Abstract
Granulocytes generate a “respiratory burst” of NADPH oxidase-dependent superoxide anion (O2−∙) production that is required for efficient clearance of bacterial pathogens. Hv1 mediates a voltage-gated H+ channel activity that is proposed to serve a charge-balancing role in granulocytic phagocytes such as neutrophils and eosinophils. Using mice in which the gene encoding Hv1 is replaced by β-Geo reporter protein sequence, we show that Hv1 expression is required for measurable voltage-gated H+ current in unstimulated phagocytes. O2−∙ production is substantially reduced in the absence of Hv1, suggesting that Hv1 contributes a majority of the charge compensation required for optimal NADPH oxidase activity. Despite significant reduction in superoxide production, Hv1−/− mice are able to clear several types of bacterial infections.
Keywords: HV1−/−, immunity, voltage-gated ion channel, innate immunity
The voltage-gated H+ channel Hv1 (also termed VSOP or BTS) was identified by its homology to the voltage sensor domain of voltage-gated cation channels and a voltage-regulated phosphatase Ci-VSP (1–4). Hv1 is encoded by the Hvcn1 gene, and both protein function and genetic sequences are well conserved from primitive chordates to mammals (1, 2). Expression of Hv1 in either mammalian cells or Xenopus oocytes is sufficient to reconstitute the hallmark biophysical properties of native voltage-dependent H+ conductances (GvH+): activation by transmembrane depolarization or net intracellular acidification, exquisite H+-selective conductance, and sensitivity to inhibition by micromolar Zn2+ (1, 2, 5–8). Under resting physiological conditions in most cells (pHi ≈ pHo), the channel's voltage-dependent gating dictates that protons flow only outward through Hv1, suggesting that a prominent physiological role for the channel is to secrete H+ (5).
Voltage-gated H+ currents and Hv1 protein are prominently expressed in granulocytic phagocytes such as neutrophils and eosinophils (1, 3, 5, 9); granulocytes are characterized by their ability to mount a “respiratory burst” of high-level NADPH oxidase-dependent superoxide anion (O2−∙) production that is required for clearance of bacterial pathogens (10). NADPH oxidase would cause a rapid intracellular acidification and profound depolarization of the cell membrane in the absence of a balancing charge movement, and GvH+ mediated by Hv1 is ideally suited to meet this physiological requirement (11–14). To investigate whether Hv1 is required for innate immune function, we generated an Hv1 knockout mouse (Hv1−/−) line and assessed voltage-gated H+ current, O2−∙ production, and bacterial clearance in Hv1−/− mice and their wt/wt littermates. Our results demonstrate that Hv1 is required for GvH+, a robust respiratory burst, and efficient bacterial clearance in vitro.
Results
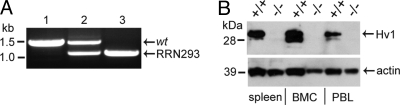
The Bay Genomics genetrap of the Hvcn1 locus leads to transcription of a transgenic mRNA that contains a β-Geo reporter gene sequence in place of the Hv1 protein coding sequence, such that only the 6 N-terminal residues of Hv1 are translated (Fig. S1). RRN293 ES cells bearing the Hvcn1 genetrap allele were used to generate a transgenic mouse line that that is expected to lack Hv1 protein expression and voltage-gated proton channel activity. Triplex genomic PCR demonstrated the expected Hvcn1 size difference for the wt and transgenic alleles (Fig. 1A), and was used in conjunction with quantitative genomic PCR for genotypic screening during breeding. Homozygous RRN293 mice lack detectable levels of Hv1 protein in spleen, bone marrow cells (BMC), and peripheral blood leukocytes (Fig. 1B), indicating that the genetrap strategy results in an effective Hv1 knockout (Hv1−/−). Consistent with the reported expression of GvH+ in alveolar epithelial cells (15, 16), we detected Hv1 protein in lung homogenates from wt/wt but not Hv1−/− mice (Fig. S1).
Fig. 1.
Generation of Hv1−/− mice. RRN293 ES cells contain a Bay Genomics genetrap vector insertion in the Hvcn1 gene. The transgene serves as a surrogate splice acceptor for wt exon 2 that produces an in-frame chimeric mRNA encoding Hvcn1 exons 1 and 2 followed by β-Geo sequence. (A) Triplex genomic PCR using reverse primers specific for both Hvcn1 and β-Geo amplifies a ≈1.5-kb band from the wt allele (lane 1) and a ≈1.0-kb band from the transgenic allele (lane 3); both alleles are detected in heterozygous wt/RRN293 mice (lane 2). (B) Expression of Hv1 protein (32 kDa) is readily detected in spleen (lanes 1 and 2), BMC (lanes 3 and 4), and PBL (lanes 5 and 6) isolated from wt/wt mice (+/+, lanes 1, 3, and 5) but is absent in RRN293/RRN293 mouse tissue (−/−, lanes 2, 4, and 6).
