Abstract
Considered a chemopreventive agent, genistein’s ability to modulate the progression of existing prostate cancer (CaP) is not clear. We show here, that the consumption of genistein (250 mg/kg diet) by 12-weeks-old Transgenic Adenocarcinoma Mouse Prostate (TRAMP-FVB) mice harboring prostatic intraepithelial neoplasia lesions until 20 weeks of age induces an aggressive progression of CaP, as evidenced by a 16% increase in the number of well and poorly-differentiated prostates, coinciding with a 70% incidence of pelvic lymph node metastases as opposed to 0 and 10% in 0 and 1000 mg/kg groups, concomitant with elevated osteopontin (OPN) expression in prostates and lymph nodes. Equivalent nanomolar (500 nM) concentrations of genistein recapitulated these effects in human PC3 prostate cancer cells as evidenced by increased proliferation, invasion and MMP-9 activity (∼2-fold), accompanied by an upregulation of OPN expression and secretion, as compared to vehicle-treated cells. A pharmacological dose (50 μM) decreased proliferation, invasion and MMP-9 activity (>2.0-fold) concomitant with OPN reduction. Upon OPN knockdown by shRNA, genistein was no longer effective in upregulating PC3 cell proliferation, invasion and MMP-9 activation, which were significantly reduced in the absence of OPN, highlighting the requirement for OPN in mediating genistein’s effects. Proliferation, invasion and OPN levels were also non-significantly induced by genistein in the presence of ICI 182,780 or Wortmannin, indicating a dependence on PI3K and estrogen signaling. Our results suggest the presence of a biphasic regulation of CaP growth and metastasis by genistein, warranting careful examination of genistein’s effects on hormone-dependent cancers in a chemotherapeutic setting.
Keywords: Genistein, TRAMP-FVB, metastasis, Osteopontin
Introduction
An estimated ∼186,320 cases of prostate cancer (CaP) will be diagnosed in the United States in 2008. About 1 man in 6 will be diagnosed with CaP during his lifetime, but only 1 man in 35 will succumb to it, making CaP accountable for ∼9% of cancer-related deaths in men (1). Initially treatable when localized, CaP is known to progress to a clinically hormone-refractory cancer and metastatic disease to bone, lungs and regional lymph nodes, when no effective treatment is available (2).
Observations have suggested that osteopontin (OPN) expression may be significantly altered in cancer and playing a role in bone metastasis (3). OPN expression has been shown to be increased upon neoplastic transformation (4), and induced by tumor promoters (5) as well as estrogens (6). Specifically to CaP, OPN is elevated in androgen-independent and tumorigenic prostatic cell lines as compared to normal prostate epithelial and benign prostatic hyperplasia cells. Increased expression is associated with elevated Gleason scores (7), increased tumor burden (8) and reduced patient survival (9). Serum OPN levels are substantially elevated in the blood of patients with metastatic cancer (10); rendering OPN a useful diagnostic marker in metastasis detection and an attractive potential target for CaP therapy.
Epidemiological observations linking increased soy consumption and decreased incidence of clinically relevant CaP have generated a lot of attention regarding the chemopreventive/chemotherapeutic effects of genistein, a major component in soy, on the initiation and progression of CaP (11). We and others have previously shown that dietary genistein (250 and 1000 mg/kg diet) inhibits the incidence of poorly-differentiated cancer when consumed by tumor-free TRAMP (transgenic adenocarcinoma mouse prostate) mice; dose-dependently (12, 13). More recently, we have examined the effect of consumption of previously-established chemopreventive genistein doses on CaP progression in mice with prostatic intraepithelial neoplasia (PIN) and observed a surprising increase in CaP growth at the lower dose used (14). This study represents a closer examination of dietary genistein’s effect on TRAMP CaP progression when consumed after tumor initiation. We show that the 250 mg/kg dose, which resulted in nanomolar serum genistein concentrations, induced a 70% incidence of pelvic lymph node metastases, concomitant with activation of Akt and upregulation of OPN. In vitro studies using PC3 cells, recapitulated the induction of proliferation and invasion by nanomolar but not micromolar doses of genistein, concomitant with Akt activation, increased OPN expression and MMP-9 activation, suggesting a biphasic modulation of proliferation and invasion by genistein in CaP cells. These effects were no longer observed upon knockdown of OPN by shRNA, treatment of PC3 cells with the estrogen receptor (ER) antagonist, ICI 182,780, or PI3K inhibition by Wortmannin. This is the first report demonstrating that consumption of genistein, resulting in nanomolar concentrations, can enhance the proliferative and metastatic potential of undiagnosed early stage CaP, and thereby exacerbate it, via an estrogen and PI3K-dependent mechanism, involving the upregulation of the metastasis promoter, osteopontin.
Materials and Methods
Animal handling and treatment
TRAMP (The Jackson laboratory, Bar Harbor, Maine) and FVB mice (Charles River Laboratories, Wilmington, MA) colonies were maintained at Georgetown University animal facilities in accordance with approved protocol guidelines. Heterozygous male offspring, from male and female TRAMP mated with FVB, were confirmed by genotyping as described (15). Four or 12 weeks-old transgenic males were fed purified AIN-76A pellets (Harlan Teklad, Indianapolis, IN) supplemented with 0, 250 and 1000 mg genistein per kilogram diet (n=15/diet group) (Sigma, St. Louis, MO) until 20 weeks of age. A 3rd group was fed a regular diet and mice were sacrificed at 5, 9, 18 and 24 weeks of age (n=10/age group).
Tissue processing and histopathological evaluation
Mice were euthanized, blood collected, major organs and lymph nodes dissected out, fixed and paraffin-embedded. Histopathological evaluation/scoring were done as previously described (12), and were reflective of prostatic lobes minus the anterior prostate.
