Abstract
New therapeutic approaches in Alzheimer's disease are urgently needed. The normal plasma protein, serum amyloid P component (SAP), is always present in cerebrospinal fluid (CSF) and in the pathognomonic lesions of Alzheimer's disease, cerebrovascular and intracerebral Aβ amyloid plaques and neurofibrillary tangles, as a result of its binding to amyloid fibrils and to paired helical filaments, respectively. SAP itself may also be directly neurocytotoxic. Here, in this unique study in Alzheimer's disease of the bis(d-proline) compound, (R)-1-[6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid (CPHPC), we observed depletion of circulating SAP and also remarkable, almost complete, disappearance of SAP from the CSF. We demonstrate that SAP depletion in vivo is caused by CPHPC cross-linking pairs of SAP molecules in solution to form complexes that are immediately cleared from the plasma. We have also solved the structure of SAP complexed with phosphothreonine, its likely ligand on hyperphosphorylated τ protein. These results support further clinical study of SAP depletion in Alzheimer's disease and potentially other neurodegenerative diseases.
Keywords: bis(d-proline), clinical study, neurodegenerative disease
The pathogenesis of neurodegeneration responsible for cognitive impairment in Alzheimer's disease is poorly understood. The relative importance of soluble oligomers of Aβ, of insoluble Aβ amyloid fibril deposits, and of hyperphosphorylated τ is controversial. Aβ accumulation and amyloid deposition precede cognitive decline in Alzheimer's disease while τ pathology comes later and is also associated with non-Alzheimer dementias. However, the universal presence of serum amyloid P component (SAP) (1) in cerebrospinal fluid (CSF) (2) and bound to cerebral and cerebrovascular amyloid deposits and to neurofibrillary tangles in Alzheimer disease (3–9) is consistent with a role of SAP in pathogenesis. SAP is highly resistant to proteolysis (10) and its binding stabilizes amyloid fibrils (11), enhances their formation in vitro (12), and contributes to their pathogenic deposition and/or persistence in vivo in systemic amyloidosis (13). Furthermore, human SAP has been reported to bind to and enter neurons in culture and in rat brain in vivo, to cause apoptotic cell death (14–18), and to activate human microglia synergistically with Aβ and C1q in vitro, provoking increased production of pro-inflammatory cytokines and Aβ itself (19). Current therapeutic developments for Alzheimer's disease, largely focused on Aβ, have so far shown modest if any clinical benefit (20). Targeting SAP for reversal of its known pro-amyloidogenic and fibril protective effects, and for inhibition of its possible direct neurotoxicity, is an attractive alternative approach. The presence of tangles composed of hyperphosphorylated τ protein, to which SAP binds in non-Alzheimer dementias, also raises the possibility of targeting SAP in those conditions.
We developed the novel palindromic bis-d-proline drug, (R)-1-[6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid (CPHPC), as a ligand for SAP intended to inhibit and dissociate SAP binding to amyloid fibrils and tangles (21). However, unexpectedly, administration of CPHPC to humans produced swift hepatic uptake of SAP leading to remarkable ≈99% depletion of SAP from the circulation, a unique effect of a small molecule drug (21). Although there are conflicting reports about both the CSF concentration of SAP (2, 22) and local synthesis of SAP in the brain (23, 24), the sustained profound depletion of circulating SAP induced by CPHPC should remove SAP from the CSF, while penetration of CPHPC itself should block SAP binding to autologous ligands in the brain.