Hv1 protein was not detectable in hippocampal microsomes, suggesting that the lack of detectable voltage-gated H+ current in mammalian neurons results from low Hv1 expression (17). Consistent with previous reports (18, 19) we measured GvH+ in acutely isolated non-neuronal hippocampal cells (presumably microglia) but not in Hv1−/− cells. The relative paucity of microglia could account for undetectably low levels of Hv1 protein in brain, and future studies should investigate Hv1 expression and function in microglia in situ. We next measured voltage-gated whole-cell H+ currents in leukocytes isolated from wt/wt or Hv1−/− mice as described (1). Purified resting (round and nonadherent) granulocytes exhibited GvH+ at varying levels, but Hv1−/− mice lacked detectable H+ current (Fig. 2 B and D). Consistently larger currents were measured in cells displaying the morphological characteristics (presence of pseudopodia and a uropod, spontaneous adherence and cytokinesis on glass substrate) of circulating neutrophils (Fig. 2A). The apparent threshold for voltage-dependent activation (Vthr) of H+ currents elicited by depolarizing voltage steps was approximately +40 mV under symmetrical recording conditions (Fig. 2C; pHi = pHo = 6.5). As expected for authentic GvH+, Vthr shifted negatively ≈40 mV when a 1-log unit outward [H+] gradient (pHi = 6.5, pHo = 7.5) was imposed (Fig. 2C) (5, 7). In no instance were we able to measure detectable voltage-activated H+ currents in cells isolated from Hv1−/− mice (Fig. 2 B and D), indicating that Hv1 expression is required for GvH+. In combination with previous results demonstrating that Hv1 is sufficient to reconstitute GvH+ (1, 2), these results suggest that Hv1 intrinsically forms the voltage-dependent proton channel that underlies GvH+ in various cell types. The existence of additional genes encoding voltage-gated proton channels therefore seems unlikely. This conclusion is further supported by the observation that native GvH+ and expressed Hv1 share unique biophysical properties (especially the invariant coupling of Vthr to the pH gradient) that are conserved in a wide variety of cell types (5, 7). Cell-specific factors are likely to modulate Hv1 channel properties, particularly in cells such as neutrophils, where GvH+ appears to be posttranslationally regulated by signaling cascades (20–22).
Fig. 2.
RRN293/RRN293 leukocytes lack detectable voltage-gated H+ currents. (A and B) Representative whole-cell currents (Lower) resulting from the indicated voltage steps (Upper) in neutrophils (Lympholyte M) purified from wt/wt (+/+, Left) or RRN293/RRN293 (−/−, Right) B5 mouse blood. (Inset) The pH of solutions (TMA6.5 pipette, TMA7.5 bath) is shown. (C) Representative ISTEP vs. voltage relations for the cells shown in A under symmetrical [H+] conditions and after bath superfusion to impose a 1 pH unit outward [H+] gradient (○, +/+ TMA6.5 bath; ●, +/+ TMA7.5 bath; □, −/− TMA6.5 bath; ■, −/−TMA7.5 bath). (D) Whole-cell current (ISTEP, +60 mV) in granulocytes (Robbins PMN) purified from wt/wt (+/+) or RRN293/RRN293 (−/−) mouse blood (Na6.5 pipette and Na7.5 bath solutions). Solid line indicates the mean current level, and box indicates SEM (+/+, 58.6 ± 10.5 pA; −/−, 2.7 ± 0.4 pA; n = 14 cells per genotype).
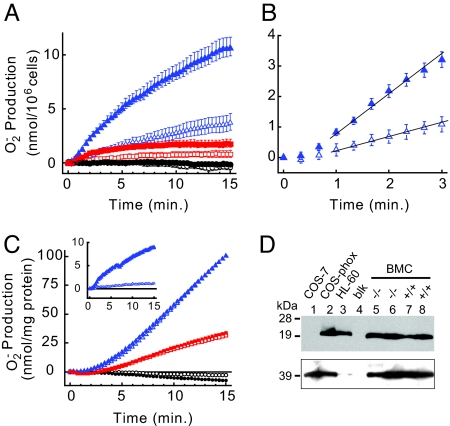
Two lines of evidence suggested that GvH+ might be necessary for sustained O2−∙ production during the respiratory burst: first, outward H+ current through Hv1 should balance the outward e− current produced by the active NADPH oxidase, thereby limiting membrane depolarization that would otherwise inhibit NADPH oxidase activity (11–14); second, Hv1 allows for extrusion of intracellular protons that are produced by NADPH oxidase, thus limiting intracellular acidosis and maintaining optimal O2−∙ production (23–25). To test the hypothesis that Hv1 is required for high-level superoxide production, we isolated BMC from wt and Hv1−/− mice and measured phorbol 12-myristate 13-acetate (PMA)-stimulated O2−∙ and hydrogen peroxide (H2O2) production spectrophotometrically (Fig. 3 and Fig. S2). PMA (200 nM) elicited a robust time-dependent increase in O2−∙ production that was absent in vehicle-treated control cells and largely blocked by dephenylene iodonium (DPI; 10 μM), an inhibitor of NADPH oxidases (Fig. 3A). After an initial delay, the rate of O2−∙ production (measured 1–3 min after PMA addition at 37 °C) was attenuated ≈65% in cells from Hv1−/− mice compared with wt/wt littermates (Fig. 3B), and a similar decrement was observed 15 min after PMA addition (Fig. 3A). DPI (10 μM) inhibited 79% and 73% of the PMA-stimulated O2−∙ production (at 15 min) in wt/wt and Hv1−/− BMC, respectively (Fig. 3A). Similar results were observed in peripheral blood leukocytes (PBL) purified from either Lympholyte M or discontinuous Percoll gradients, and BMC isolated from Hv1−/− mice with mixed C57BL/6J host and 129 ES cell genetic background were not significantly different from back-crossed Hv1−/− B5 mice. Finally, PMA-stimulated H2O2 production was also substantially decreased in Hv1−/− BMC compared with wt/wt controls (Fig. S2).