Serum genistein levels
Serum was extracted and total genistein was unconjuguated as previously described (12) and measured by Time-Resolved FluoroImmunoAssay according to the manufacturer’s protocol (Labmaster TRF-genistein, Turku, Finland).
Immunohistochemistry
Lymph node sections were stained with mouse anti-OPN (Santa Cruz Biotechnologies, Santa Cruz, CA), mouse anti-SV40-Tag (Neomarkers, Fremont, CA) overnight at 4°C; followed by incubation with biotin-labeled (SV40-Tag) (Invitrogen, Carlsbad, CA) or Alexa-fluor-tagged antibody (OPN) for 1hr. Slides were incubated in Vectastain ABC-Peroxidase (Vector Laboratories, Burlingame, CA) for 30min, developed with 3, 3′-diaminobenzidine, counterstained with hematoxylin or propidium iodide. Slides were photographed using a camera-equipped microscope (ZEISS AxioPlan2 Imaging System, Jena, Germany).
Cell lines
PC3, MCF-7 (ATCC, Manassas, VA) or PC3 (OPN-) were maintained at 37°C with 5% CO2 in phenol red-free IMEM with 2 mM glutamine, penicillin-streptomycin and 10% fetal bovine serum unless otherwise specified. For OPN knockdown stable cell line (PC3 (OPN-)), 4 different OPN-shRNA and 2 scrambled shRNA constructs (OriGene Technologies, Inc Rockville, MD) were transfected into retroviral packaging Phoenix™ Ampho cells (gift from Dr. Dean Rosenthal, Georgetown University Medical Center, Washington, DC) with GeneJammer. Forty-eight hrs post-transfection, media was filtered through 0.45μm and added to PC3 cells with polybrene (5 μg/ml). Forty eight hrs post-infection, cells were selected with puromycin, individually cloned and screened for OPN expression.
Proliferation assay
PC3 or PC3 (OPN-) cells, in triplicates, were treated with 1) 0, 500, 1,000 and 50,000 nM genistein for 72hrs or 2) ± genistein (500nM) ± ICI 182,780 (50nM) for 72hrs or 3) ± genistein (500nM) ± Wortmannin (50nM) for 72hrs and living cells were counted.
Invasion assay
A PC3 suspension (60,000 cells) in SFM treated 1) ± genistein (500, 1,000, and 50, 000 nM) or 2) ± genistein (500nM) ± ICI 182,780 (50nM) or 3) ± genistein (500nM) ± Wortmannin (50nM), or 4) PC3 (OPN-) ± genistein (500nM), for 72hrs, were subjected to the Boyden chamber assay (BD Biosciences) as described (16).
Estrogen response element reporter assay
PC3 or MCF-7 cells, in triplicates, were transfected with ERE-TATA luciferase (200ng) and Renilla luciferase (20 ng) reporter constructs. Forty-eight hrs post-transfection, cells were treated with 5 pM estradiol or 500 nM genistein for 5hrs. Luciferase activity was measured in cell lysates using the Dual Luciferase Assay kit (Promega, Madison, WI) following the manufacturer’s protocol and normalized to Renilla luciferase activity.
Reverse transcription polymerase chain reaction (RT-PCR)
RNA extracted from prostatic tissue or lymph nodes as described (12) was subjected to RT-PCR using mOPN-F:5′-TGGCAGCTCAGAGGAGAAGCTTTA-3′, mOPN-R:5′-TCCTGGCTCTCTTTGGAATGCTCA-3′and mGAPDH-F: 5′-GGCCATGTCTGAGAGATTGGTCTT-3′, mGAPDH-R:5′-AATAGAAAGGCACAGAGCCAGAGG-3′. PCR reactions were as follows (1 min 94°C, 28 cycles of 94°C for 30 sec, annealing temp (58 °C) 30 sec, 72°C for 45 sec, extension at 72°C for 5 min. Yielded PCR products were of 627 and 259 bp, respectively.
Western blot analysis
Protein isolation from prostates (minus anterior prostate lobe) and cell lines was performed as described (12). Membranes were probed with anti-OPN antibody, Akt and p-Akt (Ser473) (Cell signaling, Danvers, MA) and re-probed for GAPDH (Abcam, Cambridge, MA) to ensure for equal loading.
Human OPN immunoassay
Secreted OPN was measured with Quantikine Human OPN ELISA (R&D Systems, Inc, Minneapolis, MN) according to the manufacturer’s protocol. PC3 cells were treated with 0, 500, 1000 and 50,000 nM genistein for 72hrs. Media was collected every 24hrs, pooled together and concentrated (1/10) using an Amicon concentrator with a 50 kDa cutoff (Millipore, Danvers, MA). Samples and standards were run in triplicates.
Zymography
Conditioned media collected and pooled from PC3 cells treated with 1) genistein (0, 500, 1000 and 50,000 nM), 2) scrambled shRNA or OPN shRNA stable PC3 cells ± genistein (500 nM) and 3) vehicle- and genistein-treated (500nM) PC3 cells ± ICI 182,780 or ± Wortmannin for 72hrs were concentrated (∼10-fold) and loaded onto SDS-PAGE containing 0.1% gelatin for electrophoresis as described in (17).
Statistical analyses
Histological data was evaluated using χ2 analysis and Fisher exact test. Western blots; agarose gels and gelatin zymography band intensities were quantified with ImageJ software (NIH, Bethesda, MD) and presented as means ± SEM from 3 independent experiments. One-way ANOVA in prism 3 (GraphPad Software, Inc., San Diego, CA) was used to compare OPN levels across: age groups, diet groups in vivo, genistein treatments/inhibitors in WB analyses and ELISA and active MMP-9 across genistein doses, ± inhibitors and in PC3 (OPN-). Cells were counted in triplicates and invaded cells were counted from all filters, from 3 independent experiments and analysis was carried out between different treatments using One-way ANOVA, followed by Dunnett test with a confidence interval of 95%.
