produced by dmnX injections of D-B, visualized by DAB, in conjunction with other permanent staining protocols. Specifically, efferent projections were labeled in wholemounts along with myenteric neurons stained with either the pan-neuronal marker Cuprolinic Blue or the selective stain NADPHd. D-B was compatible with both protocols as extensive networks of innervation could be seen, including efferent terminals encircling neurons in the myenteric ganglia and distinctive axons coursing through connectives between neighboring ganglia. The tracer could be seen spanning the entire tissue specimen. At lower magnification varicosities were distinguishable, and at higher magnification, the high resolution of D-B provided the clearest visualization of axonal varicosities and putative neuronal contacts, as axon terminals unmistakably contacted NADPHd positive neurons (Fig. 2C–G). D-B proved to be a versatile tracer as it allowed for visualization of vagal efferents using either permanent (Fig. 2) or fluorescent (Fig. 3) protocols depending on the needs of the specific study. Contrary to previous studies that some anterograde tracers are less effective in aged subjects, in aging rats the high resolution of D-B was still evident as axon varicosities could be seen in the presence of NADPHd neurons, while dystrophic axons characteristic of age-related axonal degeneration were also distinguishable (Fig. 2G).
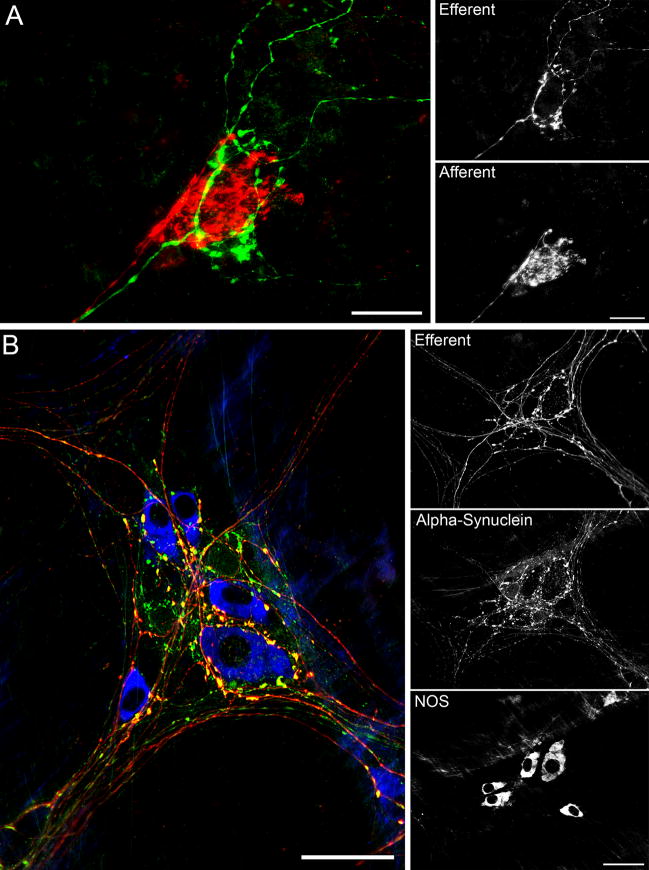
Figure 3.
A: Double labeling of vagal efferent (green) and vagal afferent (red) innervation of myenteric ganglia was readily achieved by injecting dextran-biotin into the dmnx and dextran-Texas Red into the nodose ganglia. Whole mounts were further processed in the presence of streptavidin Alexa Fluor 488. B: Triple labeling was accomplished using dextran-Texas Red tracing of vagal efferents (red) combined with alpha-synuclein (green) and NOS (blue) immunohistochemical labeling of myenteric neurons in the stomach. Prominent yellow varicosities indicate co-localization of alpha-synuclein with dextran-Texas Red in putative vagal efferent terminals. Scale bars for A = 30 μm; Scale bars for B = 50 μm.
