Abstract
The polyclonal cytokine-induced killer (CIK) cells exhibit potent cytotoxicity against a variety of tumour cells including autologous and allogeneic acute myeloid leukaemic (AML) targets. At maturity, three lymphocyte subsets: CD3− CD56+, CD3+ CD56− and CD3+ CD56+, constitute the bulk of the CIK cell culture. The CD3− CD56+ subset behaves like classical natural killer (NK) cells where cytotoxicity is potentiated by blocking the human leucocyte antigen Class I molecules in the AML targets. Both the CD3+ CD56+ and CD3+ CD56− subsets, though known to kill autologous and allogeneic targets to a comparable degree and therefore non-major histocompatibility complex (MHC)-restricted, nevertheless require the presence of the MHC molecule on the target, which interacts with their CD3–T-cell receptor complex. Although CIK cells are often termed ‘NK-like’ T cells, we have demonstrated that the well-characterized NK receptors KIR, NKG2C/E, NKG2D and DNAM-1 are not involved in the process of AML recognition for the CD3+ CD56− and CD3+ CD56+ subsets. The CD3+ CD56+ and CD3+ CD56− subsets express a polyclonal and comparable TCRVβ repertoire in a Gaussian distribution. The CD3+ CD56+ subset kills AML targets more efficiently than its CD3+ CD56− counterpart because of the presence of a higher proportion of CD8+ cells. The CD3+ CD56+ subset comprise more terminally differentiated late effector T cells that bear the CD27+ CD28− or CD27− CD28− phenotype, with a higher granzyme A content. In comparison, the phenotype of the CD3+ CD56− subset is consistent with early effector T cells that are CD27+ CD28+ and CD62L+, known to be less cytotoxic but possess greater proliferative potential.
Keywords: adoptive cellular immunotherapy, cytokine-induced killer cells, natural killer receptors, natural killer-like T cells, non-major histocompatibility complex-restricted T cells
Introduction
Expanded marrow or peripheral blood-derived cytokine-induced killer (CIK) cells are polyclonal T cells with potent non-major histocompatibility complex (MHC)-restricted cytotoxicity against a variety of tumour target cells.1–10 Earlier work had established the superiority of CIK cells over lymphokine-activated killer cells in terms of expansion and cytotoxicity. Potent in vitro cytotoxicity against various chemoresistant tumour cell lines,1 lymphoma2 and primary acute myeloid leukaemia (AML) cells3 had been reported. Furthermore, adoptive transfer of expanded CIK cells in mouse models could confer protection against lymphoma2–4 and chronic myeloid leukaemia (CML).5 Over the last few years, bioluminescent imaging has demonstrated the persistence of CIK cells in vivo and their ability to home to sites of tumour to eradicate tumour cells.6 In mismatched allogeneic transplant mouse models, allogeneic CIK cells caused relatively limited graft-versus-host disease compared to allogeneic splenocytes.10 Under stimulation by cytokine, it was feasible to expand CIK cells from peripheral blood of healthy donors and cancer patients who had undergone prior chemotherapy.11 Similar ex vivo protocols have been adopted to expand CIK cells from peripheral blood of patients under good manufacturing practice (GMP) conditions for clinical trials. These trials have reported the safety and efficacy of CIK cells in the treatment of patients with metastatic diseases,12 lymphoma13 and acute leukaemia.14
We had previously reported the potent cytotoxicity of CIK cells against AML targets.3 It was observed that a similar level of cytotoxicity could be demonstrated for CIK cells against autologous and allogeneic target cells of disparate MHC backgrounds.3 Similar to natural killer (NK) cells, prior immunogenic priming is not required for the observed cytotoxicity and the expanded CIK cells have therefore often been referred to as ‘NK-like T cells’.3,5,8,10 The bulk CIK culture is a heterogeneous population consisting of over 90% CD3+ cells, of which 35% coexpresses CD56 and the remaining cells are CD3+ CD56−. There is also a relatively small subset of CD3− CD56+ NK cells. Although the CIK cells are able to lyse autologous and allogeneic AML targets in a non-MHC-restricted fashion, we had earlier demonstrated that pre-absorption with K562 cells could only partially reduce the cytotoxicity of the bulk CIK cell culture against these targets.3 This indicates that the observed cytotoxicity exhibited by the CIK cells towards leukaemic target cells could not be totally accounted for by its NK-like activity, and that T cells also play a role in this process. Understanding the unique function of the various cell subsets and their mechanism of tumour recognition is important for the optimal application of CIK cell therapy in clinical practice. In this report, we further study the mechanism of AML target recognition and characterize the unique feature of each subset.
Materials and methods
CIK culture
Marrow aspirate of patients with AML collected at diagnosis was obtained with informed consent and in accordance with guidelines set forth by the Institutional Review Board. The CIK cells were cultured as previously described.3 In brief, mononuclear cells were cultured in 10% fetal calf serum/RPMI-1640 with interferon-γ (IFN-γ; Biodesign, Meridian Life Science, Saco, ME) added at the initiation of culture. OKT3 (Ortho Biotech, Raritan, NJ) and interleukin-2 (IL-2; Chiron, Amsterdam, the Netherlands) were added 24 hr later and the culture was maintained by addition of fresh medium every 3 days and of IL-2 every 6–7 days. The CIK cells were harvested and assays were performed at maturity after between 3 and 4 weeks of culture.
Flow cytometry
Cells before culture or CIK cells harvested at different time-points were washed, stained with various monoclonal antibodies (mAbs) at 4° for 15 min, and analysed by flow cytometry.
The following mAbs were used: anti-fluorescein isothiocyante (FITC) and anti-phycoerythrin (PE) immunoglobulin G (IgG) controls, anti-CD3 FITC, anti-CD56 PE, anti-CD8 FITC, anti-CD4 PE, anti-CD158a PE, anti-CD158b FITC, anti-CD94 FITC, anti-NKp30 PE, anti-NKp44 PE, anti-NKp46 PE and anti-CD107a peridinin chlorophyll protein (PerCP)-Cy5 (BD Pharmingen, San Diego, CA). Secondary FITC-labelled goat anti-mouse mAb was used after primary staining with purified anti-NKG2D, anti-DNAM-1 and anti-NKG2C antibodies. For the quantification of T-cell receptor (TCR) Vβ subfamilies, an IO test® Beta Mark TCRVβ repertoire kit was employed (Beckman Coulter, Inc., Fullerton, CA). For the phenotyping of memory T-cell subsets, mAbs used included anti-CD45RO, anti-CD62 ligand (CD62L), anti-CCR7, anti-CD27, anti-CD28, anti-CD127 (all PE-conjugated), and stained with either anti-CD56 FITC or anti-CD45RA FITC for coexpression. Additional assays involved the use of anti-CD3, anti-CD4, anti-CD8 or anti-CD45 PerCP-Cy5 as the third fluorescent colour for gating on the particular T-cell subset. For OKT3 stimulation of CIK cells, OKT3 at 50 ng/ml was added to effector cells. For intracellular staining of granzyme A, cells were first stained with anti-CD56 PE and then with anti-granzyme A FITC following permeabilization.
