Abstract
Integrins interact with extracellular matrix (ECM) and deliver intracellular signaling for cell proliferation, survival and motility. During tumor metastasis, integrin-mediated cell adhesion to and migration on the ECM proteins are required for cancer cell survival and adaptation to the new microenvironment. Using SILAC-MS, we profiled the phosphoproteomic changes induced by the interactions of cell integrins with type I collagen, the most common ECM substratum. Integrin-ECM interactions modulate phosphorylation of 517 serine, threonine, or tyrosine residues in 513 peptides, corresponding to 357 proteins. Among these proteins, 33 key signaling mediators with kinase or phosphatase activity were subjected to siRNA-based functional screening. Three integrin-regulated kinases, DBF4, PAK2 and GRK6, were identified for their critical role in cell adhesion and migration possibly through their regulation of actin cytoskeleton arrangement. Altogether, we not only depict an integrin-modulated phosphorylation network during cell-ECM protein interactions but also reveal novel regulators for cell adhesion and migration.
Keywords: phosphoproteomics, integrin, adhesion, metastasis, signaling
Introduction
Cell adhesion to the extracellular matrix (ECM) is critical for cell survival and growth (1, 2). Cell attachment to, spreading on, and movement along the ECM are integrin dependent (1, 2). Integrins are a large family of transmembrane receptors consisting of two heterodimeric, noncovalently-linked α and β chains (3, 4). Eighteen α chains and eight β chains have been identified and different pairing of α and β chains can yield more than 20 receptors that bind to different ECM proteins including collagen, fibronectin and laminin (1, 2, 4). Integrins bridge the cell to ECM at specialized cell membrane structures called focal adhesions (4). Integrins play indispensable roles in delivering extracellular signals across the cell membrane into the cell interior through various kinases and phosphatases which modulate the phosphorylation status of their targets (4–6). Moreover, integrins contribute to reorganization of cytoskeletal components (2, 7), and regulation of cell survival, proliferation, differentiation, cell cycle and migration (8, 9). Understanding the mechanism by which integrins modulate these cellular activities is of significant biological importance.
The most common cancers in human include breast cancer, prostate cancer, lung cancer, colon caner, and ovarian cancer (10, 11), and their metastasis is the leading cause of mortality in cancer patients, causing 90% of deaths from solid tumors (11, 12). During the process of metastasis, tumor cells leave the primary site, travel via blood and/or lymphatic circulatory systems, attach to the substratum of ECM at a distant site, and establish a secondary tumor, accompanied by angiogenesis of the newly formed neoplasm (12). Two of the critical steps of metastasis are adhesion and migration of the primary tumor cell on the ECM at the distant site and thus, integrin-mediated cell-ECM interactions are essential for the cell to adapt to the new microenvironment (3, 13). Integrin loss, overexpression or mutations have been indicated in the pathogenesis and development of benign and malignant tumors(14, 15) and integrin-regulated signaling pathways significantly influence the survival, morphology and migratory properties of tumor cells (3, 8). Conversely, intracellular signals can also regulate the affinity of integrins for ECM molecules (16). Therefore, blocking the key molecules in the integrin-modulated signaling cascades represents a potential means to inhibit tumor cell migration and adhesion to the new site, which could ultimately reduce metastasis (17).
The most frequent site of metastasis for several common cancers (breast, lung and prostate cancers) is bone, where type I collagen is the most abundant ECM protein and comprises more than 90% of the total protein within bone(18). The most common cell surface receptors for type I collagen are integrins α1β1 and α2β1 (19). Type I collagen and collagen-derived fragments are chemotactic for bone metastatic prostate cancer cells (18) and other tumor cells (20). Studying integrin-collagen I interactions should help reveal the integrin-dependent signaling mechanisms involved in cancer metastasis.
Here, we employed the SILAC-MS (Stable-Isotope Labeling by Amino acids in Cell culture-Mass Spectrometry) strategy to characterize the integrin-modulated phosphoproteomic network(21, 22). We identified 357 proteins with significant phosphorylation changes during cell adhesion to, and spreading on, type I collagen. Of the proteins identified, we were particularly interested in the kinases and the phosphatases due to their regulatory roles in signaling. By siRNA-based functional screening we revealed the critical roles of three kinases in cell adhesion and motility. Essentially, this integrin phosphoproteome data substantially expands our knowledge of intracellular molecules regulated by integrin-ECM interactions, as well as provides information which may lead to the discovery of potential therapeutic targets for preventing cancer metastasis.
Materials and methods
Wound healing assay
Scratch wound healing assays were performed in 24-well tissue culture plates. Cells at 30–40% confluence were transfected with siRNA to knockdown protein expression, if applicable. After 24 hours (cell confluence reached ~70%), scratches were made using 1 ml pipette tips and the wells were washed twice with medium. Cells were allowed to grow for additional 48 hr, then fixed in 3.7% paraformaldehyde and stained with 1% crystal violet in 2% ethanol. Photographs were taken on a Zeiss Axiovert 100 TV inverted microscope. Gap distance of the wound was measured using Photoshop software, and the data were normalized to the average of the control. When samples were compared with the control cells, differences were considered significant if P < 0.01.
