Abstract
Oral tolerance promotes a generalized decrease in specific immune responsiveness to proteins previously encountered via the oral route. In addition, parenteral immunization with a tolerated protein also triggers a significant reduction in the primary responsiveness to a second unrelated antigen. This is generally explained by ‘innocent bystander suppression’, suggesting that the transient and episodic effects of inhibitory cytokines released by contact with the tolerated antigen would block responses to the second antigen. In disagreement with this view, we have previously shown that: (i) these inhibitory effects do not require concomitance or contiguity of the injections of the two proteins; (ii) that intravenous or intragastric exposures to the tolerated antigen are not inhibitory; and (iii) that the inhibitory effect, once triggered, persists in the absence of further contact with the tolerated protein, possibly by inhibition of secondary responsiveness (immunological memory). The present work confirms that immunological memory of the second unrelated antigen is hindered by exposure to the tolerated antigen and, in addition, shows that this exposure: (i) inhibits the inflammation triggered by an unrelated antigen through the double effect of inhibiting production of leucocytes in the bone marrow and blocking their migration to inflammed sites; and (ii) significantly blocks footpaw swelling triggered by carrageenan. Taken together, these results conclusively demonstrate that inhibitory effects of parenteral injection of tolerated antigens are much more general than suggested by the ‘innocent bystander suppression’ hypothesis.
Keywords: bystander suppression, delayed-type hypersensitivity, eosinophils, inflammation, mucosal immunity, oral tolerance
Introduction
Oral tolerance is a T-cell-mediated phenomenon defined by a general decrease in immune responses to a protein previously encountered via the oral route.1,2 Oral tolerance prevents increases in serum-specific antibodies and also impairs cell-mediated phenomena such as contact dermatitis and delayed-type hypersensitivity (DTH) reactions.3–5 Oral tolerance also prevents the development of experimental asthma,6 peritonitis7 and some experimental autoimmune diseases.8 Unexpectedly, parenteral re-exposure to a tolerated antigen was found to block the initiation of immune responses to a second unrelated antigen.9–11 Thus, injection of a tolerated protein into orally tolerant mice inhibits immunological responses to unrelated proteins and block severe chronic inflammatory reactions of immunological origin, such as autoimmune reactions and granulomatous lesions around Schistosoma mansoni eggs.12 The mechanism proposed to explain this inhibitory effect was called ‘bystander supression’.10
Working on the assumption that the inhibition of unrelated immunological responses by tolerated proteins is attributable to ‘bystander suppression’, most investigations on this subject were performed by injecting the two proteins together. However, we have shown that the inhibition of serum antibody responses to unrelated antigens by injection of tolerated proteins does not require concomitance or contiguity of the injections of the two proteins.13 In addition, once triggered, the inhibitory effect persists in the absence of further contact with the tolerated protein.11 Thus, we suggested that the inhibitory effects triggered by parenteral injection of tolerated antigen are better understood when the systemic and indirect effects of the injected antigen are considered. Notwithstanding, inhibitory effects triggered by parenteral injection of tolerated antigens would improve the clinical use of oral tolerance to prevent inflammatory responses in situations where cognate antigens are not known.
Here we used three inflammatory models to study the indirect inhibitory effects of tolerated antigens: DTH reactions, antigen-induced peritonitis and paw oedema induced by carrageenan. DTH reactions are paradigmatic of ‘cellular’ immunity and can be transferred to genetically compatible recipients by T helper type 1 (Th1) lymphocytes. An experimental DTH reaction may be developed after subcutaneous (s.c.) injection of heat-denatured antigen into animals previously sensitized with the antigen in complete Freund’s adjuvant (CFA). During a DTH reaction, cytokines found to be present in lesions include interleukin-2 (IL-2), interferon-gamma (IFN-γ), tumour necrosis factor (TNF) and IL-5.14 The evidence that these cytokines are associated with the clinical inflammatory response is supported by the fact that the reaction can be inhibited by the injection of cytokines that inhibit their production, such as IL-10.15
In the peritonitis model, two s.c. injections of the antigen in Al(OH)3, given 7 days apart, sensitize the animals; subsequent intraperitoneal (i.p.) challenge with a small dose of the antigen results in accumulation of leucocytes in the peritoneum.16,17 Eosinophils are prominently involved in this inflammatory reaction, which also produce significant bone marrow eosinophilia involving T-cell-derived cytokines such as IL-5, IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF).18,19
Carrageenan-induced oedema in the paw is a widely used model to investigate the physiopathology of an acute local inflammation.20 Mediators involved in this model of acute inflammation include biogenic amines, bradykinin, prostaglandins21,22 and nitric oxide23 and, more recently, hydrogen sulphide has been shown to be involved.24
The present experiments show that the inhibitory effect triggered by tolerated antigen injection is able to block inflammatory responses in all models tested, including the footpaw swelling elicited by carrageenan, which do not require antigen sensitization. In addition, the results confirm and expand our previous observations showing that these inhibitory effects do not require concomitance or contiguity of tolerated antigens and a second protein.
Materials and methods
Experimental animals
Female 8-week-old (C57BL/6 × DBA/2)F1 or B6D2F1 mice, bred and maintained in our animal breeding unit at the Institute of Biological Sciences, Federal University of Minas Gerais, Brazil, were used. In experiments with carrageenan, female Swiss mice were used. The animals were handled according to the rules established by the ethical committee. Experimental groups contained five to eight mice. The experiments were repeated at least two times.
