Abstract
Changes in the glycan structures of some glycoproteins have been observed in autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis. A deficiency of α-mannosidase II, which is associated with branching in N-glycans, has been found to induce SLE-like glomerular nephritis in a mouse model. These findings suggest that the alteration of the glycosylation has some link with the development of SLE. An analysis of glycan alteration in the disordered tissues in SLE may lead to the development of improved diagnostic methods and may help to clarify the carbohydrate-related pathogenic mechanism of inflammation in SLE. In this study, a comprehensive and differential analysis of N-glycans in kidneys from SLE-model mice and control mice was performed by using the quantitative glycan profiling method that we have developed previously. In this method, a mixture of deuterium-labelled N-glycans from the kidneys of SLE-model mice and non-labelled N-glycans from kidneys of control mice was analysed by liquid chromatography/mass spectrometry. It was revealed that the low-molecular-mass glycans with simple structures, including agalactobiantennary and paucimannose-type oligosaccharides, markedly increased in the SLE-model mouse. On the other hand, fucosylated and galactosylated complex type glycans with high branching were decreased in the SLE-model mouse. These results suggest that the changes occurring in the N-glycan synthesis pathway may cause the aberrant glycosylations on not only specific glycoproteins but also on most of the glycoproteins in the SLE-model mouse. The changes in glycosylation might be involved in autoimmune pathogenesis in the model mouse kidney.
Keywords: isotope-tagging method, liquid chromatography/multiple-stage mass spectrometry, systemic lupus erythematosus
Introduction
Glycosylation is one of the most common post-translational modifications1,2 and contributes to many biological processes, including protein folding, secretion, embryonic development and cell–cell interactions.3 Alteration of glycosylation is associated with several diseases, including inflammatory responses and malignancies;4–6 for instance, significant increases in fucosylation and branching are found in ovarian cancer and lung cancer.7 Additionally, the carbohydrate structure changes from type I glycans (Galβ1-3GlaNAc) to type II glycans (Galβ1-4GalNAc) in carcinoembryonic antigen in colon cancer.8 Furthermore, an increase in biantennary oligosaccharides lacking galactose (Gal) was found on immunoglobulin G (IgG) in systemic lupus erythematosus (SLE) and rheumatoid arthritis,9–11 and agalactoglycans are used for the early diagnosis of rheumatoid arthritis.12
Systemic lupus erythematosus is an autoimmune disease characterized as chronic and as a systemic disease, with symptoms such as kidney failure, arthritis and erythema. In addition to the known changes in glycosylation on IgG, there have been several reports on the association between glycosylation and inflammation in SLE and rheumatoid arthritis.13–15 A deficiency of α-mannosidase II (αM-II), which is associated with branching in N-glycans, has been found to induce human SLE-like glomerular nephritis in a mouse model.16 Green et al. reported that branching structures of N-glycan in mammals are involved in protection against immune responses in autoimmune disease pathogenesis.17 Although there is no direct evidence that alteration of glycosylation is the upstream event in the pathogenesis of SLE, these findings suggest that changes in the glycan structure may be involved in the inflammatory-related autoimmune disorder. Glycosylation analysis may lead to the development of improved diagnostic methods and may help to clarify the carbohydrate-related pathogenic mechanism of inflammation in SLE.
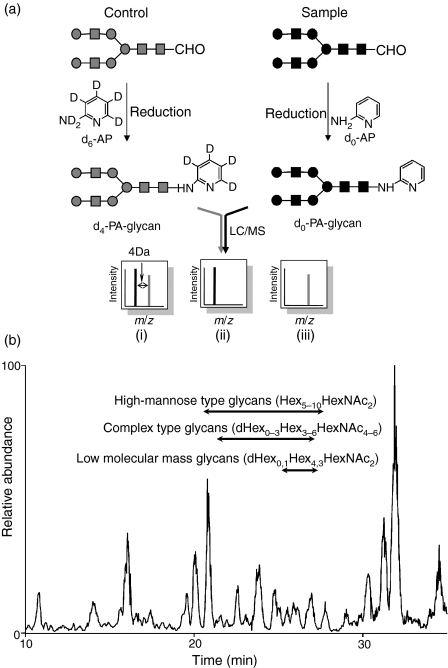
Mass spectrometry (MS) and liquid chromatography/mass spectrometry (LC/MS) are the most prevalent strategies for identifying disease-related glycans in glycomics.18–20 Aberrant glycosylations in some disease samples have been found by comparing mass spectra or chromatograms between normal and disease samples; however, because of the tremendous heterogeneities of the sugar moiety in glycoprotein as well as the low reproducibility of LC/MS, accurate quantitative analysis is difficult using MS and LC/MS alone. To overcome these problems, we previously developed the stable isotope-tagging method for the quantitative profiling of glycans using 2-aminopyridine (AP).21 After the glycans are released from sample and the reference glycoproteins are derivatized to pyridyl amino (d0-PA) glycans and to tetra-deuterium-labelled pyridyl amino (d4-PA) glycans, respectively, a mixture of both d0-PA and d4-PA glycans was subjected to LC/MS, and the levels of individual glycans were calculated from the intensity ratios of d0-glycan and d4-glycan molecular ions (Fig. 1). Recently, alternative isotope-tagging methods using deuterium-labelled compounds, such as 2-aminobenzoic acid its derivatives, and permethylation, have been proposed by other groups.22–24 All of these studies prove the utility of isotope-tagging methods for the quantitative analysis of glycosylation.
