Abstract
Vitamin D is known for its role in calcium homeostasis for optimal skeletal health. It was previously believed that only elderly or hospitalized patients were at risk for vitamin D insufficiency, but many people in the general US population have insufficient levels of 25-hydroxyvitamin D (25[OH]D). According to the Third National Health and Nutrition Examination Survey, 61% of white and 91% of black Americans suffer from vitamin D insufficiency (25[OH]D < 32 ng/mL). Recent studies have demonstrated that a minimum 25(OH)D level of 32 ng/mL is necessary for optimal protection from fracture and intestinal absorption of calcium. Recently, vitamin D has been recognized as important for extraskeletal functions such as immune function, cancer prevention, and hypertension prevention. We review the role of vitamin D in skeletal health and present data on vitamin D in other extraskeletal diseases, with special emphasis on the rheumatology patient.
Introduction
The importance of vitamin D for bone health and prevention of rickets has been known since the early 1930s. Calcium is actively absorbed from the small intestine in the presence of vitamin D. Calcium and phosphorus form hydroxyapatite crystals to mineralize and strengthen bones. Thus, a diet containing both optimal vitamin D and calcium is important for proper mineralization of bone.
Vitamin D's classic role is to increase the intestinal efficacy of calcium absorption, but other nonclassic roles for vitamin D have been investigated over the past several decades. In fact, vitamin D receptors are found in many tissues other than bone and the small intestine, such as in type 1 helper T cells, macrophages, the prostate, the brain, and other tissues [1••]. This review summarizes the evidence for vitamin D and calcium for optimal skeletal health in adults and provides evidence for emerging extraskeletal benefits of vitamin D and calcium.
Vitamin D and Calcium Physiology
Vitamin D metabolism
Vitamin D refers to vitamin D2 (ergocalciferol) or vitamin D3 (cholecalciferol). Ergocalciferol is produced from irradiated fungi or yeast. Cholecalciferol is produced in skin or found naturally in fatty fish such as salmon or mackerel. Both forms of vitamin D can be used to fortify food; however, only cholecalciferol can be made endogenously in skin. When the skin is exposed to ultraviolet B (UVB) radiation between the wavelengths of 290 and 315 nm, 7-dehydrocholesterol, a compound present in the skin, is converted to previtamin D3, which isomerizes to form vitamin D3. The amount of vitamin D3 made in the skin can be affected by an individual's skin color, age, and use of sunscreen products, as well as the time of day, season, and latitude [1••].
Once vitamin D is made in the skin (D3) or obtained in the diet (D2 or D3), it enters the circulation bound to vitamin D–binding protein. This complex is transported to the liver, where vitamin D undergoes hydroxylation in the 25 position to form 25-hydroxyvitamin D (25[OH]D), which then circulates to the kidney and is hydroxylated at the 1 position by the 1-α-hydroxylase to form the hormonal form of vitamin D, 1,25-dihydroxyvitamin D (1,25[OH]2D). 1,25(OH)2D circulates bound to vitamin D–binding protein, enters the target cell, and binds to the vitamin D receptor (VDR) in the cytoplasm, which then enters the nucleus and heterodimerizes with the retinoic acid X receptor to increase transcription of vitamin D–dependent genes important for bone metabolism, calcium absorption, and other nonclassical functions (eg, inhibition of genes important in cancer growth) [2].
Circulating 1,25(OH)2D is catabolized by the 24-hydroxylase to form 1,24,25(OH)2D, an inactive vitamin D compound. 1,25(OH)2D increases its own catabolism by increasing expression of the 24-hydroxylase. Recently, researchers discovered that medications such as anticonvulsants and rifampin can increase the catabolism of 1,25(OH)2D by activating the pregnane X receptor, resulting in increased expression of the 24-hydroxylase [3•].
Intestinal calcium absorption
The major function of vitamin D is to optimize intestinal calcium and phosphorus absorption for proper formation of the bone mineral matrix. Calcium is transported across the intestine by a paracellular or transcellular pathway. The paracellular pathway is predominately a passive process, whereas the transcellular process is highly regulated by 1,25(OH)2D. Several vitamin D–dependent calcium transport proteins regulate intestinal calcium absorption. Transient receptor potential cation channel, subfamily V, member 6 (TRPV6) is a calcium channel located on the luminal surface of the enterocyte. This channel facilitates calcium entry into the enterocytes. TRPV6 is highly regulated by 1,25(OH)2D and is most highly expressed in the duodenum compared to the jejunum and ileum [4]. In women, TRPV6 levels decline with aging, which partially explains the age-associated decline in calcium absorption [5]. Calbindin 9k is a calcium transport protein that shuttles calcium from the luminal surface to the basal surface of the enterocyte. Finally, two additional vitamin D-regulated cation exchange proteins, PMCA1b and NCX1, exist on the basal surface of the enterocyte to extrude calcium into the circulation [6].