Antigens
Ovalbumin (OVA, grade V; Sigma, St Louis, MO) and haemoglobin (Hb) from the snail Biomphalaria glabrata kindly supplied by Professor Marcelo Matos Santoro of our department were used in experiments with peritonitis. Dinitrophenylated (DNP) conjugates of OVA (DNP-OVA) and keyhole limpet haemocyanin (DNP-KLH) were used in experiments with DTH. The KLH was obtained from Sigma. We prepared DNP conjugates by coupling with dinitrophenyl sulphonate as described by Ovary and Benacerraf.25
Oral tolerance induction
Oral tolerance to OVA was induced by adding a one-fifth solution of hen egg white to the drinking water for three consecutive days and allowing the mice to drink ad libitum. Oral treatment was discontinued 7 days before parenteral immunization. The egg white solution was prepared in our laboratory from eggs commercially available at supermarkets. Daily estimated average consumption was 20 mg of OVA per mouse and this resulted in significant levels of tolerance. Bottles were changed every day to avoid contamination. Control groups received filtered tap water.
Parenteral immunizations and DTH
Mice were actively sensitized by one s.c. injection at the base of the tail with 100 μg of DNP-OVA + 50 μg of DNP-KLH in 0·2 ml of CFA (1 : 1) and challenged 28 days later with 100 μg of heat-aggregated DNP-OVA and DNP-KLH (50 μg) in the right and left rear footpad, respectively; increases in footpad thickness were measured 24 hr later and results are expressed as the percentage of increment in relation to footpad thickness before antigen injection. The increase in footpad thickness was expressed using the formula:
Parenteral immunizations and antigen-induced peritonitis
Mice were actively sensitized by two s.c. injections of 0·2 ml of a suspension containing 100 μg of OVA and 50 μg of Hb and 1·6 mg of Al(OH)3 given 7 days apart. One week thereafter, peritonitis was induced by the i.p. injection of 1 μg of OVA plus 1 μg of Hb or 1 μg of Hb only. Additional control animals sensitized s.c. with OVA plus Hb received i.p. sterile saline.
Peripheral blood cell counts
At 24 hr after i.p. antigen challenge, mice were bled under ether anaesthesia from the axillary plexus and blood was collected without anticoagulant for antibody assays and smears, or with ethylenediaminetetraacetic acid (EDTA) for global leucocyte counts. The total white blood cell count was obtained using a Neubauer chamber. Peripheral blood smears were stained with May–Grünwald–Giemsa and the differential white blood cell count was determined under oil immersion (×1000). At least 200 cells were counted and results are expressed as the number of eosinophils per millilitre of blood.
Peritoneal lavage fluid (PLF)
At 24 hr after i.p. antigen challenge and after bleeding, animals were killed by cervical displacement, and the peritoneal cavity was opened and washed with 3 ml of Hanks’ balanced salt solution (HBSS) containing heparin (10 U/ml); approximately 90% of the initial volume was recovered, and in cases when haemorrhages were detected in the peritoneal cavity the animals were discarded. The total cell count in the PLF was determined in a haemocytometer. Differential cell counts were determined after cytocentrifugation and staining with May–Grünwald–Giemsa under oil immersion (×1000). At least 400 cells were counted and results are expressed as the number of cells per millilitre of PLF.
Bone marrow (BM)
At 24 hr after i.p. antigen challenge, bone marrow cells were obtained by flushing the femur with 5 ml of HBSS containing heparin (50 U/ml). The cell suspensions were gently homogenized to break large clumps, centrifuged and resuspended in HBSS to give a final volume of 1 ml. The total cell count of the BM cell suspensions was determined microscopically by haemocytometer. Differential cell counts were determined after cytocentrifugation and staining with May–Grünwald–Giemsa under oil immersion (×1000). At least 400 cells were counted and results are expressed as the number of BM eosinophils per millilitre of cell suspension.
Parenteral immunizations and carrageenan-induced paw oedema
OVA orally tolerant female Swiss mice and controls not tolerant to OVA were i.p. immunized with 10 μg of OVA plus 5 mg of Al(OH)3; 24 hr thereafter the animals received one intradermal injection (maximal volume 50 μl) of carrageenan (300 μg/paw) in the right hindpaw. The contralateral paw received 50 μl of saline and was used as a control. To test the effects of the immunization time on the inhibition efficiency of tolerated antigen in carrageenan-induced mouse paw oedema, mice submitted to oral OVA treatment were immunized i.p. with OVA plus Al(OH)3 24 hr before, 4 hr before, or 4 hr after the paw oedema initiation with carrageenan. Carrageenan was dissolved in sterile Dulbecco’s phosphate-buffered saline (PBS). Paw oedema was measured by plethysmometry, as previously described,26,27 at the indicated time intervals. The difference in volume between the right and left hindpaws was taken as paw oedema and was expressed as Δ paw oedema in microlitres (μl).