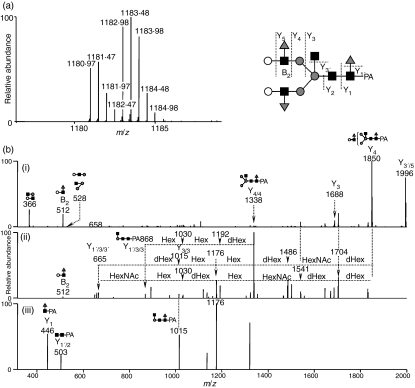
Figure 1.
(a) Quantitative glycan profiling using the stable isotope-tagging method and liquid chromatography/mass spectrometry (LC/MS). (i) sample = control, (ii) sample > control, (iii) sample < control. (b) Total ion chromatogram obtained by a single scan (m/z 700–2000) of the d0-glycan and d4-glycan mixture.
In the present study, we used the isotope-tagging method to analyse changes in N-glycosylation in the disordered kidney in an SLE mouse model. We used an MRL/MpJ-lpr/lpr (MRL-lpr) mouse which lacks the Fas antigen gene.25–27 The MRL-lpr mouse is known to naturally develop SLE-like glomerular nephritis and is widely used in SLE studies. MRL/MpJ-+/+ (MRL-+/+) mice were used as controls.
Materials and methods
Materials
The kidneys of the SLE-model mice (MRL-lpr) and control mice (MRL-+/+) (n = 3) were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Thermolysin (EC 3.4.24.27), originating from Bacillus thermoproteolyticus Rokko, was purchased from Daiwa Kasei (Shiga, Japan). Glycopeptidase A (PNGase A) was obtained from Seikagaku Kogyo Corporation (Tokyo, Japan). Non-deuterium-labelled 2-aminopyridine (d0-AP) and deuterium-labelled 2-aminopyridine (d6-AP) were purchased from Takara Bio (Otsu, Japan) and Cambridge Isotope Laboratories (Andover, MA), respectively.
Sample preparation
Mouse kidneys were filtered using a cell strainer (70 μm; BD Biosciences, San Jose, CA) and contaminating blood cells in the kidney cells were burst in 140 mm NH4Cl–Tris buffer (pH 7·2). The surviving kidney cells were washed three times with phosphate-buffered saline containing a mixture of protease inhibitors (Wako, Tokyo, Japan) and dissolved in guanidine–HCl buffer (8 m guanidine–HCl, 0·5 m Tris–HCl, pH 8·6) containing a mixture of protease inhibitors by vortexing at 4°. The protein concentration was measured using a 2-D Quant Kit (GE Healthcare Bio-Sciences, Uppsala, Sweden). The protein solution (200 μg proteins) was incubated with 40 mm dithiothreitol at 65° for 30 min. Freshly prepared sodium iodoacetate (final concentration, 96 mm) was added to the sample solution, and the mixture was incubated at room temperature for 40 min in the dark. The reaction was stopped by adding cystine (6 mg/ml in 2 m HCl) in an amount equal to the amount of dithiothreitol. The solution containing carboxymethylated proteins was diluted in four times its volume of H2O, and the mixture was incubated with 0·1 μg of thermolysin at 65° for 1 hr. After terminating the reaction by boiling, the reaction mixture was diluted in four times its volume of 0·2 m acetate buffer. The N-linked glycans were released by treatment with PNGase A (1 mU) at 37° for 16 hr and were desalted using an EnviCarb C cartridge (Supelco, Bellefonte, PA).
Labelling of N-glycans with d0-AP and d6-AP
Glycans released from the SLE-model mouse cells were incubated in acetic acid (20 μl) with 12·5 m d0-AP at 90° for 1 hr. Next, 3·3 m borane–dimethylamine complex reducing reagent in acetic acid (20 μl) was added to the solution and the mixture was incubated at 80° for 1 hr. Excess reagent was removed by evaporation, and d0-PA glycans were desalted using an EnviCarb C cartridge, concentrated in a SpeedVac and reconstituted in 20 μl of 5 mm ammonium acetate (pH 9·6). Glycans released from the control mouse were labelled with d6-AP in a similar manner. The resulting d4-PA glycans were combined with d0-PA glycans, which were prepared from an equal amount of proteins.
On-line liquid chromatography/mass spectrometry
The sample solution (4 μl) was injected into the LC/MS system through a 5-μl capillary loop. The d0-PA and d4-PA glycans were separated in a graphitized carbon column (Hypercarb, 150 × 0·2 mm, 5 μm; Thermo Fisher Scientific, Waltham, MA) at a flow rate of 2 μl/min in a Magic 2002 LC system (Michrom Bioresources, Auburn, CA). The mobile phases were 5 mm ammonium acetate containing 2% acetonitrile (pH 9·6, A buffer) and 5 mm ammonium acetate containing 90% acetonitrile (pH 9·6, B buffer). The PA-glycans were eluted with a linear gradient of 5–45% of B buffer for 90 min.