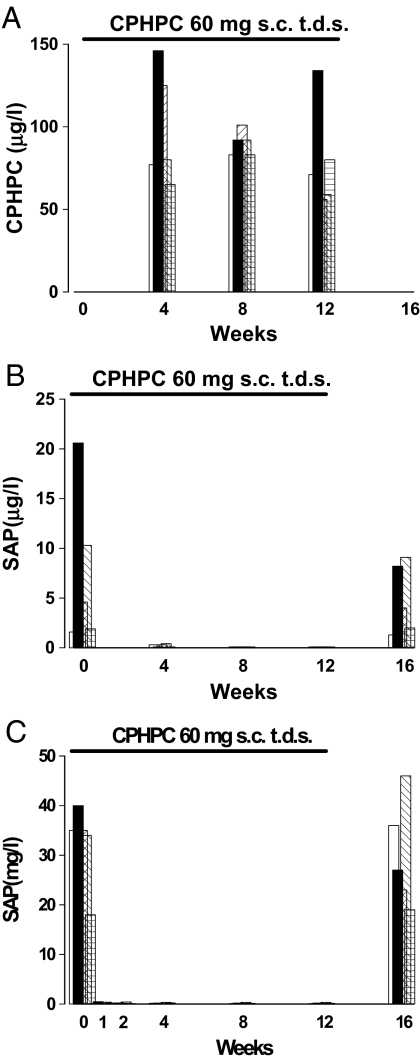
We therefore conducted a pilot proof of concept study in 5 patients aged 53–67 years with mild to moderate probable Alzheimer's disease who received 60 mg CPHPC by s.c. injection 3 times daily for 12 weeks. The drug was well tolerated with no adverse effects other than transient local discomfort on injection. Compliance was confirmed by the presence of CPHPC in all serum samples during the treatment period, and somewhat surprisingly as it is a dicarboxylic acid, CPHPC was also present in the cerebrospinal fluid (Fig. 1A). The concentration of SAP in the serum fell dramatically from mean (SD) of 32.4 (8.4) mg/L before treatment to 0.25 (0.16) mg/L 1 week after starting CPHPC, remained around this value throughout the treatment period, and had risen to 30.2 (10.8) mg/L at 4 weeks after drug discontinuation (Fig. 1B). The SAP concentration in the CSF also fell remarkably, from 32.4 (8.4) μg/L before treatment to 0.2 (0.2) μg/L after 1 week, and then remained scarcely detectable throughout the treatment period (Fig. 1C). Because depletion of circulating SAP by CPHPC involves clearance by the liver (21), these observations are most consistent with CSF SAP being derived from the blood. They suggest that entry of CPHPC into the brain is not required for removal of intracerebral SAP but the presence of CPHPC in CSF should additionally block effects of any residual or locally produced SAP.
Fig. 1.
CPHPC administration in Alzheimer's disease. CPHPC concentration in cerebrospinal fluid (A) and SAP concentrations in the plasma (B) and cerebrospinal fluid (C) in 5 patients with Alzheimer's disease are shown before, during, and after treatment with CPHPC for 12 weeks.
There was no significant difference between the clinical measures before and after CPHPC and importantly no deterioration, in the mini-mental state examination (MMSE), the Alzheimer's disease (AD) assessment scale–cognitive subscale (ADAS-Cog), or the clinician's interview-based impression of change-plus (CIBIC+) (Table 1), nor any structural change in MRI brain scans. There were also no significant changes before, during, and after treatment in CSF concentrations of Aβ40, Aβ42, total or phosphorylated τ, or S100B or in any of the comprehensive routine hematological, biochemical, endocrine, or serological blood tests. In view of the long presymptomatic duration of neuropathology and the slow progression of Alzheimer's disease, it would have been astonishing if there had been any improvement in cognition or change in the CSF biomarkers during this brief study that aimed to demonstrate safety, tolerability, and proof of biochemical concept. However, the clinical stability and absence of biochemical signs of cerebral damage importantly confirm the safety in patients with dementia both of CPHPC itself and of profound depletion of systemic and cerebral SAP and support longer-term studies of clinical efficacy. Encouragingly, continuous administration of CPHPC, producing sustained plasma SAP depletion for up to 2 years, in 31 middle-aged and elderly patients with systemic amyloidosis has had no adverse effects, including no clinically evident neurological or cognitive effects (25).
Table 1.