Myeloperoxidase (MPO) and N-acetyl-glucosaminidase (NAG) assays
Twenty-four hours after paw oedema initiation, animals were killed by cervical displacement and paws were removed for enzyme activity assays. The subcutaneous tissue of the paws was homogenized in 1·5 ml of cooled (4°) phosphate buffer (80 mm, pH 5·4) containing 0·5% weight/volume (w/v) hexadecyltrimethylammonium bromide (HTAB), and centrifuged at 4° for 20 min at 10 000 g. The supernatants were saved and used for the measurement of MPO and NAG activities as indexes of neutrophil and macrophage influx, respectively, as previously described.28 The reagents 3,3-5,5-tetramethylbenzidine (TMB) and p-nitrophenyl-N-acetyl-b-d-glucosaminide were used as MPO and NAG enzyme substrates, respectively. The results are expressed in optical density (OD) units (OD 450 nm for MPO and OD 405 nm for NAG) per mg protein.
Antibody assays
Antibody titres were determined by standard enzyme-linked immunosorbent assay (ELISA) using an automatic ELISA reader (Bio-Rad, Hercules, CA). In short, plates (Nunc, Roskild, Denmark) were coated overnight with 2 μg of OVA or 1 μg of Hb in 100 μl/well of sodium carbonate buffer, pH 9·6, at 4°. The plates were then washed with PBS containing 0·05% Tween-20 and blocked for 1 hr at room temperature (RT) with PBS containing 0·25% casein. The plates were incubated for 1 hr at RT with six dilutions of mouse serum samples starting at 1/100 or 1/400 in PBS-casein for the anti-OVA and anti-Hb assays, respectively. The plates were washed six times and incubated with goat anti-mouse immunoglobulin (IgM + IgG + IgA, H + L) labelled with horseradish peroxidase (HRP; Southern Biotechnology Associates, Birmingham, AL) for 1 hr at 37°. The plates were then washed a further six times, and incubated in the dark with H2O2 in the presence of orthophenylene-diamine (OPD; Sigma) in sodium citrate buffer, pH 5·0, for 20 or 10 min for the anti-OVA and anti-Hb assays, respectively. The reaction was stopped by the addition of 20 μl of 2N H2SO4. Colour development was measured at OD 492 nm. ELISA scores were computed by running sums of ODs between 1 : 100 and 1 : 3200 of serum dilutions in individual mice. Each score is show as a mean ± standard error of the mean (SEM) for groups of five to eight animals, as indicated in the figure legend.
Statistical analysis
Statistical analyses were performed using Sigma XL Statistical software (SigmaFlow, Plano, TX) and the statistical significance was determined using the Student–Newman–Keuls comparison test. In the experiments with carrageenan, statistical analysis was performed using two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc t-test. P-values of 0·05 or less were considered significant. The results are expressed as the mean ±SEM.
Results
Injection of DNP-OVA into OVA-tolerant mice inhibits DTH reactions to DNP-KLH
We previously showed that parenteral immunization of OVA orally tolerant mice with small doses of OVA or DNP-OVA inhibits serum antibody responses to DNP-KLH.9,13 Here we investigated whether cellular activities, such as DTH responses to DNP-KLH, can also be blocked by injection of DNP-OVA into OVA orally tolerant mice.
To induce oral tolerance to OVA, B6D2F1 mice were made to drink an egg white solution for 3 days; control mice drank tap water. Seven days after oral treatment, control and tolerant mice were doubly immunized, at the base of the tail, with 100 μg of DNP-OVA plus 50 μg of DNP-KLH in 0·2 ml of CFA (1 : 1). DTH reactions were evoked 28 days thereafter with 100 μg of heat-aggregated DNP-OVA injected into the right rear footpad and 20 μg of heat-aggregated DNP-KLH injected into the left rear footpad.
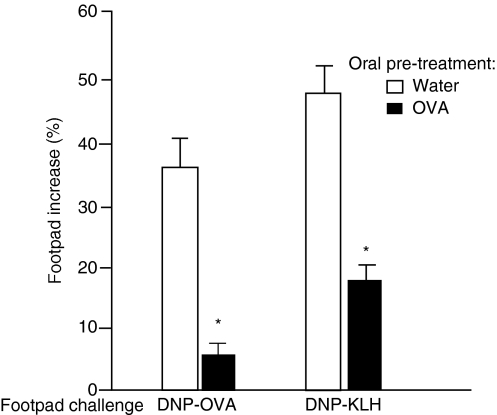
Results are shown in Fig. 1. Mice not tolerant to OVA displayed significant DTH responses to both DNP-OVA and DNP-KLH. As expected, reactions to DNP-OVA were significantly weaker in animals tolerant to OVA. In addition, reactions to DNP-KLH were also significantly inhibited, probably as a consequence of a lasting reduction of the immune responsiveness to DNP-KLH triggered by the previous s.c. immunization with DNP-KLH in the presence of DNP-OVA.
Figure 1.
Injection of ovalbumin (OVA) into OVA orally tolerant mice inhibits delayed-type hypersensitivity (DTH) triggered by dinitrophenylated OVA (DNP-OVA) or dinitrophenylated keyhole lympet haemocyanin (DNP-KLH). The footpad increased in sensitized mice 24 hr after challenge with DNP-OVA in the right footpad and DNP-KLH in the left footpad, as indicated in the figure. Open bars: control, non-tolerant mice, subcutaneously (s.c.) immunized with DNP-OVA plus DNP-KLH in complete Freund’s adjuvant (CFA). Solid bars: OVA orally tolerant mice s.c. immunized with DNP-OVA plus DNP-KLH in CFA. Values are mean ± standard error of the mean (for five or six animals). *P<0·05 compared with non-tolerant mice (given water).