Mass spectrometric analysis of PA glycans was performed using a Fourier transform ion cyclotron resonance/ion trap mass spectrometer (FT-ICR-MS, LTQ-FT; Thermo Fisher Scientific) equipped with a nanoelectrospray ion source (AMR, Tokyo, Japan). For MS, the electrospray voltage was 2·0 kV in the positive ion mode, the capillary temperature was 200°, the collision energy was 25% for MSn experiment, and the maximum injection times for FT-ICR-MS and MSn were 1250 and 50 milliseconds, respectively. The resolution of FT-ICR-MS was 50 000, the scan time (m/z 700–2000) was approximately 0·2 seconds, dynamic exclusion was 18 seconds, and the isolation width was 3·0 U (range of precursor ions ± 1·5).
Results
Quantitative profiling of kidney oligosaccharides in the SLE-model mouse
The recovery of oligosaccharides from whole tissues and cells is generally low because of the insolubility of the membrane fraction and possible degradation of the glycans. To improve the recovery of N-glycans from kidney cells, whole cells were dissolved in guanidine hydrochloride solution, and all proteins, including membrane proteins, were digested into peptides and glycopeptides with thermolysin. The N-glycans were then released from the glycopeptides with PNGase A, which is capable of liberating N-linked oligosaccharides even at the N- and/or C-terminals of peptides. The N-linked oligosaccharides from the SLE-model mice and control mice were labelled with d0-AP and d6-AP, respectively. The mixture of labelled glycans derived from an equal amount of proteins was subjected to quantitative glycan profiling using LC/MSn.
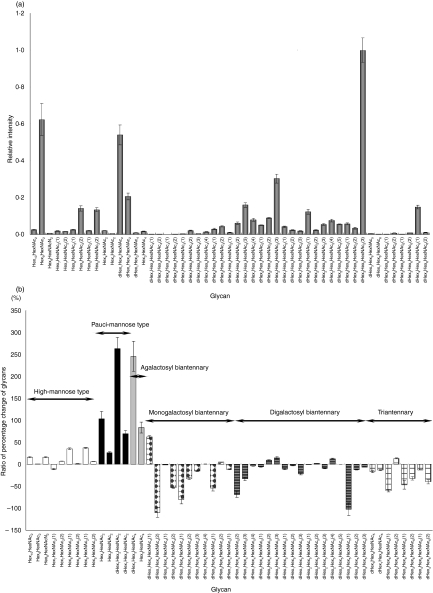
Figure 1(b) shows the total ion chromatogram obtained by a single mass scan (m/z 700–2000) of the glycan mixture in the positive ion mode. Although the MS data contain many MS spectra derived from contaminating low-molecular-weight peptides, the MS/MS spectra of oligosaccharides could be sorted based on the existence of carbohydrate-distinctive ions, such as HexHexNAc+ (m/z 366) and Hex(dHex)HexNAc+ (m/z 512). The monosaccharide compositions of the precursor ions were calculated from accurate m/z values acquired by FT-ICR-MS. Oligosaccharides found at 25–27 min were assigned to low-molecular-mass glycans consisting of dHex0,1Hex4,3 HexNAc2 (dHex, deoxyhexose; Hex, hexose; HexNAc, N-acetylhexosamine). High-mannose-type glycans, which consist of Hex5–10HexNAc2, were located at 20–28 min; complex-type glycans (dHex0–3Hex3–6HexNAc4–6) were found at 21–27 min. Figure 2(a) shows the relative intensities of the molecular ions of N-glycans in the SLE-model mouse, which may correspond roughly to the levels of individual N-glycans. More than half of all glycans were complex-type oligosaccharides, and the most prominent glycan was dHex3Hex5HexNAc5. Man-9 (Hex9HexNAc2) was the second most common oligosaccharide. Nearly one-quarter of the glycans were low-molecular-mass glycans, and dHex1Hex2HexNAc2 was the third most abundant glycan in the SLE-model mouse. The rate of percentage change in individual glycans between the SLE-model mice and control mice was calculated from the intensity ratio of d0-glycan and d4-glycan molecular ions (Fig. 2). The significant changes found in many glycans are described below.
Figure 2.
(a) Relative intensities of the molecular ions of d0-pyridyl amino (PA) glycans from the systemic lupus erythematosus (SLE) model mouse. The intensity of the most intense ion ([M + 2H]2+of d4-PA dHex3Hex5HexNAc3(3), m/z 1180·97) was taken as 1·0. (b) Rate of percentage change of d0/d4-glycans. Each value is the average of three biological repeats. Error bars correspond to the standard deviation. The numbers in parentheses show the isomers.
