Abstract
The interferon (IFN)-γ component of the immune response plays an essential role in combating infectious and non-infectious diseases. Induction of IFN-γ secretion by human T and natural killer (NK) cells through synergistic costimulation with interleukin (IL)-12 and IL-18 in the adaptive immune responses against pathogens is well established, but induction of similar activity in macrophages is still controversial, with doubts largely focusing on contamination of macrophages with NK or T cells in the relevant experiments. The possible contribution of macrophages to the IFN response is, however, an important factor relevant to the pathogenesis of many diseases. To resolve this issue, we analysed the production of IFN-γ at the single-cell level by immunohistochemistry and by enzyme-linked immunosorbent spot (ELISPOT) analysis and unequivocally demonstrated that human macrophages derived from monocytes in vitro through stimulation with a combination of IL-12 and IL-18 or with macrophage colony-stimulating factor (M-CSF) were able to produce IFN-γ when further stimulated with a combination of IL-12 and IL-18. In addition, naturally activated alveolar macrophages immediately secreted IFN-γ upon treatment with IL-12 and IL-18. Therefore, human macrophages in addition to lymphoid cells contribute to the IFN-γ response, providing another link between the innate and acquired immune responses.
Keywords: cytokines, human, inflammation, innate immunity, monocytes/macrophages
Introduction
The principal source of interferon (IFN)-γ in the human immune response is generally believed to be T and natural killer (NK) cells,1 although more recent work has demonstrated that dendritic cells also secrete IFN-γ.2 The possibility of a role for cells of myeloid origin as an additional source of IFN-γ is either controversial3–6 or simply ignored.
There is, however, increasing evidence of IFN-γ secretion by cells of the myeloid lineage in response to a wide variety of pathogens and cytokines,6 such as interleukin (IL)-12 and IL-18.3 These two cytokines contribute to the polarization of T cells to a T helper type 1 (Th1) phenotype and may also induce the differentiation of monocytes to macrophages while simultaneously triggering the production of chemokines attracting monocytes and granulocytes, such as CXC chemokine ligands (CXCL)8, CXCL9 and CXCL10,6 as well as stimulating the phosphorylation of the signal transducer and activator of transcription 4 (STAT-4) protein.7
Nevertheless, the production of IFN-γ by monocytes or macrophages has not always been completely reproducible, probably as a consequence of technical issues8 such as the purity of the isolated populations, the levels of stimulating cytokines and the variety of methods employed for detection of IFN-γ, the latter including enzyme-linked immunosorbent assay (ELISA), western blot and analysis of intracellular cytokine staining by flow cytometry.6 More importantly, the suspicion that small numbers of NK or T cells contaminating macrophage cultures could account for the observed levels of IFN-γ has been a frequent criticism.8 And, indeed, in our hands a contamination of 1 in 500 of NK cells in freshly isolated monocyte cultures stimulated with IL-12 and IL-18 can account for significant amounts of IFN-γ in culture supernatants (our unpublished data). Demonstration of IFN-γ production by macrophages at the single-cell level would unequivocally resolve this issue. However, when this has been attempted using flow cytometry, non-specific staining and autofluorescence of macrophages have contributed to unacceptable fluorescence background levels. As a further complication, costimulation with IL-12 and IL-18 through up-regulation of intercellular adhesion molecule 1 (ICAM-1)9 creates macrophage aggregates that are difficult to dissociate and thus analyse by flow cytometry.7 Finally, it has been suggested that the IFN-γ detected in macrophages could have been the secreted product of contaminating lymphoid cells that subsequently bound to the IFN-γ receptor on macrophages and then became internalized.10
As IFN-γ plays an important role in both innate and acquired immunity, its cellular origin is a critical question. In order to resolve whether monocytes and macrophages can also be a source of IFN-γ upon IL-12 and IL-18 stimulation, we have studied the production of IFN-γ by macrophages at the single-cell level, as previously reported for dendritic cells,2 specifically using both immunohistochemistry and enzyme-linked immunosorbent spot (ELISPOT) techniques. This experimental design has the advantage of specifically detecting as few as 1 in 1000 contaminating cells secreting IFN-γ.11 Freshly isolated human macrophages were first derived from monocytes by costimulation in vitro with either IL-12 plus IL-187 or macrophage colony-stimulating factor (M-CSF). The cells secreting IFN-γ in these cultures, clearly identified as macrophages by their size, morphology and expression of CD68,11 were unequivocally demonstrated to secrete IFN-γ by further culture with IL-12 plus IL-18.