Optimal vitamin D levels are necessary to increase the efficiency of calcium absorption. Without adequate vitamin D, the body absorbs no more than 10% to 15% of dietary calcium. In the vitamin D–sufficient state, the intestinal calcium absorption increases to 30% to 40% [1••]. In rodent models, vitamin D receptor knockout mice develop osteomalacia, which can be reversed with a rescue diet containing increased calcium and lactose [7]. This finding establishes that the major function for vitamin D is to increase intestinal calcium absorption for proper mineralization of bone.
Definition of Vitamin D Insufficiency
The definition of vitamin D insufficiency has changed over the past few years from less than 20 ng/mL to less than 32 ng/mL [8]. This higher level of 25(OH)D has been proposed to prevent subclinical secondary hyperparathyroidism and to maximize intestinal calcium absorption. Chapuy et al. [9] demonstrated in 1569 adults that parathyroid hormone levels begin to rise when 25(OH)D concentrations fall below 31 ng/mL. Heaney et al. [10] established that optimal calcium absorption occurs with a 25(OH)D level greater than 32 ng/mL. Some clinical trials [11-14], though not all of them [15], have suggested that the most protection from fracture comes from a 25(OH)D level greater than 30 ng/mL. Given the presented evidence, an optimal 25(OH)D should be at least 32 ng/mL to prevent secondary hyperparathyroidism and fracture.
Prevalence of Vitamin D Insufficiency
Vitamin D insufficiency was first recognized as a major health concern in hospitalized medical inpatients and institutionalized elderly subjects. An evaluation of 290 consecutively admitted medical inpatients found that 57% of subjects were severely vitamin D deficient (25[OH]D < 15 ng/mL) [16]. Vitamin D deficiency in the elderly is highly prevalent: most community-dwelling elderly have inadequate 25(OH)D levels [17].
A large percentage of patients with osteoporosis also suffer from vitamin D insufficiency. A study of 1536 postmenopausal osteoporotic women from osteoporosis clinics, evenly distributed across southern and northern latitudes of the United States, found that over half of these women (52%) were vitamin D insufficient (25[OH]D < 30 ng/mL) [18]. Vitamin D insufficiency is not limited to elderly and osteoporotic patients. A study from Boston demonstrated that over two thirds of healthy young adults were vitamin D insufficient [19]. Using cross-sectional data from the Third National Health and Nutrition Examination Survey, we found that more than 92% of black and 61% of white Americans had vitamin D insufficiency defined by 25(OH)D less than 32 ng/mL. Thus, vitamin D insufficiency is highly prevalent in the US population (Table 1).
Table 1.
Prevalence of vitamin D insufficiency (25-hydroxyvitamin D < 32 ng/mL) and associated levels of calcium in US adults from National Health and Nutrition Examination Survey III (1988−1994)
| Race | White | Black | Mexican American | Other |
| Prevalence of vitamin D insufficiency | 61% | 92% | 81% | 79% |
| Body mass index | Normal weight | Overweight | Obese | |
| Prevalence of vitamin D insufficiency | 71% | 76% | 85% | |
| Season | Winter | Spring | Summer | Fall |
| Prevalence of vitamin D insufficiency | 83% | 73% | 70% | 78% |
| Age | Age 19−49 years | Age 50 years or older | ||
| Prevalence of vitamin D insufficiency | 77% | 75% | ||
| Mean calcium intake among those with vitamin D sufficiency | 943 mg | 817 mg | ||
| Mean calcium intake among those with vitamin D insufficiency | 767 mg | 656 mg | ||
Skeletal Benefits of Calcium and Vitamin D
Several randomized, placebo-controlled trials in both institutionalized and ambulatory elderly subjects have demonstrated that vitamin D with or without calcium reduced the incidence of hip and/or nonvertebral fractures by 20% to 30% (Table 2) [11-15,20-24]. A recent meta-analysis found that vitamin D supplementation significantly reduces the risk of hip fractures (by 18%) and other nonvertebral fractures (by 12%) when taken together with calcium [25•]. Most trials used at least 800 IU of vitamin D and the minimum 25(OH)D level where antifracture efficacy was observed of 29.7 ng/mL (74 nmol/L), suggesting a threshold for optimal vitamin D status for fracture protection [26].