Increased oligosaccharides in the SLE-model mouse
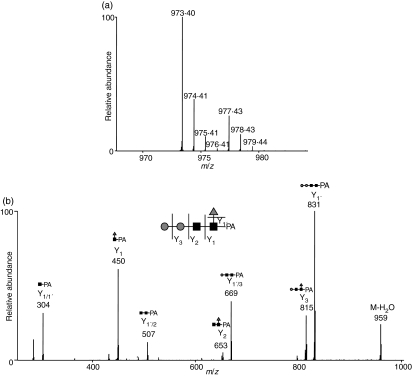
Figure 3(a,b) show the mass and MS/MS spectra of the most increased glycan, which showed a notable increase in the SLE-model mouse. Based on m/z values of molecular ions and differences of 1·00 U in m/z values among monoisotopic ions, the intense ion (m/z 973·40) and its neighbour ion (m/z 977·43) were assigned to [M+H]+ of d0-PA dHex1Hex2HexNAc2, and d4-PA dHex1Hex2HexNAc2, respectively (Fig. 3a). The intensity ratio of these ions suggested that the level of dHex1Hex2HexNAc2 increased 3·6-fold in the SLE-model mouse. The structure of this oligosaccharide was estimated to be a core-fucosylated trimannosyl core lacking a Man residue from the successive cleavages of Man (Y3: m/z 815), Man (Y2: m/z 653), GlcNAc (Y1: m/z 450) and Fuc (Y1/1′: m/z 304) (inset in Fig. 3b). Such a defective N-glycan is known as a paucimannose-type glycan, and is rarely found in vertebrates. All paucimannose-type glycans, such as dHex1Hex3HexNAc2 (a core-fucosylated trimannosyl core) and Hex3HexNAc2 (a non-fucosylated trimannosyl core) were increased in the SLE-model mouse. Furthermore, a two-fold increase was found in Hex4HexNAc2 (Man-4).
Figure 3.
Mass (a) and mass spectrometry (MS)/MS (b) spectra of the most increased glycan (dHex1Hex2HexNAc2). Precursor ion, m/z 973·4; grey circle, mannose; grey triangle, fucose; black square, N-acetylglucosamine.
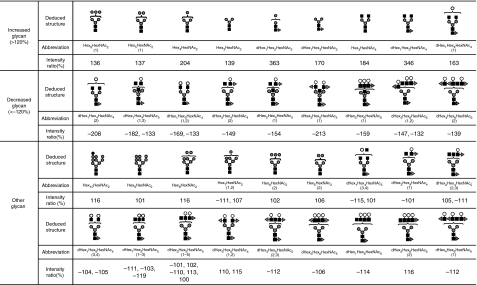
Figure 4 shows the molecular ratios of individual N-glycans between the SLE-model mice and control mice. A remarkable increase (3·5-fold) was also found in dHex1Hex3HexNAc4, which is assigned to a core-fucosylated biantennary oligosaccharide lacking two non-reducing terminal Gal residues; its non-fucosylated form (Hex3HexNAc4) was also increased 1·8-fold in the SLE-model mouse. In other complex-type glycans, dHex1Hex4HexNAc4 (1), which is assigned to a biantennary oligosaccharide lacking one molecule of Gal, increased 1·6-fold. Interestingly, a significant decrease was found in dHex1Hex4HexNAc4 (2), a positional isomer of dHex1Hex4HexNAc4 (1); this might have been caused by galactosylation on either GlcNAc-Manα1–3 or GlcNAc-Manα1–6. In contrast, no change was found between fucosylated and non-fucosylated oligosaccharides, nor between bisected and non-bisected oligosaccharides.
Figure 4.
Summary of quantitative analysis of the systemic lupus erythematosus (SLE) model mouse against control mice. Values of relative ratios are the averages of three biological repeats. Grey circle, mannose; white circle, galactose; grey triangle, fucose; black square, N-acetylglucosamine.
A significant increase was found in some high-mannose-type oligosaccharides, such as Hex5HexNAc2 (Man-5; + 137%) and Hex6HexNAc2 (1) (Man-6; + 136%), while Hex7HexNAc2 (1,2) (Man-7) and a positional isomer of Hex6HexNAc2 (1) [Hex6HexNAc2 (2)] remained unchanged in the SLE-model mouse. A slight increase was found in Hex8HexNAc2 (Man-8; + 116%) and Hex10HexNAc2 (possibly assigned to Man-9 plus Glc; + 116%).
Decreased oligosaccharides in the SLE-model mouse
The mass spectrum of the most decreased glycan is shown in Fig. 5(a). Based on differences of 0·5 U in m/z values among monoisotopic ions, molecular ions at m/z 1180·97 and 1182·98 are estimated to be [M + 2H]2+ of d0-PA and d4-PA dHex3Hex5HexNAc5 (1), respectively. The intensity ratio of d0 : d4 glycans suggests that this glycan in the SLE-model mouse was decreased to 47% of the amount found in the control mouse. Figure 5 shows the MS2–4 spectra of d0-PA dHex3Hex5HexNAc5 (1) (precursor ion, m/z 1180·97). The fragment ion at m/z 512 in MS/MS (i) and MS/MS/MS (ii) spectra, which corresponds to dHex1Hex1HexNAc1+, suggests the attachment of two Lewis motifs on the side chains of the glycan. The presence of dHex1HexNAc1PA+ (m/z 446) and dHex1Hex1HexNAc3PA+ (m/z 1015) reveals the linkages of a core fucose and a bisecting GlcNAc. Based on these fragments, this decreased glycan is estimated to be a Lewis-motif-modified, core-fucosylated and bisected biantennary oligosaccharide (inset in Fig. 5).