Fig. 3.
Superoxide anion (O2−∙) production is decreased in Hv1−/− BMC. O2−∙ secretion was measured by using the cytochrome c reduction assay in intact BMC isolated from wt/wt (+/+, filled symbols) or RRN293/RRN293 (−/−, open symbols) B5 mice in the absence (black circles) or presence of 200 nM PMA (blue triangles) or 200 nM PMA + 10 μM DPI (red squares). (A) Time course of O2−∙ production after vehicle or PMA addition at t = 0 min in intact BMC. O2−∙ production after 10-min incubation (37 °C) in the presence of 200 nM PMA was 9.93 ± 1.24 nmol/106 cells (+/+) and 3.60 ± 0.18 nmol/106 cells (−/−); mean ± SEM, n = 4 mice per genotype). (B) The rates of PMA-stimulated O2−∙ production in intact BMC between 1 and 3 min after PMA addition were: +/+, 1.39 ± 0.23 nmol/min per 106 cells;−/−, 0.47 ± 0.04 nmol/min per 106 cells; mean ± SEM, n = 4 (solid lines). Data and symbols are as shown in A. (C) Time course of O2−∙ production after vehicle or AA (100 μM) addition at t = 0 min in a representative pair of wt/wt and RRN293/RRN293 littermate BMC sonicates assayed in parallel. (Inset) Time course of O2−∙ production after vehicle or PMA addition at t = 0 min in intact BMC before sonication. Data are expressed as nmol O2−∙/min per 106 cells. Open and filled symbols are as in A. (D) Western blot showing p22phox expression in lysates prepared from nontransfected COS-7, COS-phox, or HL-60 cells (lanes 1–3) or BMC isolated from 2 pairs of RRN293/RRN293 (−/−, lanes 5 and 6) or wt/wt (+/+, lanes 7 and 8) mouse littermates.
In contrast to intact cells, O2−∙ production was not different in a broken-cell preparation made from either wt/wt or Hv1−/− BMC (Fig. 3C). Consistent with the idea that Hv1 is required for charge compensation and H+ efflux in intact cells but is irrelevant in membrane fragments that do not experience changes in voltage or pH gradients after NADPH oxidase activation. In addition, we observed similar levels of p22phox expression in wt/wt and Hv1−/− BMC microsomes (Fig. 3D). Together, these results suggest that attenuated O2−∙ production in Hv1−/− cells is unlikely to result from a decrease in the activity or expression of NADPH oxidase complex components per se. Although our data do not rule out the possibility that other gene products or ion transport pathways also contribute to charge compensation during the phagocyte respiratory burst, they suggest that Hv1 is likely to play a central role in electrical and H+ gradient homeostasis that is required for optimal NADPH oxidase activity under physiological conditions in mouse granulocytes (12, 25–32).
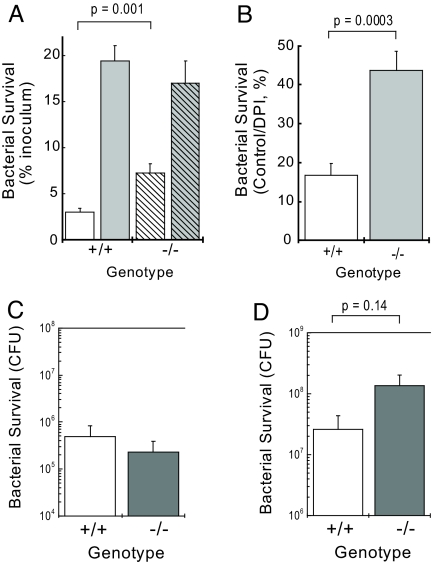
The role of the respiratory burst in innate immune function is illustrated by the observation that deletion or mutation in any of several protein components comprising the NADPH oxidase complex impairs bacterial clearance in mice and causes chronic granulomatous disease in humans (10). To determine whether the attenuated O2−∙ production that we observed in Hv1−/− cells would adversely affect bacterial clearance, we incubated wt or Hv1−/− BMC with live, serum-opsonized Staphylococcus aureus and assayed bacterial survival. BMC from both wt/wt and Hv1−/− mice cleared >90% of the added bacteria (Fig. 4A), suggesting that the viability and bacterial killing function of BMC isolated from both wt/wt and Hv1−/− mice is similar. NADPH oxidase-dependent bacterial killing was significantly attenuated in Hv1−/− compared with wt/wt mice (Fig. 4 A and B). Bacterial survival was similar in the presence of DPI, suggesting that NADPH oxidase-independent mechanisms of bacterial clearance were unchanged in Hv1−/− cells and that Hv1 deletion selectively affects NADPH oxidase-dependent killing. Furthermore, phagocytosis of heat-inactivated, fluorescently-labeled, serum-opsonized S. aureus or E. coli was not different in Hv1−/− and wt/wt cells, suggesting that the observed defect in bacterial clearance is caused by reduced O2−∙ production and not by a change in the abilty of Hv1−/− cells to phagocytose bacteria.
Fig. 4.
