Abstract
TRAF2 is an adaptor protein that modulates the activation of the JNK/c-Jun and IKK/NF-κB signaling cascades in response to TNFα stimulation. Although many serine/threonine kinases have been implicated in TNFα-induced IKK activation and NF-κB-dependent gene expression, most of them do not directly activate IKK. Here we report that PKCζ phosphorylates TRAF2 at serine 55 — within the protein’s RING domain — after TNFα stimulation. Although this phosphorylation event has a minimal effect on induction of the immediate/transient phase of IKK and JNK activation by TNFα, it promotes the secondary/prolonged phase of IKK activation and inhibits that of JNK. Importantly, constitutive TRAF2 phosphorylation increased both basal and inducible NF-κB activation and rendered Ha-Ras-V12 transformed cells resistant to stress-induced apoptosis. Moreover, TRAF2 was found to be constitutively phosphorylated in some malignant cancer cell lines and Hodgkin’s lymphoma. These results reveal a new level of complexity in TNFα-induced IKK activation modulated by TRAF2 phosphorylation, and suggest that TRAF2 phosphorylation is one of the events that are responsible for elevated basal NF-κB activity in certain human cancers.
Keywords: Apoptosis, JNK, NF-κB, Phosphorylation, TRAF2
INTRODUCTION
The tumor necrosis factor (TNF) receptor (TNFR) associated factor (TRAF) family consists of six members and is characterized by a highly conserved TRAF domain at the protein C-terminus. With the exception of TRAF1, the TRAFs contain an N-terminal RING finger domain followed by five or seven zinc-finger motifs (1, 2). Whereas the TRAF domain is required for interactions with the receptors and effectors of the signaling pathway, the RING domain is believed to be essential for activation of downstream signaling pathways (2). TRAF2 is a prototypical member of the TRAF family, and regulates signals that emanate from several members of the TNFR superfamily, resulting in the sequential activation of mitogen-activated protein kinase kinase kinase (MAPKKK such as MEKK1/3), MAPKK (e.g. MKK4/7) and MAPK (e.g. c-Jun N-terminal kinase, JNK), as well as in activation of TGF-β-activated kinase 1 (TAK1), receptor interacting protein 1 (RIP1) and IκB kinase (IKK).
MAPK and IKK activate AP-1 (e.g., c-jun/ATF2) and NF-κB transcription factors. Activated AP-1 and NF-κB in turn induce the expression of genes involved in inflammation, the immune response, cell proliferation and cell differentiation, as well as genes that act to suppress death receptor- and stress-induced apoptosis (1, 3). Gene knockout and transgenic studies have firmly established that the activation of the NF-κB pathway is essential for cancer cell progression and metastasis (4–7). In addition, many types of cancer cells exhibit elevated basal NF-κB activity and inhibition of NF-κB sensitizes cancer cells to stress-induced apoptosis (4, 6, 7). However, the mechanism underlying the constitutive activation of NF-κB in human tumors is still not fully understood (4, 6, 7).
Knockout of TRAF2 impairs TNFα-induced activation of JNK, but not of IKK (8). Tada et al. have reported that TRAF2 and TRAF5 double-knockout (TRAF2/5 DKO) mouse embryonic fibroblasts (MEFs) exhibit an almost complete defect in TNFα-induced IKK activation (9), suggesting that TRAF2 has a non-redundant role in JNK activation but is redundant with TRAF5 with regard to IKK activation. Although the mechanisms underlying signal transduction from IKK to NF-κB and from MEKK1/3 to c-Jun are better understood, the receptor proximal events that trigger IKK versus MEKK1/3 activation by TRAF2 remain largely elusive.
In this study, we show that TRAF2 is phosphorylated on residue Ser-55, and that this modification has opposite effects on the prolonged phase of TNFα-induced IKK and JNK activation. In addition, we show that TRAF2 is constitutively phosphorylated at Ser-55 in some cancer cell lines and that this phosphorylation is significant for cancer cell resistance to stress-induced apoptosis.
MATERIALS AND METHODS
Cell lines, plasmids and reagents
Wild-type (WT) MEFs, TRAF2/5 DKO MEFs, HeLa, 293T and NIH 3T3 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine calf serum (BCS; Hyclone, Logan, UT) and antibiotics. Antibodies (Abs) and reagents were purchased as follows: anti-TRAF2, anti-JNK1, anti-IKKγ, anti-IKKβ and anti-TNFR1 Abs from Santa Cruz (Santa Cruz, CA); mouse and human TNFα (hTNFα and mTNFα) from Roche (Indianapolis, IN); anti-Flag Ab, hydroxyurea, etoposide and phorbol-12-myristate-13-acetate (TPA; synonym PMA) from Sigma (St. Louis, MO); Halt cocktails of protease and phosphatase inhibitors from Pierce (Rockford, IL); pRL-TK Renilla luciferase-encoding plasmid and PKC mixture containing PKCα, β, γ, δ and ζ from Promega (Madison, WI). Constructs encoding Flag-TRAF2, constitutively active PKC (CA-PKC) or Akt1 (Myr-Akt1) as well as those encoding the NF-κB or c-Jun firefly luciferase reporter gene (NF-κB-Luc and Jun2-Luc) have been described (10, 11). Mutations were introduced into the Flag-TRAF2 expression vector using the Quick Change Site-Directed Mutagenesis Kit (Stratagene) and were confirmed by DNA sequencing. Retroviral vectors for the transduction of Flag-TRAF2 and Ha-Ras-V12 constructs were generated by subcloning the TRAF2 and Ha-Ras-V12 cDNAs into a pBabe-puro and pQCXIH-hygro plasmids, respectively.
32P-orthophosphate labeling and two-dimensional separation of phosphoamino acids
In vivo 32P-orthophosphate labeling and two-dimensional separation of amino acids on TLC plate were performed exactly as described previously (10).