Cell migration assay
Cell migration assays were performed using Transwell chambers (24-well, 8 μm pore size, Corning Incorporated, Corning, NY). DMEM medium containing 40 μg/ml type I collagen and 0.5 % serum was used as attractant in the lower chamber. About 1 × 105 cells in DMEM medium containing 0.5 % serum were added to the upper compartment of the insert and allowed to migrate toward the underside of the insert filter at 37 °C for 4 hr (HeLa cells) or 2.5 hr (PC3 and MB231 cells). Cells that did not migrate through the pores were gently removed with a cotton swab. Cells on the lower side of the insert filter were fixed by 5% glutaraldehyde and stained with 1% crystal violet in 2% ethanol. Numbers of cells on the underside of the filter from five randomly selected microscopic views were counted.
Cell adhesion assay
For cell-substratum adhesion assays, 96-well tissue culture plates were coated with 40 μg/ml type I collagen in PBS at 4 °C for 12 hr, air-dried and rinsed once with PBS. After being serum deprived at 37 °C for 8 hr, cells were detached with 10 mM EDTA in DMEM, washed twice with DMEM, and plated in quadruplicate onto the collagen-coated wells in serum-free DMEM containing 0.1% BSA at 2 × 104 cells per well. Cells in three wells of the quadruplicate were allowed to adhere to the collagen I-coated surface for 20 min, followed by four intensive washes with DMEM medium to remove non-adherent cells, and then incubated in 5 μg/ml MTT (Sigma-Aldrich, St. Louis, MO) in complete medium at 37 °C for 1 hr. One of the quadruplicate wells was used for a cell number standard. Serum was added into this well to yield complete medium, and the cells therein grown for 4 hours for complete adhesion to the plates, before addition of MTT for an additional 1 hr. Next, MTT-treated cells were lysed in DMSO and absorbance was measured on a Bechman DU-600 spectrophotometer at 560 nm with background subtraction at 680 nm. Values for the triplicate wells were divided by the corresponding cell number standard value to yield relative OD560, which were subsequently normalized to the average of the control for comparison purposes.
SILAC sample preparation and LC/LC-MS/MS analysis
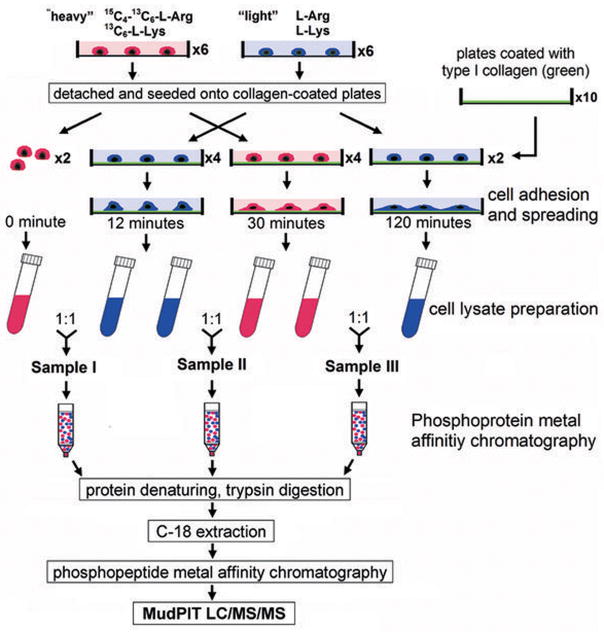
HeLa cells were grown in “heavy” or “light” medium for at least 6 generations (> 15 days). Cells were grown to ~90% confluence in 15-cm tissue culture dishes and serum-starved overnight in the respective media. The cells were detached with 10 mM EDTA in serum-free media for 10 minutes at 37 °C, centrifuged and washed once with media to remove the EDTA. The cells were then resuspended in respective serum-free media, immediately plated on the collagen-coated plates, and incubated at 37 °C for the indicated times. At the end of the incubation, the cells were washed twice with ice-cold PBS and scraped into extraction Buffer A (Clontech, Mountain View, CA) containing Protease inhibitor cocktail (Roche, Indianapolis, IN) and Halt™ phosphatase inhibitor cocktail (Pierce, Rockford, IL). After incubation on ice for 10 minutes, the samples were centrifuged to remove cell debris and the supernatants for each time point were pooled and mixed as following: the heavy supernatant from two plates of HeLa cells collected at time 0 was mixed 1:1 [wt/wt] with the light supernatant collected at 12 min to yield sample I; similarly, the light supernatant (two plates of cells) harvested at 12 min was mixed with the heavy supernatant at 30 min in equal ratio to create sample II; and sample III contains the heavy supernatant at time 30 min and the light supernatant at 120 min.