Cell sorting
The CIK cells were washed twice, incubated with CD56-conjugated microbeads (Miltenyi Biotec, Auburn, CA) and passed through an LS+ magnetic cell sorting separation column according to the manufacturer’s instructions (Miltenyi). The eluant contained the CD3+ CD56− subset while the positively selected CD56+ cells were collected by flushing the column. In some experiments, the CD56+ fraction was further sorted by flow cytometry into the CD3− CD56+ and CD3+ CD56+ subsets. This was achieved by staining the CD56+ cell fraction with FITC-conjugated anti-CD5 (anti-CD3 was not used in this situation to avoid activation of the CD3/TCR molecules). Purity of the sorted cells was verified by fluorescence-activated cell sorting (FACS) and was found to be consistently greater than 90%.
Cytotoxicity and blocking assays
Cytotoxicity was measured using a standard 51Cr-release assay. Effector cells were either bulk CIK cell culture or sorted cell subsets harvested at between 3 and 4 weeks of culture. Cryopreserved autologous or allogeneic AML blasts were thawed and cultured for 48 hr in medium containing granulocyte–macrophage colony-stimulating factor. Effector cells were added to target cells at E : T ratios of 1 : 1, 2·5 : 1, 10 : 1 and 40 : 1.3 In assays involving the blocking of effector cells, the following blocking mAbs were used: anti-CD158a (EB6), anti-CD158b (GL183), anti-CD94 (HP-3D9), anti-NKG2D (1D11), anti-DNAM-1 (DX11), anti-TCR-αβ (T10B9). Pooled human IgG (Intragam® P; CSL Ltd, Broadmeadows, Australia) was used to block FcγRIII on the NK subset to exclude antibody-dependent cell-mediated cytotoxicity (ADCC). These were added at concentrations of 20 μg/ml of effector cells for 30 min before plating. Blocking of the human leucocyte antigen (HLA) class I molecules on the target cells was achieved using W6/32 antibody added to 51Cr-labelled target cells 30 min before the final washing.
The cytotoxicity readings for each pair of assays without and with mAbs blocking at each E : T ratio were analysed by two-way paired Student’s t-test for any statistically significant difference (defined as P < 0·05) and the results are stated in the respective figure legends.
TCRVβ spectratyping
Complexity of the TCRVβ family repertoire was studied by CDR3 size spectratyping.15 Five micrograms total RNA purified from either mononuclear cells from fresh marrow samples or cultured CIK cells was reverse transcribed using SuperScript™ II reverse transcriptase with 1 μg oligo-dT and 100 ng random hexamer (Invitrogen Life Technologies Ltd, Strathclyde, UK). A constant 5′ FAM-labelled forward primer was paired with each of 26 Vβ region-specific reverse primers and employed for polymerase chain reaction (PCR).15 The PCR products were then analysed by capillary gel electrophoresis on the ABI prism® 3100-Avant (Applied Biosystems, Foster City, CA) using genescan analysis software. Routinely, 1 μl of the PCR product was added to a mixture of 0·15 μl Genescan Rox size standard and 9·85 μl HiDi Formamide in each tube, vortexed, and plated in a 96-well plate for counting. The resultant readout for each PCR-amplified Vβ family is represented by a histogram of 8–10 peaks each spanned by a space of three nucleotides. The pattern gives a qualitative representation of the T-cell repertoire either in a normal polyclonal or skewed oligoclonal distribution.
Quantification of NKG2C and NKG2E gene expression by real-time PCR
Real-time PCR assays were carried out using the RotorGene 2000 Real Time instrument (Corbett Research, Mortlake, NSW, Australia) in combination with the QuantiTect SYBR Green kit (Qiagen Inc., Valencia, CA) as the signal reporter. Firstly, 1 μg DNase-treated total RNA was reverse-transcribed to generate the first-strand complementary DNA (cDNA). This first-strand cDNA was then used as the template for subsequent PCR amplification either with the NKG2C or NKG2E specific primers pair.
The nucleotide sequences of the primers were as follows:
NKG2C: forward: 5′-GCTTTAGAAGTAAAGCATTTGCG-3′; NKG2C: reverse: 5′-GTCTGTACTTTAGTAATTGTGTGC-3′; NKG2E: forward: 5′-GGGATTACTGACACAAGCCA-3′; and NKG2E: reverse: 5′-TTTTCCAATCATAACGGTCTGC-3′.
The results obtained were analysed using the rotorgene 4.0 software. Gene expression level was calculated using the Relative Standard Curve method and the target values were normalized to β-actin, the endogenous control.
Results
Cytolysis of K562 cells by the expanded CIK cells is predominantly mediated by the CD3− D56+ subset
The expanded bulk CIK cell culture was a heterogeneous population consisting of a small fraction (2·23%) of CD3− CD56+ cells and > 90% CD3+ cells, of which 35% expressed CD56 while the remaining cells were CD3+ CD56−.
Expanded CIK cells were able to lyse the NK-sensitive K562 cells to variable degrees. However, the degree of lysis of K562 cells did not correlate with that of leukaemic target cells by the same effector. When the specific lysis of K562 was analysed in the context of the CD3− CD56+ subset, it was apparent that lysis of the K562 cells was linearly proportional to the percentage of CD3− CD56+ cells present in the bulk CIK cell culture, at various E : T ratios. The K562 cells were efficiently lysed by the relatively small fraction of CD3− CD56+ cells present (Fig. 1a). In contrast, there was no correlation between the % of CD3+ cells and K562 killing. When sorted CD3+ CD56+ and CD3+ CD56− cell subsets were studied, the observed cytotoxicity obtained against K562 cells was low, with medians of 7% and 12% for the CD3+ CD56+ and CD3+ CD56− subsets respectively at E : T of 40 : 1 (Fig. 1b). These observations suggest that the killing of the K562 is mediated by the small fraction of CD3− CD56+ NK subset in the bulk CIK culture, whereas the predominant CD3+ subset does not have any significant activity against this classical NK target which lacks HLA class I.
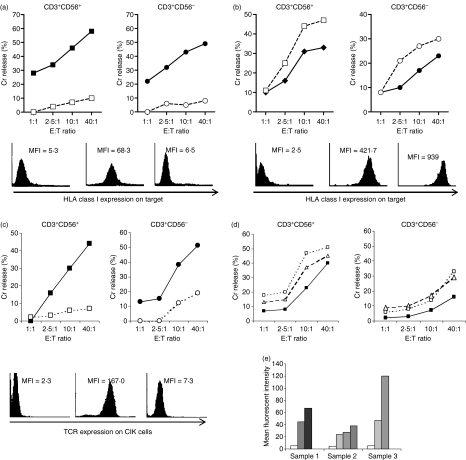
Figure 1.