Preparation of retroviral supernatants and infection of TRAF2/5 DKO cells
TRAF2/5 DKO cells that stably express WT or phosphomutant TRAF2 were generated as described previously (10).
Phosphoantibody and Immunoblotting
Phosphopeptide (GHRYCpS55FCLAS) synthesis, rabbit immunization and antibody purification were performed by ABGENT Envision Proteomics (San Diego, CA). For the detection of TRAF2 phosphorylation, cells were treated as indicated and protein samples were extracted with TNE lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM NaF, 1.0 % NP-40, 2 mM EDTA, 1 mM DTT, 0.5 mM PMSF, 1 × Halt cocktails of protease and phosphatase inhibitors) for 30 min on ice. 30 μg of cleared lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The blots were blocked with 0.2 % Tween 20/TBS containing 3% BSA for 4 hrs and incubated with TRAF2 phosphoantibody overnight at 4°C. The phosphorylation status of TRAF2 was then assessed using HRP-labeled secondary antibody and ECL solution. The same membranes were then stripped and reprobed with anti-TRAF2 antibody.
Real-time RT-PCR
TRAF2/5 DKO cells reconstituted with TRAF2-WT, -S55A or -S55D were treated with mTNFα (10 ng/ml), and total RNA was prepared using the RNeasy Mini Kit (Qiagen). Real-time PCR assays for the quantification of NF-κB-dependent gene expression were also performed exactly as described previously (10).
RESULTS
TRAF2 is phosphorylated at Ser-55
In a previous study, we identified the Ser-11 residue of TRAF2 as one of the protein’s phosphorylation sites, and developed a phosphospecific antibody (pTRAF2-Ser11) that recognizes this modification (10). In an in vivo 32P-orthophosphate labeling experiment, co-expression of a constitutively active form of either PKCα or Akt1 (CA-PKCα and Myr-Akt1) with Flag-TRAF2 in NIH 3T3 cells increased overall TRAF2 phosphorylation (Fig. 1A). However, Western blot analysis with pTRAF2-Ser11 phosphoantibody revealed that co-expression of CA-PKCα or Myr-Akt1 with Flag-TRAF2 does not increase TRAF2 phosphorylation at Ser-11 in vivo (Fig. S1A). Further analyses by in vivo 32P-orthophosphate labeling approaches revealed that overexpression of either CA-PKCα or Myr-Akt1 with Flag-TRAF2 in which the Ser-11 residue is mutated to alanine (TRAF2-S11A) increases the phosphorylation of this mutant TRAF2 (Fig. S1B), suggesting that PKCα and Akt1 induce TRAF2 phosphorylation at a different site.
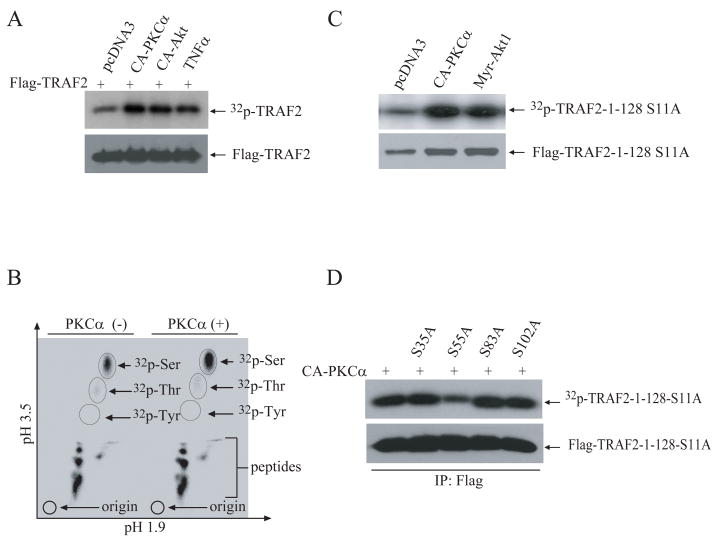
Figure 1. Expression of CA-PKCα induces TRAF2 phosphorylation at Ser-55 in vivo.
A, NIH 3T3 cells were co-transfected with Flag-TRAF2 (1.0μg) or empty pCDNA3 (1.0μg) and HA-CA-PKCα or HA-Myr-Akt1 (0.5 μg/ml). 36 hrs after transfection, cells were labeled with 32P-orthophosphate and either mock treated or treated with TNFα for 15 min. 32P-labeled Flag-TRAF2 was then immunoprecipitated, separated by SDS-PAGE and transferred onto a PVDF membrane. The membrane was exposed to X-ray film for 6 hrs, and was then probed with anti-Flag antibody.
B, NIH 3T3 cells were co-transfected with Flag-TRAF2 (1.0 μg) and pCDNA3 (1.0 μg) or HA-CA-PKCα (0.5 μg). At 36 hrs after transfection, 32P-labeled Flag-TRAF2 was purified and hydrolyzed in 6N HCl. Hydrolyzed 32P-Flag-TRAF2 was then separated on a TLC plate, and exposed to X-ray film for 7 days.
C, NIH 3T3 cells were co-transfected with the Flag-TRAF2-1-128-S11A mutant (1.0 μg) and either HA-Myr-Akt1 (0.5 μg) or HA-CA-PKCα (0.5 μg) as indicated. At 36 hrs after transfection, overall TRAF2 phosphorylation was detected as in A.
D, WT and mutant forms of Flag-TRAF2-1-128-S11A were co-expressed with CA-PKCα in NIH 3T3 cells and their phosphorylation was assessed as in A.