Injection of OVA into OVA-tolerant mice blocks peritonitis triggered by a second antigen [snail haemoglobin (Hb)]
B6D2F1 mice were rendered orally tolerant to OVA, as described above; control mice drank tap water. Seven and 14 days after oral treatment, control and tolerant mice were doubly immunized s.c. with a mixture of 100 μg of OVA and 50 μg of Hb in Al(OH)3 adjuvant. One week thereafter, allergic peritonitis was induced in control and tolerant mice by the i.p. injection of a mixture of 1 μg of Hb plus 1 μg of OVA; to test if the inhibitory effect would persist in the absence of orally tolerated antigen, some groups were challenged with 1 μg of Hb only. An additional control group of mice not tolerant to OVA were immunized s.c. and received an i.p. injection of sterile saline. The magnitude of the inflammatory cellular infiltration was determined by cell counts in the peritoneal lavage fluid 24 hr thereafter. To monitor the magnitude of immunization and oral tolerance induction, levels of serum antibodies to OVA and to Hb were determined by ELISA.
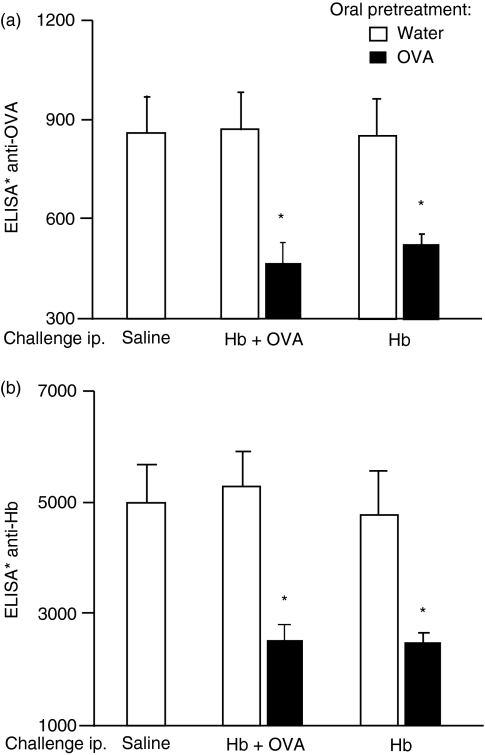
Oral pretreatment with OVA resulted in significant inhibition of anti-OVA antibody formation, showing the establishment of oral tolerance to OVA (Fig. 2a) and, in accordance with previously published results, injection of OVA into OVA orally tolerant mice also inhibited antibody responses to Hb (Fig. 2b).
Figure 2.
Injection of ovalbumin (OVA) into OVA orally tolerant mice inhibits anti-haemoglobin (Hb) antibody production. Total levels of serum anti-OVA (a) and anti-Hb (b) antibodies from sensitized mice killed 24 hr after intraperitoneal (i.p.) challenge with the antigens indicated in the figure were determined. Open bars: control, non-tolerant mice, subcutaneously (s.c.) immunized with OVA plus Hb. Solid bars: OVA orally tolerant mice s.c. immunized with OVA plus Hb. Values are mean ± standard error of the mean (for five or six animals). *P<0·05 compared with non-tolerant mice (given water).
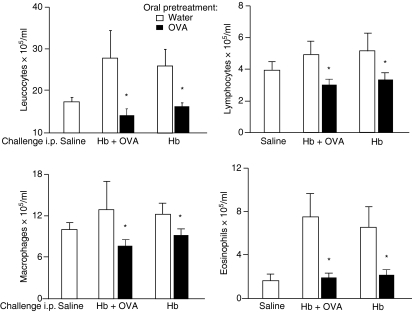
Cell counts performed on the peritoneal lavage fluid 24 hr after i.p injection of sterile saline (Fig. 3), in mice previously immunized s.c with OVA plus Hb, showed no signal of inflammation compared with normal mice (results not shown). In control mice, which were not tolerant to OVA and immunized s.c with OVA plus Hb, the i.p. injection of Hb alone or Hb plus OVA resulted in significantly increased leucocyte counts (Fig. 3). Differential cell counts showed increased eosinophils after i.p. challenge with either OVA plus Hb or Hb, compared with the saline control group (Fig. 3). In OVA-tolerant mice the cellular infiltration triggered by i.p. challenge with Hb was damped even in the absence of tolerated antigen (OVA) in the peritoneal cavity; actually, the addition of OVA to the i.p. challenge did not significantly increase the inhibition seen with Hb alone. This probably resulted from a weaker immune responsiveness to Hb triggered by the presence of OVA following s.c. immunizations (Fig. 3).
Figure 3.
Injection of ovalbumin (OVA) into OVA orally tolerant mice inhibits peritonitis triggered by snail haemoglobin (Hb). Total and differential leucocyte counts (× 105 cells/ml) in the peritoneal lavage fluid 24 hr after intraperitoneal (i.p.) challenge with the antigens indicated in the figure were determined. Open bars: control, non-tolerant mice, subcutaneously (s.c.) immunized with OVA plus Hb. Solid bars: OVA orally tolerant mice s.c. immunized with OVA plus Hb. Values are mean ± standard error of the mean (for five or six animals). *P<0·05 compared with non-tolerant mice (given water).