To assess whether D-B resulted in comparable visualization for fluorescent studies, a streptavidin tag was bound to the D-B molecule. The quality of the label was still apparent following this fluorescent protocol. To further assess the versatility of this tracer, along with the fluorescent tag on D-B, the efferent projections were visualized with other extrinsic patterns of innervation to the myenteric plexus (i.e., D-TR labeled vagal afferents). The efferents characteristic patterns of encircling neurons were observed with the prototypical afferent pattern in the presence of unlabeled myenteric neurons (Fig. 3A).
D-TR was used in a triple labeling protocol to visualize the vagal efferent association with NOS positive neurons in the myenteric plexus, along with the co-expression of the protein alpha-synuclein. The D-TR could be seen passing through the ganglion and encircling the subpopulation of neurons expressing NOS. Some of the varicosities of the D-TR axons that appeared to be in contact with NOS positive neurons co-expressed alpha-synuclein (Fig. 3B). D-TR proved a versatile tool for vagal studies using double and triple labeling protocols.
Coverslipping, Storage and Preservation of Neuroanatomical Material
The fluorescent conjugates D-TR, D-TMR-B, and D-RG were resistant to solvents, and could thus be dehydrated in a graded series of alcohols, cleared in xylene, and coverslipped usign xylene based mounting mediums. Once these mounting mediums (i.e., either DPX or DePeX) had cured, whole mounts were then stored at room temperature in slide boxes. With the use of non-aqueous mounting protocols (Nance and Burns, 1990; Espada et al., 2005), all of the fluorescent dextran conjugates maintained their fluorescent intensity and crisp pattern of labeling, and remained stable for greater than 12 months with no noticeable increase in background fluorescence. Not surprisingly, similar results were obtained for the permanently visualized biotinylated dextrans.
DISCUSSION
Extracellular injections of anterograde tracers into the dorsal motor nucleus of the vagus (dmnX) have been used to describe both the morphology and the organization of vagal motor endings in the GI tract (e.g., Kirchgessner and Gershon, 1989; Berthoud et al., 1991; Berthoud, 1995, 1996; Holst et al., 1997; Phillips and Powley, 2001; Hayakawa et al., 2006). The fine caliber and exceptional length of these preganglionic axons, as well as the dimensions of the dmnX, however, have complicated such experiments, and the three types of tracers used in the early experiments have each proved to have significant limitations that curbed full structural analyses of the motor projections. Two of the tracers previously employed, HRP conjugates and carbocyanine dyes, have proved particularly unsatisfactory. HRP conjugates provide inadequate morphological definition owing to the coarse, granular labeling patterns commonly obtained (Neuhuber, 1987; Phillips et al., 1997; Wang and Powley, 2000) and also tend, when injected into the dorsal vagal complex, to label afferents as well as efferents. Carbocyanine dyes tend to be unstable, subject to fading, hard to use for permanent labeling, and especially unsuited for combination with other staining or immunohistochemical protocols (Berthoud, 1995; 1996).
The lectin PHA-L, the third of the tracers previously used to characterize dmnX projections, has proved the most successful of the labels originally used. Under optimal circumstances, PHA-L can provide superior definition of vagal axonal processes, and it can be combined with a variety of staining and immunohistochemical procedures. Nonetheless, for labeling vagal efferents, PHA-L has proved to have some of the unreliability described for its labeling of other pathways (e.g., Schmued et al., 1990; Reiner et al., 2000) and to require the extensive, time-consuming processing previously described. Even more limiting, though, when the lectin is used for the vagus, it is especially time consuming and difficult to label much or most of the vagal preganglionic motor neuron pool because of the need for many iontophoretic injections into the elongated dorsal motor nucleus.
Given the success of the dextran amines as anterograde tracers both in the central nervous system (Lanciego and Wouterlood, 2006) and in visceral afferents of the peripheral nervous system (Nance and Burns, 1990; Powley and Phillips, 2005; Phillips et al., 2008), the present experiment was designed to assess whether these polysaccharides would solve the difficulty of extensively labeling the preganglionic motor neuron pool of the vagus, while at the same time matching or surpassing the standards of morphological definition and versatility provided by PHA-L. Since not only the vagus but also, for example, sympathetic preganglionic neurons are distributed as elongated columnar pools of neurons, this feature of extensive or complete labeling would presumably have multiple applications.