Figure 5.
(a) Mass spectrum of the most decreased glycan [dHex3Hex5HexNAc5 (1)] (b-i) Mass spectrometry (MS)/MS spectrum of m/z 1181·0; (b-ii) MS/MS/MS spectrum of m/z 1849·7; (b-iii) MS/MS/MS/MS spectrum of m/z 1338·3. Grey circle, mannose; white circle, galactose; grey triangle, fucose; black square, N-acetylglucosamine; dHex, deoxyhexose (fucose); Hex, hexose (mannose and galactose); HN, N-acetylhexosamine (N-acetylglucosamine).
As shown in Figs 2(b) and 4, oligosaccharides lacking one molecule of Gal with and without bisecting GlcNAc [dHex1Hex4HexNAc4 (2) and dHex1Hex4HexNAc5 (1)] were decreased to 48% and 55%, respectively. A significant decrease was also found in other monogalacto-biantennary oligosaccharides, such as dHex2Hex4HexNAc4 (2) (a Lewis-motif-modified, core-fucosylated monogalacto-biantennary) and dHex2Hex4HexNAc5 (1) (a Lewis-motif-modified core-fucosylated and bisected monogalacto-biantennary).
The oligosaccharides, non-reducing ends of which are fully galactosylated, were decreased in the SLE-model mouse. For example, monofucosyl biantennary dHex1Hex5HexNAc4 (1) and (2) were decreased 59% and 75%, respectively. The di-, tri- and tetra-fucosylated oligosaccharides, dHex2Hex6HexNAc6 (1), dHex3Hex6HexNAc6 (1,2) and dHex4Hex6HexNAc6 (1,2), which were estimated to be tri- and tetraantennary forms, were also significantly decreased. These results show that oligosaccharides with a complicated structure, such as high branching oligosaccharides and di- and tri-fucosylated oligosaccharides, were decreased in the SLE-model mouse.
Discussion
Using the isotope-tagging method, we demonstrated aberrant N-glycosylation on the kidney proteins of a SLE-model mouse. We found increases in low-molecular-mass glycans with simple structures, including paucimannose-type glycans, agalacto-biantennary oligosaccharides, Man-5 and Man-6, and decreases in glycans which have a complicated and diverse structure, such as digalacto-biantennary oligosaccharides and highly fucosylated glycans (Fig. 4). An increase in agalacto-biantennary oligosaccharides on IgG has been reported in the sera of patients with autoimmune diseases, including SLE, rheumatoid arthritis and IgA nephropathy.9,11,28 The present findings show that abnormal glycosylation occurs not only in IgG in serum but also in several glycoproteins in the SLE-model mouse kidney.
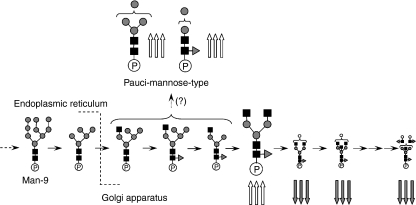
Figure 6 shows the biosynthesis pathway of N-linked oligosaccharides in mammalian cells. Man-9, a product in the early stage of the pathway, is processed to Man-5 in the endoplasmic reticulum, and a GlcNAc and Fuc are added to Man-5 in the Golgi apparatus. After the removal of two Man residues by αM-II, GlcNAc, Gal and Fuc are further added to oligosaccharides by several glycosyltransferases. There have been a few reports on paucimannose-type oligosaccharides in vertebrates;29 however, these glycans are common oligosaccharides in other multicellular organisms such as insects and Caenorhabditis elegans.30,31 The membrane protease β-N-acetylglucosaminidase is thought to mediate the synthesis of paucimannose-type oligosaccharides.32 Based on core fucosylation on some paucimannose-type oligosaccharides, it was deduced that β-N-acetylglucosaminidase might act on glycan synthesis after N-acetylglucosaminyltransferase I, core fucosyltransferase and αM-II.32 The synthesis of paucimannose-type oligosaccharides may be involved in the suppression of growing diversity and complexity of glycan structures.
Figure 6.
Biosynthesis pathway of N-linked oligosaccharides in mammalian cells. Triple up-arrow, increases of more than +2·0; triple down-arrow, decreases of not more than −2·0. Grey circle, mannose; white circle, galactose; grey triangle, fucose; black square, N-acetylglucosamine. ‘P’ is protein portion.