The relevance of this finding is emphasized by the demonstration that naturally activated bronchoalveolar lavage (BAL) macrophages produced high levels of IFN-γ directly upon similar stimulation with IL-12 and IL-18. Thus, the contribution of IFN-γ to the immune response must now be extended to include a role for macrophages, which in turn must be taken into account in understanding the interaction of the innate and acquired immune responses in health and disease.
Materials and methods
Subjects
Peripheral blood mononuclear cells (PBMC) were obtained from 10 donors and BAL from five individuals who underwent flexible bronchoscopy for diagnosis of lung cancer but were subsequently diagnosed as negative. Recovery of BAL was performed using three lavages of 50-ml aliquots of sterile saline solution. The liquid recovered after instillation of the first aliquot was discarded, so that the fluid analysed was not contaminated with bronchial cells. Differential counts were performed using flow cytometry.7
The study was approved by the Ethical Committee of the Hospital Germans Trias i Pujol, and the subjects gave written consent. The procedures followed in the study were in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Cell isolation using magnetic beads and phenotypic analysis
PBMC were obtained using Ficoll-Paque density gradient centrifugation. The CD4+ T-cell fraction and alveolar macrophages were purified from PBMC and BAL, respectively, by negative selection (StemCell Technologies Inc., Vancouver, Canada). Monocytes were purified from PBMC by positive selection using Macs CD14 microbeads (Miltenyi Biotech SL, Madrid, Spain) according to the manufacturer’s instructions.7 The purity of the populations was assessed by flow cytometry. The cells were stained with anti-CD14, anti-CD3 and anti-CD56 antibodies (BD Biosciences, Madrid, Spain) (Fig. 1). A minimum of 3 × 103 cells were acquired with a FacsCalibur (BD Biosciences). The lymphocyte, monocyte and macrophage regions were identified by forward and size scatter (Fig. 1, first row). After purification, the monocyte populations were shown to consist of > 98% CD14+ cells, < 0·5% T cells and < 0·05 CD56+ NK cells (Fig. 1, second column). Furthermore, differential counts on the isolated monocytes were performed on cytospin preparations stained with May-Grünwald-Giemsa (Merk, VWR, Barcelona, Spain), with 500 cells being counted. Cytospins of the cell cultures were routinely stained with anti-CD68 (Dako, Barcelona, Spain) prior to analysis of production of IFN-γ, and all cultures contained > 98% CD68-positive cells.
Figure 1.
Purity of monocyte cultures. Peripheral blood mononuclear cells (first column), freshly isolated monocytes (second column) and monocytes induced to differentiate in vitro in the presence of macrophage colony-stimulating factor (M-CSF), granulocyte–monocyte colony-stimulating factor (GM-CSF) plus interleukin (IL)-4, and IL-12 plus IL-18 for 1 week (third to fifth columns) were stained with anti-CD14, anti-CD3 and anti-CD56 [for monocytes, T cells and natural killer (NK) cells, respectively] and 3 × 103 cells were acquired as described in the Materials and methods. The forward and side scatter dot plots show lymphocytes and monocytes (top panels), CD3 T cells (middle panels) and NK cells (bottom panels) present in the cultures.
Differentiation of monocytes
Purified monocytes were cultured at a concentration of 1 × 106 cells/ml in RPMI-1640 medium (Gibco BRL, Rockville, MD) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen, Madrid, Spain), 100 U/ml penicillin and 100 μg/ml streptomycin, and kept at 37° in 5% CO2. The cells were induced to differentiate in the presence of IL-12 (100 ng/ml; Peprotech, London, UK) plus IL-18 (100 ng/ml; Bionova, Madrid, Spain), M-CSF (100 ng/ml; Peprotech), or granulocyte–monocyte colony-stimulating factor (GM-CSF) (1000 U/ml; Peprotech) plus IL-4 (1000 U/ml, Peprotech). After 6 days, the cells incubated with IL-12 plus IL-18 or with M-CSF alone contained monocyte-derived macrophages, whilst those incubated with GM-CSF contained immature dendritic cells. The differentiated monocytes were washed and used in further experiments. The purity of these cell populations was assessed by flow cytometry. The cells were detached with a cell scraper and stained with anti-CD14, anti-CD3 and anti-CD56 (Fig. 1, last three columns). The levels of NK or T cells in these cultures were < 0·1%, indicating that there was no selective growth of NK or T cells in these cultures. Furthermore, the macrophage nature of these cells was confirmed by their morphology after staining of cytospins with May-Grünwald-Giemsa (Merk, VWR) and positive staining with anti-CD68 (Fig. 2g).