A total of 13 mg proteins in sample I, II, or III were loaded onto one Phosphoprotein Metal Affinity Chromatography (PMAC) resin columns (Clontech). The purified phosphoproteins were then subjected to trypsin (Promega, sequencing grade) digestion at enzyme:substrate ratio of 1:60 at 37°C O/N. Digested peptides were loaded onto IMAC spin columns (Phosphopeptide Isolation Kit, Pierce) for the purification of phosphopeptides. Purified phosphopeptide samples were centrifuged to remove any particulate matter and then were analyzed by LC/LC-MS/MS using an online HPLC system (Nano LC-2D, Eksigent) coupled with a hybrid LTQ Orbitrap mass spectrometer (Thermo Electron). The LC/LC and MS/MS were performed as previously described (23). Acquired MS/MS spectra were subjected to SEQUEST searches against the EBI-IPI human database (version 3.23 released 11-02-2006) attached with common contaminants (e.g. proteases and keratins) with its reverse decoy for the assessment of false positive rates. Phosphorylation searches were performed where serine, threonine, and tyrosine were allowed to be differentially modified as previous described (23). The SEQUEST identifications were additionally filtered using a false positive cut-off by the DTASelect program based on XCorr and DeltaCN scores using a reversed database approach (24). All identified phosphopeptides were subjected to relative quantification analysis using the program Census (21). The Census program quantifies relative abundances of light and heavy versions of precursor peptides identified by MS2 spectra. The Debunker algorithm (25) was used to validate the phosphopeptide identifications. Detailed descriptions are provided in the Supplementary Materials and Methods.
Results
Morphological changes of cell upon adhering to and spreading on collagen I
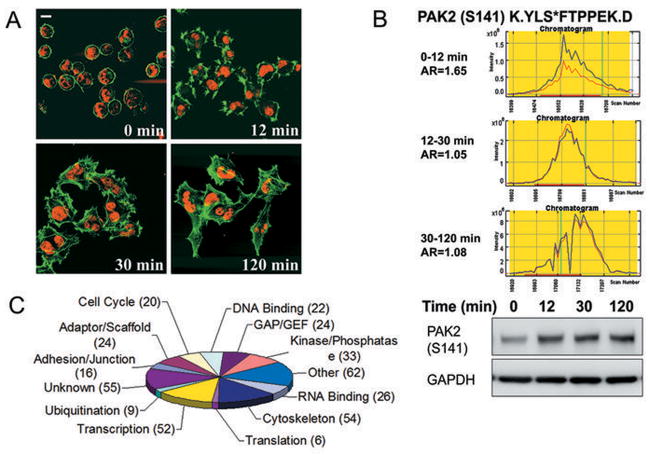
For profiling the integrin-mediated phosphoproteomic changes during the process of cell adhering to and spreading on type I collagen, we observed that, in 12 min, more than 95% of cells originally in suspension adhered to and started to spread on the collagen I-coated plates. By 30 min, more than 85–95% of the cells were fully stretched out and these cells clearly stopped spreading after 120 min in contact with collagen I. Thus, we chose 0–12 min (phase I), 12–30 min (phase II), and 30–120 min (phase III) after integrin receptor interaction with type I collagen as the three time periods (Figure 1 & 2A) and profiled the phosphorylation changes in cellular proteins during these periods using SILAC-MudPIT (multidimensional liquid chromatography coupled with tandem mass spectrometry) (6, 21, 24). Cells that had been allowed to attach to, and spread on, the substratum for the indicated time periods were harvested and subjected to further enrichment and analysis (Figure 1, Supplementary Materials and Methods).
Figure 1.
Experimental design for phosphoproteomic analysis of integrin signaling. Flowchart of sample preparation for SILAC-mass spectrometry profiling. Cells were grown in medium containing either “heavy” (red) or “light” (blue) L-Lysine and L-Arginine.
Figure 2.
MS data analyses of integrin phosphoproteomics. A, mass spectrometry experiment was designed based on the morphological changes of HeLa cells during adhesion to and spreading on type I collagen-coated surface. Cells expressing GFP-actin fusion protein (green) were fixed at the indicated times and stained with TO-PRO-3 iodide (red) for nuclei. B, phosphorylation status of S141 of PAK2 during the three indicated time points in chromatogram and in Western blot. AR is the ratio of the “light” peak area (blue) over the “heavy” peak area (red) in chromatogram. The phosphorylation of PAK2 at Serine 141 is detected by antibody against pS141-PAK2 in Western Blot. C, categorization of proteins according to biological activity. One protein may be involved in multiple activities.
MS results, validation, and analyses
We identified 1763, 1894 and 2044 phosphorylation sites (pS + pT + pY) in the 0–12 min (I), 12–30 min (II) and 30–120 min (III) samples, corresponding to 1726, 1877 and 2035 phosphopeptides or 889, 945 and 1034 phosphoproteins, respectively. Of these, 762 phosphopeptides were common in all three samples, meaning that the phosphorylation status of these peptides was tracked throughout the process of cell adhesion and spreading (Table SI). These phosphopeptide identifications were validated using the Debunker algorithm(25) that computed a phosphorylation probability score for each identified peptide (Table SI). To quantify the phosphorylation change for each phosphopeptide, we used the Census software to calculate area ratio (AR) (21), defined as the ratio of the “light” peak area over the “heavy” peak area in chromatogram (exampled in Figure 2B). The Census software also calculated an R-square statistic for each area ratio measurement (Table SI), indicating how good the heavy and light peptide chromatograms fit with each other. When we set AR threshold to be 1.4 (or 0.71=1/1.4) (26), 249 peptides show no significant change in phosphorylation (0.71<AR<1.4), whereas phosphorylation of the rest 513 peptides were either up-regulated, down-regulated or both during the three time points, suggesting that integrin-modulated intracellular phosphoproteomic changes occurred in a time-dependent manner and were well orchestrated. To validate the data, we examined the phosphorylation status of S141 of PAK2 using immunoblotting assay, and the results were consistent with the observation from MS chromatograph (Figure 2B).