Cognitive assessments before and after treatment with CPHPC in Alzheimer's disease
| Test | Mean score (SD) |
|
|---|---|---|
| Baseline | Posttreatment | |
| MMSE | 21 (4) | 19 (5) |
| ADAS-Cog | 27 (10) | 26 (10) |
| CIBIC+ | Not applicable | 0.0 (0.7) |
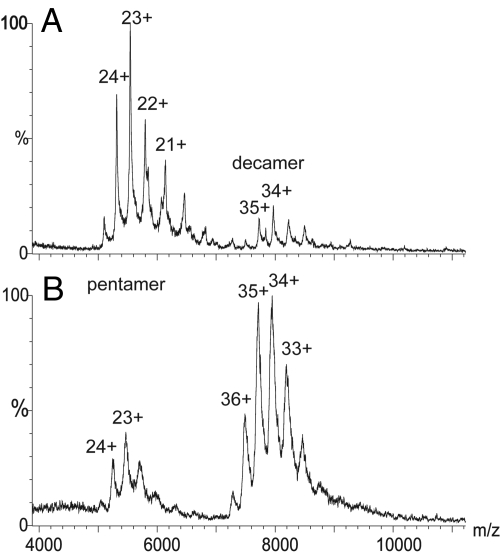
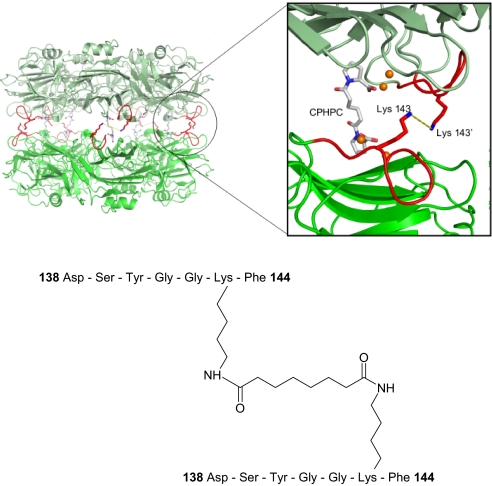
To elucidate the molecular mechanism responsible for SAP depletion, we analyzed the structure of complexes of SAP with CPHPC in solution. The 3D crystal structure of this complex contains 2 intact native pentameric SAP molecules cross-linked by 5 drug molecules (21). Here SAP-CPHPC complexes were formed in solution and isolated by size exclusion chromatography, eluting as a symmetrical peak at the volume corresponding precisely to the decameric assembly of a pair of pentameric SAP molecules (21). These complexes were then covalently cross-linked with bis(sulfosuccinimidyl)suberate (BS3), a bifunctional molecule of precisely the correct length to react at each end with the ε-amino groups of SAP residue Lys-143 in apposed pentamers if the soluble complex has the same arrangement as in the crystals. Mass spectrometry analysis under conditions designed to preserve noncovalent interactions confirmed that after treatment with BS3, >80% of the SAP population was in the decameric form (Fig. 2). To locate the site of covalent intermolecular cross-linkage the complex was digested with the protease Asp-N and the products were analyzed by mass spectrometry. Two unique peptides were identified that were not observed in mass spectra of AspN-digested SAP alone (929.4 and 1683.6 Da) [supporting information (SI) Fig. S1]. The smaller peptide corresponds to 1 molecule of BS3 attached via the ε-amino group of the Lys-143 residue in the SAP heptapeptide 138–144 (Fig. S2). The larger species corresponds to 2 copies of the same SAP sequence (138–144) covalently attached to one BS3 molecule (Fig. S3, Fig. 3). This confirms that the structure of the SAP-CPHPC complex in solution is the same as that reported in the x-ray crystal structure (21) (Fig. 3).
Fig. 2.
Mass spectrometry of SAP under nondissociating conditions before (A) and after (B) cross-linking with CPHPC followed by bis(sulfosuccinimidyl) suberate (BS3). A very small amount of decamer is seen in the native SAP preparation, reflecting the known tendency of isolated pure human SAP to autoaggregate in this way (37). After cross-linking by CPHPC and BS3, >80% of the SAP was in the decameric assembly.
Fig. 3.
Structure of the SAP-CPHPC complex in solution. SAP decamer cross-linked by CPHPC and BS3 is shown, with (Inset) a close-up of the SAP dimer interface showing the position of residue Lys-143 in relation to the CPHPC binding site. The bis(sulfosuccinimidyl)suberate (BS3) cross-linker is shown as a yellow dashed line, residues 138 to 144 are shown in red, and the calcium atoms are shown in orange. The sequence of the unique peptide produced by proteolysis of the complex after covalent cross-linking by BS3 is shown below.