Results
A chemopreventive dose of genistein induces pelvic lymph node metastasis in TRAMP-FVB mice harboring PIN lesions
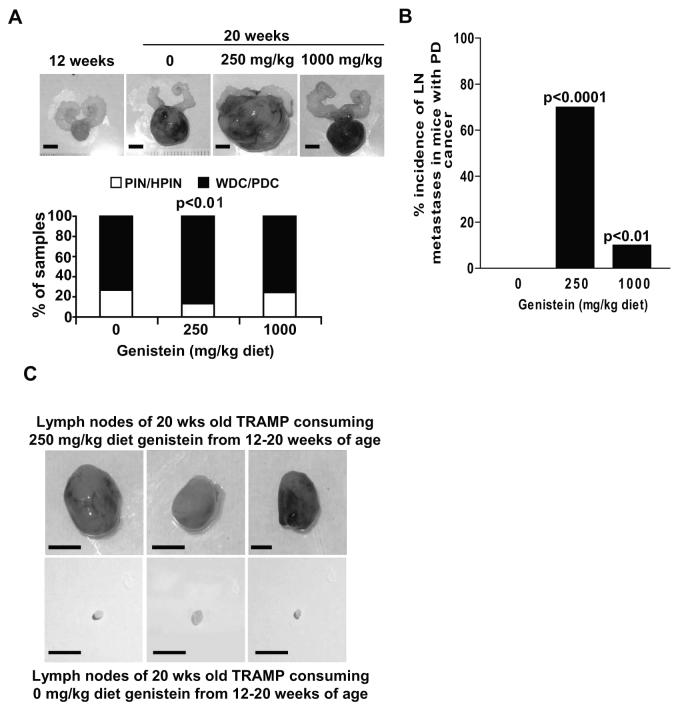
We have previously shown that genistein (250mg/kg diet), when consumed by TRAMP-FVB mice with PIN, from 12-20 weeks of age, results in growth stimulation as evidenced by increased prostate weights, compared to control, independently of SV40-Tag modulation, while resulting in serum genistein concentrations of 429.732 ± 83.709 nmol/L (14 and Figure 1A upper and Supp. Figs.1A and 1B). This aggressive phenotype is supported by a 16% increase in prostates with well- and poorly-differentiated cancer in the 250 mg/kg diet group as compared to 0 and 1000 mg/kg groups, and a 2-fold reduction in prostate numbers at PIN stage (p<0.01) with a higher incidence of expression of the neuroendocrine marker synaptophysin (Figure 1A, lower and data not shown); suggesting an accelerated cancer progression in the 250 mg/kg group. Carcass examination revealed that the 250 mg/kg dose resulted in a 70% incidence of pelvis lymph node (LN) metastasis in TRAMP-FVB mice with poorly-differentiated cancer as opposed to 0 (no enlarged lymph nodes) and 10% of TRAMP-FVB mice in the 0 and 1000 mg/kg diet groups (Figure 1B); representative photographs of 3 LNs from 3 TRAMP-FVB mice consuming 250 mg/kg diet are shown alongside 3 LNs from control TRAMP-FVB mice for comparison (Figure 1C). LN sections from the 250 mg/kg diet group revealed consistent SV40-Tag expression demonstrating that metastasizing prostatic cells underlie LN enlargement (Supp. Fig.1C).
Figure 1. Genistein induces the metastasis of prostate cancer cells to pelvic lymph nodes.
A) Photographs (upper) and histology (lower) of prostates from TRAMP-FVB mice (n=15/group) fed a diet containing 0, 250 and 1000 mg genistein/kg AIN-76A from 12–20 weeks of age, with a photograph representing beginning of treatment (12 weeks). (PIN: prostatic intraepithelial neoplasia, HPIN: high PIN, WDC: well-differentiated cancer, PDC: poorly-differentiated cancer). B) Pelvic lymph node incidence in TRAMP-FVB mice consuming 0, 250 and 1000 mg genistein/kg diet from 12-20 weeks of age, with a PDC histopathology (C) Photographs of pelvic lymph nodes removed from 20 weeks-old TRAMP-FVB mice fed control (lower) and 250 mg genistein per kilogram diet (upper) from 12 weeks of age, scale bar =1cm
Dietary genistein differentially regulates osteopontin expression depending on exposure time in TRAMP-FVB mice
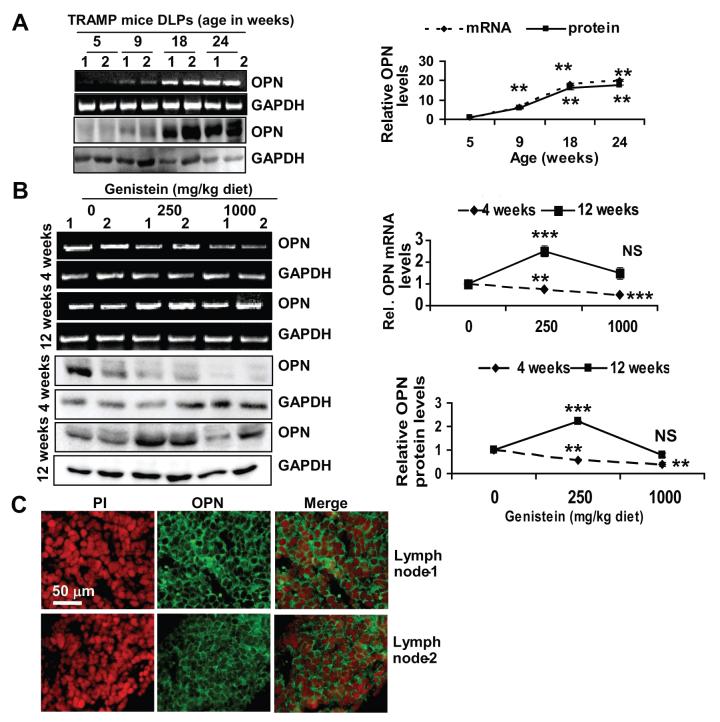
With this work in progress, Mentor-Marcel and colleagues reported OPN reduction as a possible mechanism by which genistein reduces CaP metastasis and increases TRAMP survival (18). To determine its possible involvement in genistein-induced metastasis, we examined OPN expression in CaP progression of TRAMP-FVB mice on a regular diet at 5, 9, 18 and 24-weeks of age. OPN levels were barely detectable at 5 weeks at mRNA and protein levels. By 9 weeks, levels increased by 2-fold and continued to increase up to 10-fold by 24-weeks (Figure 2A). We then examined OPN levels in the prostates of TRAMP-FVB mice consuming genistein at 4 or 12-weeks of age. Mice consuming 250 and 1000 mg/kg diets from 4-20 weeks of age (prevention regimen) displayed a dose-dependent reduction in OPN mRNA and protein expression, (∼2-fold in the 1000 mg/kg group) (Figure 2B), corroborating above-mentioned