The 513 phosphopeptides that underwent significant phosphorylation modification were assigned to 357 proteins, for which we categorized into 12 activity groups excluding 55 proteins without known function (Figure 2C). 54 proteins showed cytoskeleton-related activity, which is not surprising since cytoskeleton reorganization is a key intracellular activity during cell attachment, spreading and migration. 52 proteins are transcription-related regulators, suggesting that integrin-collagen interaction triggered gene expression, possibly for cell survival and growth. In addition, we detected 33 proteins with kinase or phosphatase activities (Figure 2C). As kinases and phosphatases are key mediators for signaling, these enzymes should be critical for the integrin-modulated signaling and the consequent biological functions.
Identification of signaling mediators critical for cell adhesion and migration
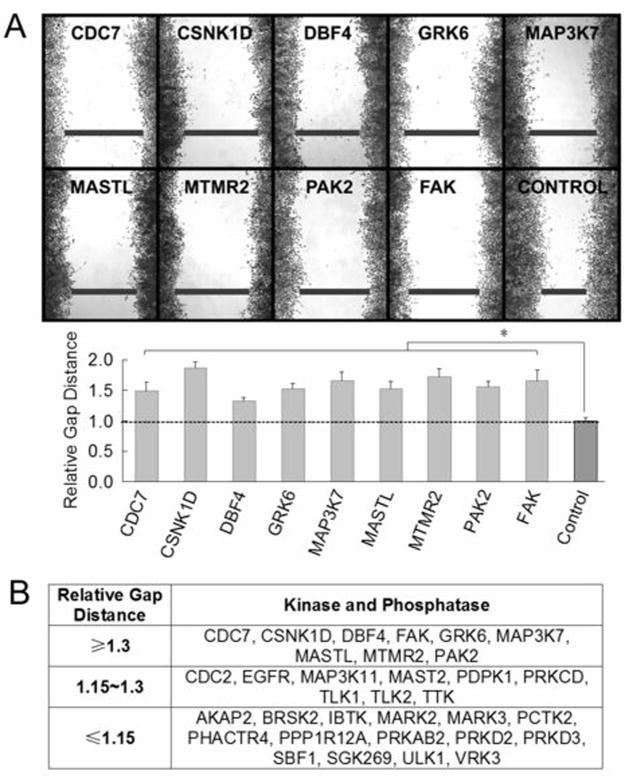
Integrin-dependent signaling pathways are critical not only for cell survival and proliferation, but also for cell attachment and motility, which are directly linked to tumor metastasis and invasion (2, 17). As kinases and phosphatases are critical signaling mediators, we suspected that these 33 newly identified integrin-regulated kinases and phosphastases might have a role in modulating cell motility. We found that depleting 9 of them significantly slowed down the rate of wound healing of cells by more than 30% (relative gap distance > 1.3) (Figure 3A & 3B), compared with the cells that were treated with control siRNA.
Figure 3.
Screening integrin-regulated kinases and phosphatases for their roles in cell motility. A, indicated integrin-regulated proteins were depleted in HeLa cells using siRNA followed by wound healing assay. Samples were fixed and stained with crystal violet (top panel). The wound gap values were normalized to the average of the control that was treated with control siRNA whose relative gap distance was taken as 1. Each value represents the average ± s.d. of 6 measurements from 3 different microscopic views. The experiment was repeated twice. *, p < 0.01 compared to the control. B, a summary of the wound healing ability of cells deficient in the indicated kinases and phosphatases.
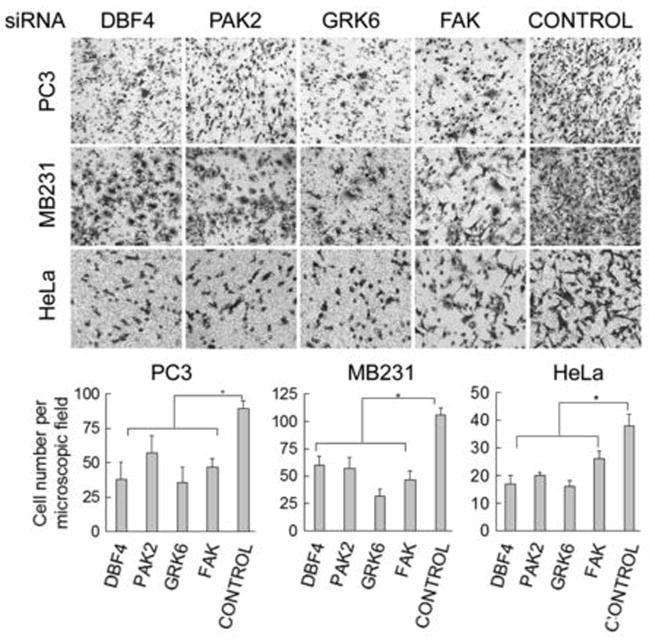
Prostate and breast cancers are the main malignant cancers in male and female patients (10). The tumor cells of these cancers preferably metastasize to the bone, where type I collagen account for 90% of the total protein (18). Interactions between the tumor cells and the bone ECM proteins, mainly collagen I, can be a prerequisite for the cells to survive in the new microenvironment (20). Therefore, we further examined the role of the abovementioned kinases and phosphatases in cell migration in prostate, breast, and cervical cancer cells by Transwell assays, using type I collagen as attractant. The depletion of DBF4, GRK6, PAK2, or FAK/PTK2 by siRNAs method significantly retarded cell movement (2–3 fold) across the Transwell membrane (Figure 4), while knockdown of CSNK1D, MAP3K7, MASTL or MTMR2 did not or only slightly slowed down cell motility (data not shown). In addition, depleting DBF4, PAK2 and GRK6 kinases in these cell lines also reduced cell attachment to the collagen I-coated surface (Figure 5A & 5B), implying that the integrin-collagen I interactions at cell surface were affected by knocking-down these proteins. We obtained similar results from cell migration and adhesion experiments when using at least two distinct siRNA duplexes for depleting these kinases (Figure S1A & S1B).