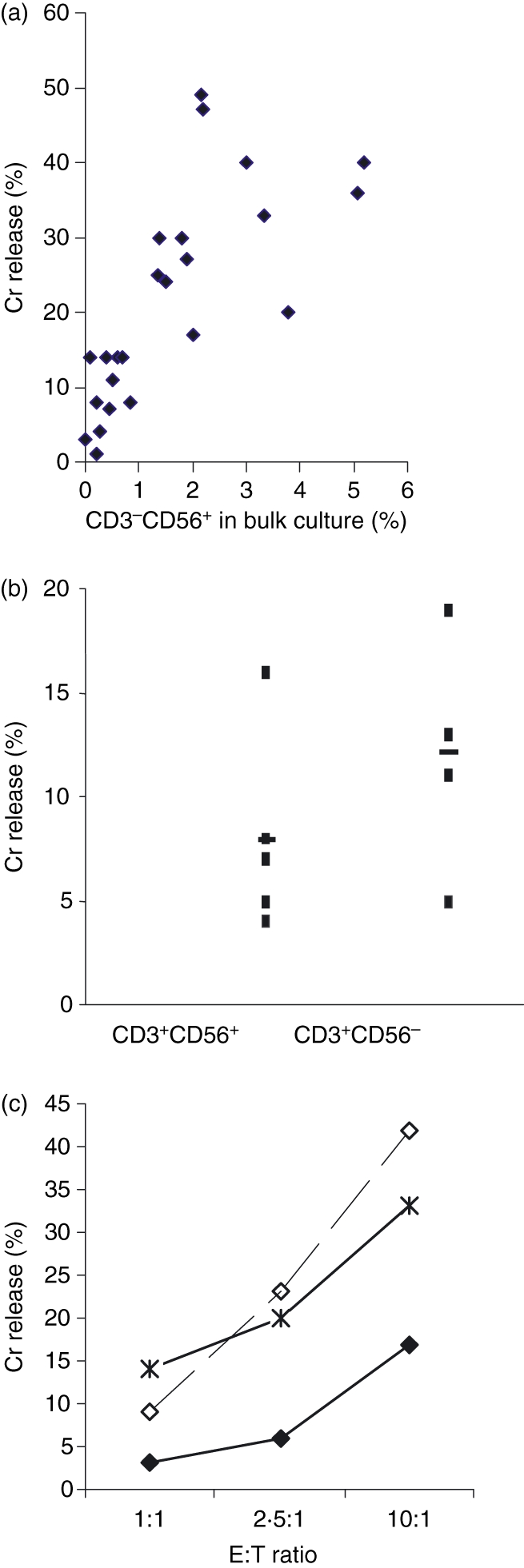
(a) Cytolysis of K562 target cells by cytokine-induced killer (CIK) cells was directly correlated with the proportion of CD3− CD56+ natural killer cell subset in the bulk CIK culture. A total of 27 cytotoxicity assays were performed and each dot represents the result of one reading of a CIK cell culture against K562 target at an effector to target (E : T) ratio of 10 : 1. Coefficient of correlation = 0·64. (b) Neither purified CD3+ CD56+ nor CD3+ CD56− cell subsets killed K562 target cells. Five separate experiments are shown for both cell subsets against K562 target cells at E : T ratio of 40 : 1, P = 0·224. (c) Cytotoxicity of the CD3− CD56+ cell subset (♦) against acute myeloid leukaemia target cells was enhanced when human leucocyte antigen class I molecules on the target cells were blocked (◊), to a degree similar to allogeneic killing ( ). Figure represents one of the two comparison sets done, P < 0·05 for both.
). Figure represents one of the two comparison sets done, P < 0·05 for both.
The CD3− CD56+ NK subset recognizes AML target by a classical NK mechanism
Since the small NK subset exhibits potent killing against K562 we expect it to kill AML target with the same classical NK mechanism. Sorted CD3− CD56+ subset showed modest cytotoxicity against the autologous AML target. When the class I molecules on autologous target were blocked by mAb W6/32, the cytotoxicity increased significantly to a level comparable to that against an allogeneic AML target. This is consistent with the mechanism of target recognition of the classical NK cell, where, because of the missing self MHC class I molecule, cytotoxicity is not suppressed by the inhibitory KIR on the NK cell (Fig. 1c). The enhanced killing after W6/32 blockade was not mediated by ADCC because it was not inhibited by the blocking of FcγRIII on the NK effector subset using pooled human IgG. Others have also shown that W6/32 binding on HLA class I of leukaemic target does not mediate ADCC by NK cells.16
The ‘NK-like’ CD3+ T cells recognize AML targets by a T-cell mechanism
We have previously shown that CIK cells are able to kill both autologous and allogeneic AML target cells equally well regardless of HLA type.3 In the above section we have shown that the small CD3− CD56+ NK subset in the bulk CIK culture is truly independent of MHC and recognizes AML target by an NK mechanism. Following this, we further investigated the CD3+ subsets, consisting of CD3+ CD56+ and CD3+ CD56− subsets, which made up the main bulk of CIK. The following series of complementary experiments on both CD3+ CD56+ and CD3+ CD56− subsets show that although these two CD3+ subsets were able to kill allogeneic AML targets without HLA-specificity, they were nevertheless dependent on the presence of MHC for induction of killing:
Blocking of HLA class I on AML targets by the pan-class I mAb W6/32 completely inhibited killing of AML targets by both CD3+ CD56+ and CD3+ CD56− subsets (Fig. 2a). Conversely, killing was enhanced when HLA class I expression on AML targets was up-regulated by prior treatment with IFN-γ (Fig. 2b).
Blocking of TCR on both CD3+ CD56+ and CD3+ CD56− subsets by T10B9, a blocking mAb for TCR, significantly reduced cytotoxicity of both against AML targets (Fig. 2c). Conversely addition of stimulating anti-TCR-αβ mAb or OKT-3 enhanced the cytotoxicity of both the CD3+ CD56+ and CD3+ CD56− subsets against AML targets (Fig. 2d).
The CD3+ subset degranulated in response to ligation by OKT3. The surface expression of CD107a, which reflects the degranulation process following T-cell activation, increased over time after OKT3 ligation (Fig. 2e). This is consistent with our observation of OKT3 potentiating CIK killing. All the above experiments were performed for CIK against both autologous and allogeneic AML targets.
Figure 2.