The present results, which include observations with four conjugates of dextran, can be considered in terms of the criteria just mentioned, starting with the issue of complete labeling and then discussing the quality of the labeling and the versatility of the protocols.
Labeling Elongated, Columnar Distributions of Preganglionic Neurons
Injections of dextrans easily, reliably and quickly delineated networks of vagal efferent endings in the myenteric plexus. These tracer-defined networks (see Fig. 1) were as extensive or more extensive than those that have been previously obtained with even exceptional injections of PHA-L (see Kirchgessner and Gershon, 1989; Holst et al., 1997).
A consideration of the architecture of the dmnX and a review of the PHA-L protocol both explains the limitations of the lectin for vagal analyses and highlights the utility of the dextrans for tracing vagal motor fibers. The dmnX, like other autonomic motor nuclei, is an elongated (roughly 6 mm long in the rat), narrowly columnar structure on either side of the brainstem. To inclusively label the efferent motor neurons, injections must effectively infiltrate these extended, columnar motor neuron pools on either side of the brainstem. The fact that PHA-L must be injected iontophoretically (Reiner et al., 2000) explains why extensive or complete labeling of vagal motor neurons with the lectin is difficult. The maximum size of such an injection is limited to a diameter of about 200 μm (Lanciego and Wouterlood, 2006), even with the prolonged (i.e. 20 min) iontophoretic applications that are typically recommended. Thus, in comparison to expedient large pressure injections of dextran, time-consuming small iontophoretic injections of PHA-L take orders of magnitude longer to approximate complete labeling and complicate the surgery required.
In contrast to the limitations of PHA-L, the properties of dextrans make them ideal for injecting such distributed pools of neurons. These polysaccharides are hydrophilic and highly soluble in water, which promotes their distribution through the tissue parenchyma. They can be readily pressure injected, filling injection sites 800 μm or more in diameter (Lanciego and Wouterlood, 2006). They also have low toxicity and low immunogenicity (Reiner and Honig, 2006). The biological inertness of the dextrans means that even large injections can be done in sensitive areas such as the brainstem without significant tissue reaction or necrosis.
Furthermore, in the case of the dmnX, the fact that injection leakage or spillover into the nucleus of the solitary did not lead to labeling of the afferent terminals in the gut (at least for the transport time used in the present experiment to label the efferent endings), meant that large injections of tracer into the brainstem could be used without the complications of inadvertent labeling other projections to the same peripheral sites. Another explanation for the lack of co-labeling of vagal afferent projections is the route of dextran incorporation into neurons is at the cell body (Reiner et al., 2000; Raju and Smith, 2006). Along with this, the dextrans are not incorporated by fibers of passage (unless damaged during the injection process), and they do not escape from labeled neurons or synaptic membranes (Wouterlood and Jorritsma- Byham, 1993). Whatever the mechanism, however, the low toxicity and lack of afferent labeling that the dextran injections produce make it practical to work with large injections of the polysaccharides into the brainstem.
High Resolution Golgi-like Labeling of Preganglionic Terminals
As the examples of permanent labeling in Figures 1 and 2 and the examples of fluorescent labeling in Figure 3 illustrate, the dextrans routinely provide smooth, complete labeling of vagal efferent axons and their terminal ramifications. Notably, this superior quality of labeling defined the structural continuity and detail sufficiently well that it was easily practical to evaluate the shape and size of individual terminal varicosities (e.g., Fig. 2A and 2B), follow individual axonal processes over long distances (e.g. Fig. 1; Fig 2D and 2F; Fig. 3B), and distinguish swellings or dystrophic axons (e.g., Fig. 2G) from normal profiles (e.g., Fig. 2C, 2D, or 2F).
The present dextran-labeled profiles appear to equal or exceed in definition the labeling previously obtained for vagal endings with WGA-HRP and carbocyanine compounds; dextran-defined terminals also compared favorably with even exceptional PHA-L labeling (compare the present results with, for example, those of Kirchgessner and Gershon, 1989; Berthoud et al., 1991; Berthoud, 1995, 1996; Holst et al., 1997; Phillips and Powley, 2001; Hayakawa et al., 2006). This superiority of labeling is also in consistent with experiments which have observed that DB provides a denser label and transports more effectively over greater distances in the peripheral nervous than PHA-L (e.g., dorsal root ganglion projections--Novikov, 2001). Overall, then, the exquisite detail and comprehensive labeling observed through the tissues should prove to be a useful tool for quantitative analysis of morphological features of vagal efferents.