study (18). Alternatively, prostates of mice consuming 250 and 1000 mg/kg from 12-20 weeks of age (intervention regimen), displayed an increase (∼2.5 fold) in OPN (mRNA and protein levels) in the 250 mg/kg group, whereas in 1000 mg/kg group OPN levels were not significantly altered, statistically, with only a single mouse showing elevated OPN, comparable to 250 mg/kg group levels and coinciding with LN metastasis (Figure 2B and data not shown). LNs derived from TRAMP-FVB mice consuming 250 mg/kg genistein from 12-20 weeks of age expressed OPN (Figure 2C), as opposed to lymphatic vessels from control TRAMP-FVB mice that didn’t (Supp. Fig.2).
Figure 2. Age-dependent expression of OPN and its modulation by genistein (prevention vs. intervention regimens).
A) RT-PCR and Western blot analysis of OPN levels in two representative samples/age group (1 and 2) of TRAMP-FVB mice on a regular diet. B) RT-PCR (upper) and Western blot analysis (lower) showing OPN expression in prostates of TRAMP-FVB mice consuming dietary genistein from 4 or 12 weeks until 20 weeks of age. Quantifications from 3 independent experiments are shown in respective right panels. NS, ** and ***, indicate p>0.05, p<0.01 and p<0.001, respectively. C) OPN expression in lymph node (1 and 2) sections derived from TRAMP-FVB mice fed genistein (250 mg/kg) from 12-20 weeks of age, by immunofluorescence with Alexa-fluor-tagged antibodies with propidium iodide counterstaining.
Genistein biphasically regulates PC3 cell proliferation and invasion in vitro
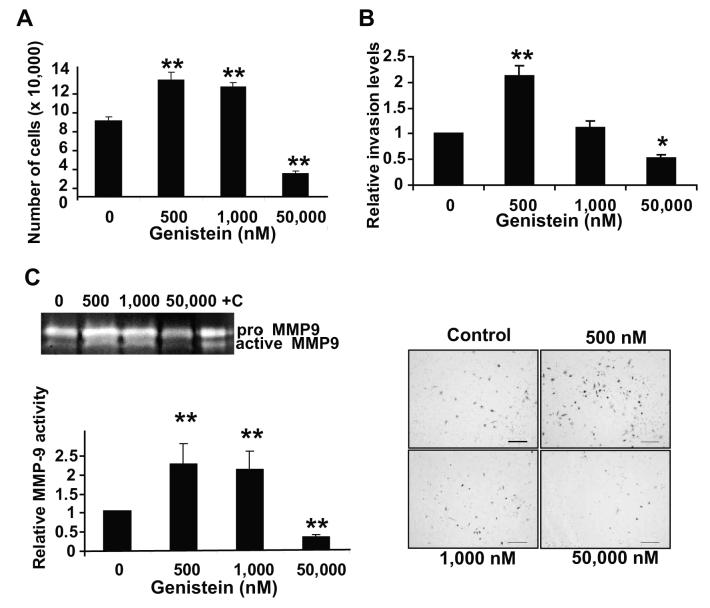
To delineate whether the genistein-induced metastasis in TRAMP-FVB is an inherent characteristic of the model or a consistent biphasic effect of genistein at low versus high concentrations, human PC3 cells were treated with various concentrations of genistein (0, 500, 1,000 nM representing physiologically achievable concentrations in vivo (12) and 50,000 nM (pharmacological dose) for 72hrs and counted. We observed a biphasic effect of genistein on PC3 proliferation; 500-1,000 nM induced a significant 1.5-fold increase in cell number, as opposed to >3.0-fold decrease with 50,000 nM genistein, as compared to vehicle-treated cells (p<0.01) (Figure 3A).
Figure 3. Low doses of genistein increase the proliferation and invasion of PC3 cells.
A) Quantification of genistein-treated PC3 cells for 72hrs. Viable cells (as assessed by trypan blue) were counted and plotted as mean cell number ± SEM, based on 3 independent experiments. ** indicates p<0.01 compared to control. B) Quantification of invaded genistein-treated PC3 cells for 72hrs. Forty thousand cells were placed in invasion chambers with 10% FBS as chemoattractant for 24hrs. All invaded cells-stained with Toluidine blue, from all filters, were viewed and counted at a 2.5X magnification. Graphs are representative of 3 independent experiments; with values normalized to vehicle-treated cells and represented as mean fold ± SEM. * and **, indicate p<0.05 and 0.01, respectively. Representative filters are in lower panel. Scale bar=200 μm. C) Zymogram of pro and active MMP-9 levels in conditioned media from genistein-treated PC3 cells for 72hrs. +C indicates MMP-9 standards (positive control). Quantification of active MMP-9 levels in above-mentioned samples from 3 independent experiments (lower), presented as mean activity ± SEM. **, indicates p<0.01 compared to vehicle-treated cells.