Table 2.
Summary of randomized controlled trials examining the effect of calcium and vitamin D supplementation on fracture risk
| Study | n | Dose | Adherence | Fracture (95% CI) |
|---|---|---|---|---|
| Vitamin D3 alone | ||||
| Meyer et al. [24] | 1144 | 400 IU | 33% | Hip RR = 1.09 (0.73−1.63); nonvertebral RR = 0.92 (0.66−1.27) |
| Lips et al. [23] | 2578 | 400 IU (cod liver oil) | 63% | Hip HR = 1.18 (0.81−1.71); nonvertebral HR = 1.03 (0.75−1.4) |
| Trivedi et al. [13] | 2686 | 100,000 IU every 4 months | 80% | All RR = 0.78 (0.61−0.99); nonvertebral RR = 0.67 (0.46−0.99) |
| Vitamin D3 < 800 IU plus calcium ≤ 1000 mg | ||||
| Dawson-Hughes et al. [14] | 389 | 700 IU, 500 mg | 93% | All fractures RR = 0.4 (0.2−0.8); nonvertebral RR = 0.4 (0.2−1) |
| Larsen et al. [15] | 9605 | 400 IU, 1000 mg | Not reported | Hospitalized osteoporotic RR = 0.78 (0.63−0.96) |
| Jackson et al. [20] | 36,282 | 400 IU, 1000 mg | 59% | Hip HR = 0.88 (0.72−1.08) |
| Vitamin D3 800 IU plus calcium ≥ 1000 mg | ||||
| Chapuy et al. [11] | 3270 | 800 IU, 1200 mg | 83% | Hip OR = 0.57 (0.37−0.9); nonvertebral OR = 0.75 (0.61−0.91) |
| Chapuy et al. [12] | 583 | 800 IU, 1200 mg | 95% | Hip OR = 0.59 (0.33−1.04); nonvertebral not significant |
| Grant et al. [22] | 5292 | 800 IU, 1000 mg | 47% | All fractures HR = 1.01 (0.88−1.17) |
| Porthouse et al. [21] | 3314 | 800 IU, 1000 mg | 59% | Hip OR = 0.75 (0.31−1.43); nonvertebral OR = 0.89 (0.56−1.44) |
Of importance, two randomized controlled trials included in this meta-analysis failed to show an effect of either vitamin D or calcium supplementation on fracture risk [21,22]. Both trials included community-dwelling women with one or more fracture risks, including previous history of fractures. The negative findings of these trials could be explained by low adherence rates (< 60%) and inadequate supplementation with vitamin D. In the Randomized Evaluation of Calcium or vitamin D (RECORD) trial [22], vitamin D status was measured in a subgroup of subjects who completed the study. Mean 25(OH)D levels (25 ng/mL) did not reach the threshold of 30 ng/mL for optimal fracture protection.
The 2007 meta-analysis also included the Women's Health Initiative (WHI) study [20], a large randomized controlled trial with a 7-year follow-up, which showed a nonsignificant decrease in hip fractures (RR = 12%). However, when the study was analyzed to exclude nonadherent subjects (those who took vitamin D and calcium < 80% of the time), calcium and vitamin D intake decreased the relative risk of hip fractures by 29%, which was statistically significant. Of note, the amount of vitamin D assigned to subjects in this study was low (ie, 400 IU/d), and 25(OH)D levels were not assessed at the end of this 7-year study. Given the clinical trial data and the level demonstrated to maximally absorb calcium, the authors recommend an intake of vitamin D of at least 800 IU and calcium of at least 1000 to 1200 mg daily [25•].
Extraskeletal Benefits of Calcium and Vitamin D
Muscle function and fall prevention
Severe vitamin D deficiency causes a reversible myopathy characterized by muscle weakness, wasting, and instability of gait. The etiology of this myopathy is multifactorial and attributed to secondary hyperparathyroidism, hypocalcemia, hypophosphatemia, and calcitriol deficiency itself [27]. The direct association between vitamin D deficiency and myopathy was underscored with the finding of VDR in skeletal muscle [27].