Figure 4.
Novel integrin-regulated kinases are critical for cell migration. The prostate (PC3), breast (MB231) and cervical (HeLa) cancer cells were depleted of the indicated proteins by siRNA and subsequently were examined for cell motility using Transwell experiments. Lower panel, each column represents the average cell number ± s.d. from five different microscopic views; *, p < 0.01 compared to the control.
Figure 5.
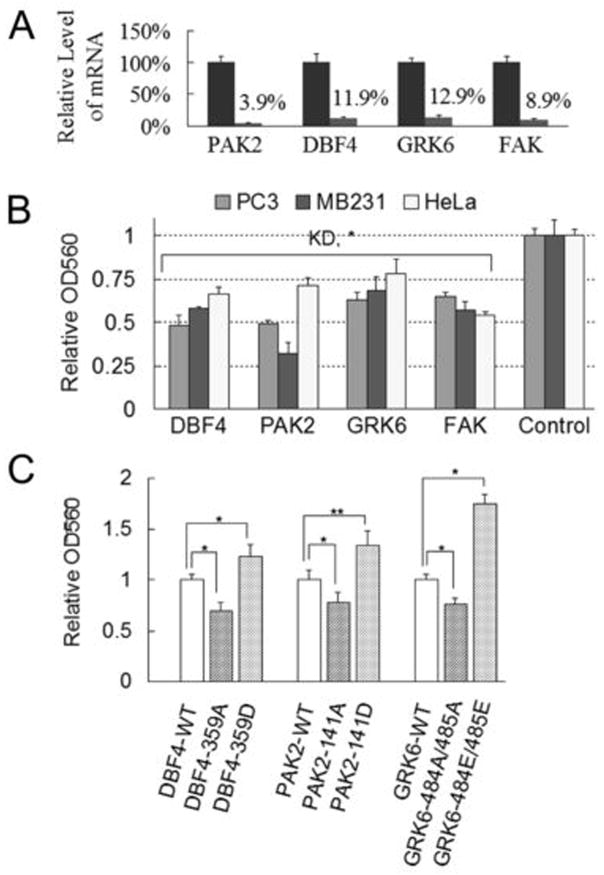
Novel integrin-regulated kinases are critical for cell adhesion. A, real-time qPCR was used to monitor the levels of mRNA for the indicated proteins before (normalized to be 1) and after siRNA treatment in HeLa cells. Each column is the average ± s.d. from three experiments. B, siRNA-treated cells were tested for adhesion ability to the surfaces that were coated with type I collagen. Each value is the average ± s.d. of 3 experiments, each done in triplicate. KD, knockdown. *, p < 0.01 compared to the group that was treated with control siRNA whose relative OD560 was taken as 1. C, HeLa cells that express either wild-type or indicated mutant forms of DBF4, PAK2 or GRK6 proteins were examined for their adhesion ability to collagen I coated surfaces. Each value is the average ± s.d. of 2 experiments, each done in triplicate. *, p < 0.01, **, p < 0.05, compared to the WT groups.
To examine the function of integrin-regulated phosphorylation sites in these kinases, we expressed wild-type or mutant forms of the three kinases in HeLa cells. Expressing mutant forms of DBF4, PAK2 and GRK6, in which the Ser or Thr residues were substituted with Ala, not only slowed cell migration (Figure S1C) but also inhibited cell adhesion to collagen I substratum (Figure 5C), compared to those of their respective wild-type forms. In contrast, substituting the same Ser or Thr residues with Asp or Glu increased cell migration and adhesion. Taken together, these data confirmed that integrin-dependent phosphorylation of kinases DBF4, PAK2 and GRK6 play critical roles through in cancer cell adhesion and migration.