Soluble complexes of SAP with CPHPC formed from SAP trace radiolabeled with 125I, either with or without additional cross-linking by BS3, were cleared from the circulation within 20 min after i.v. injection into mice, compared to the ≈3-h plasma half-life of native human SAP in mice (26). Circulating SAP is normally cleared in vivo exclusively by hepatocytes (26) but not via the asialoglycoprotein receptor (27) and the rapid clearance of the SAP-CPHPC complex in mice was also not affected by concurrent administration of desialylated orosomucoid to block this receptor (27). Circular dichroism measurements showed no significant secondary or tertiary structural change in the SAP-CPHPC complex compared to native SAP alone (Fig. S4). The immediate clearance of the SAP-CPHPC complex in vivo therefore indicates that dimerization of SAP molecules is sufficient to trigger their removal from the plasma, a unique molecular mechanism responsible for this previously uncharacterized example of targeted pharmacological knockout of a pathogenic protein by a small molecule drug.
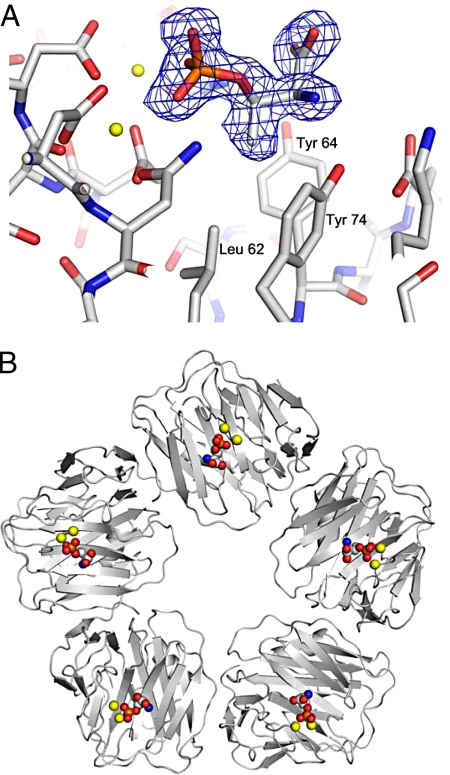
The intractability of insoluble amyloid fibers for high-resolution structural studies has hitherto prevented identification of their molecular ligands recognized by SAP. Although paired helical filaments have recently been shown to have cross-β structure typical of amyloid fibrils (28), the pathognomonic hyperphosphorylation of serine and threonine residues in τ protein (29), associated with formation of neurofibrillary tangles, provides potential small molecule ligands for investigation. We found that in the presence of calcium, human SAP bound O-phospho-l-threonine but not O-phospho-l-serine. Although binding to O-phospho-l-threonine was relatively weak, Kd = 380 μM by isothermal titration calorimetry, multivalent binding of the pentameric SAP molecule provides profound enhancement of avidity (21), which is likely to be responsible for the universal presence of SAP on tangles in vivo. We cocrystallized SAP with O-phospho-L-threonine and the 3D x-ray structure of the complex showed a molecule of ligand in each of the 5 identical calcium-dependent ligand-binding pockets in the SAP molecule (Fig. 4). Multivalent binding by SAP must stabilize the filaments, as we have previously demonstrated with Aβ and other types of amyloid fibrils (11), and may also inhibit phosphatase activity by masking phosphate residues. SAP may thus make a significant contribution to in vivo persistence and potential pathogenicity of tangles (30).
Fig. 4.
3D x-ray crystal structure of O-phospho-l-threonine bound by SAP. (A) There is clear electron density (Fo-Fc omit map contoured at 3σ) for 1 molecule of O-phospho-l-threonine bound in the double-calcium site of each subunit of SAP through the phosphate oxygens. The Cγ-methyl group of the amino acid side chain is positioned within the adjacent Leu-62/Tyr-64/Tyr-74 pocket that is also occupied by the proline ring of bound CPHPC (21). The amino and carboxylate components are positioned so that a turn with O-phospho-l-threonine at the apex might visit the site, with NH and CO forming hydrogen bonds with Tyr-74-OH and Gln-148. Calcium atoms are shown in yellow and phosphorus in orange. (B) SAP pentamer showing the 5 calcium binding sites occupied by O-phospho-l-threonine with calcium atoms shown in yellow.