We found a number of changes in the levels of monogalacto-biantennary oligosaccharides in the SLE mouse. Galactosylation to agalacto-biantennary oligosaccharides is mediated by β-1,4-galactosyltransferase (β-1,4-GalTase).33 Previous studies suggested that translational repression of β-1,4-GalTase in lymphocytes is associated with an increase in agalacto-oligosaccharides on IgG in the serum of the MRL-lpr mouse.34 Although the activity of β-1,4-GalTase remains unknown in the SLE-model mouse, the increase in agalacto forms and the decrease in digalacto forms imply changes in β-1,4-GalTase activity. The present results suggest a decrease in diverse and complex glycans, which are synthesized at a late stage in the N-glycan synthesis pathway, and an increase in the simple glycans appearing at an early stage in the SLE-model mouse.
The activation of complements is involved in glomerular nephritis of SLE.35–37 The complements are activated through three pathways: a classical pathway, an alternative pathway and a lectin pathway. In the classical pathway, a binding of C1q to an immune complex triggers the activation of C1r and C1s. Activated C1s cleaves C4 and C2, generating C3 convertase (C4b2a), which generates C3b. The complement component subsequently produces C5b-9 complex, which leads to an inflammatory response on host tissues.38–41 The excess deposition of immune complexes followed by a sustained immune response triggers tissue disorders, including lupus nephritis.42–45 In the lectin pathway, mannose-binding lectin (MBL) is associated with the activation of complements. Two forms of MBL (MBL-A and MBL-C) are present in complexes with MBL-associated serine proteases (MASPs) in mice. The MASPs are activated by binding MBL to Man or GlcNAc on the surface of the antigen in a calcium-dependent manner.46–49 Like C1s in the classical pathway, activated MASPs cleave C4 and C2.50,51 In lupus nephritis, MBL-A and MBL-C in the immune complex bind to GlcNAc residues at the reducing ends of agalacto-biantennary oligosaccharides in IgG,52 and subsequently activate the complements.53,54 In αM-II-deficient mice, which suffer from SLE-like syndromes including kidney disorders, the majority of glycans are hybrid-type oligosaccharides because of the failure of Man trimming by the lack of αM-II.16 Green et al. concluded that MBL recognized Manα1–3 and Manα1–6 linkages in hybrid-type oligosaccharides,17 and glycans lacking normal side chains, including agalacto-biantennary oligosaccharides, might be involved in the aberrant immune response in autoimmune diseases. Paucimannose glycans, which contain exposed Manα1–3 or Manα1–6 linkages, may be recognized as ligand carbohydrates by MBL. Our present finding, an increase in paucimannose oligosaccharides and agalacto forms, might result from an alteration of the biosynthesis pathway of N-glycans. The alterations may cause the aberrant glycosylations on most of the glycoproteins rather than some glycoproteins in the SLE-model mouse. The changes in glycosylation might be involved in an autoimmune pathogenesis in the SLE-model mouse kidney.
The continuous production of aberrant antibodies that react with components from self-tissue and accumulation in the immune complex are thought to promote tissue damage in autoimmune disease.55,56 The mechanism of localized accumulation in the immune complex in some tissues remains unknown in SLE. We found an increase in glycans that may bind to MBL and subsequently promote complement activation via the lectin pathway in the mouse kidney. Our present results suggest that an aberrant N-glycan synthesis pathway as well as an abnormal immune system may be involved in the damage caused by glomerular nephritis in the SLE-model mouse.
Acknowledgments
This study was supported in part by a Grant-in-Aid from the Ministry of Health, Labor, and Welfare, and Core Research for the Evolutional Science and Technology Program (CREST), Japan Science and Technology Corp (JST).
References
- 1.Dwek RA. Glycobiology: toward understanding the function of sugars. Chem Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 2.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–9. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 3.Zak I, Lewandowska E, Gnyp W. Selectin glycoprotein ligands. Acta Biochim Pol. 2000;47:393–412. [PubMed] [Google Scholar]
- 4.Axford JS. Glycosylation and rheumatic disease. Biochim Biophys Acta. 1999;1455:219–29. doi: 10.1016/s0925-4439(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 5.Feizi T, Gooi HC, Childs RA, Picard JK, Uemura K, Loomes LM, Thorpe SJ, Hounsell EF. Tumour-associated and differentiation antigens on the carbohydrate moieties of mucin-type glycoproteins. Biochem Soc Trans. 1984;12:591–6. doi: 10.1042/bst0120591. [DOI] [PubMed] [Google Scholar]