Figure 2.
Production of interferon (IFN)-γ by mononuclear cells stimulated with anti-CD3 and anti-CD28 and by monocyte and macrophage cultures stimulated with interleukin (IL)-12 plus IL-18. The figure shows cytospins of (a, b) peripheral blood mononuclear cells (PBMC) stimulated with anti-CD3 and anti-CD28 plus IL-2 and IL-4 for 1 week and then re-stimulated for another 4 hr with phorbol myristate acetate (PMA), ionomycin and monensin; (c, d) control freshly isolated monocytes, and (e, h) macrophages induced to differentiate from monocyte cultures with IL-12 plus IL-18 for 1 week and then re-stimulated with IL-12 plus IL-18 for 72 hr. The cytospins were stained with (a, c, e) anti-IFN-γ (clone B27), (g) anti-CD68, or (b, d, f, h) an isotype control mouse immunoglobulin, and counterstained with haematoxylin. Cells were micrographed using a Zeiss Axioscop (Zeiss Spain, Barcelona, Spain) coupled to a Hamamatsu C5810 (Hamamatsu Spain, Cerdanyola, Spain). Magnification ×400. Note the difference in size and morphology between PBMC and monocyte/macrophage lineage cells.
Stimulation of macrophages to secrete IFN-γ
Macrophages derived in vitro with IL-12 and IL-18 or M-CSF or recovered in BAL (see above) were cultured in the presence or absence of IL-12 (100 ng/ml) or IL-18 (100 ng/ml) or IL-12 (100 ng/ml) plus IL-18 (100 ng/ml) for 72 hr.
Detection of IFN-γ by immunohistochemistry
As a positive control, PBMC were cultured in plates (BD Biosciences) coated with anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) in 0·05 m carbonate buffer, pH = 9·6 in the presence of recombinant IL-2 (10 units/ml; Roche, Barcelona, Spain) and recombinant IL-4 (1000 units/ml; Peprotech). The cells were then cultured for 48 hr, washed and cultured for an additional 3 days. Finally, the cultures were stimulated for another 4 hr with phorbol myristate acetate (PMA) at 5 ng/ml (Sigma, Barcelona, Spain) and ionomycin at 500 ng/ml (Sigma) in the presence of monensin (Golgi Stop; BD Biosciences), as recommended by the manufacturer.
For detection of IFN-γ, the cells were harvested, cyto-centrifuged and fixed with 5% formaldehyde in phosphate-buffered saline (PBS), and the production of IFN-γ was detected by immunohistochemistry using a cytokine detection kit (Anti-mouse IG HRP detection Kit; BD Biosciences). Two different clones of anti-IFN-γ (clone B27 and clone K3; BD Biosciences) were tested and gave identical results. After the primary antibodies had been washed off, the slides were incubated with biotinylated secondary goat anti-mouse immunoglobulin (BD Biosciences) followed by horseradish peroxidase (HRP)-labelled streptavidin (BD Biosciences), visualized with diaminobenzidine (DAB) solution containing hydrogen peroxide (BD Biosciences) and counterstained with haematoxylin (Dako). Appropriate isotype-matched purified mouse immunoglobulins were used as negative controls (BD Biosciences).