To map the TRAF2 phosphorylation site modified by PKCα and Akt1, we first co-expressed Flag-TRAF2 with CA-PKCα and examined which type of phosphoamino acid is modified. To this end, we carried out two-dimensional separation of amino acids (aa) on a TLC plate after 32P-labeled Flag-TRAF2 was hydrolyzed with 6N HCl. As shown in Fig. 1B, both basal and inducible TRAF2 phosphorylation took place at serine residues. TRAF2 has been reported to be phosphorylated at Thr-117 (12). However, we were not able to detect TRAF2 phosphorylation at threonine residues in NIH 3T3 cells. In a previous study, we had found that TRAF2 phosphorylation occurs primarily in the N-terminal region, between aa 1-128 (10). As expected, an in vivo 32P-labeling analysis revealed that CA-PKCα and Myr-Akt1 induce TRAF2 phosphorylation on the N-terminal region, at a site other than Ser-11 (Fig. 1C). Analysis of the TRAF2-1-128 aa sequence with the Scansite program revealed that serine residues 35, 55, 83 and 102 fit the consensus phosphorylation sites for PKC, Akt1 and CK1 (data not shown). We thus mutated each of these sites individually to alanine using the TRAF2-1-128-S11A plasmid as template, and then co-expressed these mutants with CA-PKCα in NIH 3T3 cells. 32P-labeling analysis revealed that mutation of Ser-55 to alanine significantly, but not completely, inhibits TRAF2-1-128-S11A phosphorylation, suggesting that Ser-55 is at least one target of PKCα (Fig. 1D). In fact, Ser-55 (RYCS55F) is a consensus PKC phosphorylation site (RxxS/T, RxS/T or S/TxR) as well as a consensus Akt1 phosphorylation site (RxxS/TF/L), and is conserved between mouse and human (Fig. S2B). Notably, Ser-55 resides in the middle of the TRAF2 RING domain (Fig. S2A).
TRAF2 Ser-55 phosphorylation increases NF-κB activation but inhibits c-Jun activation
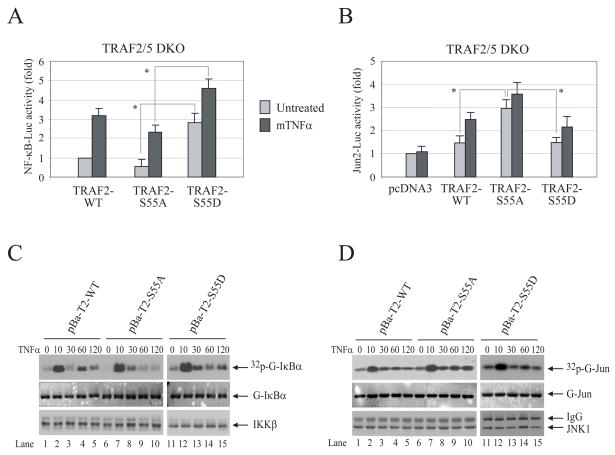
To assess the role of TRAF2 phosphorylation in TNFα-induced c-Jun and NF-κB activation, we generated two phosphomutant TRAF2 plasmids: TRAF2-S55A in which Ser-55 is mutated to alanine to abolish phosphorylation; and TRAF2-S55D in which Ser-55 is mutated to aspartic acid to mimic phosphorylation. In luciferase reporter gene assays performed in WT MEFs, TRAF2-S55A expression reduced NF-κB activity by 20–30%, but increased c-Jun activity by 20–30%, compared to that measured in cells transfected with TRAF2-WT (Fig. S3A and B). To examine the role of TRAF2 Ser-55 phosphorylation in the absence of interference from endogenous TRAF2 and TRAF5, we performed luciferase reporter gene assays in TRAF2/5 DKO MEFs. As shown in Fig. 2A and B, the expression of TRAF2-S55D significantly increased both the basal and inducible NF-κB activities compared to those measured in TRAF2-S55A-transfected cells, whereas the expression of TRAF2-S55A significantly increased basal c-Jun activity compared to that induced by TRAF2-WT and -S55D expression. However, none of the constructs tested exhibited dominant negative effect to block TNFα-induced c-Jun and NF-κB activation. Overall, these findings suggest that TRAF2 phosphorylation at Ser-55 contributes to, but is not essential for, TNFα-induced activation of NF-κB and c-Jun.
Figure 2. TRAF2 Ser-55 phosphorylation increases NF-κB activity but decreases c-Jun activity.
A, B, TRAF2/5 DKO MEFs were co-transfected with NF-κB-Luc or Jun2-Luc, pRL-TK and pCDNA3, TRAF2-WT, -S55A or -S55D as indicated. 36 hrs after transfection, the cells were either mock treated or treated with mTNFα (5ng/ml) for 4 hrs, after which the NF-κB-Luc or Jun2-Luc activity was measured and normalized to pRL-TK activity. Data shown are the mean ± SD of three experiments that were done in triplicate. “*” indicates p<0.05.
C, D, TRAF2/5 DKO cells reconstituted with TRAF2-WT (pBa-T2-WT), -S55A (pBa-T2-S55A) or -S55D (pBa-T2-S55D) were treated with mTNFα (10ng/ml) for the indicated times. The IKK complex or JNK1 was immunoprecipitated with anti-IKKγ or anti-JNK1 antibody, respectively, and subjected to in vitro kinase assays in which GST-IκBα1–55 served as substrate for IKK and GST-jun1–87 served as substrate for JNK1. Reaction mixtures were separated by SDS-PAGE, transferred onto a nitrocellulose membrane and exposed to X-ray film for 6 hrs (32p-G-IκBα or 32p-G-jun). The same membrane was stained with Ponceau S (G-IκBα or G-jun) and then immunoblotted with anti-IKKβ or anti-JNK1 antibody (IKKβ or JNK1).