The present series of injections employing the four different dextran conjugates did suggest a salient footnote concerning the quality of labeling obtained. In our series, all four conjugates produced labeling, but there were conspicuous differences in the quality and/or contrast of the labeling, with D-B and D-TR giving superior definition and contrast. We did not investigate how much of these differences could be attributed to the choice of filter sets of fluorescence or other protocol details, but some of the differences may reflect fundamental differences in the efficacies of different conjugates. Indeed, the observed variability in resolution among the tracers that were employed may reflect inherent differences among the conjugates, as other studies have found differences in labeling among conjugated dextrans (Dolleman-van der Weel, 1994; Kaneko, 1995).
Versatility of the Dextran Conjugates
Dextran labeling of vagal efferent endings proved exceptionally versatile as well as practical. On these criteria, the polysaccharides appear to match or even surpass the performance of PHA-L.
The biotinylated dextrans (D-B and D-TMR-B in the present series) when processed with DAB, yielded superior, permanent label that was readily combined with counterstains (e.g., NADPHd, see Fig 2C, 2D, 2E and 2F; Cuprolinic blue, see Fig 2A and 2B). The smooth and complete labeling of the axoplasm of even the finest fibers and their terminal processes provided excellent structural detail, and the combined permanence and quality of the label indicate that dextran-labeled preganglionic terminals could readily be inventoried or quantified as well as evaluated morphometrically. Since biotinylated dextrans processed with DAB have proved practical in electron as well as light microscopy (see Wouterlood and Jorritsma-Byham, 1993; Powley et al., 2008), the permanent labeling feature of the polysaccharide conjugates for ultrastructural assessments should presumably apply to vagal motor fibers.
Dextran conjugated to fluorochromes (i.e., D-TR, D-TMR-B, and D-RG) provided fluorescent labeling of the preganglionic terminals that was readily combined with double- and triple-labeling immunohistochemistry (Fig. 3). It was also practical to inject two different fluorescent conjugates in different pools of neurons to examine patterns of interaction of the two projections to a common site (e.g., the dmnX efferent-nodose afferent labeling in Fig. 3A). The resulting labeling was useful for both conventional (Fig. 3B and 3A, respectively) and confocal fluorescence microscopy (Phillips et al., 2008). Another dimension of the versatility of the fluorescent conjugates was that of the three compounds tested, all succeeded in labeling the vagal preganglionics. As described, clear differences in the quality of the fluorescent labeling we achieved did occur, but the patterns suggest that a number of fluorescent conjugates could be used and that experimenters should be able, through choosing the appropriate conjugate as well as optimal filter combinations and protocols, to effectively label vagal and other autonomic preganglionic processes.
Dextran labeling also provided a useful hybrid procedure involving both fluorescence and permanent labeling of the same endings. Specifically, biotinylated dextran conjugated with tetramethylrhodamine (D-TMR-B) could first be examined and evaluated as a fluorescent label, and then as needed subsequently processed with DAB to produce a permanent reaction product.
Other features of the conjugated dextrans also contributed to their versatility. Their low toxicity and water solubility made it practical to do the multiple relatively large injections into the brain without compromising the health or physiology of the animals. The compounds worked equally well in young as well as older animals (see also Rajakumar et al., 1993 for a CNS example), indicating that questions of development or aging could be readily addressed. With the fixation protocols employed in the present experiment, the four lysine fixable conjugates examined all proved particularly stable, evidencing no diffusion of the label from the injected neurons and remaining stable even in long-term storage when coverslipped.
In summary, the observed labeling and versatility of the dextrans—and notably of D-B and D-TR--suggest that they are a new standard for vagal tract tracing studies.