Novel integrin-regulated kinases are involved in actin cytoskeleton organization
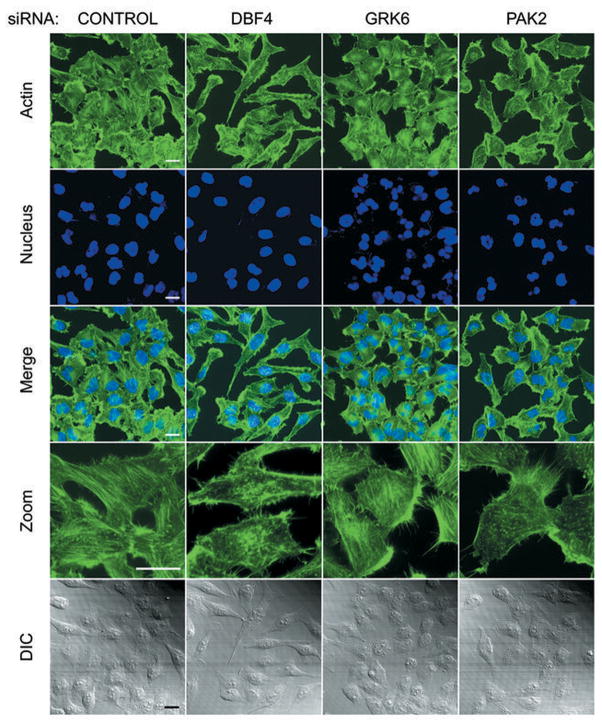
Cell adhesion and migration require dynamic interactions not only between the ECM and the cell surface molecules (e.g., integrin receptors), but also between the surface molecules and cytoskeleton components (4, 7). Many cellular proteins are implicated in controlling cell polarity, shape and motility through their regulation of cytoskeletal organization. We suspected that integrin-regulated proteins critical for cell migration such as DBF4, PAK2 and GRK6 may also be involved in actin cytoskeleton arrangement. To test this hypothesis, we depleted these proteins individually in cells, followed by actin staining using phalloidin. Compared with the control, cells with DBF4 knockdown were more elongated; the actin stress fibers were sparse and largely perturbed (Figure 6). GRK6-depleted cells were smaller than the control cells and had a more circular/oval-like shape and rounded edges. The central stress fibers were still maintained in these cells, yet more actin was reorganized into cortical bundles. We also noticed that many of the GRK6-knockdown cells contained polymorphonuclear-like nuclei, and have bigger gaps between cell-cell boundaries (Figure 6). The PAK2 knockdown cells were also smaller, indicating that these cells did not spread as well as the control cells. In addition, much less actin network was observed in these cells, and the actin fibers were thinner (Figure 6). These observations suggest that knockdown of these integrin-regulated kinases altered the assembly and/or organization of actin cytoskeleton within the cells, which may account for the attenuated attachment and migration of these cells.
Figure 6.
Integrin-regulated kinases, DBF4, GRK6 and PAK2, are involved in actin cytoskeleton organization. HeLa cells seeded on collagen I-coated coverslips were transfected with siRNA to knockdown the indicated kinases. The cells were stained with Oregon Green 488-phalloidin (green) for visualization of the actin cytoskeleton. Nuclei were costained with DAPI (blue). Photographs were taken with a 60x objective lens on a BioRad MRC-2100 confocal microscope. Zoom, 3x magnified. Differential Interference Contrast (DIC) photos were used to monitor cell integrity and outline. Scale bar, 20 μm.
Integrin-regulated molecules and human cancer
Integrin-dependent signaling pathways not only transmit survival and growth signals but also augment the tumorigenic and metastatic potential of the cancer cells(3). Therefore, molecules identified in the integrin-modulated phosphoproteome may contribute directly or indirectly for tumorigenesis and metastasis. We analyzed the potential correlations between these molecules and known cancer occurrence by checking each integrin-modulated protein against the Swissprot database, Cancer Gene Census(27), and available publications. Proteins in which mutation, deletion, and/or overexpression were detected in tumors were considered cancer-related. We determined that 68 of the 357 integrin-regulated proteins correlated with different types of human cancers (Table SII). For example, RB1(28) and BRAF(29) are well-known cancer-related genes and mutations in these genes have been found in breast, lung, prostate, lung, ovary and other cancers. This database not only provides important information about the possible mechanisms by which human cancer cells take control of the components in integrin-signaling network to boost their malignancy such as uncontrolled proliferation and metastatic potential but also offers possible targets for therapeutic intervention to stop or slow down the growth and spread of human cancer.
Discussion
Integrins and the molecules they regulate have long been the targets of numerous studies directed at understanding tumor pathogenesis, especially the mechanisms involved in ECM-dependent tumor cell survival and metastasis (17). In addition, reversible phosphorylation of serine, threonine and tyrosine residues is a crucial means of regulating signaling pathways in mammalian cells (6, 30). For these reasons, integrin-modulated signaling by phosphorylation inevitably attracts general interest. Traditional biochemical and genetic analyses can only focus on one or a few proteins in integrin signaling at a time, thereby giving us a very limited view of the signaling network of integrins. The rapid advances in analytical technology, such as mass spectrometry, combined with genome databases, make studying many molecules simultaneously readily possible (6, 30, 31). Our strategy described here incorporates the abovementioned considerations, thereby providing a platform to observe and investigate integrin-modulated cellular signaling in a macroscopic view.
We used network modeling with the Ingenuity software (32) to evaluate the interactions of DBF4, GRK6 or PAK2 with other integrin-regulated kinases and phosphatases. PAK2 has been reported to induce cell retraction and cytoskeletal rearrangements (33). Our modeling results showed that PAK2 interacts with a variety of molecules, including the nonreceptor tyrosine kinase ABL1 that regulates cell adhesion, morphogenesis and motility (34), and the Rho family GTPase, CDC42, that regulates cytoskeletal rearrangements to form actin-rich filopodia during cell spreading (35) (Figure S2A). In supporting our observations, Coniglio et al. reported recently that PAK2 was needed to generate new focal adhesions and to limit the sizes of focal adhesions (36). They demonstrated that PAK2 inhibited phosphorylation of myosin light chain (MLC), partially through downregulating RhoA, and blocking RhoA-mediated signaling restored invasion in PAK2-depleted tumor cells. Moreover, Koh et al. showed that PAK2 acted downstream of Cdc42, Rac1, and PKC isoforms to regulate endothelial cell lumen and tube formation as well as endothelial cell invasion in three-dimensional collagen matrices (37). Together, above evidence may explain the mechanism by which PAK2 modulates cell adhesion and motility.