TRAF2 Ser-55 phosphorylation has opposite effects on the prolonged phase of TNFα-induced IKK and JNK activation
To further examine the role of TRAF2 Ser-55 phosphorylation in TNFα-induced JNK and IKK activation, we established TRAF2/5 DKO cell lines that stably express Flag-TRAF2-WT (pBa-T2-WT), -S55A (pBa-T2-S55A) or -S55D (pBa-T2-S55D) at physiological levels as described previously (10). Immunokinase assays revealed that stable expression of TRAF2-WT in TRAF2/5 DKO cells restores TNFα-induced transient, as well as secondary/prolonged, IKK activation. However, stable expression of TRAF2-S55A restored the transient, but not the prolonged, phase of IKK activation (Fig. 2C; compare lane−4 and −5 with lane−9 and −10). Stable expression of TRAF2-S55D also altered the oscillation of IKK activity following TNFα stimulation (Fig. 2C). On the other hand, stable expression of TRAF2-S55A enhanced TNFα-induced prolonged JNK activation, although it had no effect on transient JNK activation (Fig. 2D; compare lanes−4 and −5 with lanes−9 and −10). In vitro IKK and JNK kinase assays were repeated three times, and average kinase activities are summarized in Fig. S4A and B. Collectively, these data suggest that TRAF2 phosphorylation at Ser-55 positively regulates the prolonged phase of IKK activation while inhibiting the prolonged phase of JNK activation, which explains why TRAF2-S55A expression partially inhibits NF-κB activity and increases c-Jun activity.
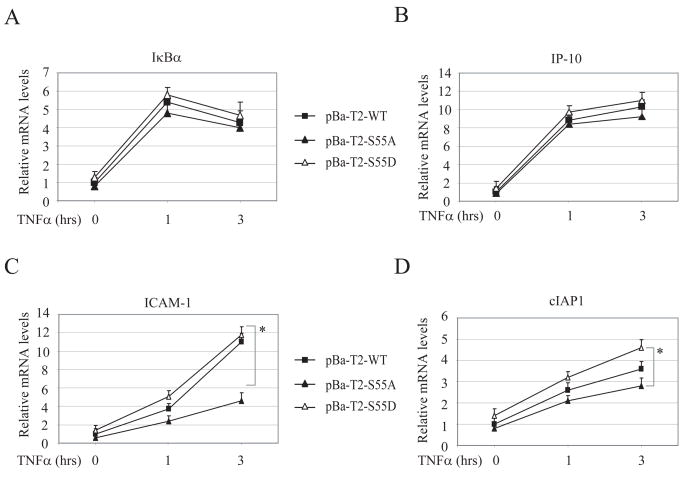
TRAF2 Ser-55 phosphorylation is essential for the efficient expression of a subset of NF-κB target genes in response to TNFα stimulation. To examine the role of TRAF2 Ser-55 phosphorylation in TNFα-induced NF-κB activation in a physiological setting, we analyzed the expression of NF-κB target genes in pBa-T2-WT, pBa-T2-S55A and pBa-T2-S55D cells by real-time RT-PCR. As shown in Fig. 3A and B, there were no significant differences in the expression levels of IκBα and IP-10 in all three cell lines before and after TNFα stimulation. On the other hand, TNFα-induced expression of ICAM-1, RANTES, cIAP1, cIAP2, cFLIP and Mn-SOD was significantly enhanced in pBa-T2-S55D cells compared to that in pBa-T2-S55A cells (Fig. 3C and D and Fig. S5A–D). In pBa-T2-WT cells, only ICAM-I and RANTES expression was significantly higher than that in pBa-T2-S55A cells. These data indicate that TRAF2 Ser-55 phosphorylation is essential for the efficient expression of certain NF-κB target genes, such as ICAM-1 and RANTES, in response to TNFα stimulation.
Figure 3. TRAF2 Ser-55 phosphorylation is essential for efficient TNFα-induced expression of a subset of NF-κB target genes.
A—D, pBa-T2-WT, pBa-T2-S55A and pBa-T2-S55D cells were treated with mTNFα (10ng/ml) as indicated, and the expression levels of IκBα, IP-10, ICAM-I and cIAP1 were determined by real-time PCR. The relative expression level of each gene is presented as the ratio between it and the reference gene GAPDH, as an average from four independent experiments. “*” represents p<0.05.
TRAF2 Ser-55 phosphorylation is induced by TNFα and UV
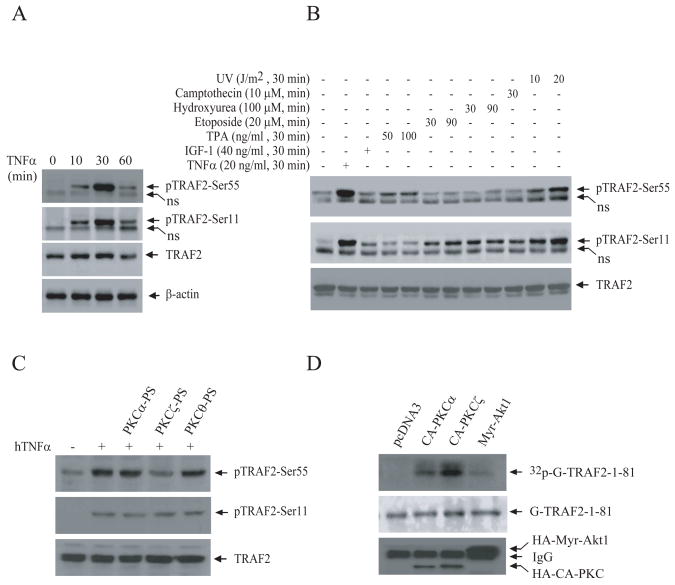
To analyze endogenous TRAF2 phosphorylation at Ser-55, we generated a phosphoantibody (pTRAF2-Ser55) directed against TRAF2 Ser-55. As shown in Fig. S6A, pTRAF2-Ser55 antibody specifically recognized TRAF2-1-128-WT and -S11A, but not TRAF2-1-128-S55A, expressed in NIH 3T3 cells. Treatment of immunoprecipitated Flag-TRAF2-1-128-WT with calf intestinal alkaline phosphatase completely blocked the recognition of TRAF2-1-128-WT by pTRAF2-Ser55 antibody (Fig. S6B), confirming that this antibody recognizes only TRAF2 that is phosphorylated at Ser-55. Next, we examined endogenous TRAF2 phosphorylation in HeLa cells following TNFα stimulation. As shown in Fig. 4A, TNFα treatment immediately induced TRAF2 phosphorylation in HeLa cells, with peak induction occurring at 30 min after stimulation. We also examined TRAF2 phosphorylation in response to growth factors and various inducers of cellular stress. In addition to TNFα, UV strongly induced TRAF2 phosphorylation at Ser-55 in HeLa cells (Fig. 5B). Unexpectedly, a potent activator of PKCα (TPA) only weakly induced TRAF2 Ser-55 phosphorylation, and a potent activator of Akt1 (IGF-I) did not induce TRAF2 Ser-55 phosphorylation at all in HeLa cells. These data suggest that even though PKCα and Akt1 induce TRAF2 Ser-55 phosphorylation in transient overexpression system, they may not be involved in the phosphorylation of endogenous TRAF2.