Detection of IFN-γ by ELISPOT
Freshly purified PBMC and CD4+ T cells were cultured in triplicate at 105, 104 and 103 cells/well and differentiated monocytes and alveolar macrophages were similarly cultured at 104, 103 and 102 cells/well in 96-well PVDF plates (Millipore, Barcelona, Spain) sensitized with anti-IFN-γ at 1 μg/ml in PBS (Diaclone, Besancon, France) and stimulated with IL-12 (100 ng/ml), IL-18 (100 ng/ml), or IL-12 plus IL-18. Production of IFN-γ in the ELISPOT assay was detected using a biotinylated anti-IFN-γ antibody (100 ng/ml), followed by an alkaline phosphatase streptavidin conjugate (Amersham Biosciences, Uppsala, Sweden), and visualized with a solution of 4-nitro-bleu tetrazolium chloride and 5-bromo-4 chloro-3-indolyl phosphate, as recommended by the manufacturer (Sigma). As a positive control, PBMC and isolated CD4 T cells were stimulated with phytohaemagglutinin (PHA), at 1 μg/ml (Sigma).11
Detection of IFN-γ by ELISA
To compare the levels of total secreted IFN-γ with the results of the immunohistochemistry and the ELISPOT analysis, supernatants from five independent experiments were analysed for secreted IFN-γ by conventional sandwich ELISA (OptEIA; BD Biosciences) according to the manufacturer’s instructions.
Results
Isolated monocytes produce low levels of IFN-γ upon stimulation with IL-12 and IL-18
As a positive control, IFN-γ production was clearly demonstrated by immunocytochemistry in T cells stimulated for 72 hr with anti-CD3, anti-CD28, recombinant IL-2 (rIL-2) and rIL-4 followed by 4 hr of incubation with PMA, ionomycin and monensin (Fig. 2a). The simultaneously examined isotype control was negative (Fig. 2b). Similarly, using the ELISPOT technique, while PBMC cultured in medium alone did not yield any IFN-γ (Fig. 3, lane 1), activation of PBMC or freshly isolated CD4+ T cells with PHA or with IL-12 and IL-18 for 72 hr was so efficient that essentially confluent positivity was observed on the IFN-γ ELISPOT membrane. As cells activated either with PHA or with IL-12 plus IL-18 gave very similar results, only data for cells activated with IL-12 plus IL-18 are shown (Fig. 3, lanes 2 and 3).
Figure 3.
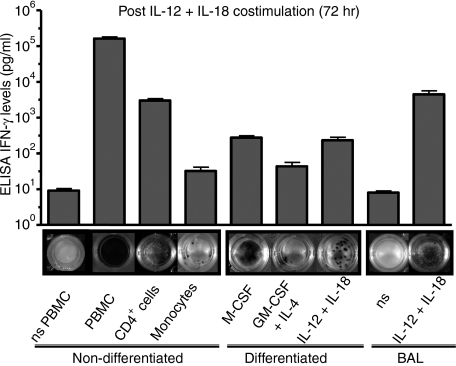
Production of interferon (IFN)-γ by different cell subsets subjected to various stimuli measured by enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunosorbent spot (ELISPOT) assays. The figure shows the production of IFN-γ, determined by ELISA analysis of supernatants (vertical bars) and ELISPOT analysis of cells (the photographs below), for peripheral blood mononuclear cells (PBMC) (lanes 1 and 2), CD4 T cells (lane 3), and monocytes/macrophages (lanes 5–9). The cells were cultured at 104 cells per well for 72 hr in the presence of medium [non-stimulated (ns)] (lanes 1 and 8) or interleukin (IL)-12 and IL-18 (lanes 2–7 and 9). The first lane shows the production of IFN-γ by freshly isolated PBMC cultured with medium. The next three lanes show the production of this cytokine by PBMC (lane 2), CD4 T cells (lane 3) and monocytes (lane 4) cultured in the presence of IL-12 and IL-18. The next three lanes show the production of IFN-γ by monocytes previously induced to differentiate into macrophages with macrophage colony-stimulating factor (M-CSF) (lane 5) or IL-12 plus IL-18 (lane 6), and monocytes induced to differentiate into dendritic cells with granulocyte–monocyte colony-stimulating factor (GM-CSF) plus IL-4 and then re-stimulated with IL-12 and IL-18 for 72 hr (lane 7). Lanes 8 and 9 show the production of IFN-γ by bronchoalveolar lavage (BAL) macrophages, cultured in medium alone (lane 8) or with IL-12 plus IL-18 for 72 hr. The bars shows the mean and standard error of the mean for five experiments. The ELISPOT is for one representative experiment of these five experiments. Plates were photographed with a Gel Logic 440 Imaging system (Kodak).
In contrast, monocytes cultured for 3 days in the presence of IL-12 and IL-18 produced insignificant amounts of IFN-γ, as determined by immunocytochemistry staining (Fig. 2c and d). These latter results were also confirmed by the detection of corresponding levels of IFN-γ in culture supernatants by ELISA (n = 5) (Fig. 3, lane 4).