(a) Cytotoxicity of purified CD3+ CD56+ (▀) and CD3+ CD56− cells (•) against acute myeloid leukaemia (AML) targets were abrogated when the human leucocyte antigen (HLA) class I molecules on the target cells were blocked by the W6/32 monoclonal antibody (mAb; □,○). Figure shows one representative assay. Eight comparison sets were performed for the CD3+ CD56+ subset, P < 0·05 in seven out of eight sets. Eight comparison sets were performed for the CD3+ CD56− subset, P < 0·05 for all eight sets. Histograms show isotype control (left) and HLA class I expression on blasts without (middle) and with W6/32 blocking (right) respectively, of one representative assay. (b) Cytotoxicity of the CD3+ CD56+ cells (▀) and CD3+ CD56− cells (•) was enhanced when HLA class I molecules on the AML target cells were up-regulated by preincubation with interferon-γ (IFN-γ; □ and ○, respectively). Figure shows one representative assay of two comparison sets carried out for both CD3+ CD56+ and CD3+ CD56− subsets, P < 0·05 for both. Histograms show isotype control (left) and HLA class I expression on blasts without (middle) and with preincubation with IFN-γ (right) respectively, of one representative assay. (c) Cytotoxicity of the CD3+ CD56+ cells (▀) and CD3+ CD56− cells (•) was inhibited when the CD3/T-cell receptor (TCR) complexes on the effector cells were blocked by a TCR-blocking mAb T10B9. The figure shows one representative assay. Four comparison sets were made for the CD3+ CD56+ subset, P < 0·05 in three out of four sets. Four comparison sets were made for the CD3+ CD56− subset, P < 0·05 for all. Histograms show isotype control (left) and TCR expression on cytokine-induced killer cells without (middle) and with T10B9 blocking (right) respectively, of one representative assay. (d) Cytotoxicity of the CD3+ CD56+ cells (▀) and CD3+ CD56− cells (•) was enhanced when the CD3/TCR complexes on the effector cells were either stimulated with OKT3 (Δ) or with a stimulating anti-TCR mAb (□). Two comparison sets were carried out for the CD3+ CD56+ subset and three comparison sets for the CD3+ CD56− subset, P < 0.05 for all. (e) Mean fluorescent intensity of degranulation marker CD107a over time after ligation with OKT3. There were three samples at serial time-points: 0 min (□), 30 min ( ), 60 min (
), 60 min ( ), 120 min (
), 120 min ( ), 240 min (▀).
), 240 min (▀).
Based on these evidence we have no doubt that the CD3+ subsets of CIK cells require interaction of their CD3/TCR complex with MHC class I of the AML target for cytotoxicity.
The well-characterized NK-related receptors are not involved in the recognition of target cells by the CD3+ cell subsets
In addition to the CD3/TCR complex for which we have demonstrated involvement in AML target killing, we investigated whether the well-characterized NK receptors were involved in the recognition of AML targets.
The killer immunoglobulin-like receptors (KIRs) are present in NK cells and some T-cell subsets and produce either activation or inhibitory signals upon binding with the appropriate HLA ligand on target cells. Before culture, marrow cells obtained from patients at diagnosis were mostly leukaemic blast cells. CD3− CD56+ NK cells constituted a median of 15·3% (range 7·6–20·7%, n= 9) within the lymphocyte compartment. A median of 1·73% (range 1·4–3·3%) CD158a+ cells and 8% CD158b+ cells (range 1·1–16·6%) could be detected for both CD3− CD56+ NK cells and CD3+ T cells. After culture for 3–4 weeks, there was a marked expansion of the CD3+ T cells. The overall NK cell subset was reduced to 2·23% (range 1·14–4·8%, n= 9). At this time, 0·9% (range 0·3–1·4%) of CD158a+ cells and 4·4% (range 0·5–19·7%) of CD158b+ cells were present within the bulk CIK culture. However, these KIRs did not seem to interact with the target cells and blocking these KIRs with blocking mAb did not result in any noticeable alteration in the cytotoxicity of the CD3+ CD56+ cells against autologous and allogeneic AML targets (Fig. 3a).
Figure 3.
(a) One representative cytotoxicity assay of CD3+ CD56+ subset against acute myeloid leukaemia (AML) target cells (▀) in the presence of anti-CD158a and anti-CD158b monoclonal antibody (mAb; Δ), and with anti-CD94 mAb (□). Three comparison sets comparing the blocking with anti-CD158a/anti-CD158b and anti-CD94 were done for the CD3+ CD56+ and CD3+ CD56− subsets respectively, P = NS for all. (b) Real-time polymerase chain reaction analyses of the relative expression level of NKG2C and NKG2E transcripts after normalization to β-actin. Analysis was done on CD3+ CD56+ subsets from two independent AML samples (AML#1 and AML#2) and two independent acute lymphoblastic leukaemia (ALL) samples (ALL#1 and ALL#2), before and after coincubation with their respective autologous targets. Relative expression level of CD3+ CD56+ subset at baseline (open column) is compared with that after 4 hr of incubation with the respective AML or ALL targets (grey column). (c) Histogram showing one representative fluorescence-activated cell sorting (FACS) analysis of the expression of NKG2D receptor in CD3+ CD56+ cells after cytokine-induced killer cell (CIK) cell culture. One representative cytotoxicity assay of CD3+ CD56+ cells against autologous AML target cells in the absence (▀) and presence (□) of blocking anti-NKG2D mAb (right panel). There were five comparison sets, P = NS for all. (d) Histogram showing one representative FACS analysis of the expression of DNAM-1 receptor in CD3+ CD56+ cells after CIK cell culture. One representative cytotoxicity assay of CD3+ CD56+ cells against autologous AML target cells in the absence (▀) and presence (□) of blocking anti-DNAM-1 mAb (right panel). Three comparison sets were done, P = NS for all.
The heterodimer formed by CD94 and the various NKG2 molecules is a C-type lectin receptor expressed by NK cells and a subset of CD8+ T cells. Although CD94 could be detected at a median of 21% (0−70%, n = 14) in the CD3+ CD56+ and CD3− CD56+ cell subsets, it did not play any significant role in the cytolysis of target cells by the CD3+ CD56+ cells (Fig. 3a).
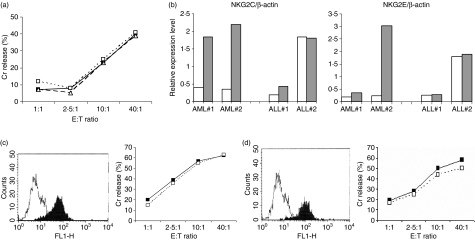
Previously, we demonstrated through global gene profiling that the NKG2C and NKG2E messenger RNA were up-regulated in CD3+ CD56+ cells upon interaction with susceptible AML targets but not resistant acute lymphoblastic leukaemia (ALL) targets, suggesting the possibility that these receptors could be involved in target recognition.17 We therefore measured the surface expression of NKG2C receptor on CD3+ CD56+ cells (there is no commercially available NKG2E mAb), in the presence or absence of the appropriate AML target. However, we were surprised to find that NKG2C could not be detected on the CIK cells. To validate our gene expression findings, we therefore performed a real-time PCR analysis and confirmed our previous observation of the up-regulation of the NKG2C and NKG2E genes in CD3+ CD56+ cells after interactions with susceptible AML target cells. We also confirmed the absence of up-regulation of these two genes in CD3+ CD56+ cells upon meeting with resistant ALL targets (Fig. 3b).