- 6.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–84. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodarzi MT, Turner GA. Decreased branching, increased fucosylation and changed sialylation of alpha-1-proteinase inhibitor in breast and ovarian cancer. Clin Chim Acta. 1995;236:161–71. doi: 10.1016/0009-8981(95)06049-j. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita K, Fukushima K, Sakiyama T, Murata F, Kuroki M, Matsuoka Y. Expression of Sia alpha 2→6Gal beta 1→4GlcNAc residues on sugar chains of glycoproteins including carcinoembryonic antigens in human colon adenocarcinoma: applications of Trichosanthes japonica agglutinin I for early diagnosis. Cancer Res. 1995;55:1675–9. [PubMed] [Google Scholar]
- 9.Tomana M, Schrohenloher RE, Reveille JD, Arnett FC, Koopman WJ. Abnormal galactosylation of serum IgG in patients with systemic lupus erythematosus and members of families with high frequency of autoimmune diseases. Rheumatol Int. 1992;12:191–4. doi: 10.1007/BF00302151. [DOI] [PubMed] [Google Scholar]
- 10.Mizuochi T, Hamako J, Nose M, Titani K. Structural changes in the oligosaccharide chains of IgG in autoimmune MRL/Mp-lpr/lpr mice. J Immunol. 1990;145:1794–8. [PubMed] [Google Scholar]
- 11.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 12.Das H, Atsumi T, Fukushima Y, et al. Diagnostic value of antiagalactosyl IgG antibodies in rheumatoid arthritis. Clin Rheumatol. 2004;23:218–22. doi: 10.1007/s10067-003-0860-9. [DOI] [PubMed] [Google Scholar]
- 13.Raghav SK, Gupta B, Agrawal C, Saroha A, Das RH, Chaturvedi VP, Das HR. Altered expression and glycosylation of plasma proteins in rheumatoid arthritis. Glycoconj J. 2006;23:167–73. doi: 10.1007/s10719-006-7922-6. [DOI] [PubMed] [Google Scholar]
- 14.Elliott MA, Elliott HG, Gallagher K, McGuire J, Field M, Smith KD. Investigation into the concanavalin A reactivity, fucosylation and oligosaccharide microheterogeneity of alpha 1-acid glycoprotein expressed in the sera of patients with rheumatoid arthritis. J Chromatogr B Biomed Sci Appl. 1997;688:229–37. doi: 10.1016/s0378-4347(96)00309-x. [DOI] [PubMed] [Google Scholar]
- 15.Rops AL, van den Hoven MJ, Bakker MA, et al. Expression of glomerular heparan sulphate domains in murine and human lupus nephritis. Nephrol Dial Transplant. 2007;22:1891–902. doi: 10.1093/ndt/gfm194. [DOI] [PubMed] [Google Scholar]
- 16.Chui D, Sellakumar G, Green R, et al. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc Natl Acad Sci USA. 2001;98:1142–7. doi: 10.1073/pnas.98.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green RS, Stone EL, Tenno M, Lehtonen E, Farquhar MG, Marth JD. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity. 2007;27:308–20. doi: 10.1016/j.immuni.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Wada Y. Mass spectrometry in the detection and diagnosis of congenital disorders of glycosylation. Eur J Mass Spectrom (Chichester, Eng) 2007;13:101–3. doi: 10.1255/ejms.836. [DOI] [PubMed] [Google Scholar]
- 19.Faid V, Chirat F, Seta N, Foulquier F, Morelle W. A rapid mass spectrometric strategy for the characterization of N- and O-glycan chains in the diagnosis of defects in glycan biosynthesis. Proteomics. 2007;7:1800–13. doi: 10.1002/pmic.200600977. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto S. Clinical applications of glycomic approaches for the detection of cancer and other diseases. Curr Opin Mol Ther. 2006;8:507–13. [PubMed] [Google Scholar]
- 21.Yuan J, Hashii N, Kawasaki N, Itoh S, Kawanishi T, Hayakawa T. Isotope tag method for quantitative analysis of carbohydrates by liquid chromatography-mass spectrometry. J Chromatogr A. 2005;1067:145–52. doi: 10.1016/j.chroma.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Manilla G, Warren NL, Abney T, Atwood J, III, Azadi P, York WS, Pierce M, Orlando R. Tools for glycomics: relative quantitation of glycans by isotopic permethylation using 13CH3I. Glycobiology. 2007;17:677–87. doi: 10.1093/glycob/cwm033. [DOI] [PubMed] [Google Scholar]
- 23.Kang P, Mechref Y, Kyselova Z, Goetz JA, Novotny MV. Comparative glycomic mapping through quantitative permethylation and stable-isotope labeling. Anal Chem. 2007;79:6064–73. doi: 10.1021/ac062098r. [DOI] [PubMed] [Google Scholar]
- 24.Bowman MJ, Zaia J. Tags for the stable isotopic labeling of carbohydrates and quantitative analysis by mass spectrometry. Anal Chem. 2007;79:5777–84. doi: 10.1021/ac070581b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 26.Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci USA. 1993;90:1756–60. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merino R, Iwamoto M, Fossati L, Izui S. Polyclonal B cell activation arises from different mechanisms in lupus-prone (NZB × NZW)F1 and MRL/MpJ-lpr/lpr mice. J Immunol. 1993;151:6509–16. [PubMed] [Google Scholar]
- 28.Homma H, Tozawa K, Yasui T, Itoh Y, Hayashi Y, Kohri K. Abnormal glycosylation of serum IgG in patients with IgA nephropathy. Clin Exp Nephrol. 2006;10:180–5. doi: 10.1007/s10157-006-0422-y. [DOI] [PubMed] [Google Scholar]