Higher 25(OH)D concentrations (> 16 ng/mL) are associated with better lower extremity muscle function in active and inactive ambulatory elderly patients ages 60 years and older [28]. Furthermore, vitamin D improves postural and dynamic balance, both of which are independent and significant fall predictors [29]. A 2004 meta-analysis found that vitamin D supplementation as compared with calcium alone or placebo, reduced the risk of falls significantly (22%) in both ambulatory and institutionalized elderly subjects [29].
Immune system
The ameliorating effect of vitamin D on autoimmune diseases such as multiple sclerosis (MS), rheumatoid arthritis (RA), inflammatory bowel disease, and systemic lupus erythematosus (SLE) has been shown in murine disease models [30,31]. In humans, higher vitamin D intake was associated with a lower incidence of RA in one large, prospective, cohort study [32]. However, vitamin D intake was not associated with decreased risk of developing SLE or RA in the Nurses’ Health Study of 186,389 women followed for up to 22 years [33•]. Vitamin D intake has been associated with lower risk of developing MS. A recent prospective, case-control study found a significantly lower incidence of MS in white subjects with 25(OH)D levels greater than 41 ng/mL (OR = 0.38) [34].
The exact mechanism of vitamin D–mediated immune modulation remains unclear. The VDR is expressed in peripheral mononuclear cells and in both T-helper 1 (Th1) and T-helper 2 (Th2) cells. 1,25(OH)2D3 reduces the inflammatory response of Th1 cells and suppresses antigen presentation by dendritic cells, both of which are involved in the autoimmune response [31]. 1,25(OH)2D3 increases expression of cathelicidin (LL-37), an antimicrobial peptide thought to be important for the innate immune system, especially against mycobacterium tuberculosis [35].
Diabetes
Vitamin D deficiency increases the risk for type 1 and type 2 diabetes mellitus [36•]. In vivo studies have shown that vitamin D deficiency leads to decreased insulin production from pancreatic β cells, leading to impaired glucose tolerance. In the Third National Health and Nutrition Examination Survey, lower vitamin D status was associated with higher fasting glucose and 2-hour glucose after oral glucose tolerance test [37]. In case-control studies in children, vitamin D supplementation has been protective against developing type 1 diabetes later in life [1••]. In addition, several case-control studies have shown that vitamin D supplementation has improved insulin sensitivity in adults [36•].
Cardiovascular disease and hypertension
Lower 25(OH)D concentrations have been associated with increased risk of the metabolic syndrome, including hypertension [38,39]. Restoring vitamin D levels can reduce systolic blood pressure and thus reduce the risk of cardiovascular disease [40].
Studies examining the role of calcium alone on cardiovascular mortality and morbidity have shown a trend toward decreased stroke and coronary heart disease events. However, all studies relied on food recall questionnaires assessing dietary calcium intake, which is an inferior method limited by recall bias. The WHI, which is the only randomized controlled trial to date to examine this issue, found no difference in cardiovascular or cerebrovascular risk when comparing calcium plus vitamin D to placebo. However, the study was limited by low vitamin D doses (400 IU daily) and a low adherence rate of 59% by the end of the study [41]. A recent meta-analysis evaluated the effect of vitamin D on all-cause mortality in 18 randomized controlled trials and found a 7% relative risk reduction for subjects randomized to a mean dose of 528 IU daily of vitamin D [42••].
Cancer
VDR and 1-α-hydroxylase are expressed in normal and malignant breast, colon, prostate, and pancreatic tissue, among other tissues. Numerous in vivo and in vitro studies have shown that treatment of these malignant cells with 1,25(OH)2D3 or 1,25(OH)2D3 analogues decreases their proliferation, whereas vitamin D deficiency promotes their proliferation [2].
The antineoplastic effect of vitamin D is the result of inhibition of cell cycle genes, induction of apoptosis, and reduction in tumor invasiveness and angiogenesis. Colon cancer cell cultures have significant reduction in proliferation in the presence of 25(OH)D3 or 1,25(OH)D3 [2]. Of significance, the antiproliferative action of vitamin D is postulated to occur in an autocrine and paracrine fashion via local conversion of 25(OH)D to its hormonally active form through the local 1-α-hydroxylase [1••]. The optimal circulating level of 25(OH)D for extrarenal tissues to have antiproliferative activity is unknown but is thought to be higher than the levels necessary for skeletal health.