Hv1 deletion decreases bacterial clearance in vitro. (A) Mouse BMC (5 × 105) from wt/wt (solid columns, n = 8) or RRN293/RRN293 (hatched columns, n = 7) mice were incubated (30 min, 37 °C) with S. aureus in the absence (white columns) or presence of DPI (10 μM; gray columns) before addition of gentamicin and washing. Cell pellets were lysed in 1% saponin, and surviving bacteria were serially diluted and elaborated overnight on selective agar plates. (B) Data shown as the fraction of surviving CFU in the absence of DPI (control) divided by the CFU surviving the presence of DPI to isolate the DPI-sensitive component of bacterial clearance (wt/wt, white column; RRN293/RRN293, gray column). (C) Bacterial survival 24 h after i.p. inoculation of 1 × 108 CFU S. aureus in wt/wt (white column, n = 6) or RRN293/RRN293 (gray column, n = 8) mice. (D) Bacterial survival 6 h after i.p. inoculation of 5 × 109 CFU S. aureus in wt/wt (white column, n = 7) or RRN293/RRN293 (gray column, n = 7) B5 mice. Data represent mean values ± SEM; indicated P values are from Student's unpaired t test.
We next tested whether Hv1−/− mice are able to clear bacterial infections in vivo. Hv1−/− or wt/wt mice were inoculated i.p. with S. aureus, and surviving bacteria were elaborated on selective agar from peritoneal lavage fluid h 6–24 later. In mice inoculated with 1 × 108 S. aureus we observed a >2-log unit decrease in bacterial CFU after a 24-h incubation in vivo (Fig. 4C). Whereas mice that received 1 × 108 CFU of S. aureus did not visibly manifest sepsis, mice of either genotype that received 5 × 109 CFU were typically moribund within 6 h of inoculation. Although bacterial load in the peritoneal lavage from Hv1−/− mice suggested a trend toward decreased bacterial clearance, the data did not reach statistical significance. We also observed robust clearance of experimental pneumonia 24 h after intranasal inoculation of 1 × 108 CFU Pseudomonas aeurginosa or Burkholderia cepacia in both wt/wt and Hv1−/− mice. Although Hv1 deletion reduces Hv1 protein and GvH+ to undetectably low levels, decreases O2−∙ production by ≈75%, and significantly attenuates bacterial killing in vitro, we were unable to see a significant effect on bacterial clearance in vivo under our experimental conditions.
Discussion
In summary, we demonstrate here that Hv1 is necessary for measurable GvH+ in a variety of leukocytes, and previous studies showed that expression of Hv1 is sufficient to reconstitute the hallmark biophysical properties of GvH+ in heterologous systems (1, 2, 5–8). Superoxide production by intact (but not broken) cells is substantially reduced in the absence of Hv1. The simplest interpretation of these results is that Hv1 serves to provide a majority of the charge compensation needed to support NADPH oxidase-mediated e− export. Despite a significant reduction in superoxide production, wt/wt and Hv1−/− mice were similarly able to clear S. aureus infections in vivo, but differences in innate immune function may become apparent under different experimental conditions.
Similar to human patients with chronic granulomatous disease, mice bearing targeted deletions or mutations of genes encoding NADPH oxidase components manifest symptoms (e.g., dramatically decreased or undetectable O2−∙ production and decreased bacterial clearance in vitro and in vivo) (33–36). Interestingly, mice lacking either gp91phox (the product of the Nox2 gene) or inducible nitric oxide synthase (the Nos2 gene product) exhibited a similarly modest impairment in the innate immune response to S. aureus challenge. In vivo, Nox2−/−/Nos2−/− (double-knockout) mice required administration of oral antibiotics for survival even in the absence of bacterial challenge, suggesting that normal innate immune function relies on at least partially redundant systems for bacterial clearance (37). Thus, superoxide- and nitric oxide-dependent systems appear to function synergistically for bacterial clearance in vivo. Reconstitution of a small fraction of the total superoxide-generating capacity of the innate immune system in gp91phox (Nox2−/−) knockout mice was sufficient to significantly restore bacterial clearance, suggesting the presence of enormous excess capacity for NADPH oxidase-dependent superoxide production in normal granulocytes (38–40). Because O2−∙ generation was decreased ≈75% in Hv1−/− mice, the lack of a significant in vivo impairment in bacterial clearance in these mice is perhaps not surprising. Hv1−/− mice should be useful for investigating the physiological role of Hv1 in cells and tissues outside of the innate immune system in the future.
Materials and Methods
Hv1 Knockout Mice.
Mice lacking functional Hv1 protein were derived from the Bay Genomics genetrap of the Hvcn1 locus (dbGSS7585445; gi37500117). The exogenous genetrap vector sequence, containing an in-frame splice acceptor site followed by coding sequence for a β-galactosidase/neomycin (β-Geo) fusion protein, was inserted into a large (13.6 kb) intron following exon 2, which contains the Hv1 translation initiation codon. Chimeric male mice were derived from sequence-verified RRN293 genetrap ES cells and used to establish heterozygous wt/RRN293 founders; homozygous offspring were generated from heterozygous mating pairs (Mutant Mouse Regional Resource Center, Davis, CA). Hv1−/− mice backcrossed against C57/BL6J for 5 generations are designated B5. All animal husbandry and experimental procedures were approved by and performed in accordance with guidelines of the Children's Hospital Boston Institutional Animal Care and Use Committee.