Figure 4. TNFα-induced TRAF2 Ser-55 phosphorylation is mediated by PKCζ.
A, B, HeLa cells were left untreated or were treated with hTNFα, IGF-I, TPA, etoposide, hydroxyurea, camptothecin or UV as indicated, and TRAF2 phosphorylation was monitored by Western blotting using TRAF2 phosphoantibodies. The same membranes were then stripped and reprobed with an anti-TRAF2 or an anti-β-actin antibody.
C, HeLa cells were pretreated with myristoylated PKC isotype-specific pseudosubstrates (PS) for 60 min before being stimulated with hTNFα (20 ng/ml), and TRAF2 phosphorylation was then detected as in A.
D, Bacterially expressed and purified GST-TRAF2-1-81-WT, -S11A and -S55A were subjected to an in vitro kinase assay in the presence of CA-PKCα, CA-PKCζ or Myr-Akt1 purified from 293T cells, and phosphorylation of TRAF2 was monitored as described in Fig. 2C.
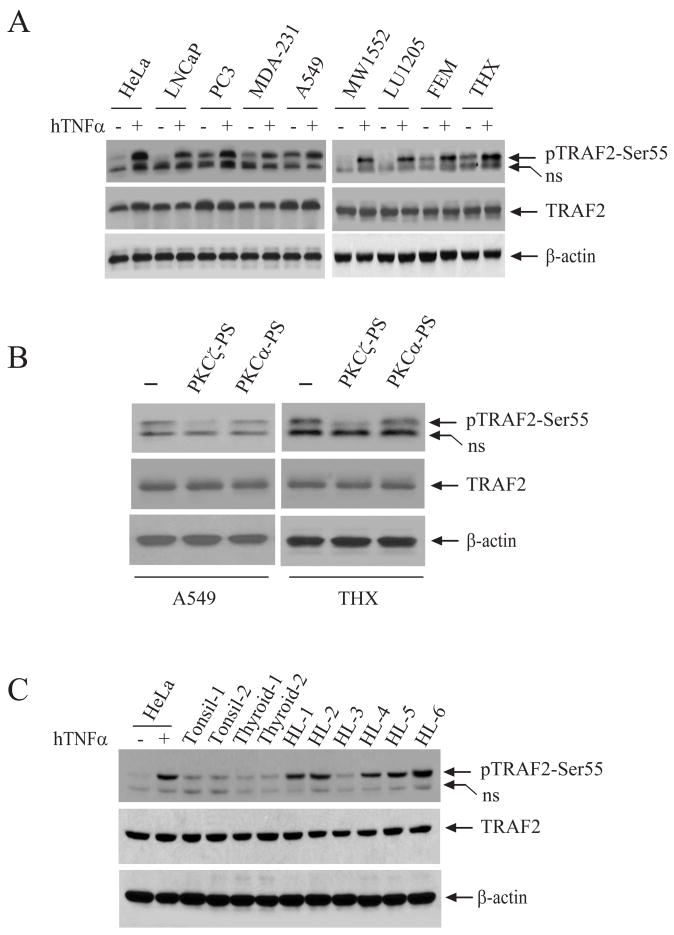
Figure 5. TRAF2 is constitutively phosphorylated in some cancer cell lines and in Hodgkin’s lymphoma.
A, TRAF2 Ser-55 phosphorylation in several cancer cell lines, before and after TNFα stimulation, was monitored by Western blotting with a TRAF2 Ser-55-specific phosphoantibody.
B, A549 and THX cells were treated with isotype-specific PKC PS for 60 min, and TRAF2 phosphorylation was then detected as in A.
C, TRAF2 Ser-55 phosphorylation in normal tonsil, thyroid and Hodgkin’s lymphoma (HL) was monitored by Western blotting. Lysates from HeLa cells mock treated or treated with TNFα were used as controls.
TNFα-induced TRAF2 Ser-55 phosphorylation is mediated by PKCζ
To identify the kinases involved in TRAF2 phosphorylation at Ser-55, we pre-treated HeLa cells with various kinase inhibitors before stimulating them with TNFα. As shown in Fig.S7A, an inhibitor of both PKCα and PKCζ (Go6983) significantly reduced TNFα-induced TRAF2 phosphorylation at Ser-55 but not at Ser-11, whereas a PKCα-specific inhibitor (Go6976) failed to inhibit TRAF2 phosphorylation at either site. In line with this, PKCζ-specific pseudosubstrate (PS) blocked TNFα-induced TRAF2 phosphorylation at Ser-55 but not at Ser-11, whereas PKCα-specific PS did not block phosphorylation at either site (Fig. 4C). These data suggest that PKCζ is involved in TRAF2 phosphorylation at Ser-55. To examine whether PKC directly phosphorylates TRAF2, we expressed and immunopurified HA-CA-PKCα, HA-CA-PKCζ and HA-Myr-Akt1 from 293T cells and used these preparations as kinase sources for an in vitro kinase assay in which bacterially expressed and purified GST-TRAF2-1-81 was used as a substrate. Interestingly, both PKCα and PKCζ, but not Myr-Akt1, phosphorylated GST-TRAF2-1-81 (Fig. 4D). Mutation of Ser-55 to alanine almost completely abolished PKCζ-mediated TRAF2 phosphorylation in vitro (Fig. S7B). Collectively, these data suggest that PKCζ directly phosphorylates TRAF2 at Ser-55 in response to TNFα stimulation.