A study of 1179 postmenopausal women randomized to calcium, calcium plus vitamin D, or placebo, noted a significant reduction in all-cancer incidence in the calcium plus vitamin D–treated group after 4.1 years of follow-up [43•]. Unlike the WHI, which did not show a reduction in cancer incidence, this study used higher amounts of daily vitamin D (1100 IU vs 400 IU per day in the WHI) that allowed 25(OH)D levels to reach values greater than 32 ng/mL after 1 year of treatment. Interestingly, there was also a nonsignificant trend toward lower all-cancer incidence in the calcium only–treated group (1400−1500 mg/d) [43•].
Recommended Optimal Intake
All the studies that demonstrated efficacy of vitamin D to prevent fracture used at least 800 IU of cholecalciferol in largely vitamin D–deficient populations. Although ergo-calciferol is likely to be effective in preventing fractures, evidence is limited. In addition, 25(OH)D levels should be maintained above at least 32 ng/mL. Vitamin D should also be taken along with 1000 to 1200 mg per day of calcium to have maximum antifracture efficacy [25•]. Adherence to a daily calcium and vitamin D regimen is extremely important and must be stressed to each patient.
Whether calcium absorption differs among common preparations of calcium (ie, calcium citrate or calcium carbonate) is a matter of controversy. Most forms of calcium are best absorbed in divided dosages of 500 mg or less given two to three times daily [44]. Calcium citrate may have a slightly better absorption than calcium carbonate in patients with achlorhydria [45]. A meta-analysis of 15 studies demonstrated an approximately 20% superior absorption of calcium citrate as compared with calcium carbonate [46]. However, only 5 of the 15 studies included in this meta-analysis calculated calcium absorption using dual calcium isotope measurements, which are considered the gold standard. For most healthy adults, calcium carbonate or citrate supplements should adequately satisfy the daily calcium requirement. If cost is an issue, it may be more cost-effective to recommend calcium carbonate as the primary form of calcium supplementation [47].
Calcium absorption does not seem to differ among different food sources such as milk, yogurt, or cheese [48]. Dairy products contain 200 to 300 mg of calcium per serving (1 cup of milk, three quarters of a cup of yogurt, 1.25-inch cube of cheese). Absorption of calcium from cow's milk does not differ from soy milk, an increasingly popular drink, when the latter is fortified with calcium carbonate.
Cholecalciferol has been reported to be more effective in raising 25(OH)D levels compared to ergocalciferol when given in supraphysiologic dosages [49]. Of importance, with advancing age, vitamin D production in the skin declines, making elderly populations more dependent on dietary vitamin D. For the average older person, higher dietary intake of vitamin D may be required to achieve optimal serum levels of 25(OH)D. Most dietary sources of vitamin D do not contain sufficient amounts of vitamin D to satisfy daily requirements. Foods thought to contain high amounts of vitamin D3 include fortified milk and oily fish such as salmon, mackerel, and blue fish [1••].
Additional recommendations for intake in the rheumatology patient
Several rheumatologic diseases, including RA and SLE, are independent risk factors for fracture development. Glucocorticoids, which are frequently employed in the treatment of these diseases, have an additive effect on fracture risk. Vitamin D and calcium play pivotal roles in the prevention of fractures [25•], and higher vitamin D intake may be associated with a decreased incidence of RA [32]. The prevalence of vitamin D deficiency is as high as 67% in SLE patients [50]. In these patients, renal disease, photosensitivity, and African American race are strong predictors of low 25(OH)D levels (< 10 ng/mL) [50].
We recommend that all patients with autoimmune diseases such as SLE or RA as well as those with malabsorption (eg, Crohn's disease or ulcerative colitis) be screened for vitamin D deficiency. If insufficient (< 32 ng/mL), 50,000 IU ergocalciferol once or twice a week should be prescribed for up to 3 months. A follow-up 25(OH)D level should be determined to ensure that optimal 25(OH)D levels have been achieved. After correction of vitamin D insufficiency, we recommend ergocalciferol 50,000 IU once a month or 1000 IU of cholecalciferol daily to maintain optimal 25(OH)D levels. Physicians should consider checking vitamin D status annually or semiannually to ensure optimal circulating 25(OH)D levels year-round, especially in those patients with osteoporosis or risk for falls. Figure 1 presents our proposed algorithm to maintain optimal vitamin D status.
Figure 1.