Mouse Genotyping.
The genomic locus of the Bay Genomics genetrap vector (pGTOLxf) insertion was identified by PCR using a series of forward primers spaced at 1- to 2-kb intervals in the predicted intronic Hvcn1 sequence, along with a β-Geo-specific reverse primer (β-GeoR: 5′-GACAGTATCGGCCTCAGGAAGATCG-3′) to amplify a product containing the pGTOLxf insertion site. DNA sequencing of the PCR product (Mental Retardation/Developmental Disabilities Research Center Molecular Genetics Core Facility at Children's Hospital) indicated that the pGTOLxf vector was inserted after nucleotide 13899 (of 17054) in predicted intron 3 (variant 1, Fig. S1A). Genotypes were confirmed by triplex PCR performed on genomic DNA isolated from mouse tails using 2 gene-specific primers (Hvcn1F: 5′-GAGATCCATCTGCCTCCGTTATGAGTG-3′; Hvcn1R: 5′-CATGGTCTCAGTGATGTTAGGACGTC-3′) and β-GeoR. The genotypes of mice used for breeding were determined by quantitative genomic PCR (Transnetyx) using primers specific to Hvcn1 intron 3 and pGT0Lxf; results from the Transnetyx genotyping strategy were verified by triplex genomic PCR. Although quantitative RT-PCR confirmed that the RRN293 transgene was expressed at the expense of the wt Hvcn1 allele (Fig. S1C), we were unable to detect either β-galactosidase protein [using several different anti-β-galactosidase antibodies in Western blot analysis, immunocytochemistry (ICC) or immunohistochemistry (IHC)] or activity (using X-gal staining) in spleen or BMC from Hv1−/− mice.
Quantitative RT-PCR.
Total RNA (500 ng) extracted from mouse BMC (Rneasy; Qiagen) was reverse-transcribed by using an oligo(dT) primer (SuperScript 3; Invitrogen). Quantitative PCR (Real time PCR systems 7300; Applied Biosystems) was performed in a final volume of 25 μL containing 40 ng of cDNA, 300 μM primers (Hvcn1 Ex1 Fwd: 5′-TGCAAAGGAGTGCTGCAAACTA-3′; Hvcn1 Ex1 Rev: 5′-TCGAGTAGACGCTCCGCAAT-3′; Hvcn1 Ex2–3 Fwd: 5′-CTCTCACTGTTGGATCTTGAGACAA-3′, Hvcn1 Ex2–3 Rev: 5′-GGGAGCCACCTTGGTTCTG-3′; β-Geo Fwd: 5′-GGACGCGCGAATTGAATTA-3′, β-Geo Rev: 5′-CTGTTGACTGTAGCGGCTGATG-3′), and 12.5 μL of SybrGr PCR master mix (Applied Biosystems). Triplicate quantitative PCRs were performed by using BMC cDNA synthesized from wt/wt or RRN293/RRN293 mRNA isolated from 3 mice of each genotype. Normalized values for cycle threshold (ΔCtwt/wt and ΔCtRRN/RRN) were calculated by subtracting the observed cycle threshold (Ct) for each cDNA and primer pair from the Ct observed for L8 ribosomal RNA primers (2). Relative expression of β-Geo (Fig. S1, primers H and I) or Hvcn1. Relative expression of β-Geo mRNA (Fig. S1, primers H and I) in wt/wt BMC and Hvcn1 mRNA in RRN293/RRN293 BMC [Fig. S1, primers D and E (Hvcn1 Ex 1′, 1″) or primers F and G (Hvcn1 Ex 2–3)] was calculated by using Eqs. 1 and 2.
Eq. 1.
Fractional β-Geo expression in wt/wt = 2−ΔΔCtβ-Geo, where ΔΔCtβ-Geo = ΔCtwt/wt − ΔCtRRN/RRN (primers H and I) and ΔCtwt/wt = CtHvcn1Ex1 − CtL8RNA and ΔCtwt/wt = Ctβ-GeoRNA − CtL8RNA.
Eq. 2.
Fractional Hvcn1 expression in RRN293/RRN293 = 2−ΔΔCtHvcn1, where ΔΔCt = ΔCtRRN/RRN − ΔCtwt/wt (primers D, E and F, G).
Isolation of Mouse Leukocytes.
For measurement of O2−∙ and H2O2, phagocytosis, and in vitro bacterial killing assays, BMC were dispersed by trituration through a 22-G needle in ice-cold Dulbecco's Ca2+- and Mg2+-free PBS (D-PBS) or Ca2+-free modified Tyrode's Solution (TS+EGTA: 138 NaCl, 5 KCl, 1 MgCl2, 1 EGTA, 10 Hepes, pH 7.4 at 23 °C) and passed through a 40-μm mesh filter (Becton-Dickinson). Cells were centrifuged (250 × g, 10 min, 4 °C), resuspended to 5 × 106/mL in TS, and kept on ice until use. In some experiments, whole blood (collected in 2–10 mM EGTA and diluted in D-PBS) or BMC were layered onto 1.5 vol PMN isolation medium (Robbins Scientific) or 0.75 vol Lympholyte M (Cedarlane), or before centrifugal sedimentation. For Western blot analysis, the mixture of PBL contained in the fractions above the erythrocyte pellet were removed, diluted into 20 vol ice-cold D-PBS containing 1 mM EGTA, pH 7.4 (PBS/EGTA), and centrifuged for 10 min at 250 × g. Differentiated HL-60 and COS-phox cells (gift of M. Dinauer, Indiana University School of Medicine) were cultured as described (1, 41).