Similarly-treated cells were subjected to the Boyden Chamber with Matrigel assay with 10% FBS media as a chemoattractant for 24hrs, to assess invasion. A 2.0-fold increase in invaded cell number was observed with 500 nM genistein as compared to vehicle (p<0.01); whereas 50,000 nM genistein reduced invasion by 2.0-fold (p<0.05) (Figure 3B). The 1,000 nM dose failed to significantly increase invasion (Figure 3B). Representative photographs of filters with toluidine blue-stained invading cells are shown (Figure 3B, lower). Zymography analysis of conditioned media from these samples revealed that genistein (500 nM and 1,000 nM) induced a ∼2.0-fold increase in active MMP-9 as compared to vehicle, whereas 50,000 nM decreased MMP-9 activity >2-fold (p<0.01) (Figure 3C).
Physiologically achievable doses of genistein increase OPN levels that are necessary for upregulation of proliferation and invasion of PC3 cells
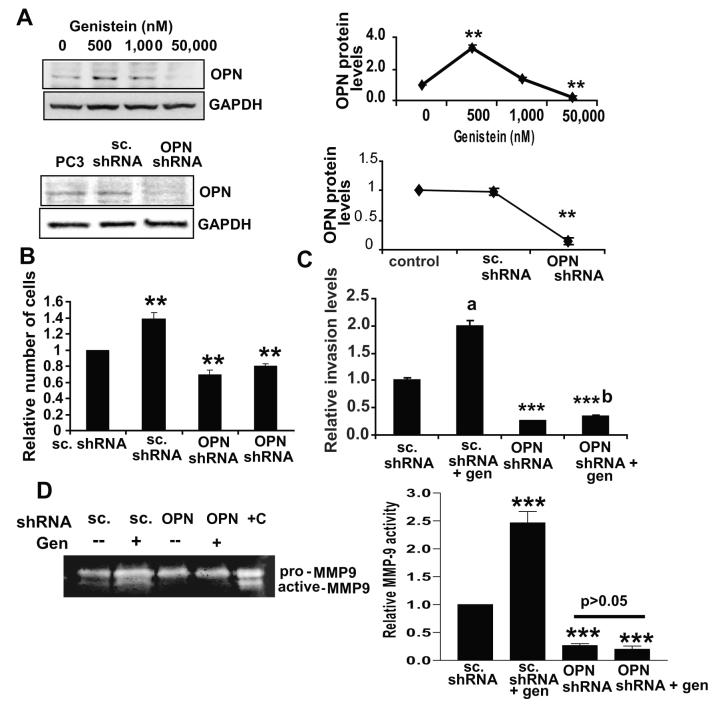
To examine whether PC3 cells recapitulate genistein’s effects on OPN, expression and secreted levels of OPN in PC3 cells treated with 0, 500, 1,000 and 50,000 nM genistein for 72hrs were examined. Genistein (500 nM) resulted in a 2.0-fold increase in OPN protein expression (Figure 4A) as well as secretion as detected by ELISA (Supp. Fig.3). To determine the requirement for OPN in the genistein-induced increase in proliferation and invasion, scrambled and OPN shRNA stable cell lines were established via retroviral infection. The OPN shRNA stable cell line had undetectable OPN levels which were not altered by genistein (Figure 4A, lower and Supp. Fig.4).
Figure 4. Induction of osteopontin by genistein is necessary for the increase in proliferation and invasion of PC3 cells.
A) Western blot of OPN levels in genistein-treated PC3 cells for 72hrs (upper) and in PC3, scrambled shRNA and OPN shRNA stably-transfected PC3 cells (lower) with respective quantification (right). B) Quantification of cell count from 3 independent experiments of scrambled plasmid and OPN shRNA-stably transfected PC3 cells treated ± genistein (500 nM) for 72hrs in triplicates. Values were normalized to vehicle-treated scrambled shRNA PC3 cells. Quantification is plotted as mean fold ± SEM, **, indicates p<0.01 C) Quantification of invaded scrambled shRNA and OPN shRNA-stably transfected PC3 cells treated ± genistein (500 nM) for 72hrs. After treatment, 60,000 cells were placed in Boyden chambers with Matrigel, with 10% FBS as chemoattractant for 24hrs and counted as earlier. Values were normalized to scrambled-transfected, vehicle-treated PC3 cells and represented as mean fold ± SEM from 3 independent experiments. a, indicates significantly different from scrambled-transfected, vehicle-treated PC3 cells with p< 0.01. b, indicates p<0.05 as compared to vehicle-treated OPN-shRNA cells. D) Zymogram of pro and active MMP-9 levels in conditioned media from scrambled and OPN shRNA stably-transfected PC3 cells treated ± 500 nM genistein for 72hrs. +C indicates MMP-9 standards (positive control) with quantification of active MMP-9 levels in above-mentioned samples from 3 independent experiments, presented as mean activity ± SEM. ***, indicates p<0.001 compared to vehicle-treated cells (right).
An equal number of OPN shRNA and scrambled shRNA-expressing cells were seeded and treated ± genistein (500 nM) for 72hrs, living cell number was recorded and 60,000 cells/cell type or treatment were transferred to the Boyden Chamber with Matrigel and allowed to invade for 24hrs. Genistein treatment (500 nM) resulted in a 1.5-fold increase in the number of scrambled shRNA-expressing cells (Figure 4B), recapitulating genistein’s effect on paternal PC3 cells. Basal proliferation of OPN shRNA-expressing cells was reduced as compared to scrambled shRNA cells by ∼35%, and genistein induced a 1.16-fold induction of proliferation as compared to vehicle (Figure 4B), suggesting that OPN induction by genistein contributes to the proliferation increase. Knockdown of OPN also resulted in a 75% reduction of invasion compared to scrambled shRNA cells, and genistein (500 nM) resulted in a ∼1.4-fold increase in invasion compared to vehicle-treated OPN shRNA cells, which is significantly lower than the 2-fold increase observed in the genistein-treated scrambled shRNA cells (Figure 4C). OPN knockdown also resulted in a 70% reduction in MMP-9 activity (p<0.001), which was no longer inducible by genistein (500 nM) (Figure 4D).These results suggest that OPN not only contributes to basal PC3 invasion, but that its induction is one of the mechanisms mediating the genistein-induced invasion.