We also studied the presence of NKG2D and DNAM-1 receptors on the CIK cells. Both the NKG2D and DNAM-1 receptors were present on 100% of the CIK cells before culture. After culture, the mean fluorescent intensity of both receptors increased more than twofold. However, blocking of either the NKG2D or DNAM-1 receptor on the CD3+ CD56+ subset with mAb did not affect the cytolysis of leukaemic targets (Fig. 3c,d). As a positive control, CIK cells were shown to be able to kill the Jurkat cell line, which expresses ligands to both NKG2D and DNAM-1, and cytotoxicity was inhibited to about half when either the NKG2D or the DNAM-1 receptor was blocked by the respective mAb.
As expected, the NK cell-specific natural cytotoxicity receptors NKp30, NKp44 and NKp46 were absent on CIK cells (five CIK samples tested, data not shown).
The anti-leukaemic cytotoxicity of the CD3+ CD56+ subset is superior to its CD3+ CD56− counterpart
Having demonstrated the dependence on CD3/TCR receptor and not other NK-related receptors in AML target recognition, we further studied the difference between CD3+ CD56+ and CD3+ CD56− subsets. Serial cytotoxicity assays performed with bulk CIK cells demonstrated that cytotoxicity against both autologous and allogeneic target cells increased over time and correlated well with the increase in the percentage of CD3+ CD56+ cells in the bulk culture (Fig. 4a). When the cytotoxicity of purified CD3+ CD56+ and CD3+ CD56− cells obtained after cell sorting was tested against autologous and allogeneic AML target cells, the cytolytic activity of the CD3+ CD56+ cell subset was significantly higher than that of the CD3+ CD56− cell subset in the majority of the cultures (Fig. 4b).
Figure 4.
(a) Cytotoxicity of the bulk cytokine-induced killer (CIK) cell culture against acute myeloid leukaemia (AML) target cells was correlated with the increase in the percentage of CD3+ CD56+ cells upon culture. The percentage of CD3+ CD56+ cells in the bulk CIK cell culture was shown in brackets; D denotes days in culture for the CIK cells. (b) Cytotoxicity of the CD3+ CD56+ subset (▀) against autologous AML target cells was superior to that of the CD3+ CD56− cell subset (□), at all effector to target ratios. *P < 0·05 using a two-tailed paired Student’s t-test performed on 20 independent sets of cytotoxicity assays. (c) Frequency histogram showing intracellular granzyme A expression intensity. Mean fluorescence intensity 4·2 for isotype control, 11.2 for CD3+ CD56− subset and 28·7 for CD3+ CD56+ subset.
In agreement with this, the intracellular granzyme A content was also found to be higher with the CD3+ CD56+ cells than the CD3+ CD56− cells. Mean fluorescent intensity expression was sixfold over control levels for the CD3+ CD56+ cells as compared to two or threefold for the CD3+ CD56− cells (Fig. 4c).
Both CD3+ CD56+ and CD3+ CD56− subsets exhibit polyclonal TCR repertoire
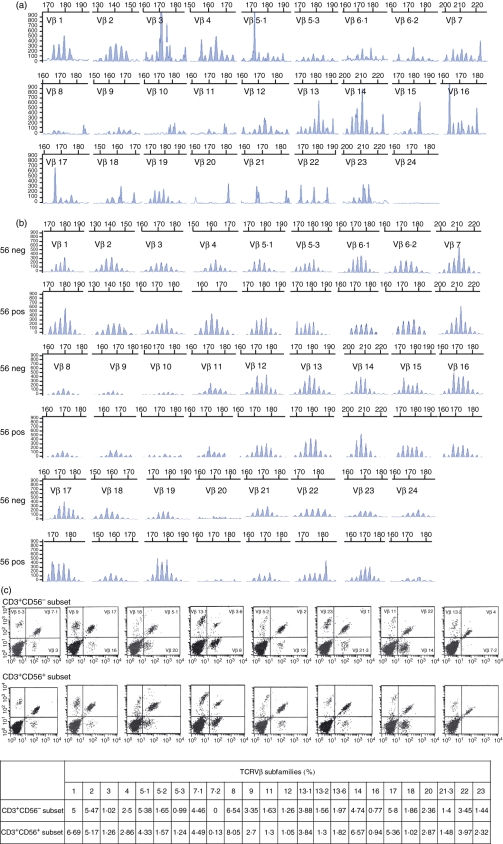
In an attempt to explain the difference in cytotoxicity of CD3+ CD56+ and CD3+ CD56− subsets, we analysed the TCR repertoire of the two subsets for any differences. Before culture, CD3+ T cells constituted only a small fraction of the marrow cells obtained from patients with newly diagnosed AML. This heterogeneous T-cell population showed a skewed TCRVβ repertoire as demonstrated by TCRVβ CDR3 spectratyping (Fig. 5a). After culture for 3–4 weeks, a normalized polyclonal spectrum of T-cell repertoire could be demonstrated within the expanded CD3+ T-cell population for both the CD3+ CD56+ and CD3+ CD56− subsets (Fig. 5b). These observations could further be confirmed by quantification of the various TCRVβ sub-families by flow cytometry (Fig. 5c).
Figure 5.
(a) T-cell receptor Vβ (TCRVβ) spectratyping of T lymphocytes obtained from the marrow sample of patient with acute myeloid leukaemia (AML) at diagnosis. (b) TCR repertoire of cytokine-induced killer (CIK) T cells at maturity. Fifty-six neg denotes CD3+ CD56− cells; 56 pos denotes CD3+ CD56+ cells. (c) Flow cytometric analyses of the composition of the different TCRVβ subfamilies for the CD3+ CD56− (upper panel) and CD3+ CD56+ cell subsets (middle panel). The table summarizes the % of each of the TCRVβ subfamilies within the two T-cell subsets (lower panel).
The CD3+ CD56+ subset has a higher proportion of CD8+ cells that account for its higher cytolytic activity against AML targets
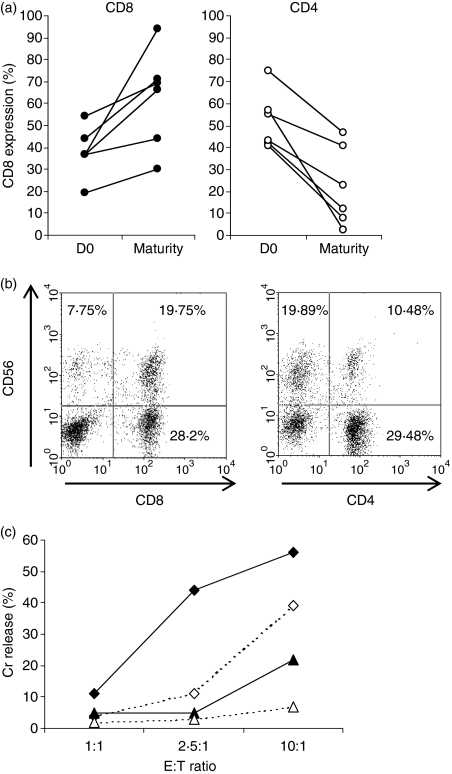
Both the CD3+ CD56+ and CD3+ CD56− cell subsets were comprised of a mixture of CD4+ and CD8+ cells. The proportion of CD8+ cells preferentially increased during CIK cell culture, from a median of 37% (range 19–54%) within the starting lymphocyte population to 67·5% (range 30–94%) at maturity, while CD4+ cells decreased over time (Fig. 6a). Since CD8+ cells are the cytotoxic effectors, we analysed the CD3+ CD56+ and CD3+ CD56− subsets for their CD8 proportion. The CD3+ CD56+ cell subset consisted of a higher proportion of CD8+ cells than CD4+ cells. In contrast, the CD3+ CD56− cell subset consisted of a higher proportion of CD4+ cells (Fig. 6b).