We have reported 3 original findings here: (i) the unprecedented, profound depletion of a potentially pathogenic protein, SAP, from the CSF in patients with Alzheimer's disease produced by parenteral administration of a unique small molecule drug, CPHPC; (ii) the unique molecular mechanism by which CPHPC depletes SAP from the plasma and thus the CSF; and (iii) the previously uncharacterized molecular structure of SAP bound to a putative pathophysiologic ligand. These observations strongly support further studies of SAP depletion in neurodegenerative diseases associated with SAP accumulation in the brain.
Methods
Clinical Study of CPHPC in Alzheimer's Disease.
The open label study was approved by the institutional review board and all patients signed informed consent. Lumbar puncture and venous blood sampling were performed at baseline and at 4-week intervals until 4 weeks after drug discontinuation. SAP and CPHPC concentrations were measured (2, 21) in all plasma samples, together with routine hematological, biochemical (renal, liver, and bone profiles), endocrine (glucose, thyroid), and serological (rheumatoid factor, autoantibodies, and C-reactive protein) tests. SAP and CPHPC concentrations were also measured (2, 21) in all cerebrospinal fluid samples as well as lumbar puncture opening pressure, white cell count, red cell count, total protein, and glucose by routine methods in all samples. CSF concentrations of Aβ40, Aβ42, total τ, and phospho-τ were measured by commercial ELISA (Biosource Europe) and S100B by in-house ELISA (31, 32). Cognition was assessed by MMSE (33), ADAS-Cog (34), and CIBIC+. Brain MRI scans were obtained before and after treatment.
Structure of the SAP-CPHPC Complex in Solution.
Isolated pure human SAP (35, 36) at 1.782 mg/mL, 70 μM with respect to protomer (Mr 25,462), in 10 mM Tris, 140 mM NaCl, pH 8.0, was mixed with CPHPC (80 μM) in 10 mM Tris, 140 mM NaCl, 2 mM CaCl2, pH 8.0, in a total volume of 0.5 mL and incubated for 1 h at 37 °C before fractionation on a Superdex 200 column in the Åkta Explorer system (GE HealthCare) eluted with 10 mM Tris, 140 mM NaCl, 2 mM CaCl2, pH 8.0. The column was calibrated with standard globular markers and with human SAP in a known decameric assembly and the closely related pentraxin protein, human C-reactive protein, which is known to be a single pentamer (37). All of the SAP that had been preincubated with CPHPC eluted as a single symmetrical peak at the volume corresponding to SAP decamers and was then dialyzed extensively against 20 mM Hepes, 2 mM CaCl2, 140 mM NaCl, pH 7.3, before addition, in the same Hepes buffer, of bis(sulfosuccinimidyl)suberate (Pierce) at 10- to 50-fold molar excess over SAP. After 30 min at room temperature and then 2 h at 4 °C, the reaction was terminated by adding 1 M Tris·HCl, pH 7.5, to a final concentration of 50 mM. The covalently cross-linked protein no longer bound to phosphoethanolamine immobilized on Sepharose beads (36), consistent with occlusion of the binding (B) face of the molecule (38). Retention of decameric assembly was confirmed by the elution profile on Superdex 200 and increased anodal migration relative to that of unmodified SAP on 1% wt/vol agarose in 0.07 M sodium barbitone, 10 mM EDTA, pH 8.6, consistent with involvement of lysine residues in the covalent cross-linking reaction. Mass spectrometry also confirmed that the majority of cross-linked SAP was in the decameric form (Fig. 2). The residues involved in the covalent cross-linking of the SAP decamers produced by binding of CPHPC were identified by mass spectrometry following proteolysis of the complex at 40 μM SAP protomer by Asp-N protease (Roche) at 1:100 in 50 mM ammonium acetate, pH 7.0, overnight at 37 °C.
Mass Spectrometry.
SAP alone and after complexing with CPHPC followed by covalent cross-linking with BS3 were diluted to 80 μM and buffer-exchanged into 200 mM ammonium acetate, pH 8.0, using microbiospin columns (Bio-Rad). Aliquots (2 μL) of each were analyzed under identical mass spectrometry conditions on a modified Q-ToF MS under instrument parameters designed to preserve noncovalent interactions (39) (Fig. 2). After treatment of cross-linked SAP with Asp-N, the digest was mixed 1:1 vol/vol with cyano-4-hydroxycinnamic acid matrix solution (10 mg/ml in 50% CH3CN containing 0.1% vol/vol TFA) and then spotted directly onto the target plate and allowed to air dry. Using the MALDI Tof/Tof 4700 (Applied Biosystems), an average of 1000 laser shots were applied to obtain the MS and MS/MS spectra, using a 200-Hz frequency-tripled Nd:YAG laser operating at a wavelength of 355 nm. For MALDI-MS/MS sequencing experiments the precursor ions were selected by a timed-ion selector and activated inside the collision cell with argon at a pressure of 2 × 10−6 Torr with the collision energy set at 1 kV.