Immunoblotting.
Membranes were prepared from cell pellets or tissues by homogenization (1–2 min; Polytron) in ice-cold homogenization solution, 150 mM NaCl, 10 mM Tris·HCl (pH 7.2), 1 mM EDTA containing 0.3 M sucrose and protease inhibitor mixture (Complete mini; Roche; mammalian cell protease inhibitor mixture; Sigma) and sonicated (3 × 5 s, 40% maximum power, Heat Systems–Ultrasonics W225) before centrifugation (10 min, 4,000 × g, 4 °C) to clear nuclei and particulate material. The supernatant was then centrifuged (30 min, 25,000 × g, 4 °C; Sorvall Ultrafuge) to pellet cell membranes. Membranes were solubilized by sonication (3 × 5 s, 25% maximum power) in 0.2 mL of ice-cold lysis buffer (TS+EGTA containing 1% Triton X-100 and protease inhibitors). Protein concentration was determined (Bradford, BioRad protein assay) and 30 μg per lane was loaded onto 4–12% Bis-Tris Novex Nu-PAGE gels (Invitrogen) for electrophoresis in Mops running buffer before transfer to a PVDF membrane. Western blots were incubated with rocking in PBST (D-PBS containing 0.5% Tween-20) blocking solution containing 5% nonfat dry milk (60 min, 24 °C). Blots were first incubated with an anti-actin (Chemicon) primary antibody (1:1,000, 2 h, 24 °C) followed by secondary antibody (goat anti-mouse HRP-conjugated IgG; Zymed; 1:100,000, 45 min, 24 °C). Immunoreactivity was detected by using ECL Advance (Amersham). Blots were stripped (20 min, 50 °C) in 67.5 mM Tris·HCl (pH 6.8), 100 mM β-mercaptoethanol, and 2% SDS and washed with PBST before sequential incubation with anti-Hv1 primary (4234, 2 h) (1) and secondary (goat anti-rabbit HRP-conjugated IgG; Zymed; 1:100,000, 45 min) antibodies.
Electrophysiology.
Mouse PBL or PMN were resuspended in either TMA6.5 [100 mM Bis·Tris (pH 6.5), 80 mM TMA-MeSO3, 2 mM MgCl2, 4 mM HCl, 1 mM EGTA] or Na6.5 [100 mM Bis·Tris (pH 6.5), 80 mM NaCl, 10 mM MgCl2, 1 mM EGTA]. Cells were visually selected and subjected to whole-cell voltage clamp under symmetrical conditions (pipette solution same as bath solution). We also recorded currents from either crude BMC or Percoll gradient-purified BMC neutrophils; in no case did we measure voltage-dependent H+ current in cells isolated from RRN293/RRN293 mice.
O2−∙ and H2O2 Production Assay (Cuvette Format).
BMC were resuspended in modified Tyrode's solution [TS: 138 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 10 Hepes (pH 7.4) at 23 °C] on ice. Phase-dark cells were counted (after 5 min at 24 °C) with a glass hemacytometer and diluted to 5 × 106/mL in ice-cold TS. Cells (0.1× final volume) were added to individual cuvettes containing TS plus 100 μM cytochrome c (or 5 μM Amplex Ultra Red and 0.5 unit/mL horseradish peroxidase for H2O2 production assay) with or without DPI (10 μM) or ZnCl2 (1 mM). O2−∙ and H2O2 production were measured at 550 nm, 37 °C, in 1.0 mL of cuvettes by using a temperature-controlled spectrophotometer fitted with an automated 6-cuvette sample changer (DU800; Beckman–Coulter). Medium containing assay reagent and indicated drugs was preincubated (37 °C) for 10 min; cells were added 5 min before addition of PMA (200 nM) or arachidonic acid (AA, 100 μM) and absorbance was measured in a final volume of 1.0 mL (cuvette assay). O2−∙ and H2O2 production in wt/wt and Hv1−/− BMC were measured in parallel assays conducted simultaneously using the automated sample changer.
H2O2 Production Assay (96-Well Format).
BMC or HL-60 cells were centrifuged (250 × g, 10 min) and resuspended in TS on ice. Cells were counted and diluted to 5 × 106/mL in ice-cold TS. Cells (20 μL) were added to individual wells containing ice-cold TS plus indicated assay reagent with or without PMA (200 nM), DPI (10 μM), or ZnCl2 (1 mM). Assay plates containing added assay reagents and indicated drugs (minus PMA) were preincubated in a warm-air incubator (37 °C) for 10 min Cells were added and incubated (37 °C) for an additional 5 min. Plates were removed and t = 0 assigned as immediately after PMA addition. H2O2 secretion was measured at room temperature by using a plate-reading spectrophotometer/fluorimeter (550 ± 8-nm filter, Victor3; Wallac) in a final volume of 0.2 mL per well. All assay wells contained 5 μM Amplex Ultra Red (Invitrogen) and 0.5 unit/mL horseradish peroxidase (Zymed). In control experiments where PMA-stimulated O2− (cytochrome c reduction) and H2O2 (Amplex Ultra red) were measured side by side in a heated cuvette-reading spectrophotometer (1.0-mL assay volume), we observed only a small delay in H2O2 generation compared with O2− production, suggesting that the slow time course observed in some experiments (Fig. S2) was attributable to slow heating and reheating of the reagents and plates in the 37 °C warm air incubator between time points that were measured using the nonheated spectrophotometric plate reader.