Macrophages derived from monocytes produce high levels of IFN-γ upon in vitro stimulation with IL-12 and IL-18
As monocytes failed to secrete significant amounts of IFN-γ when stimulated for 3 days with IL-12 and IL-18, we then tested the capacity of macrophages derived from monocytes to respond to these two cytokines. Monocytes were induced to differentiate into macrophages by culture for 7 days either with IL-12 plus IL-18 or with M-CSF as previously described.7 Monocytes similarly cultured with GM-CSF plus IL-4 yielded immature dendritic cells. The purity of the resulting population was > 98%, demonstrating that there was no selective growth of T or NK cells during the culture period (Fig. 1, last three columns). Most importantly, unequivocal identification of the cells induced to differentiate with IL-12 plus IL-18 or with M-CSF as macrophages was confirmed by (i) their morphology, as monocyte-derived macrophages have three times the diameter of T cells or large granular lymphocytes (Fig. 2e–h), and (ii) positive staining with anti-CD68 antibody specific for macrophages (Fig. 2g). After this initial week of culture, the cells induced to differentiate with IL-12 plus IL-18 stained only weakly for IFN-γ (Fig. 4b), while IFN-γ was undetectable in the cells cultured with M-CSF or with GM-CSF plus IL-4 (Fig. 4c and d). Re-stimulation with IL-12 and IL-18 of macrophages derived from monocytes by culture either with IL-12 plus IL-18 or with M-CSF clearly resulted in the stimulation of IFN-γ synthesis, as observed in both immunohistochemistry (Fig. 4g and h) and ELISPOT assays (Fig. 3, lanes 5 and 7). Control cultures without cytokines (Fig. 4f), or monocytes induced to differentiate into immature dendritic cells with GM-CSF and IL-4, showed low expression of IFN-γ (Fig. 4i), and yielded relatively few ELISPOTs compared with the macrophage cultures (Fig. 3, lane 6). Similarly, the macrophages differentiated from monocytes were never stimulated to secrete IFN-γ when cultured with medium and IL-12 or IL-18 alone (data not shown); only the combination of the two cytokines was effective in stimulating macrophages to secrete IFN-γ.
Figure 4.
Production of interferon (IFN)-γ by cells of the monocyte lineage at different stages of differentiation demonstrated by immunohistochemistry staining. Freshly isolated monocytes were incubated in medium (first column) or with interleukin (IL)-12 plus IL-18 (second column) or macrophage colony-stimulating factor (M-CSF) (third column) or were induced to differentiate into dendritic cells with granulocyte–monocyte colony-stimulating factor (GM-CSF) plus IL-4 (fourth column) for 7 days as described in the Materials and methods. All these populations, as well as freshly isolated macrophages obtained from bronchoalveolar lavages (fifth column), were cultured for another 72 hr in the presence of medium (a–e) or with IL-12 plus IL-18 (f–j). The cells were then cytospinned and immunostained with anti-IFN-γ antibodies (clone B27) (a–j) and clone K3 (not shown; staining was identical for the two antibodies). There was no staining of duplicate macrophage cultures with an isotype-matched mouse immunoglobulin control (k–o). This is a representative experiment of five. Plates were photographed with a Gel Logic 440 Imaging system (Kodak). Cells were micrographed using a Zeiss Axioscop (Zeiss Spain) coupled to a Hamamatsu C5810 (Hamamatsu Spain). Magnification ×100.
The results from the immunohistochemistry and ELISPOT analyses were consistent with parallel measurements of secreted IFN-γ in the culture supernatants (n = 5). Specifically, the mean [± standard error of the mean (SEM)] concentration of IFN-γ detected by ELISA was 29·0 ± 17·6 pg/ml for cells cultured with medium alone (data not shown); 275·0 ± 88·6 pg/ml for macrophages induced to differentiate with M-CSF; 231·7 ± 123·5 pg/ml for macrophages induced to differentiate with IL-12 + IL-18, and 43·44 ± 25·4 pg/ml for macrophages induced to differentiate with GM-CSF + IL-4 (Fig. 3).