Exposure to OVA blocks peripheral blood and bone marrow eosinophilia triggered by Hb in OVA-tolerant/Hb-immunized mice
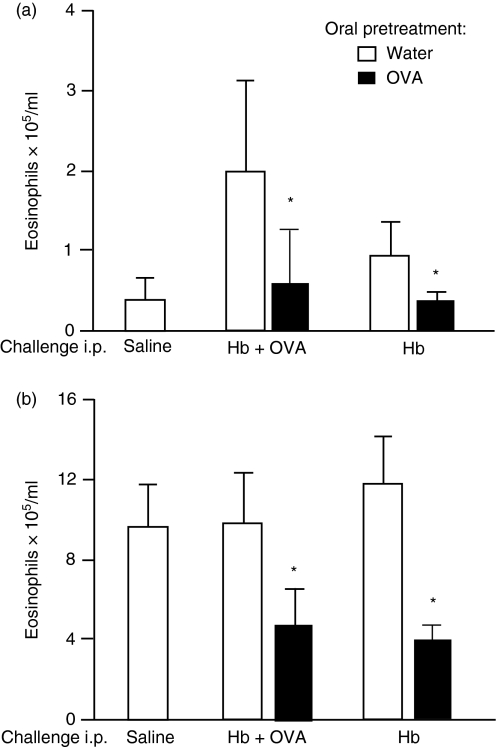
Local inflammatory reactions demand cellular migration. This is reflected in increased blood leucocyte counts and, as previously demonstrated with peritonitis, it may also result in elevated bone marrow eosinopoiesis.7 To assess the inhibitory effect of OVA injection on cell traffic in OVA-tolerant mice, we performed differential blood cell counts and bone marrow cell counts for eosinophils. As shown in Fig. 4a, significant blood eosinophilia was triggered by i.p. injection of Hb alone, or Hb plus OVA, in mice previously immunized with OVA + Hb. Oral tolerance to OVA blocked blood eosinophilia in both cases. The bone marrow eosinophilia present at 24 hr after i.p. challenge with OVA + Hb was similarly suppressed in OVA-tolerant mice (Fig. 4b). It is important to stress that this inhibition also occurred when peritonitis was triggered by Hb i.p. injection alone in OVA-tolerant mice, in the absence of further challenge with OVA.
Figure 4.
Injection of ovalbumin (OVA) into OVA orally tolerant mice inhibits the increase in peripheral blood and bone marrow eosinophils triggered by snail haemoglobin (Hb). Total eosinophil counts in the peripheral blood (a) and bone marrow (b) 24 hr after intraperitoneal (i.p.) challenge with the antigens indicated in the figure were determined. Open bars: control, non-tolerant mice, subcutaneously (s.c.) immunized with OVA plus Hb. Solid bars: OVA orally tolerant mice s.c. immunized with OVA plus Hb. Values are mean ± standard error of the mean (for five or six animals). *P<0·05 compared with non-tolerant mice (given water).
In conclusion, parenteral exposure to OVA, the tolerated antigen, inhibited inflammation triggered by an unrelated antigen both by blocking eosinophil migration to inflamed sites and also by inhibiting eosinophil production in the bone marrow. This inhibitory effect persisted in the absence of further contact with the orally tolerated protein.
Injection of OVA into OVA orally tolerant mice inhibits footpaw swelling triggered by carrageenan
The next experiments were conducted in order to investigate whether the indirect and systemic effects of tolerated antigen injection were also able to inhibit inflammatory reactions triggered by agents independent of immunological lymphocyte activation. We choose footpaw swelling induced by carrageenan, a widely used model of inflammation.
To induce oral tolerance to OVA, Swiss mice were made to drink an egg white solution for 3 days; control mice drank tap water. Seven days after oral treatment, control and tolerant mice were challenged s.c. with carrageenan in the right hindpaw; the contralateral paw received saline as a control.
To test the inhibitory effects of re-exposure to a tolerated antigen, mice were challenged i.p. with OVA + Al(OH)3, 24 hr before carrageenan injection; a control group received only carrageenan. Footpaw swelling was measured 24, 48 and 72 hr after carrageenan injection.
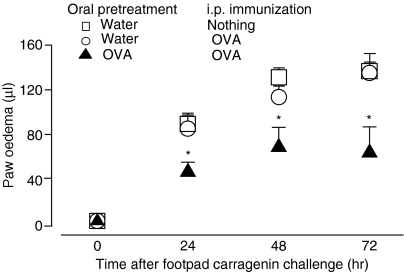
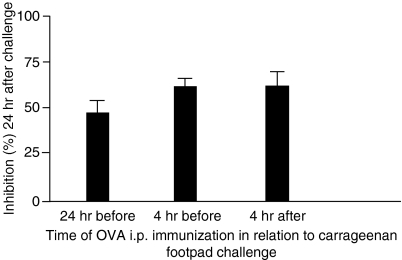
The results shown in Fig. 5 demonstrate that, in mice not tolerant to OVA and immunized i.p. with OVA 24 hr before carrageenan injection, footpaw swelling did not differ from that in controls injected with only carrageenan. However, the same treatment in mice previously made orally tolerant to OVA resulted in significant inhibition of paw oedema at all measurement times. Further experiments tested the effect of OVA injection into OVA-tolerant mice at different times, before or after carrageenan injection. Figure 6 shows that inhibition was triggered by OVA injection even 4 hr after carrageenan injection.
Figure 5.