Induction of OPN, proliferation and invasion by genistein in PC3 cells are estrogen- and PI3K-dependent
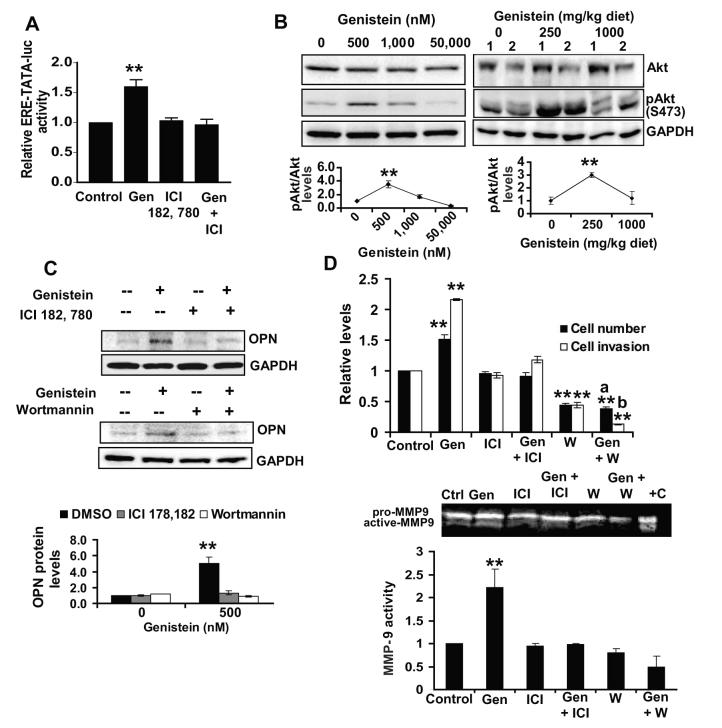
OPN expression and activity are known to be regulated by estrogen and PI3K signaling (19).Genistein’s ability to modulate these pathways was examined. PC3 cells were transfected with ERE-TATA-luciferase and treated ± 500 nM genistein. Genistein induced >1.6-fold increase in luciferase activity compared to vehicle-treated transfected cells (Figure 5A). Induction was abolished by addition of ICI 182, 780, an ER antagonist. Due to low induction levels of ERE-TATA-luciferase by genistein, we examined its modulation by estradiol in PC3 cells. Estradiol (5 pM) induced a 1.5-fold increase in luciferase activity relative to control in PC3 cells (Suppl. Fig.5A) as compared to >8-fold induction in the estrogen-responsive breast cancer cell line, MCF-7 (Suppl. Fig.5B), suggesting that genistein exerts estrogenic activity in PC3 cells similar to estradiol. Genistein (500 nM) induced a significant 2.0-fold increase in pAkt (S473) as opposed to a 2.0-fold reduction by 50,000 nM (Figure 5B, upper left); with no effect on total Akt. Interestingly, prostatic lysates from TRAMP-FVB mice consuming genistein-supplemented diets also demonstrated a biphasic pAkt regulation with a significant 2-fold upregulation in the 250mg/kg diet group and a slight non-significant decrease in the 1000 mg/kg diet compared to control (Figure 5B, upper right). Inhibition of estrogen and PI3K signaling in vitro (Figure 5A and Suppl. Fig.6) abolished OPN induction by genistein in the presence of ICI 182, 780 and Wortmannin, respectively (Figure 5C, upper and lower panels). Although PC3 proliferation was not significantly affected by ICI 182,780 alone; the increase in proliferation by genistein (500 nM) was abolished in its presence, demonstrating that estrogen signaling is necessary for the proliferation increase by genistein (Figure 5D, upper). The significant ability of genistein to increase PC3 invasion was also mitigated in the presence of ICI 182, 780 and was decreased from 2.1-fold to 1.18-fold (Figure 5D, upper). Concerning PI3K signaling, a significant 2.0-fold reduction in PC3 cell number was observed in presence of Wortmannin alone (Figure 5D, upper). Moreover, Wortmannin eliminated the 1.5-fold induction in cell number by genistein, suggesting that PI3K and/or Akt activation is needed for both basal and genistein-induced increase in PC3 proliferation. Wortmannin alone, induced a 2.0-fold decrease in invasion compared to vehicle (Figure 5D, upper). Addition of genistein (500 nM) in the presence of Wortmannin not only failed to increase invasion but further reduced invading cell numbers, suggesting that PI3K activity is necessary for the genistein-induced invasion increase but also that low genistein doses might inhibit cell invasion in the absence of PI3K activity. Zymography revealed that genistein (500 nM) failed to induce MMP-9 activity in the presence of ICI 182, 780 or Wortmannin in PC3 cells (Figure 5D, lower), suggesting MMP-9 as a possible mediator of the estrogen and PI3K-dependent effects of genistein on PC3 cells.
Figure 5. Induction of osteopontin by genistein is dependent on estrogen and PI3K signaling.