TRAF2 is constitutively phosphorylated in some cancer cell lines and in Hodgkin’s lymphoma
NF-κB is constitutively activated in many types of human cancer cells (4). We thus wanted to determine whether TRAF2 phosphorylation is correlated with NF-κB activation in cancer cells. To this end, we examined the phosphorylation of TRAF2 in an assortment of well-established cancer cell lines: LNCaP and PC3 (prostate cancer), MDA-MB-231 (breast cancer), A549 (lung cancer) and WM1552, LU1205, FEMX and THX (melanoma). As shown in Fig. 5A, TRAF2 was constitutively phosphorylated (but to varying degrees) in the PC3, MDA-231, A549, FEMX and THX cell lines, and treatment of these cells with TNFα led to a further increase in TRAF2 Ser-55 phosphorylation. Consistently, inhibition of PKCζ, but not of PKCα, with isotype-specific PS blocked constitutive phosphorylation of TRAF2 in A549 and THX cell lines (Fig. 5B), suggesting that PKCζ is responsible for basal TRAF2 Ser-55 phosphorylation in these cell lines. NF-κB is known to be constitutively activated in Hodgkin’s lymphoma (HL) and Hodgkin/Reed-Sternberg (HRS) cell lines (13). Thus, we also examined TRAF2 phosphorylation in HL tissues obtained from the Tissue Procurement Core Facility in the University of Iowa. As shown in Fig. 5C, TRAF2 was constitutively phosphorylated in 5 of 6 HL samples, but not in samples of normal tonsil and thyroid. These data demonstrate that constitutive TRAF2 phosphorylation is very common both in cancer cell lines and Hodgkin’s lymphomas.
TRAF2 Ser-55 phosphorylation protects cells from stress-induced cell death
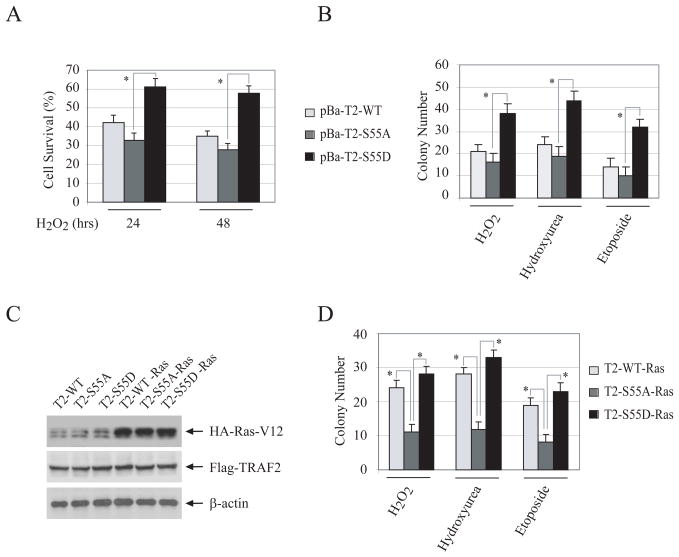
In TRAF2/5 DKO cells, TNFα stimulation causes the accumulation of reactive oxygen species (ROS) and prolonged JNK activation, both of which ultimately lead to necrotic and apoptotic cell death (14). We also observed that over 90% of TRAF2/5 DKO MEFs undergo cell death within 48 hrs of TNFα treatment (Fig. S8A). Notably, stable expression of TRAF2-WT, -S55A or -S55D in TRAF2/5 DKO MEFs completely inhibited TNFα-induced cell death, indicating that the phosphorylation of TRAF2 is not required for its inhibition of TNFα-induced cell death. On the other hand, stable expression of TRAF2-S55D in TRAF2/5 DKO cells rendered cells more resistant to H2O2-induced cell death than did the expression of TRAF2-S55A (Fig. 6A). However, we did not observe a significant difference between TRAF2-WT- and TRAF2-S55A-transfected cells with respect to their sensitivity to H2O2-induced cell death. Colony formation assays (CFA) also revealed that pBa-T2-S55D cells are significantly more resistant to hydroxyurea-, etoposide- and H2O2-induced apoptosis and/or growth arrest than are pBa-T2-S55A cells (Fig. 6B and S8B). To further assess the role of TRAF2 phosphorylation in the resistance of transformed cells to stress-induced cell death, we stably expressed Ha-Ras-V12 in pBa-T2-WT (T2-WT-Ras), -S55A (T2-S55A-Ras) and -S55D (T2-S55D-Ras) cells (Fig. 6C), and then performed cytotoxicity and CFA assays. Interestingly, both the cytotoxicity and CFA assays revealed that T2-WT-Ras cells are significantly more resistant to stress-induced cell death than are T2-S55A-Ras cells (Fig. 6D and S9). These data suggest that TRAF2 phosphorylation at Ser-55 plays a critical role in protecting cells from stress-induced apoptosis.
Figure 6. TRAF2 Ser-55 phosphorylation inhibits stress-induced cell death.