GRK6 belongs to the seven-member G-protein coupled receptor kinase family (GRKs) (38). GRKs mediate phosphorylation-dependent desensitization of the G-protein coupled receptors (GPCRs), while emerging evidence shows that the substrates of GRKs are far beyond the GPCRs (39). GRK6 has been found to participate in the regulation of chemotaxis of T/B cells and neutrophils (40, 41). Here we showed that GRK6 contributed to the adhesion and motility of several cancer cell lines. Though the mechanism used by GRK6 remains to be elucidated, by modeling we discovered several cellular proteins that may contribute to the effect of GRK6 on cell adhesion and migration (Figure S2B). For example, GRK6 interacts with the GRK-interacting protein (GIT1) that serves as an integrator and provides a scaffold for the signaling molecules to control cell adhesion and cytoskeleton organization (42). In addition, GRK6 may act through second messengers such as cAMP (39) or calmodulin (43), or indirectly transactivate EGFR, in turn affecting cancer cell migration and invasion.
DBF4 is the crucial regulatory subunit of the S phase kinase DDK that, together with the catalytic subunit CDC7, controls S phase progression by promoting assembly of the Cdc45-MCM complex for DNA replication (44). DBF4 mRNA is expressed at high levels in many cancer cell lines (45). Here we found that DBF4 contributed to cell adhesion and motility of several cancer cells, yet these results were somewhat unexpected since DBF4 is found mainly in nucleus. Possibly for this same reason, the modeling program did not yield a plausible mechanism for DBF4 to directly regulate cell adhesion/migration activities (Figure S2C). However, previous studies showed that DBF4, through its COOH-terminus, interacts with metastasis inhibition factor NM23 (46) as well as with the Myosin chain 9 (MYH9) that contributes to cytokinesis and cell shape regulation (47). Thus, DBF4 may regulate integrin-mediated cell adherence/motility through NM23 and MYH9. Further functional studies are needed to elucidate this idea.
Because PAK2, GRK6, and DBF4 may carry out their biological functions through other cellular proteins, we sought the correlations between the 357 MS-identified integrin-regulated proteins that had significant phosphorylation changes using the Ingenuity modeling software(32). When no additional linkage molecule (zero node) was applied, the program yielded a complex network of 165 paths, in which 96 of those 357 molecules directly or indirectly regulate or are being regulated by the others’ activities (Fig S2D). Several proteins, including PRKCD(48), CDC2(49), EGFR(50), and RB1(28) etc., appear at the “junctions” of multiple axes, confirming the vital contributions of these proteins to cell growth and motility. Interestingly, PAK2, DBF4 and GRK6 were suggested to be directly or indirectly correlated with one or more of these significant molecules. For example, GRK6 interacts with GIT1 and indirectly regulates EGFR, and PAK2 and DBF4 may have indirect regulations on RB1. Furthermore, when one node was available for any two molecules, the program projected a very complex interaction network (12,652 paths) with many more integrin-regulated proteins involved (data not shown). Our modeling data suggest that integrin-ECM interactions during cell adhesion and spreading can trigger a very complex and interactive signaling network. Clearly, further studies are needed to explore the correlations of these molecules and their potential roles in regulating integrin-mediated physiological and pathological cellular functions.
Collectively, results from our study provide insight into the still ill-defined integrin-modulated signaling network, as well as provide data on the integrin-dependent phosphoproteome to tackle the complexity of this regulatory mechanism. Dysfunction of integrin signaling pathways contribute to tumor proliferation, invasion and metastasis (16). Therefore, the novel signaling mediators identified by integrin phosphoproteomic profiling herein should represent potential targets for pharmacological intervention to prevent tumor growth and spread to distal sites.
Acknowledgments
This work was supported by grants from the NIH, CA079871 and CA114059, (to J.-D.L.). This research was supported by funds from the Tobacco-Related Disease, Research Program of the University of California, 15RT-0104, (to J.-D.L.).