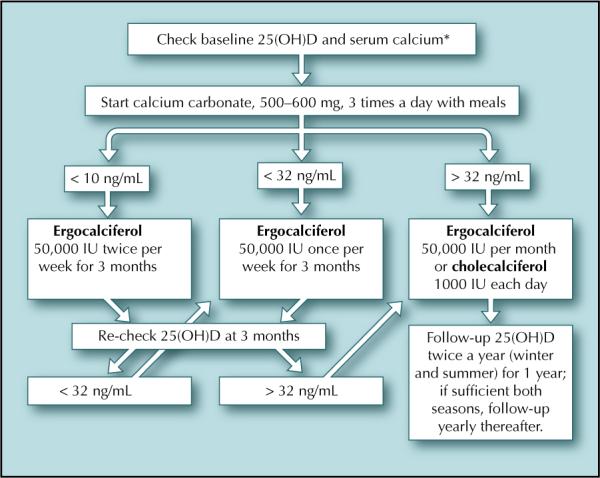
Proposed algorithm for optimal vitamin D status in patients with rheumatologic disease. 25(OH)D—25-hydroxyvitamin D. *If calcium level is elevated, evaluation for primary hyperparathyroidism may be necessary prior to repletion of vitamin D status.
Calcium supplementation should be 1000 to 1200 mg daily in the form of dairy intake or over-the-counter pills in divided doses of 300 to 600 mg twice to three times a day [51]. In patients who do not suffer from photosensitivity or who do not have risk of skin cancer, sun exposure of at least 10 to 15 minutes daily of the hands, face, and arms can be an alternative method to promote endogenous vitamin D production [27].
Patients initiating glucocorticoid therapy are at especially high risk of fractures if these drugs are continued for more than 3 months. In animal studies, these agents inhibit vitamin D–mediated intestinal TRPV6 transcription [44], which may reduce active intestinal calcium transport. Thus, if patients begin glucocorticoid treatment with an expected duration of more than 3 months, higher doses of calcium and vitamin D may be required.
Conclusions
Vitamin D and calcium play important roles in the proper mineralization of bone for optimal skeletal health. Additional benefits of vitamin D include the prevention of autoimmune diseases, decreased risk of cancer, prevention of falls, improved immunity, prevention of hypertension, and diabetes. Given the widespread prevalence of vitamin D insufficiency in the general population and in patients with rheumatologic diseases, screening for and treating vitamin D insufficiency should become standard, especially in those patients with osteoporosis and/or autoimmune diseases. Prospective studies must be conducted to determine whether vitamin D supplementation can reduce the risk of extraskeletal diseases.
Acknowledgment
This work was supported in part by grants from the University Research Committee of Emory University, UV Foundation, Atlanta Research and Education Foundation, and the US National Institutes of Health grant K23AR054334.
Footnotes
Disclosures
The authors have reported no potential conflicts of interest relevant to this article.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1••.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 2007;357:1980–1981. [Comment in. author reply 1981−1982. [Google Scholar]; N Engl J Med. 2007;357:1981. author reply 1981−1982. [Google Scholar]; N Engl J Med. 2007;357:1981. author reply 1981−1982. [Google Scholar]; N Engl J Med. 2007;357:1981. author reply 1981−1982.] [Of major importanceThis very comprehensive review of vitamin D deficiency includes 126 references.] [Google Scholar]
- 2.Tangpricha V, Spina C, Yao M, et al. Vitamin D deficiency enhances the growth of MC-26 colon cancer xenografts in Balb/c mice. J Nutr. 2005;135:2350–2354. doi: 10.1093/jn/135.10.2350. [DOI] [PubMed] [Google Scholar]
- 3•.Zhou C, Assem M, Tay JC, et al. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest. 2006;116:1703–1712. doi: 10.1172/JCI27793. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Clin Invest. 2006;116:2564. doi: 10.1172/JCI30017. Comment in. [Of importanceThis manuscript was one of the first to describe the mechanism for drug-induced osteomalacia by anticonvulsants.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walters JR, Balesaria S, Chavele KM, et al. Calcium channel TRPV6 expression in human duodenum: different relationships to the vitamin D system and aging in men and women. J Bone Miner Res. 2006;21:1770–1777. doi: 10.1359/jbmr.060721. [DOI] [PubMed] [Google Scholar]
- 5.Pattanaungkul S, Riggs BL, Yergey AL, et al. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: evidence for age-related intestinal resistance to 1,25(OH)2D action. J Clin Endocrinol Metab. 2000;85:4023–4027. doi: 10.1210/jcem.85.11.6938. [DOI] [PubMed] [Google Scholar]
- 6.Walters JR, Balesaria S, Khair U, et al. The effects of vitamin D metabolites on expression of genes for calcium transporters in human duodenum. J Steroid Biochem Mol Biol. 2007;103:509–512. doi: 10.1016/j.jsbmb.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Amling M, Priemel M, Holzmann T, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 8.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352:515–516. doi: 10.1056/NEJM200502033520521. author reply 515−516. [DOI] [PubMed] [Google Scholar]