In Vitro Bacterial Killing.
Aliquots of bacterial stocks (stored at −80 °C in 20% glycerol) on dry ice were scraped with a sterile needle and bacteria were spread on selective agar plates (S. aureus: mannitol, Molecular Toxicology; P. aeurginosa: cetrimide, PML Microbiologicals; B. cepacia: BCSA, PML Microbiologicals) and grown overnight at 37 °C. LB medium was subsequently inoculated and liquid cultures were grown with shaking at 37 °C to an OD600 ≈ 0.5. The concentration of bacterial CFU was calculated (OD600 = 0.5, CFU = 5 × 105/μL) and verified for each bacterial preparation by serial dilution and plating onto selective agar plates. Bacteria were centrifuged (10 min, 1,000 × g, 4 °C) and resuspended and opsonized in mouse serum containing 0.05% Tween-20 (30 min with rotation, 24 °C). Opsonized bacteria were then diluted into TS containing 0.05% Tween-20 to 2 × 104 CFU/μL. BMC were resuspended in ice-cold TS (106 cells per 100 μL) and added to round-bottom tubes containing TS solution with or without DPI (10 μM). The assay was initiated by addition of bacteria (50 μL) and allowed to proceed for 30 min (37 °C, orbital shaking) before addition of gentamicin (50 μg/mL; Sigma) and centrifugation (10 min, 2,800 × g, 24 °C). Cell pellets were washed in TS + EGTA and lysed by resuspension in saponin (1% in water) and 1 freeze-thaw cycle. Lysates were serially diluted and plated onto selective LB agar for overnight culture (37 °C) for manual determination of bacterial CFU. Although DPI reproducibly inhibited killing of bacteria under these experimental conditions, a large fraction of the inoculum survived even in the presence of DPI (Fig. 4A), suggesting that mouse BMC may use NADPH oxidase-independent mechanisms for bacterial killing that are not prominent in purified, circulating human neutrophils and/or that incomplete cell lysis may have led to an underestimate of the DPI-dependent fraction of bacterial killing (42). Our results should therefore be interpreted as a lower limit on the effect of Hv1 deletion on bacterial killing by the NADPH oxidase system.
In Vivo Bacterial Clearance.
Bacteria were grown to OD600nm ≈0.5, centrifuged (10 min, 1,000 × g, 4 °C), and resuspended in sterile 0.9% NaCl or D-PBS to a final concentration of 2 × 108 CFU/mL (i.p.) or 2 × 1010 CFU/ml (intranasal) for inoculation. Mice were allowed to recover for 18–24 h after i.p. or intranasal inoculation before peritoneal (3 × 5 mL of D-PBS, 4 °C) or lung lavage (3 × 0.8 mL of D-PBS, 4 °C), respectively. Lavage fluid was centrifuged (10, 1,000 × g, 4 °C) and the pellet was resuspended in D-PBS containing 0.05% Tween-20 (PBST), then serially diluted in PBST before plating on selective agar plates for culture at 37 °C for 24–48 h.
Phagocytosis Assay.
BMC (1 × 105 cells per well) were added to a 96-well plate and incubated in 0.2 mL of TS (60 min, 37 °C) to promote adherence. The supernatant was aspirated and replaced with prewarmed TS with or without DPI (10 μM) and the assay was initiated by addition of heat-inactivated Alexa Fluor 488-labeled E. coli K-12 Bioparticles (Invitrogen) resuspended in TS per the manufacturer's directions. After phagocytosis (120 min, 37° C), trypan blue solution (100 μL) was added to each well to quench fluorescence of nonphagocytosed extracellular bacterial particles and quickly (1 min, 24 °C incubation) aspirated. Fluorescence of phagocytosed bacterial particles was measured on a plate-treading fluorimeter (Wallac Victor3; excitation 488 nm, lowpass filter 520 nm).
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant T32 HLO7572 (to I.S.R. and the Department of Pediatrics, Children's Hospital). The Mental Retardation/Developmental Disabilities Research Center Molecular Genetics Core Facility at Children's Hospital is supported by National Institutes of Health Grant P30-HD18655.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902761106/DCSupplemental.