Figure 6.
(a) Comparison of the % of CD8+ and CD4+ cells in the bulk cytokine-induced killer (CIK) cultures of six independent acute myeloid leukaemia (AML) patients at diagnosis (D0) and after CIK cell culture (Maturity). (b) Flow cytometric analyses of the % of CD8+ and CD4+ cells in the CD3+ CD56+ cell subset. % of CD8+ cells = 72% [19·75/(19·75 + 7·75)] and % of CD4+ cells = 32% [10·48/(10·48 + 19·89)]. (c) Comparison of the level of cytotoxicity for the four different purified cell subsets: CD8+ CD56+(♦), CD8+ CD56− (⋄), CD4+ CD56+(▴) and CD4+ CD56− (Δ) against the same AML target cell.
When the various cell subsets were sorted from the bulk of CIK cells, the CD8+ CD56+ cells exhibited the most potent cytotoxicity against AML targets, followed by the CD8+ CD56−, CD4+ CD56+ and CD4+ CD56− cells (Fig. 6c). This provides the explanation for the superior cytotoxicity of the CD3+ CD56+ subset because it contains a higher proportion of CD8+ cell.
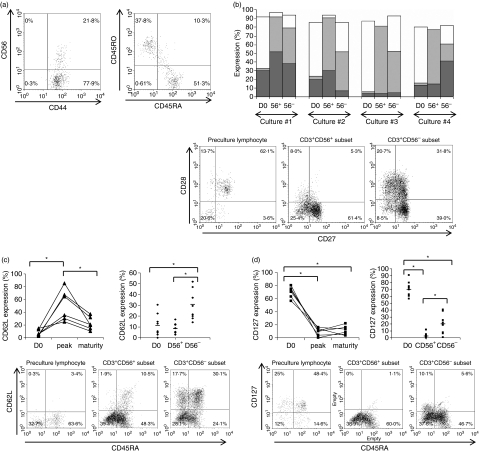
The CD3+ CD56+ subset expresses a more matured and differentiated phenotype than its CD3+ CD56− counterpart
Since the CD3+ CD56+ subset is derived from CD3+ CD56− cells, we further studied the memory T-cell markers to look for any difference between the two. FACS analysis showed that before culture, all T cells were CD44+ activated T cells (Fig. 7a). They consisted of a mixture of CD45RA+ and CD45RO+ effector T cells coexpressing variable levels of CD27 and CD28 (Fig. 7a). Expression of the homing molecules CD62L and CCR7 was generally low or absent while the expression of CD127 (IL-7Rα) was relatively high in these de novo T cells.
Figure 7.
(a) Flow cytometric analyses for the presence of memory T-cell phenotype in the leukaemic marrow sample obtained at diagnosis. (b) Proportion of early CD27+ CD28+ (□), intermediate CD27+ CD28− ( ) and late CD27− CD28− TE-cell subsets (
) and late CD27− CD28− TE-cell subsets ( ) present in the starting population (D0), within the CD3+ CD56+ (56+) and CD3+ CD56− (56−) subsets in the matured cytokine-induced killer (CIK) cell culture. Cultures #1 to #4 represent four separate CIK cell cultures of four patients with acute myeloid leukaemia (AML; bar chart). The dot plot shows the fluorescence-activated cell sorting (FACS) profiles of a representative culture, sample #2. (c) Level of CD62L expression on T cells before culture (D0), at the peak of expression (D15–23), and at maturity of CIK cell culture (D25–30) for six separate CIK cell cultures of six AML patients. Expression of CD62L at maturity was higher in the CD3+ CD56− subset than in CD3+ CD56+ subset (upper panel). The lower panel shows the FACS profiles of a representative culture sample. *Indicates statistical significance at P < 0·05 by two-way paired Student’s t-test. (d) Level of CD127 expression on T cells before culture (D0), at the peak of expression (D15–23), and at maturity of CIK cell culture (D25–30) for six separate CIK cell cultures of six AML patients. CD127 was dimly expressed at variable levels in the CD3+ CD56− subset at maturity but absent in CD3+ CD56+ subset (upper panel). The lower panel shows the FACS profiles of a representative culture sample. *Indicates statistical significance at P < 0·05 by two-way paired Student’s t-test.
) present in the starting population (D0), within the CD3+ CD56+ (56+) and CD3+ CD56− (56−) subsets in the matured cytokine-induced killer (CIK) cell culture. Cultures #1 to #4 represent four separate CIK cell cultures of four patients with acute myeloid leukaemia (AML; bar chart). The dot plot shows the fluorescence-activated cell sorting (FACS) profiles of a representative culture, sample #2. (c) Level of CD62L expression on T cells before culture (D0), at the peak of expression (D15–23), and at maturity of CIK cell culture (D25–30) for six separate CIK cell cultures of six AML patients. Expression of CD62L at maturity was higher in the CD3+ CD56− subset than in CD3+ CD56+ subset (upper panel). The lower panel shows the FACS profiles of a representative culture sample. *Indicates statistical significance at P < 0·05 by two-way paired Student’s t-test. (d) Level of CD127 expression on T cells before culture (D0), at the peak of expression (D15–23), and at maturity of CIK cell culture (D25–30) for six separate CIK cell cultures of six AML patients. CD127 was dimly expressed at variable levels in the CD3+ CD56− subset at maturity but absent in CD3+ CD56+ subset (upper panel). The lower panel shows the FACS profiles of a representative culture sample. *Indicates statistical significance at P < 0·05 by two-way paired Student’s t-test.
By maturity, a higher proportion of CD27+ CD28+ cells could be detected in the CD3+ CD56− cell subset of the CIK cells. In contrast, higher proportion of CD27+ CD28− and CD27− CD28− cells were detected in the CD3+ CD56+ subsets. This shows that a majority of the T cells in the CD3+ CD56+ cell subset are memory effector T cells at relatively later stages of differentiation in contrast to the CD3+ CD56− counterpart, which consists of a group of earlier memory T cells (Fig. 7b).
Expression of CD62L increased and peaked between 15 and 23 days of culture, with subsequent decline. The CD62L was significantly higher in the CD3+ CD56− cell subset than the CD3+ CD56+ cell subset (Fig. 7c). On the other hand, expression of CD127 decreased drastically with culture. Nevertheless, its expression was significantly higher in the CD3+ CD56− cell subset than in the CD3+ CD56+ subset (Fig. 7d).