A, pBa-T2-WT, -S55A and -S55D cells were treated with H2O2 (0.075mM) as indicated. At 24 or 48 hrs after treatment, total cell death was assessed via the trypan blue exclusion assay, and data shown represent the average of three experiments performed in triplicate. “*” indicates p<0.05.
B, pBa-T2-WT, -S55A and -S55D cells cultured in 6-well plates (500 cells/well) were left untreated or treated with H2O2 (0.1 mM), etoposide (5 μM) or hydroxyurea (0.4mM) for 6 hrs. 14 days later, colonies containing more than 50 cells were counted. The averages are represented as mean ± SD. “*” indicates p<0.05.
C, pBa-T2-WT, -S55A and -S55D cells were stably transfected with Ha-Ras-V12, and Ras expression was monitored by Western blotting.
D, Ha-Ras-V12-transformed pBa-T2-WT, -S55A and -S55D cells were cultured in 6-well plates (500 cells/well) and left untreated or treated with H2O2 (0.1 mM), etoposide (5 μM) or hydroxyurea (0.4mM) for 6 hrs. Colony formation was then assessed as in B.
DISCUSSION
The PKC family of proteins is divided into three groups (15): conventional (including α, β and γ isotypes), novel (includingη, ε, δ, and θ isotypes) and atypical (including ι/λ and ζ isotypes). These PKCs possess broadly overlapping substrates and exhibit redundancy in their biological functions (16–18). The phosphorylation of PKC substrates, in many cases, requires scaffold proteins (e.g. p62 for PKCζ and RACK for PKCα), and these scaffold proteins are believed to regulate both the subcellular localization and substrate specificity of PKC isoforms in vivo (18, 19). Several studies have shown that, whereas PKCα mediates TPA-induced NF-κB activation, PKCζ mediates TNFα-induced NF-κB activation (16, 20, 21). Thus, we speculated that PKCα may induce TRAF2 Ser-55 phosphorylation in response to TPA stimulation. However, TPA only weakly induced TRAF2 Ser-55 phosphorylation in all cell lines tested, including HeLa (Fig. 4B and data not shown). Thus, it is possible that although transiently overexpressed CA-PKCα is able to induce TRAF2 Ser-55 phosphorylation, endogenous TRAF2 may not be a physiological substrate for this kinase. Myr-Akt1 also increased TRAF2 phosphorylation in vivo (Fig. 1C). However, an in vitro kinase assay revealed that Akt1 only weakly phosphorylates TRAF2 (Fig. 4D), indicating that Akt1 may induce TRAF2 phosphorylation in vivo indirectly. One kinase can phosphorylate two or more substrates, and one protein can be phosphorylated by two or more kinases. Therefore, it is possible that TRAF2 can also be phosphorylated by other members of the PKC family or PKC-related kinases.
An early study showed that PKCζ directly activates IKK in response to TNFα stimulation (20), and many subsequent studies have shown that inhibition of PKCζ (by either a PKCζ-specific PS or antisense oligonucleotides) significantly attenuates TNFα-induced expression of NF-κB target genes (such as MMP-9 and ICAM-I) in different cell types (16, 21, 22). However, a more recent gene knockout study has demonstrated that PKCζ is not essential for TNFα-induced transient IKK activation, but is required for efficient activation of the NF-κB pathway, both upstream and downstream of IKK (23). The findings we present here demonstrate that PKCζ-mediated TRAF2 phosphorylation at Ser-55 is not essential for TNFα-induced transient IKK activation, but rather is required for the prolonged phase of IKK activation, and that this phase plays an important role in the efficient expression of a subset of NF-κB target genes.
Our analysis of the expression of well-known NF-κB target genes in TRAF2/5 DKO cells reconstituted with TRAF2-WT or -S55A revealed that TRAF2 Ser-55 phosphorylation is essential for the efficient expression of RANTES and ICAM-I, but not of IκBα and IP-10, in response to TNFα stimulation (Fig. 3A–C and S5A). Expression of IκBα and IP-10 was induced very quickly and peaked within 1 hr of TNFα stimulation, whereas the expression of RANTES and ICAM-1 rose relatively slowly. It seems that the transient activation of IKK that occurs in the absence of TRAF2 Ser-55 phosphorylation is sufficient to trigger efficient expression of IP-10 and IκBα, but the prolonged phase of IKK activation regulated by TRAF2 Ser-55 phosphorylation is required for TNFα-induced expression of RANTES and ICAM-I. Thus, our data suggest that TRAF2 Ser-55 phosphorylation represents a new level at which this pathway controls the expression of a subset of NF-κB target genes by linking certain serine/threonine kinases to the prolonged phase of IKK activation.
TNFα-induced expression of anti-apoptotic proteins such as cIAP1/2, cFLIP and Mn-SOD was slightly reduced in TRAF2-S55A-expressing cells versus TRAF2-WT-expressing cells (Fig. 3C and S5B–D), though the differences were not statistically significant. A statistical difference with respect to inducible expression of these anti-apoptotic proteins was observed only between TRAF-S55A- and -S55D-expressing cells. Consistent with this finding, TRAF2-S55D-expressing cells, but not TRAF2-WT-expressing cells, displayed significantly elevated resistance to stress-induced apoptosis compared to TRAF2-S55A-expressing cells (Fig. 6A and B). This suggests that the constitutive phosphorylation of TRAF2 plays a more important role than its inducible phosphorylation in protecting cells from stress-induced apoptosis.
The RING domain of TRAF2 has been reported to possess ubiquitin E3 ligase activity, and TRAF2-mediated RIP1 ubiquitination is currently thought to play an essential role in TNFα-induced IKK activation (24). As the Ser-55 residue lies in the middle of the TRAF2 RING domain (Fig. S2), we reasoned that TRAF2 Ser-55 phosphorylation may affect TRAF2 E3 ligase activity. However, we did not observe any difference between TRAF2-WT- and -S55A-expressing cells with respect to TRAF2 self-ubiquitination or RIP1 ubiquitination (Fig. S10A, B).