A) Forty-eight hrs post-transfection with ERE-TATA-luciferase plasmid, PC3 cells were treated with 500 nM genistein ± 50 nM ICI 182,780 for 5hrs and assayed using the Dual Luciferase Assay kit, with values normalized to Renilla and to values obtained in vehicle-treated transfected cells. Results are representative of 3 independent experiments with **, indicating p<0.01. B) Western blot of Akt and pAkt (S473) in genistein-treated PC3 cells for 72hrs (left) and 2 prostate samples of TRAMP-FVB mice consuming genistein from 12-20 weeks of age (right) with quantification from all samples below. **, indicates p<0.01 compared to control. C) Western blot of OPN levels in PC3 cells treated ± genistein (500 nM) for 72hrs ±50 nM ICI 182,780 (upper) or ± 50 nM Wortmannin (lower) with quantification from 3 independent experiments (lowest panel). Immunoblots were reprobed with GAPDH to ensure for equal loading. D) Cell numbers and invasion levels of PC3 cells treated ± genistein (500 nM) for 72hrs ± 50 nM ICI 182,780 or 50 nM Wortmannin. **, indicates p<0.01 compared to untreated PC3 cells, a, indicates non-significant compared to own control, b, indicates p<0.01 compared to own control. Representative zymogram and quantification (lower) of active MMP-9 levels in media from above-mentioned treatments from 3 independent experiments with **, indicating p<0.01 and +C indicating MMP-9 standards (positive control).
Discussion
In this study, we demonstrated that consumption of low genistein doses (250 mg/kg diet) accelerates CaP progression in TRAMP-FVB mice when consumed after PIN initiation. This phenotype was characterized by Akt activation, OPN upregulation and occurrence of pelvic LN metastases. Furthermore, the previously-established chemopreventive dose (1000 mg/kg diet) (12) lost its efficacy when consumed at 12 weeks of age. To delineate the mechanisms underlying these observations, in vitro studies using PC3 cells treated with physiological versus pharmacological genistein doses recapitulated the proliferation and invasion increases observed in vivo. These increases were dependent on OPN upregulation and required active estrogen and PI3K signaling, involving MMP-9 activation. To the best of our knowledge, this is the first report documenting an increase in metastasis by genistein in the TRAMP-FVB model while pinpointing an estrogen and PI3K-dependent induction of OPN as a necessary mechanism.
Considered potentially chemopreventive, increased usage of soy products prompted the examination of effects of physiologically achievable concentrations of genistein on breast and uterine cancer cell lines and animal models (20). At low doses (<10 μM), genistein stimulates the growth of estrogen-sensitive cell lines (21-23); while decreasing proliferation at higher doses (>10-20 μM) (22-23). This suggests that genistein exerts a biphasic effect on growth and proliferation of cancer cells, similarly to what we have observed in prostate tumors of TRAMP-FVB mice and human PC3 cells; with low nanomolar concentrations inducing growth (14) and metastasis of prostate tumor cells when treatment started after PIN in vivo as well as proliferation and invasion in vitro, and a higher dose (50,000 nM) reducing proliferation and invasion.
Increased tumor growth, metastasis and OPN upregulation were not seen when genistein is consumed (same dose) by 4-weeks old, tumor-free TRAMP-FVB mice or 12-weeks old non-transgenic C57BL/6xFVB mice (12, unpublished data); suggesting a dependence on exposure time or tumor presence and is not an inherent issue in the model used.
OPN expression concomitant with pelvic LN metastases in TRAMP-FVB mice and proliferation/invasion induction in PC3 cells by low genistein doses is significant in the context of CaP metastasis. CaP cells preferentially metastasize to bone, a process facilitated by OPN in various ways (24-25). Here, we present data with reference to LN metastasis as opposed to bone, since the former occurs more frequently in the TRAMP model with 100% incidence in mice over 28 weeks as opposed to 25% incidence of bone metastases in 32-weeks old TRAMP mice (26-27), and LN metastases were the most striking observation upon mice dissection. Interestingly, OPN expression has been correlated with LN metastases in a variety of cancers (28-31). However, the possibility that bone metastasis occurs upon OPN induction by genistein in other models of CaP or humans cannot be eliminated.
We have observed OPN increases as early as 9-weeks of age, coinciding with PIN initiation and a highly proliferative stage (12) in TRAMP-FVB mice, suggesting a role in proliferation. In fact, OPN induction promoted tumor growth, and its knockdown reduced Ras-transformed 3T3 cell growth in soft agar and animal implants (32). This is also confirmed by the decreased proliferation of PC3(OPN-) cells. Importantly, PC3 proliferation and invasion were no longer enhanced by genistein (500 nM) to the same extent in PC3(OPN-) cells, indicating that OPN and/or its downstream effectors mediate at least the stimulatory part of the proposed biphasic genistein effect.
MMP-9 activity was also increased by low genistein doses (500 and 1,000 nM). Interestingly, elevated OPN expression correlate with MMP-9 levels (33) and MMP-9 mediates OPN-induced cell migration and chemoinvasion in B16F10 cells (34). In PC3 cells, OPN overexpression increases MMP-9 activity (17), presenting strong evidence that MMP-9 is a downstream effector of OPN in invasion. Our results are in agreement with these reports in that PC3(OPN-) exhibit reduced MMP-9 activity, and inhibition of genistein-induced OPN induction by ICI 182, 780 or Wortmannin, abrogated MMP-9 activation by genistein, suggesting that genistein (500 nM) may stimulate invasion via upregulation of OPN and subsequent activation of MMP-9.
The mechanism underlying OPN induction by genistein is unknown. However, OPN increase was paralleled by an increase in Akt phosphorylation in vivo and in vitro and was consistently abrogated in vitro by Wortmannin or ICI 182, 780. OPN is a transcriptional target of the ER-related receptor alpha (ERR-α) (35) and is induced by estradiol in vivo (36). Therefore, OPN induction in prostates of TRAMP-FVB mice consuming 250 mg/kg genistein might be of estrogenic nature. OPN is also under the control of the PI3K pathway and is induced by PTEN deletion in colon cancer, whereas Ras-induced expression of OPN is PI3K-dependent (37), which is in agreement with our Wortmannin studies.