Circular Dichroism.
Secondary and tertiary structural features of native SAP and the decameric SAP-CPHPC complex with and without covalent cross-linking were analyzed by circular dichroism at 0.1 mg/mL and 2 mg/mL, in 10 mM Tris, 140 mM NaCl, pH 8.0, for far and near UV spectra, respectively (Fig. S4). Path lengths of 1 and 10 mm were used in the far (200–250 nm) and near (250–350 nm) UV ranges, respectively, in a Jasco 710 spectropolarimeter equipped with temperature control and calibrated with 0.06% wt/vol ammonium d-10-camphorosulphonate.
In Vivo Clearance Studies.
125I-SAP (40, 41) with specific activity of 2.4 MBq/μmol SAP pentamer was used in trace amounts to spike unlabeled SAP for preparation of SAP-CPHPC complexes with and without covalent cross-linking as described above. SAP and orosomucoid (Sigma-Aldrich) were desialylated as described previously (26). Male and female wild-type C57BL/6 mice were bred and housed in standard pathogen-free conditions and were used in clearance experiments at 16–20 weeks of age after supplementing the drinking water for at least 24 h beforehand with 60 mg/L potassium iodide. Mice received 100 μg of 125I-labeled native or complexed SAP, either alone or in combination with desialylated orosomucoid, intravenously via the lateral tail vein. They were bled from the tail (25–40 μL) at 2 min and at various time points up to 60 min. Samples were weighed and counted, and TCA precipitable radioactivity per gram of blood was calculated.
X-Ray Analysis.
Crystals of the SAP/O-phospho-l-threonine complex were grown by hanging drop vapor diffusion from 14.2 mg/mL SAP with a 10-fold molar excess of O-phospho-l-threonine in 60 mM Tris·HCl (pH 8.0), 10 mM CaCl2, and 16% vol/vol PEG550 MME. The crystals were monoclinic, space group P21, with unit cell dimensions a = 94.77, b = 69.43, c = 102.06 Å, β = 97°. X-ray diffraction data were collected from a single crystal at 100 K to a resolution of 1.7 Å on beam line ID14.2 (European Synchrotron Radiation Facility, Grenoble, France). Structure analysis details are provided in SI Methods and Table S1.
Supplementary Material
Acknowledgments.
We thank F. Hoffmann-La Roche for permission to undertake the clinical study with CPHPC, the staff of the European Synchrotron Radiation Facility, Grenoble, France for their support, and Beth Jones for processing the manuscript. This study was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the United Kingdom Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme. The Dementia Research Centre is an Alzheimer Research Trust Centre. This study was also supported by Medical Research Council Program Grant G97900510 (to M.B.P. and P.N.H.), by awards from the Walters Kundert Trust and the Royal Society (to C.V.R. and N.W.), and by Alzheimer Research Trust funding (to S.J.C.).
Footnotes
Conflict of interest: M.B.P. is the inventor on patents related to SAP and CPHPC that are owned by Pentraxin Therapeutics Ltd., a University College London spinout company in which he, P.N.H., and S.P.W. have shares, which owns CPHPC.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2W08).
This article contains supporting information online at www.pnas.org/cgi/content/full/0902640106/DCSupplemental.