Acknowledgments
We thank Kate Higgs and Sarah Wilder for their assistance with the processing of whole mounts. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIH DK27627 and DK61317).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berthoud HR. Anatomical demonstration of vagal input to nicotinamide acetamide dinucleotide phosphate diaphorase-positive (nitrergic) neurons in rat fundic stomach. J Comp Neurol. 1995;358:428–439. doi: 10.1002/cne.903580309. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Morphological analysis of vagal input to gastrin releasing peptide and vasoactive intestinal peptide containing neurons in the rat glandular stomach. J Comp Neurol. 1996;370:61–70. doi: 10.1002/(SICI)1096-9861(19960617)370:1<61::AID-CNE6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Jedrzejewska A, Powley TL. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J Comp Neurol. 1990;301:65–79. doi: 10.1002/cne.903010107. [DOI] [PubMed] [Google Scholar]
- Chang HT. Immunoperoxidase labeling of the anterograde tracer fluoro-ruby (tetramethylrhodamine-dextran amine conjugate) Brain Res Bull. 1993;30:115–118. doi: 10.1016/0361-9230(93)90046-e. [DOI] [PubMed] [Google Scholar]
- Deller T, Naumann T, Frotscher M. Retrograde and anterograde tracing combined with transmitter identification and electron microscopy. J Neurosci Methods. 2000;103:117–126. doi: 10.1016/s0165-0270(00)00301-0. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Wouterlood FG, Witter MP. Multiple anterograde tracing, combining Phaseolus vulgaris leucoagglutinin with rhodamine- and biotin-conjugated dextran amine. J Neurosci Methods. 1994;51:9–21. doi: 10.1016/0165-0270(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Elberger AJ, Honig MG. Double-labeling of tissue containing the carbocyanine dye DiI for immunocytochemistry. J Histochem Cytochem. 1990;38:735–739. doi: 10.1177/38.5.2110209. [DOI] [PubMed] [Google Scholar]
- Espada J, Juarranz A, Galaz S, Canete M, Villanueva A, Pacheco M, Stockert JC. Non-aqueous permanent mounting for immunofluorescence microscopy. Histochem Cell Biol. 2005;123:329–334. doi: 10.1007/s00418-005-0769-2. [DOI] [PubMed] [Google Scholar]
- Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985;341:269–282. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Fast axonal diffusion of 3000 molecular weight dextran amines. J Neurosci Methods. 1993;50:95–103. doi: 10.1016/0165-0270(93)90060-5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Sonntag R. Sequential double labelling with different fluorescent dyes coupled to dextran amines as a tool to estimate the accuracy of tracer application and of regeneration. J Neurosci Methods. 1991;39:9–17. doi: 10.1016/0165-0270(91)90088-h. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Wilm C. Dextran amines in neuronal tracing. Trends Neurosci. 1990;13:14. doi: 10.1016/0166-2236(90)90056-g. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. A method for anterograde axonal tracing of chemically specified circuits in the central nervous system: combined Phaseolus vulgaris-leucoagglutinin (PHA-L) tract tracing and immunohistochemistry. Brain Res. 1985;343:144–150. doi: 10.1016/0006-8993(85)91168-0. [DOI] [PubMed] [Google Scholar]
- Gonzalo N, Moreno A, Erdozain MA, Garcia P, Vazquez A, Castle M, Lanciego JL. A sequential protocol combining dual neuroanatomical tract-tracing with the visualization of local circuit neurons within the striatum. J Neurosci Methods. 2001;111:59–66. doi: 10.1016/s0165-0270(01)00440-x. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M. Direct synaptic contacts on the myenteric ganglia of the rat stomach from the dorsal motor nucleus of the vagus. J Comp Neurol. 2006;498:352–362. doi: 10.1002/cne.21069. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. BioMed Verlag (Worthsee) Federal Republic of Germany; 1986. Anatomy and Embryology of the Laboratory Rat. [Google Scholar]
- Herzog A, Brosamle C. ‘Semifree-floating’ treatment: a simple and fast method to process consecutive sections for immunohistochemistry and neuronal tracing. J Neurosci Methods. 1997;72:57–63. doi: 10.1016/s0165-0270(96)00156-2. [DOI] [PubMed] [Google Scholar]
- Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;381:81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Honig MG, Hume RI. Carbocyanine dyes. Novel markers for labelling neurons. Trends Neurosci. 1989;12:336–338. [PubMed] [Google Scholar]
- Jorritsma-Byham B, Witter MP, Wouterlood FG. Combined anterograde tracing with biotinylated dextran-amine, retrograde tracing with fast blue and intracellular filling of neurons with lucifer yellow: an electron microscopic method. J Neurosci Methods. 1994;52:153–160. doi: 10.1016/0165-0270(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Saeki K, Lee T, Mizuno N. Improved retrograde axonal transport and subsequent visualization of tetramethylrhodamine (TMR) -dextran amine by means of an acidic injection vehicle and antibodies against TMR. J Neurosci Methods. 1996;65:157–165. doi: 10.1016/0165-0270(95)00162-x. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Gershon MD. Identification of vagal efferent fibers and putative target neurons in the enteric nervous system of the rat. J Comp Neurol. 1989;285:38–53. doi: 10.1002/cne.902850105. [DOI] [PubMed] [Google Scholar]
- Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Chapple CC, Hunter N. Improved double immunofluorescence for confocal laser scanning microscopy. J Histochem Cytochem. 1999;47:1213–1218. doi: 10.1177/002215549904700913. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Wouterlood FG. Multiple neuroanatomical tract-tracing: Approaches for multiple tract-tracing. In: Zaborszky L, Wouterlood FG, Lanciego JL, editors. Neuroanatomical Tract-tracing 3. Springer Science +Business Media, Inc; New York: 2006. pp. 336–365. [Google Scholar]
- Lanciego JL, Wouterlood FG, Erro E, Arribas J, Gonzalo N, Urra X, Cervantes S, Gimenez-Amaya JM. Complex brain circuits studied via simultaneous and permanent detection of three transported neuroanatomical tracers in the same histological section. J Neurosci Methods. 2000;103:127–135. doi: 10.1016/s0165-0270(00)00302-2. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Wouterlood FG, Erro E, Gimenez-Amaya JM. Multiple axonal tracing: simultaneous detection of three tracers in the same section. Histochem Cell Biol. 1998;110:509–515. doi: 10.1007/s004180050312. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Brushart TM. Transganglionic and anterograde transport of horseradish peroxidase across dorsal root ganglia: a tetramethylbenzidine method for tracing central sensory connections of muscles and peripheral nerves. Neuroscience. 1979;4:1107–1117. doi: 10.1016/0306-4522(79)90192-1. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Rosene DL. Sensitivity in horseradish peroxidase neurohistochemistry: a comparative and quantitative study of nine methods. J Histochem Cytochem. 1979;27:763–773. doi: 10.1177/27.3.113450. [DOI] [PubMed] [Google Scholar]
- Nance DM, Burns J. Fluorescent dextrans as sensitive anterograde neuroanatomical tracers: applications and pitfalls. Brain Res Bull. 1990;25:139–145. doi: 10.1016/0361-9230(90)90264-z. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Kressel M, Stark A, Berthoud HR. Vagal efferent and afferent innervation of the rat esophagus as demonstrated by anterograde DiI and DiA tracing: focus on myenteric ganglia. J Auton Nerv Syst. 1998;70:92–102. doi: 10.1016/s0165-1838(98)00034-4. [DOI] [PubMed] [Google Scholar]
- Novikov LN. Labeling of central projections of primary afferents in adult rats: a comparison between biotinylated dextran amine, neurobiotin and Phaseolus vulgaris-leucoagglutinin. J Neurosci Methods. 2001;112:145–154. doi: 10.1016/s0165-0270(01)00461-7. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Afferent innervation of gastrointestinal tract smooth muscle by the hepatic branch of the vagus. J Comp Neurol. 1997 Jul 28;384(2):248–70. [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003 Jul 31;106(2):69–83. doi: 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: Autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153:733–750. doi: 10.1016/j.neuroscience.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Advances in neural tracing of vagal afferent nerves and terminals. In: Undem BJ, Weinreich D, editors. Advances in Vagal Afferent Neurobiology. CRC Press; Boca Raton: 2005. pp. 123–145. [Google Scholar]