Upon culture, 82% (range 57–97%) of the CD3+ CD56+ and 82% (range 63–96%) of the CD3+ CD56− cells became CD45RO+. Therefore, the overall phenotype of the CD3+ CD56+ cell could be summarized as CD45RO+ CD27lo CD28lo CD62L− CCR7− IL7Rα− and was consistent with the phenotype of terminally differentiated effector T cells.
Discussion
We have reported above a series of experiments in an attempt to study the anti-AML mechanism and characteristics of the three subsets constituting the heterogeneous bulk CIK culture.
The cytotoxicity exhibited by the CD3+ cell subset of the CIK culture has previously been shown to be non-MHC-restricted2–7 and hence is termed ‘NK-like’ T cells.3,5,8,10 We have shown previously that CIK cells kill autologous and allogeneic AML targets equally well, irrespective of HLA type.3 However, we have also shown here from various approaches that these T cells still depend on the interaction of the CD3/TCR complex with class I antigens on the target cells for efficient cytolysis. These observations suggest that although these T cells do not recognize by MHC specificity, the presence of the MHC molecule is nevertheless indispensable for cytotoxicity. This is contradictory to the current doctrine of target recognition by antigen-specific CTL, which states that TCR recognition of target occurs in the context of the self MHC molecule. Other groups have reported similar observations. Zhong et al.18 reported a method of T-cell expansion by sequential addition of cytokines to first convert AML blasts into dendritic cells, then further expand the coexisting T cells with IL-2, OKT3 and anti CD28 mAb. Both T cells expanded under such conditions and T cells expanded with IL-2, OKT-3 and anti-CD28 mAb only, could kill both autologous AML target as well as AML cell lines HL-60, NB4, U937, i.e. showing non-MHC-restricted target killing. Interestingly they too demonstrated that blocking of HLA class I on the target abrogated the T-cell killing of both autologous and allogeneic target, an observation consistent with our current findings. Earlier work has reported similar observations where MUC-1-specific CTL clones were able to kill MUC1+ breast and pancreatic tumours regardless of HLA type, and yet were blocked by anti-class I mAb. Involvement of MHC class I/CD8 coreceptor together with other adhesion molecules was required for such ‘MHC-unrestricted’ but ‘MHC-dependent’ cytotoxicity.19 One of the explanations of MHC unrestricted mechanism is direct TCR-αβ-mediated recognition of tumour-specific peptide epitope, e.g. MUC1, or other tumour antigens with distinct properties of multivalency, a high level of expression and an ordered conformational structure.19–21 Extrapolated to our observation, CIK cells, being a population of cytokine-activated and polyclonal T cells expressing high levels of adhesion molecules, might be in an activated state that enables target recognition much more readily without stringent MHC matching. MUC1 is expressed in a large proportion of AML blasts.22 This combination may therefore allow a non-MHC-restricted but MHC-dependent interaction between the CIK cell and its cognate target. Although our observation is proven via several approaches by ourselves and also explained by others in somewhat different settings, our inference requires further research into the basic mechanisms for verification.
We have demonstrated in this report that several of the well-established NK receptors, including the KIRs, C-type lectin receptors, NKG2D and DNAM-1 receptors, are not involved in mediating the cytotoxicity of the CIK cells, often referred to as ‘NK-like T cells’, against AML targets. Our earlier gene profiling analyses showed the up-regulation of the NKG2C and NKG2E transcripts in CIK cells upon contact with sensitive AML targets but not so when coincubated with resistant ALL targets.22 We validated this observation by real-time PCR in this study (Fig. 3b). However, this did not result in the surface expression of NKG2C when studied with a commercially available mAb, even at various time-points after coincubation with the relevant susceptible AML target cells. The absence of surface expression of these NKG2C and NKG2E receptors despite up-regulation of their transcripts has also been reported by others.23 NKG2D receptor has been found to be the mediator for non-MHC-restricted killing of CIK cell against the plasmactyoma cell line24 as well as primary ovarian cells.25 However these targets express the NKG2D ligands MICA and MICB, which are known to be poorly expressed on AML targets,26 therefore NKG2D as a CIK receptor for AML recognition is unlikely in theory and excluded by our observations. The DNAM-1 receptors have been shown to be involved in the killing of leukaemic cells by NK cells27 and they also participate in primary adhesion during CTL-mediated cytotoxicity.28 Although the ligands for the DNAM-1 receptors, PVR and nectin 2, have been reported to be highly expressed in AML blasts,27 we showed in our experiments that DNAM-1 was not involved in the killing of leukaemic cells mediated by the T cells within the CIK cell culture (Fig. 3d).
With the above series of experiments we have not identified any further specific receptors on CD3+ subsets of CIK cells besides the CD3/TCR complex for AML target recognition. We conclude that the CD3/TCR complex is the receptor responsible for AML target recognition by CIK cells, and it is the CD8+ CD56+ subset that accounts for the largest part of anti-AML cytotoxicity of the bulk CIK culture.
An earlier report demonstrated that the CD3+ CD56+ cells originate from the CD3+ CD56− cells.2 Hence, the CD3+ CD56+ subset, generated following repeated stimulations, would represent a relatively more terminally differentiated effector T-cell population.29 We showed that this cell subset has the phenotype CD45RO+ CD62L− CCR7− CD27lo CD28lo CD127−. Such late stage effector T cells are known to possess potent cytotoxicity but have low proliferative capacity.30 The presence of higher intracellular granzyme A content in this cell subset (Fig. 4c) adds further evidence to this observation. On the other hand, the CD3+ CD56− cell subset, consisting of a higher proportion of CD27+ CD28+ cells, represents early effector T cells that have reduced cytotoxicity but a higher capacity to proliferate. These CD62L+ CCR7+/− CD27+ CD28+ CD127+ early effector T cells, expressing a higher level of CD62L, have the potential to home to lymph nodes and potentially persist in vivo after adoptive transfer.31 In fact, it has been reported that a large proportion of the in vitro-expanded cultured cells could only live for a short duration in vivo because of the impairment of their homing ability to the lymph node.31 Therefore, it would be advantageous to have multiple T-cell populations at different differentiation stages to achieve potential long-term tumour protection.32
From the above observations, the expanded bulk CIK cell culture may represent an ideal cell population for adoptive immunotherapy. The CD3+ CD56+ subset could deliver potent cytotoxicity for the immediate destruction of tumour cells. The less potent CD3+ CD56− cell subset in the CIK cell population could proliferate and persist in vivo for a longer duration and therefore provide a continuous source of cells with long-term anti-tumour activities. In addition, it consists of a small NK cell subset that has been shown to efficiently lyse MHC class I-deficient tumour targets that escape T-cell recognition. The polyclonal T cell in the CIK culture with a comprehensive TCR repertoire, in contrast to antigen-specific oligoclonal T cells, has the potential capability to respond to a broad spectrum of antigen. Recent work has shown that a lymphodepleted state could favour a homeostasis-driven expansion of adoptively transferred T cells possibly as a result of reduced competition for homeostatic regulatory cytokines or the depletion of suppressor T cells.33,34 Therefore, we expect that the therapeutic effect of adoptive immunotherapy with CIK cells for AML will be maximal shortly after ablative chemotherapy treatment given for autologous peripheral blood stem cell transplant. The minimal residual disease state will be most susceptible to immunotherapy, while adoptive transfer of polyclonal T cells will facilitate a more rapid immune reconstitution.