References
- 1.Pepys MB, et al. Amyloid P component. A critical review. Amyloid: Int J Exp Clin Invest. 1997;4:274–295. [Google Scholar]
- 2.Hawkins PN, et al. Concentration of serum amyloid P component in the CSF as a possible marker of cerebral amyloid deposits in Alzheimer's disease. Biochem Biophys Res Commun. 1994;201:722–726. doi: 10.1006/bbrc.1994.1760. [DOI] [PubMed] [Google Scholar]
- 3.Coria F, et al. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest. 1988;58:454–458. [PubMed] [Google Scholar]
- 4.Duong T, Pommier EC, Scheibel AB. Immunodetection of the amyloid P component in Alzheimer's disease. Acta Neuropathol. 1989;78:429–437. doi: 10.1007/BF00688180. [DOI] [PubMed] [Google Scholar]
- 5.Kalaria RN, Grahovac I. Serum amyloid P immunoreactivity in hippocampal tangles, plaques and vessels: implications for leakage across the blood-brain barrier in Alzheimer's disease. Brain Res. 1990;516:349–353. doi: 10.1016/0006-8993(90)90941-4. [DOI] [PubMed] [Google Scholar]
- 6.Kalaria RN, Galloway PG, Perry G. Widespread serum amyloid P immunoreactivity in cortical amyloid deposits and the neurofibrillary pathology of Alzheimer's disease and other degenerative disorders. Neuropathol Appl Neurobiol. 1991;17:189–201. doi: 10.1111/j.1365-2990.1991.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 7.Duong T, Doucette T, Zidenberg NA, Jacobs RW, Scheibel AB. Microtubule-associated proteins tau and amyloid P component in Alzheimer's disease. Brain Res. 1993;603:74–86. doi: 10.1016/0006-8993(93)91301-8. [DOI] [PubMed] [Google Scholar]
- 8.Perlmutter LS, Barrón E, Myers M, Saperia D, Chui HC. Localization of amyloid P component in human brain: vascular staining patterns and association with Alzheimer's disease lesions. J Comp Neurol. 1995;352:92–105. doi: 10.1002/cne.903520107. [DOI] [PubMed] [Google Scholar]
- 9.Hamazaki H. Ca2+-dependent binding of human serum amyloid P component to Alzheimer's β-amyloid peptide. J Biol Chem. 1995;270:10392–10394. doi: 10.1074/jbc.270.18.10392. [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita CM, et al. A protease-sensitive site in the proposed Ca2+-binding region of human serum amyloid P component and other pentraxins. Protein Sci. 1992;1:700–709. doi: 10.1002/pro.5560010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tennent GA, Lovat LB, Pepys MB. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer's disease and systemic amyloidosis. Proc Natl Acad Sci USA. 1995;92:4299–4303. doi: 10.1073/pnas.92.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamazaki H. Amyloid P component promotes aggregation of Alzheimer's β-amyloid peptide. Biochem Biophys Res Commun. 1995;211:349–353. doi: 10.1006/bbrc.1995.1819. [DOI] [PubMed] [Google Scholar]
- 13.Botto M, et al. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 14.Urbányi Z, Lakics V, Erdó SL. Serum amyloid P component-induced cell death in primary cultures of rat cerebral cortex. Eur J Pharmacol. 1994;270:375–387. doi: 10.1016/0926-6917(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 15.Duong T, Acton PJ, Johnson RA. The in vitro neuronal toxicity of pentraxins associated with Alzheimer's disease brain lesions. Brain Res. 1998;813:303–312. doi: 10.1016/s0006-8993(98)00966-4. [DOI] [PubMed] [Google Scholar]
- 16.Urbányi Z, et al. Serum amyloid P component induces neuronal apoptosis and beta-amyloid immunoreactivity. Brain Res. 2003;988:69–77. doi: 10.1016/s0006-8993(03)03345-6. [DOI] [PubMed] [Google Scholar]
- 17.Urbányi Z, et al. Serum amyloid P component induces TUNEL-positive nuclei in rat brain after intrahippocampal administration. Brain Res. 2007;1145:221–226. doi: 10.1016/j.brainres.2007.01.132. [DOI] [PubMed] [Google Scholar]
- 18.Pisalyaput K, Tenner AJ. Complement component C1q inhibits β-amyloid- and serum amyloid P-induced neurotoxicity via caspase- and calpain-independent mechanisms. J Neurochem. 2008;104:696–707. doi: 10.1111/j.1471-4159.2007.05012.x. [DOI] [PubMed] [Google Scholar]
- 19.Veerhuis R, et al. Amyloid β plaque-associated proteins C1q and SAP enhance the Aβ1–42 peptide-induced cytokine secretion by adult human microglia in vitro. Acta Neuropathol. 2003;105:135–144. doi: 10.1007/s00401-002-0624-7. [DOI] [PubMed] [Google Scholar]