Interestingly, the ELISPOTs in the cultures of monocyte-derived macrophages re-stimulated with IL-12 plus IL-18 were very large, as stimulation with IL-12 and IL-18 induces such a firm aggregation of cells that it is impossible to completely disrupt them by mechanical means (Fig. 5).7,9 These aggregates, however, dissociate after longer periods of incubation with IL-12 and IL-18. To demonstrate this, monocytes were induced to differentiate in vitro with IL-12 plus IL-18 for 1 and 2 weeks and then, following the standard experimental design, the cells were re-stimulated on ELISPOT plates with IL-12 and IL-18 for a further 72 hr. As predicted, the large aggregate spots observed in monocytes stimulated for 1 week changed with time to many small spots, correlating with dissociation of the aggregated cells, and being accompanied by increased production of secreted IFN-γ with time, as measured by ELISA (Fig. 5).
Figure 5.
Time-course of interferon (IFN)-γ secretion by monocytes induced to differentiate into macrophages with interleukin (IL)-12 and IL-18 stimulation. Monocytes were cultured in the presence of IL-12 and IL-18 for periods of 1 and 2 weeks and re-stimulated with IL-12 and IL-18 for a further 72 hr. During the IL-12 and IL-18-induced differentiation of monocytes into macrophages, cells aggregated to form clusters (c) that yielded the larger positive plaques observed in the enzyme-linked immunosorbent spot (ELISPOT) assays (b). With time, and further maturation of the cells, these clusters spontaneously disaggregated (c). There was increased production of IFN-γ with time as determined by the enzyme-linked immunosorbent assay (ELISA) (a). Plates were photographed with a Gel Logic 440 Imaging system (Kodak). Cells were micrographed using a Nikon eclipse TE-200 microscope (Nikon, Barcelona, Spain) coupled to a CCD Kappa camera (Kappa, Gleichen, Germany). Magnification ×100.
Lung macrophages produce IFN-γ upon IL-12 and IL-18 stimulation ex vivo
To determine whether macrophages induced to differentiate in vivo also produce IFN-γ upon IL-12 plus IL-18 costimulation, BAL consisting largely of macrophages (Fig. 4e) was tested. These cells, like macrophages induced to differentiate from monocytes in vitro, produced IFN-γ directly when introduced into the standard 72-hr ELISPOT assay in the presence of IL-12 plus IL-18 (Fig. 3, lane 9). Moreover, these BAL cells secreted levels of IFN-γ (9449 ± 2066 pg/ml) (Fig. 3, lane 9) as high as those of cultures of stimulated CD4 T cells (Fig. 3, lane 3). Significantly, BAL cells introduced into the 72-hr ELISPOT assay without IL-12 and IL-18 did not produce IFN-γ (Fig. 3, lane 8).
These results were confirmed by intracellular staining for IFN-γ in BAL cells stimulated with IL-12 plus IL-18 (Fig. 4j). Intracellular IFN-γ was not detected in BAL cells cultured in the absence of IL-12 plus IL-18 (Fig. 4e) or in IL-12 plus IL-18-stimulated BAL cells stained with control isotype antibodies (Fig. 4o).
Discussion
In this study we demonstrate that human macrophages, in addition to T cells and NK cells,1 can participate in the immediate innate immune response through their secretion of IFN-γ upon costimulation with IL-12 and IL-18. This was unequivocally demonstrated at the single-cell level using immunohistochemical and ELISPOT analysis and using both in vitro derived and in vivo activated macrophages, the former being isolated human monocytes that were first stimulated to differentiate into macrophages with IL-12 and IL-18 or M-CSF and the latter being alveolar macrophages. The differentiated macrophage populations were unambiguously identified as such by their morphology and expression of CD68. These results extend the role of the IFN-γ response and suggest a co-ordinated role for IL-12, IL-18 and IFN-γ activity in the innate immune response. Thus, macrophages, in addition to cells of lymphoid origin, have the capacity to produce IFN-γ,4–6,12 as indeed do dendritic cells.2
Previous attempts to demonstrate the secretion of IFN-γ by macrophages have produced inconclusive results, largely because IFN-γ secretion has been generally measured in bulk cultures and contamination with NK or T cells has been suspected to account for the amounts of IFN-γ detected.8 In addition, analysis at the single-cell level by flow cytometry after staining of macrophages for intracellular IFN-γ has encountered problems caused by non-specific fluorescence. Finally, as an added problem, stimuli such as IL-12 plus IL-18 induce the up-regulation of ICAM-1,9 and the consequent aggregation of macrophages, as observed in our cultures, impedes flow cytometric analysis. In contrast, the production of IFN-γ by human monocyte-derived dendritic cells challenged with mycobacterium has been convincingly demonstrated by three different methods: cytokine secretion assays, in situ hybridization and confocal microscopy.10