- 29.Hase S, Okawa K, Ikenaka T. Identification of the trimannosyl-chitobiose structure in sugar moieties of Japanese quail ovomucoid. J Biochem. 1982;91:735–7. doi: 10.1093/oxfordjournals.jbchem.a133748. [DOI] [PubMed] [Google Scholar]
- 30.Kubelka V, Altmann F, Kornfeld G, Marz L. Structures of the N-linked oligosaccharides of the membrane glycoproteins from three lepidopteran cell lines (Sf-21, IZD-Mb-0503, Bm-N) Arch Biochem Biophys. 1994;308:148–57. doi: 10.1006/abbi.1994.1021. [DOI] [PubMed] [Google Scholar]
- 31.Natsuka S, Adachi J, Kawaguchi M, Nakakita S, Hase S, Ichikawa A, Ikura K. Structural analysis of N-linked glycans in Caenorhabditis elegans. J Biochem. 2002;131:807–13. doi: 10.1093/oxfordjournals.jbchem.a003169. [DOI] [PubMed] [Google Scholar]
- 32.Altmann F, Schwihla H, Staudacher E, Glossl J, Marz L. Insect cells contain an unusual, membrane-bound beta-N-acetylglucosaminidase probably involved in the processing of protein N-glycans. J Biol Chem. 1995;270:17344–9. doi: 10.1074/jbc.270.29.17344. [DOI] [PubMed] [Google Scholar]
- 33.Guo S, Sato T, Shirane K, Furukawa K. Galactosylation of N-linked oligosaccharides by human beta-1,4-galactosyltransferases I, II, III, IV, V, and VI expressed in Sf-9 cells. Glycobiology. 2001;11:813–20. doi: 10.1093/glycob/11.10.813. [DOI] [PubMed] [Google Scholar]
- 34.Jeddi PA, Lund T, Bodman KB, et al. Reduced galactosyltransferase mRNA levels are associated with the agalactosyl IgG found in arthritis-prone MRL-lpr/lpr strain mice. Immunology. 1994;83:484–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10:413–24. doi: 10.1681/ASN.V102413. [DOI] [PubMed] [Google Scholar]
- 36.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 37.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 38.Botto M. Links between complement deficiency and apoptosis. Arthritis Res. 2001;3:207–10. doi: 10.1186/ar301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 40.Arason GJ, Steinsson K, Kolka R, Vikingsdottir T, D’Ambrogio MS, Valdimarsson H. Patients with systemic lupus erythematosus are deficient in complement-dependent prevention of immune precipitation. Rheumatology (Oxford) 2004;43:783–9. doi: 10.1093/rheumatology/keh183. [DOI] [PubMed] [Google Scholar]
- 41.Cook HT, Botto M. Mechanisms of disease: the complement system and the pathogenesis of systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2:330–7. doi: 10.1038/ncprheum0191. [DOI] [PubMed] [Google Scholar]
- 42.Gunnarsson I, Sundelin B, Heimburger M, Forslid J, van Vollenhoven R, Lundberg I, Jacobson SH. Repeated renal biopsy in proliferative lupus nephritis – predictive role of serum C1q and albuminuria. J Rheumatol. 2002;29:693–9. [PubMed] [Google Scholar]
- 43.Buyon JP, Tamerius J, Belmont HM, Abramson SB. Assessment of disease activity and impending flare in patients with systemic lupus erythematosus. Comparison of the use of complement split products and conventional measurements of complement. Arthritis Rheum. 1992;35:1028–37. doi: 10.1002/art.1780350907. [DOI] [PubMed] [Google Scholar]
- 44.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–27. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sturfelt G. The complement system in systemic lupus erythematosus. Scand J Rheumatol. 2002;31:129–32. [PubMed] [Google Scholar]
- 46.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 47.Weis WI, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127–34. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi M, Mori S, Shigeta S, Fujita T. Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. Adv Exp Med Biol. 2007;598:93–104. doi: 10.1007/978-0-387-71767-8_8. [DOI] [PubMed] [Google Scholar]
- 49.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 50.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 51.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 52.Lhotta K, Wurzner R, Konig P. Glomerular deposition of mannose-binding lectin in human glomerulonephritis. Nephrol Dial Transplant. 1999;14:881–6. doi: 10.1093/ndt/14.4.881. [DOI] [PubMed] [Google Scholar]
- 53.Ohsawa I, Ohi H, Tamano M, et al. Cryoprecipitate of patients with cryoglobulinemic glomerulonephritis contains molecules of the lectin complement pathway. Clin Immunol. 2001;101:59–66. doi: 10.1006/clim.2001.5098. [DOI] [PubMed] [Google Scholar]
- 54.Trouw LA, Seelen MA, Duijs JM, et al. Activation of the lectin pathway in murine lupus nephritis. Mol Immunol. 2005;42:731–40. doi: 10.1016/j.molimm.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 55.Jorgensen TN, Gubbels MR, Kotzin BL. New insights into disease pathogenesis from mouse lupus genetics. Curr Opin Immunol. 2004;16:787–93. doi: 10.1016/j.coi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Lauwerys BR, Wakeland EK. Genetics of lupus nephritis. Lupus. 2005;14:2–12. doi: 10.1191/0961203305lu2052oa. [DOI] [PubMed] [Google Scholar]