References
- 1.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 3.Suenaga T, et al. Cloning of B cell-specific membrane tetraspanning molecule BTS possessing B cell proliferation-inhibitory function. Eur J Immunol. 2007;37:3197–3207. doi: 10.1002/eji.200737052. [DOI] [PubMed] [Google Scholar]
- 4.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 5.DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 6.Koch HP, et al. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci USA. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musset B, et al. Detailed comparison of expressed and native voltage-gated proton channel currents. J Physiol (London) 2008;586:2477–2486. doi: 10.1113/jphysiol.2007.149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeCoursey TE, Cherny VV. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys J. 1993;65:1590–1598. doi: 10.1016/S0006-3495(93)81198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 11.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 12.Henderson LM, Chappell JB, Jones OT. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeCoursey TE. Voltage-gated proton channels. Cell Mol Life Sci. 2008;65:2554–2573. doi: 10.1007/s00018-008-8056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rada BK, Geiszt M, Hably C, Ligeti E. Consequences of the electrogenic function of the phagocytic NADPH oxidase. Philos Trans R Soc London Ser B. 2005;360:2293–2300. doi: 10.1098/rstb.2005.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeCoursey TE. Hydrogen ion currents in rat alveolar epithelial cells. Biophys J. 1991;60:1243–1253. doi: 10.1016/S0006-3495(91)82158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy R, Cherny VV, Morgan D, DeCoursey TE. Voltage-gated proton channels help regulate pHi in rat alveolar epithelium. Am J Physiol. 2005;288:L398–L408. doi: 10.1152/ajplung.00299.2004. [DOI] [PubMed] [Google Scholar]
- 17.Cheng YM, Kelly T, Church J. Potential contribution of a voltage-activated proton conductance to acid extrusion from rat hippocampal neurons. Neuroscience. 2008;151:1084–1098. doi: 10.1016/j.neuroscience.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Eder C, DeCoursey TE. Voltage-gated proton channels in microglia. Prog Neurobiol. 2001;64:277–305. doi: 10.1016/s0301-0082(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 19.De Simoni A, Allen NJ, Attwell D. Charge compensation for NADPH oxidase activity in microglia in rat brain slices does not involve a proton current. Eur J Neurosci. 2008;28:1146–1156. doi: 10.1111/j.1460-9568.2008.06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan D, DeCoursey TE. Diversity of voltage gated proton channels. Front Biosci. 2003;8:1266–1279. doi: 10.2741/1191. [DOI] [PubMed] [Google Scholar]
- 21.Musset B, Cherny VV, Morgan D, DeCoursey TE. The intimate and mysterious relationship between proton channels and NADPH oxidase. FEBS Lett. 2009;583:7–12. doi: 10.1016/j.febslet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan D, et al. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2 α activity. J Physiol (London) 2007;579:327–344. doi: 10.1113/jphysiol.2006.124248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeCoursey TE, Cherny VV, Zhou W, Thomas LL. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci USA. 2000;97:6885–6889. doi: 10.1073/pnas.100047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson LM, Chappell JB, Jones OT. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J. 1988;255:285–290. [PMC free article] [PubMed] [Google Scholar]
- 25.Rada BK, Geiszt M, Kaldi K, Timar C, Ligeti E. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood. 2004;104:2947–2953. doi: 10.1182/blood-2004-03-1005. [DOI] [PubMed] [Google Scholar]
- 26.Essin K, et al. BK channels in innate immune functions of neutrophils and macrophages. Blood. 2009;113:1326–1331. doi: 10.1182/blood-2008-07-166660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Femling JK, et al. The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels. J Gen Physiol. 2006;127:659–672. doi: 10.1085/jgp.200609504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeCoursey TE, Morgan D, Cherny VV. The gp91phox component of NADPH oxidase is not a voltage-gated proton channel. J Gen Physiol. 2002;120:773–779. doi: 10.1085/jgp.20028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petheo GL, Demaurex N. Voltage- and NADPH dependence of electron currents generated by the phagocytic NADPH oxidase. Biochem J. 2005;388:485–491. doi: 10.1042/BJ20041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maturana A, Krause KH, Demaurex N. NOX family NADPH oxidases: Do they have built-in proton channels? J Gen Physiol. 2002;120:781–786. doi: 10.1085/jgp.20028713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banfi B, et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 32.Ahluwalia J, et al. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature. 2004;427:853–858. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knockoutmodel of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollock JD, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 35.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Dinauer MC. Disorders of neutrophil function: An overview. Methods Mol Biol. 2007;412:489–504. doi: 10.1007/978-1-59745-467-4_30. [DOI] [PubMed] [Google Scholar]
- 37.Shiloh MU, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 38.Becker S, et al. Correction of respiratory burst activity in X-linked chronic granulomatous cells to therapeutically relevant levels after gene transfer into bone marrow CD34+ cells. Hum Gene Ther. 1998;9:1561–1570. doi: 10.1089/hum.1998.9.11-1561. [DOI] [PubMed] [Google Scholar]
- 39.Dinauer MC, Li LL, Bjorgvinsdottir H, Ding C, Pech N. Long-term correction of phagocyte NADPH oxidase activity by retroviral-mediated gene transfer in murine X-linked chronic granulomatous disease. Blood. 1999;94:914–922. [PubMed] [Google Scholar]
- 40.Barese CN, Goebel WS, Dinauer MC. Gene therapy for chronic granulomatous disease. Exp Opin Biol Ther. 2004;4:1423–1434. doi: 10.1517/14712598.4.9.1423. [DOI] [PubMed] [Google Scholar]
- 41.Price MO, et al. Creation of a genetic system for analysis of the phagocyte respiratory burst: High-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood. 2002;99:2653–2661. doi: 10.1182/blood.v99.8.2653. [DOI] [PubMed] [Google Scholar]
- 42.Decleva E, Menegazzi R, Busetto S, Patriarca P, Dri P. Common methodology is inadequate for studies on the microbicidal activity of neutrophils. J Leukocyte Biol. 2006;79:87–94. doi: 10.1189/jlb.0605338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.