In this work, we have successfully measured the secretion of IFN-γ by human macrophages after costimulation with IL-12 plus IL-18 at the single-cell level using two independent techniques: immunocytochemistry and ELISPOT analysis. The immunocytochemistry procedure avoids the problem of aggregation and, in addition to quantifying the numbers of IFN-γ-positive cells, also permits confirmation of the cell type through morphology. Using this technique, the majority of cells secreting IFN-γ in IL-12 and IL-18-stimulated cultures were clearly macrophages, judging by both their size and morphology (Fig. 2). Nevertheless, this technique is at best only semiquantitative and does not eliminate the remote possibility that the IFN-γ detected in the major macrophage population was produced by a very small number of contaminating cells and subsequently captured and internalized through the IFN-γ receptor.10
As an alternative approach, therefore, the ELISPOT assay, which has the merit of detecting secretion of IFN-γ by single cells, was also employed. Here, the macrophages are first washed prior to the assay, and so the IFN-γ detected in the ELISPOT focus must be secreted and cannot be intracellular. As the majority of macrophages derived from monocytes by stimulation with IL-12 and IL-18 yielded IFN-γ-positive ELISPOTs, it is clear that the majority of the macrophage population secreted IFN-γ. In contrast, in the control monocyte cultures only 1 in 500 cells secreted IFN-γ (probably as a result of a small amount of lymphoid contamination). Thus, the possibility of minor contamination with lymphoid cells could not account for the numerous spots we observed.
We previously showed that IL-12 and IL-18 together had a direct effect on monocytes by promoting clustering and subsequent differentiation of monocytes into macrophages.7 As a result, these macrophage aggregates yielded large zones of positivity in the ELISPOT assays. With longer periods of incubation during the differentiation phase of the experiment, however, the aggregates dissociated and IFN-γ ELISPOTs corresponding to single cells were observed after further costimulation with IL-12 and IL-8.
Importantly, naturally activated bronchoalveolar macrophages were immediately sensitive to costimulation with IL-12 and IL-18 and their production of high levels of IFN-γ suggests that IFN-γ is similarly produced by alveolar macrophages in vivo, as indeed has been described for patients with pulmonary infections.13,14 As macrophages are the predominant leucocyte population in the lung,15 the observation that they produce IFN-γ upon costimulation with IL-12 and IL-18 is directly relevant to the pathogenesis of pulmonary infections. Furthermore, the elimination of intracellular parasites that escape the acquired immune response, such as Mycobacterium tuberculosis or Leishmania, is critically dependent on IFN-γ activity.16 In addition to macrophages, IL-12 is also produced by neutrophils,17 and keratinocytes, stromal cells and damaged endothelial cells are also sources of IL-18.18 Therefore, the levels of IL-12 and IL-18 found in the lung19 might be an important factor in the local activation of macrophages and hence the production of IFN-γ, a suggestion consistent with the finding that patients with deficiencies in the IFN-γ or IL-12 pathways frequently have intracytoplasmic mycobacterial infections.20
Our data also emphasize the importance of the combined synergistic effect of IL-12 and IL-18, both in the differentiation of monocytes into mature macrophages, and in subsequent stimulation of IFN-γ production. Neither IL-12 nor IL-18 alone mediated either of these processes. The requirement for two signals, as well as a threshold of responsiveness,7 provides the basis for a common control mechanism in many immunological reactions, such as T-cell activation through the T-cell receptor and CD28. An additional level of control is provided by the fact that IL-12 and IL-18 belong to two different families of cytokines, with IL-12 signalling through the STAT-4 pathway and IL-18 activating nuclear factor (NF)-κB through interleukin-1 receptor associated kinase (IRAK).21
In conclusion, this work raises the possibility that the production of IL-12 and IL-18 in response to a pathogen in vivo might be an early, specific trigger for the local production of IFN-γ by macrophages in the absence of an adaptive T-cell response or NK cells. The existence of such a component in the innate response is more than justified by the importance of IFN-γ in the control of intracellular pathogens,20 and extends the complexity and flexibility of the interactive innate/acquired immune response system, with relevance and impact in health and disease.