- 9.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 10.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 11.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 12.Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13:257–264. doi: 10.1007/s001980200023. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]; ACP J Club. 1998;128:47. Comment in. [PubMed] [Google Scholar]; N Engl J Med. 1997;337:701–702. [Google Scholar]
- 15.Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004;19:370–378. doi: 10.1359/JBMR.0301240. [DOI] [PubMed] [Google Scholar]
- 16.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 1998;339:344–345. Comment in. author reply 345−346. [Google Scholar]; N Engl J Med. 1998;339:345–346. [Google Scholar]; N Engl J Med. 1998;338:828–829. doi: 10.1056/NEJM199803193381209. [DOI] [PubMed] [Google Scholar]
- 17.van der Wielen RP, Löwik MR, van den Berg H, et al. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 19.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson RD, LaCroix AZ, Gass M, et al. Women's Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 2006;354:1102. Erratum in. [Google Scholar]; ACP J Club. 2006;145:4–5. Comment in. [PubMed] [Google Scholar]; Evid Based Nurs. 2006;9:114. doi: 10.1136/ebn.9.4.114. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 2006;354:750–752. [Google Scholar]; N Engl J Med. 2006;354:2285–2287. author reply 2285−2287. [Google Scholar]; N Engl J Med. 2006;354:2285–2287. author reply 2285−2287. [Google Scholar]; N Engl J Med. 2006;354:2285–2287. author reply 2285−2287. [Google Scholar]
- 21.Porthouse J, Cockayne S, King C, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330:1003. doi: 10.1136/bmj.330.7498.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]; ACP J Club. 2005;143:72–74. Comment in. [PubMed] [Google Scholar]; Age Ageing. 2005;34:542–544. doi: 10.1093/ageing/afi184. [DOI] [PubMed] [Google Scholar]; BMJ. 2005;331:108–109. author reply 109. [Google Scholar]; BMJ. 2005;331:108. author reply 109. [Google Scholar]; BMJ. 2005;331:108. author reply 109. [Google Scholar]
- 22.Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]; ACP J Club. 2005;143:72–74. Comment in. [PubMed] [Google Scholar]; J Fam Pract. 2005;54:658. [Google Scholar]; Lancet. 2005;366:543. author reply 543−544. [Google Scholar]; Lancet. 2005;366:543. author reply 543−544. [Google Scholar]; Lancet. 2005;366:544. [Google Scholar]; Lancet. 2005;365:1599–1600. doi: 10.1016/S0140-6736(05)66385-4. [DOI] [PubMed] [Google Scholar]
- 23.Lips P, Graafmans WC, Ooms ME, et al. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400–406. doi: 10.7326/0003-4819-124-4-199602150-00003. [DOI] [PubMed] [Google Scholar]; ACP J Club. 1996;125:16. Comment in. [PubMed] [Google Scholar]
- 24.Meyer HE, Smedshaug GB, Kvaavik E, et al. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res. 2002;17:709–715. doi: 10.1359/jbmr.2002.17.4.709. [DOI] [PubMed] [Google Scholar]
- 25•.Boonen S, Lips P, Bouillon R, et al. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92:1415–1423. doi: 10.1210/jc.2006-1404. [Of importanceThis meta-analysis demonstrates that vitamin D and calcium together are most effective in preventing hip fractures.] [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Dawson-Hughes B. Where do we stand on vitamin D? Bone. 2007;41(1 Suppl 1):S13–19. doi: 10.1016/j.bone.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]; Mayo Clin Proc. 2006;81:297–299. doi: 10.4065/81.3.297. Comment in. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 30.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(6 Suppl):1717S–1720S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 31.Vaisberg MW, Kaneno R, Franco MF, Mendes NF. Influence of cholecalciferol (vitamin D3) on the course of experimental systemic lupus erythematosus in F1 (NZBxW) mice. J Clin Lab Anal. 2000;14:91–96. doi: 10.1002/(SICI)1098-2825(2000)14:3<91::AID-JCLA2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merlino LA, Curtis J, Mikuls TR, et al. Iowa Women's Health Study Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004;50:72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]; Arthritis Rheum. 2006;54:3719–3720. doi: 10.1002/art.22191. Comment in. [DOI] [PubMed] [Google Scholar]