The proapoptotic protein Par-4 interacts with and inhibits the kinase activity of PKCζ (16). Genetic inactivation of Par-4 results in elevated NF-κB, but decreased JNK, activation in response to TNFα stimulation (16). This correlates very well with PKCζ-mediated phosphorylation of TRAF2 at Ser-55 and with the role of this phosphorylation in TNFα-induced IKK and JNK activation. In Ras-transformed cells, the Par-4 protein level is down-regulated, and restoration of Par-4 levels to normal in Ras-transformed cells makes these cells sensitive to camptothecin-induced apoptosis (25). Consistent with this finding, the expression of TRAF2-S55A in Ras-transformed TRAF2/5 DKO cells strongly sensitized cells to stress-induced cell death (Fig. 6D). PKCζ is highly expressed and constitutively activated in many types of human cancer cells (16, 25, 26). In the study presented here, we demonstrated that TRAF2 is constitutively phosphorylated at Ser-55 in several malignant cancer cell lines as well as in Hodgkin’s lymphoma (Fig. 5A, and B). Therefore, our data and findings that have been published by others suggest that elevated PKCζ activation and the consequent increase in TRAF2 Ser-55 phosphorylation are one of the causes of the elevated NF-κB activation in cancer cells, therein as well as of the resistance of cancer cells to stress-induced apoptosis.
Supplementary Material
Acknowledgments
We thank Andrew Chan (Mount Sinai Medical Center) for H-Ras-V12 plasmids, and Thomas Waldschmidt and Frederick Domann (University of Iowa) for helpful discussions. Support by NCI grant CA78419 (to HH) is gratefully acknowledged.
Footnotes
Supplementary data: Supplementary data are available at Cancer Research online.
References
- 1.Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs) Oncogene. 2001;20(44):6482–91. doi: 10.1038/sj.onc.1204788. [DOI] [PubMed] [Google Scholar]
- 2.Wajant H, Henkler F, Scheurich P. The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal. 2001;13(6):389–400. doi: 10.1016/s0898-6568(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 3.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 5.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8(8):385–9. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 8.Yeh WC, Shahinian A, Speiser D, et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7(5):715–25. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 9.Tada K, Okazaki T, Sakon S, et al. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. The Journal of biological chemistry. 2001;276(39):36530–4. doi: 10.1074/jbc.M104837200. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell K, Zhang L, Thomas GS, Sun S, Nakano H, Habelhah H. TRAF2 phosphorylation modulates tumor necrosis factor alpha-induced gene expression and cell resistance to apoptosis. Molecular and cellular biology. 2009;29(2):303–14. doi: 10.1128/MCB.00699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habelhah H, Takahashi S, Cho SG, Kadoya T, Watanabe T, Ronai Z. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-kappaB. Embo J. 2004;23(2):322–32. doi: 10.1038/sj.emboj.7600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Wang L, Berman MA, Zhang Y, Dorf ME. RNAi screen in mouse astrocytes identifies phosphatases that regulate NF-kappaB signaling. Molecular cell. 2006;24(4):497–509. doi: 10.1016/j.molcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horie R, Watanabe T, Ito K, et al. Cytoplasmic aggregation of TRAF2 and TRAF5 proteins in the Hodgkin-Reed-Sternberg cells. Am J Pathol. 2002;160(5):1647–54. doi: 10.1016/S0002-9440(10)61112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakon S, Xue X, Takekawa M, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. The EMBO journal. 2003;22(15):3898–909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moscat J, Diaz-Meco MT, Rennert P. NF-kappaB activation by protein kinase C isoforms and B-cell function. EMBO Rep. 2003;4(1):31–6. doi: 10.1038/sj.embor.embor704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscat J, Rennert P, Diaz-Meco MT. PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ. 2006;13(5):702–11. doi: 10.1038/sj.cdd.4401823. [DOI] [PubMed] [Google Scholar]
- 17.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003;376(Pt 3):545–52. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaken S, Parker PJ. Protein kinase C binding partners. Bioessays. 2000;22(3):245–54. doi: 10.1002/(SICI)1521-1878(200003)22:3<245::AID-BIES6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Schechtman D, Mochly-Rosen D. Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene. 2001;20(44):6339–47. doi: 10.1038/sj.onc.1204778. [DOI] [PubMed] [Google Scholar]
- 20.Lallena MJ, Diaz-Meco MT, Bren G, Paya CV, Moscat J. Activation of IkappaB kinase beta by protein kinase C isoforms. Mol Cell Biol. 1999;19(3):2180–8. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman A, Anwar KN, Malik AB. Protein kinase C-zeta mediates TNF-alpha-induced ICAM-1 gene transcription in endothelial cells. Am J Physiol Cell Physiol. 2000;279(4):C906–14. doi: 10.1152/ajpcell.2000.279.4.C906. [DOI] [PubMed] [Google Scholar]
- 22.Esteve PO, Chicoine E, Robledo O, et al. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J Biol Chem. 2002;277(38):35150–5. doi: 10.1074/jbc.M108600200. [DOI] [PubMed] [Google Scholar]
- 23.Leitges M, Sanz L, Martin P, et al. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Molecular cell. 2001;8(4):771–80. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 24.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nature cell biology. 2005;7(8):758–65. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barradas M, Monjas A, Diaz-Meco MT, Serrano M, Moscat J. The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. Embo J. 1999;18(22):6362–9. doi: 10.1093/emboj/18.22.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen EE, Lingen MW, Zhu B, et al. Protein kinase C zeta mediates epidermal growth factor-induced growth of head and neck tumor cells by regulating mitogen-activated protein kinase. Cancer Res. 2006;66(12):6296–303. doi: 10.1158/0008-5472.CAN-05-3139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.