Injection of ovalbumin (OVA) into OVA orally tolerant mice inhibits development of the delayed phase of carrageenan-induced paw oedema. Animals that had previously been treated with OVA orally (closed triangles) or that had been drinking only water (open circles) were injected with OVA plus Al(OH)3 intraperitoneally (i.p.) 24 hr before the intraplantar injection of carrageenan. The control group (open squares) consisted of animals that received only carrageenan. Each point represents the mean of six to eight animals and vertical lines are the standard error of the mean (SEM). *P<0·05 compared with the control group.
Figure 6.
Injection of ovalbumin (OVA) into OVA orally tolerant mice, at different time intervals, inhibits carrageenan-induced paw oedema. Mice submitted to oral OVA treatment were immunized with OVA plus Al(OH)3 at 24 hr before, 4 hr before, or 4 hr after paw oedema initiation with carrageenan. Values are mean ± standard error of the mean (six to eight animals), indicating the oedema inhibition percentage at 24 hr compared with the control carrageenan group.
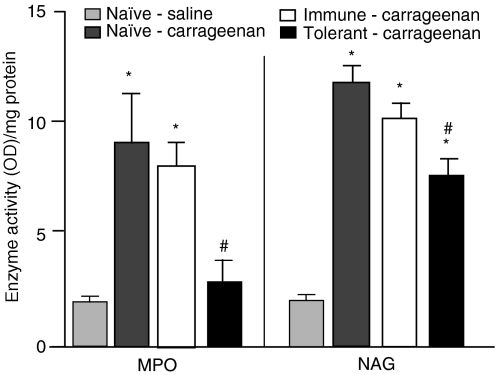
In these same experimental groups, we quantified MPO and NAG activity as indirect indicators of polimorphonuclear and mononuclear cell influx into the footpads, respectively. An additional group of naïve mice that had not received carrageenan injections was added as a control. Figure 7 shows that the i.p. injection of OVA into mice previously made orally tolerant to OVA resulted in significant inhibition of MPO and NAG activity, reflecting a reduced influx of leucocytes into the footpad. Thus, the inhibitory effect triggered by the injection of a tolerated antigen may also block unspecific inflammation.
Figure 7.
Injection of ovalbumin (OVA) into OVA orally tolerant mice inhibits polymorphonuclear (PMN) and mononuclear (MN) leucocyte influx into inflamed paws injected with carrageenan. Myeloperoxydase (MPO) and N-acetyl-glucosaminidase (NAG) activities were quantified as indirect indicators of PMN and MN cell influx, respectively. Animals that had been drinking only water (white bars) or water plus OVA (black bars) received an intraperitoneal (i.p.) injection of OVA plus Al(OH)3 24 hr before paw oedema initiation with carrageenan. Enzyme activity was measured 24 hr after carrageenan injection. Control groups were naïve animals that received only a carrageenan intraplantar injection (positive control; dark grey bars) or a saline intraplantar injection (negative control; light grey bars). Values are mean ± standard error of the mean (six to eight animals). *P<0·05 compared with the negative control group; #P<0·05 compared with the positive control group.
Discussion
Oral tolerance is quite efficient in blocking DTH reactions and IgG1 and IgE antibody formation and in preventing inflammatory immune reactions in allergy and in other models of autoimmune diseases.29 Oral tolerance to OVA also prevents OVA-induced peritonitis and its associated bone marrow eosinopoiesis.7 What is not usually taken into account is that parenteral re-exposure to a tolerated antigen triggers a wide range of inhibitory phenomena that may, for example, block the initiation of immune responses to a second unrelated antigen. This phenomenon has been named ‘innocent bystander suppression’10 and presently this seems to be the only available explanation.
Here we have shown that the inhibitory effect triggered by the injection of OVA into OVA orally tolerant mice may block DTH reactions to KLH, HB-induced peritonitis and paw oedema induced by carrageenan. What, then, is the mechanism by which tolerated antigen injection reduces inflammation?
Regardless of the experimental model chosen, the hallmark of inflammation is leucocyte migration. Using the antigen-induced peritonitis model we have previously shown that oral tolerance to OVA blocks OVA-induced eosinophil, neutrophil and macrophage increases in the peritoneal cavity.7 Oral tolerance also blocked blood neutrophil and eosinophil increases and bone marrow eosinopoiesis, but not neutropoiesis.7 Thus, it seems that injection of tolerated antigen may interfere with the three aspects of leucocyte tissue infiltration, i.e. bone marrow production, release into blood and migration into peripheral tissues. Acute inflammation triggered by carrageenan injection into the footpad is also dependent on leucocyte migration. The onset of carrageenan-induced local inflammation has been linked to neutrophil infiltration and the production of neutrophil-derived free radicals, such as hydrogen peroxide, superoxide and hydroxyl radicals, as well as to the release of other neutrophil-derived mediators.30 However, in contrast to DTH and peritonitis, where lymphocytes are clearly involved, paw oedema induced by carrageenan is a case of non-immune inflammation, in which lymphocytes seem to play only a limited role.31 In conclusion, the present observations add a new dimension to the problem by showing that the bone marrow production of eosinophils may be affected by injection of tolerated proteins into the peritoneal cavity. In addition, we have shown that unspecific inflammatory reactions, such as those triggered by carrageenan, are also inhibited by tolerated antigen injection.