Acknowledgments
This work was funded in part by the Fundacio Irsicaixa and Red Temática de Investigación en SIDA (RG GO3/173). LD was supported by the Spanish Ministry of Education & Science through the ‘Juan de la Cierva’ Programme. The authors thank the patients who volunteered for this study, and the nursing staff who assisted at the study site.
References
- 1.Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, Okamura H, Nakanishi K. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12:151–60. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- 2.Fricke I, Mitchell D, Mittelstadt J, Lehan N, Heine H, Goldmann T, Bohle A, Brandau S. Mycobacteria induce IFN-gamma production in human dendritic cells via triggering of TLR2. J Immunol. 2006;176:5173–82. doi: 10.4049/jimmunol.176.9.5173. [DOI] [PubMed] [Google Scholar]
- 3.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–8. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golab J, Zagozdzon R, Stoklosal T, Kaminski R, Kozar K, Jakobisiak M. Direct stimulation of macrophages by IL-12 and IL-18–a bridge too far? Immunol Lett. 2000;72:153–7. doi: 10.1016/s0165-2478(00)00178-4. [DOI] [PubMed] [Google Scholar]
- 5.Frucht DM, Fukao T, Bogdan C, Schindler H, O'Shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–60. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan C, Schleicher U. Production of interferon-gamma by myeloid cells – fact or fancy? Trends Immunol. 2006;27:282–90. doi: 10.1016/j.it.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Coma G, Pena R, Blanco J, et al. Treatment of monocytes with interleukin (IL)-12 plus IL-18 stimulates survival, differentiation and the production of CXC chemokine ligands (CXCL)8, CXCL9 and CXCL10. Clin Exp Immunol. 2006;145:535–44. doi: 10.1111/j.1365-2249.2006.03145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schleicher U, Hesse A, Bogdan C. Minute numbers of contaminant CD8+ T cells or CD11b+CD11c+ NK cells are the source of IFN-{gamma} in IL-12/IL-18-stimulated mouse macrophage populations. Blood. 2005;105:1319–28. doi: 10.1182/blood-2004-05-1749. [DOI] [PubMed] [Google Scholar]
- 9.Stuyt RJ, Netea MG, Geijtenbeek TB, Kullberg BJ, Dinarello CA, van der Meer JW. Selective regulation of intercellular adhesion molecule-1 expression by interleukin-18 and interleukin-12 on human monocytes. Immunology. 2003;110:329–34. doi: 10.1046/j.1365-2567.2003.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finbloom DS. Regulation of cell-surface receptors for human interferon-gamma on the human histiocytic lymphoma cell line U937. Biochem J. 1991;274:775–80. doi: 10.1042/bj2740775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slyker JA, Lohman BL, Mbori-Ngacha DA, et al. Modified vaccinia Ankara expressing HIVA antigen stimulates HIV-1-specific CD8 T cells in ELISpot assays of HIV-1 exposed infants. Vaccine. 2005;23:4711–9. doi: 10.1016/j.vaccine.2005.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietila TE, Veckman V, Kyllonen P, Lahteenmaki K, Korhonen TK, Julkunen I. Activation, cytokine production, and intracellular survival of bacteria in Salmonella-infected human monocyte-derived macrophages and dendritic cells. J Leukoc Biol. 2005;78:909–20. doi: 10.1189/jlb.1204721. [DOI] [PubMed] [Google Scholar]
- 13.Fenton MJ, Vermeulen MW, Kim S, Burdick M, Strieter RM, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–56. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson BW, McLemore TL, Crystal RG. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985;75:1488–95. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bofill M, Lipman M, McLaughlin JE, Johnson MA, Poulter LW. Changes in lung lymphocyte populations reflect those seen in peripheral blood in HIV-1 positive individuals. Eur Respir J. 1998;11:548–53. [PubMed] [Google Scholar]
- 16.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis. 2003;3:578–90. doi: 10.1016/s1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 17.Cassatella MA, Meda L, Gasperini S, D’Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 18.Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–57. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 20.Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11:321–33. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 21.Schindler H, Lutz MB, Rollinghoff M, Bogdan C. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signalling and is inhibited by IL-4. J Immunol. 2001;166:3075–82. doi: 10.4049/jimmunol.166.5.3075. [DOI] [PubMed] [Google Scholar]