- 20.St George-Hyslop PH, Morris JC. Will anti-amyloid therapies work for Alzheimer's disease? Lancet. 2008;372:180–182. doi: 10.1016/S0140-6736(08)61047-8. [DOI] [PubMed] [Google Scholar]
- 21.Pepys MB, et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417:254–259. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]
- 22.Kimura M, et al. Assessment of cerebrospinal fluid levels of serum amyloid P component in patients with Alzheimer's disease. Neurosci Lett. 1999;273:137–139. doi: 10.1016/s0304-3940(99)00631-x. [DOI] [PubMed] [Google Scholar]
- 23.Kalaria RN, Golde TE, Cohen M, Younkin LH, Younkin S. Absence of detectable mRNA of serum amyloid P component (SAP) in human brain, choroid plexus, and meninges suggests that the presence of SAP in CSF is due to transport across the blood-brain barrier. J Neuropathol Exp Neurol. 1991;50:339. [Google Scholar]
- 24.Yasojima K, Schwab C, McGeer EG, McGeer PL. Human neurons generate C-reactive protein and amyloid P: upregulation in Alzheimer's disease. Brain Res. 2000;887:80–89. doi: 10.1016/s0006-8993(00)02970-x. [DOI] [PubMed] [Google Scholar]
- 25.Pepys MB. In: Amyloid and Amyloidosis. Grateau G, Kyle RA, Skinner M, editors. Boca Raton, FL: CRC Press; 2005. pp. 488–490. [Google Scholar]
- 26.Hutchinson WL, Noble GE, Hawkins PN, Pepys MB. The pentraxins, C-reactive protein and serum amyloid P component, are cleared and catabolized by hepatocytes in vivo. J Clin Invest. 1994;94:1390–1396. doi: 10.1172/JCI117474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepys MB, et al. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Natl Acad Sci USA. 1994;91:5602–5606. doi: 10.1073/pnas.91.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berriman J, et al. Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-β structure. Proc Natl Acad Sci USA. 2003;100:9034–9038. doi: 10.1073/pnas.1530287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- 30.Goedert M, Klug A, Crowther RA. Tau protein, the paired helical filament and Alzheimer's disease. J Alzheimers Dis. 2006;9:195–207. doi: 10.3233/jad-2006-9s323. [DOI] [PubMed] [Google Scholar]
- 31.Green AJ, Keir G, Thompson EJ. A specific and sensitive ELISA for measuring S-100b in cerebrospinal fluid. J Immunol Methods. 1997;205:35–41. doi: 10.1016/s0022-1759(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 32.Petzold A, Keir G, Lim D, Smith M, Thompson EJ. Cerebrospinal fluid (CSF) and serum S100B: release and wash-out pattern. Brain Res Bull. 2003;61:281–285. doi: 10.1016/s0361-9230(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state. ” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 35.de Beer FC, Pepys MB. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50:17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins PN, Tennent GA, Woo P, Pepys MB. Studies in vivo and in vitro of serum amyloid P component in normals and in a patient with AA amyloidosis. Clin Exp Immunol. 1991;84:308–316. doi: 10.1111/j.1365-2249.1991.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchinson WL, Hohenester E, Pepys MB. Human serum amyloid P component is a single uncomplexed pentamer in whole serum. Mol Med. 2000;6:482–493. [PMC free article] [PubMed] [Google Scholar]
- 38.Ashton AW, Boehm MK, Gallimore JR, Pepys MB, Perkins SJ. Pentameric and decameric structures in solution of serum amyloid P component by X-ray and neutron scattering and molecular modelling analyses. J Mol Biol. 1997;272:408–422. doi: 10.1006/jmbi.1997.1271. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez H, Robinson CV. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat Protoc. 2007;2:715–726. doi: 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- 40.Hawkins PN, Myers MJ, Epenetos AA, Caspi D, Pepys MB. Specific localization and imaging of amyloid deposits in vivo using 123I-labeled serum amyloid P component. J Exp Med. 1988;167:903–913. doi: 10.1084/jem.167.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reay P. Use of N-bromosuccinimide for the iodination of proteins for radioimmunoassay. Ann Clin Biochem. 1982;19:129–133. doi: 10.1177/000456328201900214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.