- 33•.Costenbader KH, Feskanich D, Benito-Garcia E, et al. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.072736. [Epub ahead of print]. [Of importanceA prospective cohort study of the Nurses’ Health Study and Nurses’ Health Study II cohorts. After 22 years of follow-up, of 186,389 women, investigators found 190 new cases of confirmed SLE and 722 new cases of RA. No association was found between vitamin D intake (based on food frequency questionnaire) and incidence of SLE or RA.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 35.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 36•.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [Of importanceThis is a very well-written review for the evidence of vitamin D in the prevention of type 1 and type 2 diabetes.] [DOI] [PubMed] [Google Scholar]
- 37.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 39.Judd SE, Nanes MS, Ziegler TR, et al. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87:136–141. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 40.Pfeifer M, Begerow B, Minne HW, et al. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 41.Hsia J, Heiss G, Ren H, et al. Women's Health Initiative Investigators Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]; Circulation. 2007;115:e466. Erratum in. [Google Scholar]; Circulation. 2007;115:827–828. doi: 10.1161/CIRCULATIONAHA.106.686238. Comment in. [DOI] [PubMed] [Google Scholar]; Circulation. 2007;116:e85. author reply e87. [Google Scholar]; Circulation. 2007;116:e86. author reply e87. [Google Scholar]
- 42••.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [Of major importanceThis very intriguing manuscript reviewed all randomized placebo-controlled trials with vitamin D in which death was collected as an end point. The results show a survival advantage when taking usual over-the-counter doses of vitamin D (∼500 IU daily).] [DOI] [PubMed] [Google Scholar]
- 43•.Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]; Am J Clin Nutr. 2007;86:1804–1805. doi: 10.1093/ajcn/86.5.1804. [Comment in. author reply 1805−1806. [DOI] [PubMed] [Google Scholar]; Am J Clin Nutr. 2007;86:1549. author reply 1549−1550.] [Of importanceA double-blind, randomized controlled trial of Nebraska women with primary outcome of fractures and principal secondary outcome of cancer incidence. Subjects (n = 1179) were randomly assigned to calcium (1400−1500 mg) plus vitamin D (1100 IU), calcium alone, or placebo, with a 4-year follow-up. Treatment with vitamin D and levels of 25(OH)D were significantly associated with decreased cancer incidence, with a RR of 0.232 for cancers diagnosed 1 year after study initiation in the vitamin D and calcium group.] [Google Scholar]
- 44.Huybers S, Naber TH, Bindels RJ, Hoenderop JG. Predniso-lone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92–G97. doi: 10.1152/ajpgi.00317.2006. [DOI] [PubMed] [Google Scholar]
- 45.Recker RR. Calcium absorption and achlorhydria. N Engl J Med. 1985;313:70–73. doi: 10.1056/NEJM198507113130202. [DOI] [PubMed] [Google Scholar]
- 46.Sakhaee K, Bhuket T, Adams-Huet B, Rao DS. Meta-analysis of calcium bioavailability: a comparison of calcium citrate with calcium carbonate. Am J Ther. 1999;6:313–321. doi: 10.1097/00045391-199911000-00005. [DOI] [PubMed] [Google Scholar]; Am J Ther. 2001;8:73–74. Comment in. [Google Scholar]; Am J Ther. 2001;8:74–77. [Google Scholar]
- 47.Heaney RP, Dowell MS, Bierman J, et al. Absorbability and cost effectiveness in calcium supplementation. J Am Coll Nutr. 2001;20:239–246. doi: 10.1080/07315724.2001.10719038. [DOI] [PubMed] [Google Scholar]
- 48.Recker RR, Bammi A, Barger-Lux MJ, Heaney RP. Calcium absorbability from milk products, an imitation milk, and calcium carbonate. Am J Clin Nutr. 1988;47:93–95. doi: 10.1093/ajcn/47.1.93. [DOI] [PubMed] [Google Scholar]
- 49.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 50.Kamen DL, Cooper GS, Bouali H, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–117. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Harvey JA, Zobitz MM, Pak CY. Dose dependency of calcium absorption: a comparison of calcium carbonate and calcium citrate. J Bone Miner Res. 1988;3:253–258. doi: 10.1002/jbmr.5650030303. [DOI] [PubMed] [Google Scholar]