Bystander suppression is presently thought to be related to the effect of T regulatory cells. Transforming growth factor (TGF)-β-secreting Th3 regulatory cells have been described after oral tolerance induction and it is believed that they are triggered in an antigen-specific fashion but act in an antigen-non-specific fashion, thus mediating ‘bystander suppression’ when they encounter the fed autoantigen at the target organ.32,33 Another subtype of T regulatory cell is characterized by the expression of CD4+ CD25+ Foxp3+ molecules34 and may act either by suppressing naïve bystander T cells directly or by preventing antigen-presenting cells from priming naïve T cells.35 Yet, the cellular basis of this phenomenon remains unclear, and how naïve T cells specific for the bystander antigen can be suppressed is not known.35 Even if bystander effects can occur in the cases we have investigated, this is insufficient to explain the systemic inhibitory phenomena.
We have previously argued that these inhibitory effects do not require contiguity of the injections of the two proteins.13 Furthermore, once triggered, the inhibitory effect persists at least for 3 days in the absence of further contact with the tolerated protein.11 More revealing, perhaps, is the fact that parenteral re-exposure to tolerated antigens is unable to block ungoing (i.e. secondary) immune activities,11 which, in itself, strongly argues against the bystander hypothesis. We previously showed that even the injection of self-component in adjuvants may trigger the inhibitory effects,36 whereas re-exposure to the tolerated antigen by a non-imunogenic route (oral or soluble intravenous) has no inhibitory effects.13 It remains to be investigated whether immune responses triggered in the absence of adjuvants are inhibitory. It is possible, therefore, that the re-exposure to a tolerated antigen under immunogenic conditions triggers presently undefined systemic effects that are able to interfere with a wide array of organism activities.
In several areas of biological investigation the advantages of a systems approach are being evaluated. The main tenets of immunological theory are intended to account for ‘clonal’ or ‘specific’ cellular activities. The present phenomena, which we have called indirect effects of tolerated antigens, deal with the effect of the tolerated antigens upon the organism as a whole, interfering with its ability to mount primary immune responses to antigens not previously encountered and apparently blocking inflammatory reactions. The general significance of these findings awaits further experimentation. Lord Rayleigh, the 1904 Nobel Laureate in physics, asserted that one should ‘neither seek nor avoid complexity’ in finding appropriate solutions to problems.
Acknowledgments
We thank Dr Jamil Assreuy (Universidade Federal de Santa Catarina, Brazil) for kindly supplying carrageenan and for the use of his laboratory. The research was supported by FAPEMIG and CNPq (Brazil).
Glossary
Abbreviations
- Al(OH)3
aluminium hydroxide
- BM
bone marrow
- CFA
complete Freund’s adjuvant
- DNP
dinitrophenylated
- DTH
delayed-type hypersensitivity
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- Hb
snail haemoglobin
- HBSS
Hank’s balanced salt solution
- i.p.
intraperitoneal
- IL
interleukin
- KLH
keyhole lympet haemocyanin
- MPO
myeloperoxidase
- NAG
N-acetyl-glucosaminidase
- OPD
orthophenylene-diamine
- OVA
ovalbumin
- PBS
phosphate-buffered saline
- PLF
peritoneal lavage fluid
- RT
room temperature
- s.c.
subcutaneous
- TNF
tumour necrosis factor
References
- 1.Hanson DG, Vaz NM, Maia LC, Hornbrook MM, Lynch JM, Roy CA. Inhibition of specific immune responses by feeding protein antigens. Int Arch Allergy Appl Immunol. 1977;55:526–32. doi: 10.1159/000231966. [DOI] [PubMed] [Google Scholar]
- 2.Richman LK, Chiler JM, Brown WR, Hanson DG, Vaz NM. Enterically-induced immunological tolerance – I. Indution of supressor T limphocytes by intragastric administration of soluble protein antigens. J Immunol. 1978;121:2429–34. [PubMed] [Google Scholar]
- 3.Gautam SC, Battisto JR. Suppression of contact sensitivity and cell-mediated lympholysis by oral administration of hapten is caused by different mechanisms. Cell Immunol. 1983;78:295–304. doi: 10.1016/0008-8749(83)90284-8. [DOI] [PubMed] [Google Scholar]
- 4.Mowat AM. The role of antigen recognition and suppressor cells in mice with oral tolerance to ovalbumin. Immunology. 1985;56:253–60. [PMC free article] [PubMed] [Google Scholar]
- 5.Lamont AG, Bruce MG, Watret KC, Ferguson A. Suppression of an established DTH response to ovalbumin in mice by feeding antigen after immunization. Immunology. 1988;64:135–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Russo M, Nahori MA, Lefort J, et al. Suppression of asthma-like responses in different mouse strains by oral tolerance. Am J Respir Cell Mol Biol. 2001;24:518–26. doi: 10.1165/ajrcmb.24.5.4320. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues CM, Martins-Filho OA, Vaz NM, Carvalho CR. Systemic effects of oral tolerance on inflammation: mobilization of lymphocytes and bone marrow eosinopoiesis. Immunology. 2006;117:517–25. doi: 10.1111/j.1365-2567.2006.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–57. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaz NM, Hanson DG, Maia LCS, Lynch JL. Cross-suppression of specific immune responses after oral tolerance. Memórias do Instituto Oswaldo Cruz. 1981;76:83–91. doi: 10.1590/s0074-02761981000100009. [DOI] [PubMed] [Google Scholar]