References
- 1.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–9. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 2.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 3.Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–38. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- 4.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 5.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 6.Olsen JV, Blagoev B, Gnad F, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 7.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–82. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 8.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–23. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Fashena SJ, Thomas SM. Signalling by adhesion receptors. Nat Cell Biol. 2000;2:E225–9. doi: 10.1038/35046654. [DOI] [PubMed] [Google Scholar]
- 10.Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–52. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 12.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 14.Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. Embo J. 2002;21:3919–26. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans RD, Perkins VC, Henry A, Stephens PE, Robinson MK, Watt FM. A tumor-associated beta 1 integrin mutation that abrogates epithelial differentiation control. J Cell Biol. 2003;160:589–96. doi: 10.1083/jcb.200209016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–16. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–80. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Hall CL, Dai J, van Golen KL, Keller ET, Long MW. Type I Collagen Receptor ({alpha}2{beta}1) Signaling Promotes the Growth of Human Prostate Cancer Cells within the Bone. Cancer Res. 2006;66:8648–54. doi: 10.1158/0008-5472.CAN-06-1544. [DOI] [PubMed] [Google Scholar]
- 19.Gullberg D, Gehlsen KR, Turner DC, et al. Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell--collagen interactions: identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. Embo J. 1992;11:3865–73. doi: 10.1002/j.1460-2075.1992.tb05479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mundy GR, DeMartino S, Rowe DW. Collagen and collagen-derived fragments are chemotactic for tumor cells. J Clin Invest. 1981;68:1102–5. doi: 10.1172/JCI110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SK, Venable JD, Xu T, Yates JR., 3rd A quantitative analysis software tool for mass spectrometry-based proteomics. Nat Methods. 2008;5:319–22. doi: 10.1038/nmeth.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–7. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 23.Cantin GT, Yi W, Lu B, et al. Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J Proteome Res. 2008;7:1346–51. doi: 10.1021/pr0705441. [DOI] [PubMed] [Google Scholar]
- 24.Peng J, Schwartz D, Elias JE, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–6. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 25.Lu B, Ruse C, Xu T, Park SK, Yates J., 3rd Automatic validation of phosphopeptide identifications from tandem mass spectra. Anal Chem. 2007;79:1301–10. doi: 10.1021/ac061334v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Ding SJ, Wang W, et al. Profiling signaling polarity in chemotactic cells. Proc Natl Acad Sci U S A. 2007;104:8328–33. doi: 10.1073/pnas.0701103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenman C, Stephens P, Smith R, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chau BN, Wang JY. Coordinated regulation of life and death by RB. Nat Rev Cancer. 2003;3:130–8. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- 29.Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17:31–9. doi: 10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Mumby M, Brekken D. Phosphoproteomics: new insights into cellular signaling. Genome Biol. 2005;6:230. doi: 10.1186/gb-2005-6-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nesvizhskii AI, Vitek O, Aebersold R. Analysis and validation of proteomic data generated by tandem mass spectrometry. Nat Methods. 2007;4:787–97. doi: 10.1038/nmeth1088. [DOI] [PubMed] [Google Scholar]
- 32.Mayburd AL, Martlinez A, Sackett D, et al. Ingenuity network-assisted transcription profiling: Identification of a new pharmacologic mechanism for MK886. Clin Cancer Res. 2006;12:1820–7. doi: 10.1158/1078-0432.CCR-05-2149. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Q, Lagunoff D, Masaracchia R, Goeckeler Z, Cote G, Wysolmerski R. Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J Cell Sci. 2000;113 (Pt 3):471–82. doi: 10.1242/jcs.113.3.471. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez SE, Krishnaswami M, Miller AL, Koleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 2004;14:36–44. doi: 10.1016/j.tcb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 36.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–72. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 38.Willets JM, Challiss RA, Nahorski SR. Non-visual GRKs: are we seeing the whole picture? Trends Pharmacol Sci. 2003;24:626–33. doi: 10.1016/j.tips.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Ribas C, Penela P, Murga C, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–22. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99:7478–83. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kavelaars A, Vroon A, Raatgever RP, et al. Increased acute inflammation, leukotriene B4-induced chemotaxis, and signaling in mice deficient for G protein-coupled receptor kinase 6. J Immunol. 2003;171:6128–34. doi: 10.4049/jimmunol.171.11.6128. [DOI] [PubMed] [Google Scholar]
- 42.Premont RT, Claing A, Vitale N, et al. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc Natl Acad Sci U S A. 1998;95:14082–7. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pronin AN, Satpaev DK, Slepak VZ, Benovic JL. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J Biol Chem. 1997;272:18273–80. doi: 10.1074/jbc.272.29.18273. [DOI] [PubMed] [Google Scholar]
- 44.Sheu YJ, Stillman B. Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Mol Cell. 2006;24:101–13. doi: 10.1016/j.molcel.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumagai H, Sato N, Yamada M, et al. A novel growth- and cell cycle-regulated protein, ASK, activates human Cdc7-related kinase and is essential for G1/S transition in mammalian cells. Mol Cell Biol. 1999;19:5083–95. doi: 10.1128/mcb.19.7.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohkura N, Kishi M, Tsukada T, Yamaguchi K. Menin, a gene product responsible for multiple endocrine neoplasia type 1, interacts with the putative tumor metastasis suppressor nm23. Biochem Biophys Res Commun. 2001;282:1206–10. doi: 10.1006/bbrc.2001.4723. [DOI] [PubMed] [Google Scholar]
- 47.Obungu VH, Lee Burns A, Agarwal SK, Chandrasekharapa SC, Adelstein RS, Marx SJ. Menin, a tumor suppressor, associates with nonmuscle myosin II-A heavy chain. Oncogene. 2003;22:6347–58. doi: 10.1038/sj.onc.1206658. [DOI] [PubMed] [Google Scholar]
- 48.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–62. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 49.Juliano R. Movin’ on through with Cdc2. Nat Cell Biol. 2003;5:589–90. doi: 10.1038/ncb0703-589. [DOI] [PubMed] [Google Scholar]
- 50.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]