- Powley TL, Holst MC, Boyd DB, Kelly JB. Three-dimensional reconstructions of autonomic projections to the gastrointestinal tract. Microsc Res Tech. 1994;29:297–309. doi: 10.1002/jemt.1070290407. [DOI] [PubMed] [Google Scholar]
- Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil. 2008;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- Rajakumar N, Elisevich K, Flumerfelt BA. Biotinylated dextran: a versatile anterograde and retrograde neuronal tracer. Brain Res. 1993;607:47–53. doi: 10.1016/0006-8993(93)91488-e. [DOI] [PubMed] [Google Scholar]
- Raju DV, Smith Y. Anterograde axonal tract tracing. Curr Protoc Neurosci. 2006;Chapter 1(Unit 114) doi: 10.1002/0471142301.ns0114s37. [DOI] [PubMed] [Google Scholar]
- Reiner A, Honig MG. Dextran amines: Versatile tools for anterograde and retrograde studies of nervous system connectivity. In: Zaborszky L, Wouterlood FG, Lanciego JL, editors. Neuroanatomical Tract-tracing 3. Springer Science+Business Media, Inc; New York: 2006. pp. 304–335. [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Gladdy R, Creasy JL, Kitamura S, Smits E, Thomson DB. Efficacy of seven retrograde tracers, compared in multiple-labelling studies of feline motoneurones. J Neurosci Methods. 1994;53:35–46. doi: 10.1016/0165-0270(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Sangster CL, Galea MP, Fan R, Morrison WA, Messina A. A method for processing fluorescent labelled tissue into methacrylate: a qualitative comparison of four tracers. J Neurosci Methods. 1999;89:159–165. doi: 10.1016/s0165-0270(99)00063-1. [DOI] [PubMed] [Google Scholar]
- Schmued L, Kyriakidis K, Heimer L. In vivo anterograde and retrograde axonal transport of the fluorescent rhodamine-dextran-amine, Fluoro-Ruby, within the CNS. Brain Res. 1990;526:127–134. doi: 10.1016/0006-8993(90)90258-d. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Retrograde axonal tracing with fluorescent markers. Curr Protoc Neurosci. 2008;Chapter 1(Unit 117) doi: 10.1002/0471142301.ns0117s43. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Schofield RM, Sorensen KA, Motts SD. On the use of retrograde tracers for identification of axon collaterals with multiple fluorescent retrograde tracers. Neuroscience. 2007;146:773–783. doi: 10.1016/j.neuroscience.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. A method for combining confocal and electron microscopic examination of sections processed for double- or triple-labelling immunocytochemistry. J Neurosci Methods. 1997;73:149–157. doi: 10.1016/s0165-0270(97)02222-x. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Kamo H, Araki M, Kimura H. An economic anterograde axonal tracing method using Phaseolus vulgaris agglutinin (PHA) P-form. J Neurosci Methods. 1987;22:1–8. doi: 10.1016/0165-0270(87)90082-3. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992;41:239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Repici M, Garbossa D, Grimaldi A. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res Bull. 2000;51:11–28. doi: 10.1016/s0361-9230(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000 Jun 5;421(3):302–24. [PubMed] [Google Scholar]
- Wang FB, Powley TL. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. 2007;329:221–230. doi: 10.1007/s00441-007-0413-7. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Bol JG, Steinbusch HW. Double-label immunocytochemistry: combination of anterograde neuroanatomical tracing with Phaseolus vulgaris leucoagglutinin and enzyme immunocytochemistry of target neurons. J Histochem Cytochem. 1987;35:817–823. doi: 10.1177/35.8.2439583. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Jorritsma-Byham B. The anterograde neuroanatomical tracer biotinylated dextran-amine: comparison with the tracer Phaseolus vulgaris-leucoagglutinin in preparations for electron microscopy. J Neurosci Methods. 1993;48:75–87. doi: 10.1016/s0165-0270(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Vinkenoog M, van den Oever M. Tracing tools to resolve neural circuits. Network. 2002;13:327–342. [PubMed] [Google Scholar]