- 10.Miller A, Lider O, Weiner HL. Antigen driven bystander suppression after oral administration of antigen. J Exp Med. 1991;174:791–8. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho C, Verdolin B, Vaz N. Indirect effects of oral tolerance cannot be ascribed to bystander suppression. Scand J Immunol. 1997;45:276–81. doi: 10.1046/j.1365-3083.1997.d01-394.x. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho CR, Lenzi HL, Correa-Oliveira R, Vaz NM. Indirect effects of oral tolerance to ovalbumin interfere with the immune responses triggered by Schistosoma mansoni eggs. Braz J Med Biol Res. 2002;35:1195–9. doi: 10.1590/s0100-879x2002001000012. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho CR, Verdolin BA, Souza AV, Vaz NM. Indirect effects of oral tolerance in mice. Scand J Immunol. 1994;39:533–8. doi: 10.1111/j.1365-3083.1994.tb03410.x. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan KL, Murphy JW. Kinetics of cellular infiltration and cytokine production during the efferent phase of a delayed-type hypersensitivity reaction. Immunology. 1997;90:189–97. doi: 10.1046/j.1365-2567.1997.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Elliott JF, Mosmann TR. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J Immunol. 1994;153:3967–78. [PubMed] [Google Scholar]
- 16.Zuany-Amorim C, Leduc D, Vargaftig BB, Pretolani M. Characterization and pharmacological modulation of antigen-induced peritonitis in actively sensitized mice. Br J Pharmacol. 1993;110:917–24. doi: 10.1111/j.1476-5381.1993.tb13900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuani-Amorim C, Créminon C, Nevers MC, Nahori MA, Vargaftig B, Pretolani M. Modulation by IL-10 of antigen-induced IL-5 generation, and CD4+ T lymphocyte and eosinophil infiltration into the mouse peritoneal cavity. J Immunol. 1996;157:377–84. [PubMed] [Google Scholar]
- 18.Gaspar Elsas MI, Joseph D, Elsas PX, Vargaftig BB. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Respir Cell Mol Biol. 1997;17:404–13. doi: 10.1165/ajrcmb.17.4.2691. [DOI] [PubMed] [Google Scholar]
- 19.Cyr MM, Denburg JA. Systemic aspects of allergic disease: the role of the bone marrow. Curr Opin Immunol. 2001;13:727–32. doi: 10.1016/s0952-7915(01)00286-2. [DOI] [PubMed] [Google Scholar]
- 20.Garcia Leme J, Hamamura L, Leite MP, Rocha e Silva M. Pharmacological analysis of the acute inflammatory process induced in the rat’s paw by local injection of carrageenin and by heating. Br J Pharmacol. 1973;48:88–96. doi: 10.1111/j.1476-5381.1973.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 22.Di Rosa M, Willoughby DA. Screens for anti-inflammatory drugs. J Pharm Pharmacol. 1971;23:297–8. doi: 10.1111/j.2042-7158.1971.tb08661.x. [DOI] [PubMed] [Google Scholar]
- 23.Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, Currie MG. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 1996;118:829–38. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br J Pharmacol. 2005;145:141–4. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ovary Z, Benacerraf B. Immunological specificity of the secondary response with dinitrophenylated proteins. Proc Soc Exp Biol Med. 1963;124:72–6. doi: 10.3181/00379727-114-28589. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira SH. A new method for measuring variations of rat paw volume. J Pharm Pharmacol. 1979;31:648. doi: 10.1111/j.2042-7158.1979.tb13616.x. [DOI] [PubMed] [Google Scholar]
- 27.Henriques MG, Silva PM, Martins MA, Flores CA, Cunha FQ, Assreuy-Filho J, Cordeiro RS. Mouse paw edema. A new model for inflammation? Braz J Med Biol Res. 1987;20:243–9. [PubMed] [Google Scholar]
- 28.Barcelos LS, Talvani A, Teixeira AS, Cassali GD, Andrade SP, Teixeira MM. Production and in vivo effects of chemokines CXCL1-3/KC and CCL2/JE in a model of inflammatory angiogenesis in mice. Inflamm Res. 2004;53:576–84. doi: 10.1007/s00011-004-1299-4. [DOI] [PubMed] [Google Scholar]
- 29.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinegar R, Truax JF, Selph JL. Quantitative studies of the pathway to acute carrageenan inflammation. Fed Proc. 1976;35:2447–56. [PubMed] [Google Scholar]
- 31.Leme JG, Verissimo de Mello SB, Falcao RP, Rocha JR. Lymphocytes in non-immune inflammation: a specific subclass of lymphoid cells? Br J Exp Pathol. 1981;62:172–82. [PMC free article] [PubMed] [Google Scholar]
- 32.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 33.Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5:179–90. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- 34.Nagatani K, Sagawa K, Komagata Y, Yamamoto K. Peyer’s patch dendritic cells capturing oral antigen interact with antigen-specific T cells and induce gut-homing CD4(+)CD25(+) regulatory T cells in Peyer’s patches. Ann N Y Acad Sci. 2004;1029:366–70. doi: 10.1196/annals.1309.020. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carvalho CR, Vaz NM. Indirect effects are independent of the way of tolerance induction. Scand J Immunol. 1996;43:613–8. doi: 10.1046/j.1365-3083.1996.d01-261.x. [DOI] [PubMed] [Google Scholar]