Acknowledgments
The authors wish to express their gratitude to Dr Garnet Suck and Dr Ho Liam Pock for their helpful suggestion and advice. They also thank the research staff at the Division of Cellular and Molecular Research, National Cancer Center, and laboratory technologists at Department of Haematology, Singapore General Hospital, for their generous assistance. This study is supported by grants from the National Medical Research Council of Singapore and Department of Clinical Research, Singapore General Hospital.
References
- 1.Schmidt-Wolf IGH, Lefterova P, Johnston V, et al. Sensitivity of multidrug-resistant tumour cell lines to immunologic effector cells. Cell Immunol. 1996;169:85–90. doi: 10.1006/cimm.1996.0094. [DOI] [PubMed] [Google Scholar]
- 2.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–96. [PubMed] [Google Scholar]
- 3.Linn YC, Lau LC, Hui KM. Generation of cytokine-induced killer cells from leukemic samples with in vitro cytotoxicity against autologous and allogeneic leukemic blasts. Br J Hematol. 2002;116:78–86. doi: 10.1046/j.1365-2141.2002.03247.x. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Wolf IGH, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumour cell activity. J Exp Med. 1991;174:139–49. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3+CD56+ cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92:3318–27. [PubMed] [Google Scholar]
- 6.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–8. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Wolf IGH, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumour cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21:1673–9. [PubMed] [Google Scholar]
- 8.Ziske C, Marten A, Schottker B, et al. Resistance of pancreatic carcinoma cells is reversed by coculturing NK-like T cells with dendritic cells pulsed with tumour-derived RNA and CA 19-9. Mol Ther. 2001;3:54–60. doi: 10.1006/mthe.2000.0230. [DOI] [PubMed] [Google Scholar]
- 9.Marten A, Renoth S, von Lilienfeld-Toal M, Buttgereit P, Schakowlki F, Glasmacher A, Sauerbruch T, Schmidt-Wolf IGH. Enhanced lytic activity of cytokine-induced killer cells against multiple myeloma cells after co-culture with idiotype-pulsed dendritic cells. Haematologica. 2001;86:1029–37. [PubMed] [Google Scholar]
- 10.Baker J, Vernaris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft vs host disease induction due to interferon γ production. Blood. 2001;97:2923–31. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 11.Alvarnas JC, Linn YC, Hoppe EG, Negrin RS. Expansion of cytotoxic CD3+CD56+cells from peripheral blood progenitor cells of patients undergoing autologous haemopoietic cell transplant. Biol Blood Marrow Transplant. 2001;7:216–22. doi: 10.1053/bbmt.2001.v7.pm11349808. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Wolf IGH, Finke S, Trojaneck B, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999;81(6):1009–16. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin’s disease and non Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2005;11:181–7. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Lu K, Tong C, Jiang K, Lu D. The efficacy of chemotherapy combination with auto-cytokine-induced killer cells in acute leukemia. Chin J Intern Med. 2005;44:198–201. [PubMed] [Google Scholar]
- 15.Gorski J, Yassai M, Zhu X, Kissella B, Keever C, Flomenberg N. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDr3 size spectratyping. Correlation with immune status. J Immunol. 1994;152:5109–19. [PubMed] [Google Scholar]
- 16.Lang P, Barbin K, Feuchtinger T, et al. Chimeric CD19 antibody mediates cytotoxic activity against leukemic blasts with effector cells from pediatric patients who received T-cell-depleted allografts. Blood. 2004;103:3982–5. doi: 10.1182/blood-2003-05-1735. [DOI] [PubMed] [Google Scholar]
- 17.Linn YC, Wang SM, Hui KM. Comparative gene expression profiling of cytokine-induced killer cells in response to acute myeloid leukemic and acute lymphoblastic leukemic stimulators using oligonucleotide arrays. Exp Hematol. 2005;33:671–81. doi: 10.1016/j.exphem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhong R-K, Rassenti LZ, Kipps TJ, Chen J, Law P, Yu J-F, Ball ED. Sequential modulation of growth factors: a novel strategy for adoptive immunotherapy of acute myeloid leukemia. Biol Blood Marrow Transplant. 2002;8:557–68. doi: 10.1053/bbmt.2002.v8.pm12434951. [DOI] [PubMed] [Google Scholar]
- 19.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci USA. 1989;86:7159–65. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blander JM, Ciboroski P, Hsia S, Watkins SC, Finn OJ. Intercellular and intracellular events following the MHC-unrestricted TCR recognition of a tumor-specific peptide epitope on the epithelial antigen MUC1. J Immunol. 1998;160:3111–20. [PubMed] [Google Scholar]
- 21.Nehad MA, Schmielau J, Alter MD, Cascio M, Finn OJ. Therapeutic potential of a tumor-specific, MHC-unrestricted T-cell receptor expressed on effector cells of the innate and the adaptive immune system through bone marrow transduction and immune reconstitution. Blood. 2005;105:4583–9. doi: 10.1182/blood-2004-10-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brossart P, Schneider A, Dill P, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;61:6846–50. [PubMed] [Google Scholar]
- 23.Derre L, Corvaisier M, Pandolfino MC, Diez E, Jotereau F, Gervois N. Expression of CD94/NKG2-A on human T lymphocytes is induced by IL-12: implications for adoptive immunotherapy. J Immunol. 2002;168:4864–70. doi: 10.4049/jimmunol.168.10.4864. [DOI] [PubMed] [Google Scholar]
- 24.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–72. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 25.Chan JK, Hamilton CA, Cheung MK, et al. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study. Clin Cancer Res. 2006;12:1859–67. doi: 10.1158/1078-0432.CCR-05-2019. [DOI] [PubMed] [Google Scholar]
- 26.Pende D, Rivera P, Marcenaro S, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different hstiotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–86. [PubMed] [Google Scholar]
- 27.Pende D, Spaggiari GM, Marcenaro S, et al. Analysis of the receptor–ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the poliovirus receptor (CD155) and Nectin-2(CD112) Blood. 2005;105:2066–73. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 28.Shibuya A, Campbell D, Hannum C, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–81. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 29.Wajchman HJ, Pierce CW, Varma VA, Issa MM, Petros J, Dombrowski KE. Ex vivo expansion of CD8+ CD56+ and CD8+CD56− natural killer T cells specific for MUC 1 mucin. Cancer Res. 2004;64:1171–80. doi: 10.1158/0008-5472.can-3254-2. [DOI] [PubMed] [Google Scholar]
- 30.Powel DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–50. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo anti tumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speiser DE, Romero P. Towards improved immunocompetence of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1467–9. doi: 10.1172/JCI25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–92. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]