Estrogen sensitivity of cell lines/models used seems to determine the growth and metastasis-promoting effects of genistein. Studies reporting proliferation increase by genistein in estrogen-responsive cell lines have failed to observe similar effects in ER-negative cells (38). Consumption of 250 mg/kg diet before orthotopic implantation of PC3-M, a metastatic subline of PC3 lacking ERs (39) decreased lung metastases (40), whereas LN weights increased in genistein-treated mice harboring PC3 xenografts (41). Our study agrees with these observations in that TRAMP-FVB prostates (unpublished data) and PC3 cells express ER α and β (39). The need for estrogen signaling was also highlighted by the administration of ICI 182,780, which reduced the induction of proliferation and invasion by genistein. However, the importance of estrogen signaling on the in vivo effects of genistein remains to be determined.
We and others have pinpointed Akt inhibition by genistein as one mechanism by which genistein exerts its chemopreventive actions (42, 12). Recently, an increase in Akt phosphorylation by 10 μM genistein was reported in porcine aortic endothelial cells in vivo (43). Although not made in a tumor cell setting, this observation is in agreement with our findings regarding Akt activation by genistein in vitro and in vivo.
We have also observed that PI3K inhibition by Wortmannin abrogated the proliferation and invasion increase by genistein, suggesting that genistein acts in a PI3K-dependent manner in PC3 cells. Furthermore, the inhibitory effects on invasion by nanomolar doses of genistein in the absence of PI3K activity, suggest that there might be a balance between inhibitory and activating effects of genistein, with the balance shifted towards inhibition upon PI3K inactivation. Experiments are underway to determine whether PI3K/Akt and estrogen signaling activation by genistein are independent events or an interaction between both pathways.
The inhibition of metastasis by genistein in a chemopreventive setting has been reported extensively (44). However, genistein increased the size of LN metastases but not tumor size when administered to PC-3/nude mouse xenograft model (41); postulating that LN metastases increase is due to genistein’s antiangiogenic effects and subsequent hypoxia. However, in our study, genistein resulted in increased tumor size and metastasis, suggesting a direct effect on tumor cell proliferation when administered to TRAMP-FVB mice with PIN lesions. Furthermore, proliferation and invasion were potentiated in the same cells used for the xenografts in vitro, eliminating the hypoxia theory in our model at least.
Recent findings showed that PD carcinomas in the TRAMP-FVB strain are derived from neuroendocrine (NE) cells (45). In this study, 250 mg/kg diet genistein increased the number of synaptophysin-expressing PD carcinomas, which was also expressed in pelvic LNs (data not shown). One hypothesis is that a low genistein environment (provided by 250 mg/kg diet) targets the synaptophysin-expressing NE population in the prostate resulting in the upregulation of OPN, the positive selection of this population (considered highly-proliferative and a candidate for the transit-amplifying population in the prostate (46)) and emergence of a more aggressive phenotype in this group. More experiments characterizing the NE population in our model, its possible differences at 4 and 12-weeks of age, and its ER and Akt status would prove/disprove this hypothesis and further highlight the potential detrimental effects of low genistein doses.
In this work, we have shown that timing of genistein exposure as well as the dose used had a major impact on CaP outcome and progression in TRAMP-FVB mice. This highlights the importance of examining the effects of physiologically achievable levels of genistein and its deleterious effects on undiagnosed prostate cancer.
Supplementary Material
Acknowledgments
Grant support: This work was supported by NIH grant R01 DK060875 to Partha P. Banerjee.
References
- 1.Makarov DV, Loeb S, Getzenberg RH, Partin AW. Biomarkers for Prostate Cancer. Annu Rev Med. 2008 doi: 10.1146/annurev.med.60.042307.110714. [DOI] [PubMed] [Google Scholar]
- 2.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. Jama. 2005;293:2095–101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 3.Carlinfante G, Vassiliou D, Svensson O, Wendel M, Heinegård D, Andersson G. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin Exp Metastasis. 2003;20:437–44. doi: 10.1023/a:1025419708343. [DOI] [PubMed] [Google Scholar]
- 4.Senger DR, Perruzzi CA, Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989;9:1291–9. [PubMed] [Google Scholar]
- 5.Craig AM, Bowden GT, Chambers AF, et al. Secreted phosphoprotein mRNA is induced during multi-stage carcinogenesis in mouse skin and correlates with the metastatic potential of murine fibroblasts. Int J Cancer. 1990;46:133–7. doi: 10.1002/ijc.2910460124. [DOI] [PubMed] [Google Scholar]
- 6.Vanacker JM, Delmarre C, Guo X, Laudet V. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related alpha. Cell Growth Differ. 1998;9:1007–14. [PubMed] [Google Scholar]
- 7.Thalmann GN, Sikes RA, Devoll RE, et al. Osteopontin: possible role in prostate cancer progression. Clin Cancer Res. 1999;5:2271–7. [PubMed] [Google Scholar]
- 8.Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF. Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer. 2002;95:506–12. doi: 10.1002/cncr.10709. [DOI] [PubMed] [Google Scholar]
- 9.Forootan SS, Foster CS, Aachi VR, et al. Prognostic significance of osteopontin expression in human prostate cancer. Int J Cancer. 2006;118:2255–61. doi: 10.1002/ijc.21619. [DOI] [PubMed] [Google Scholar]
- 10.Ramankulov A, Lein M, Kristiansen G, Loening SA, Jung K. Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate. 2007;67:330–40. doi: 10.1002/pros.20540. [DOI] [PubMed] [Google Scholar]
- 11.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–8S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 12.El Touny LH, Banerjee PP. Akt GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis. 2007;28:1710–7. doi: 10.1093/carcin/bgm103. [DOI] [PubMed] [Google Scholar]
- 13.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–82. [PubMed] [Google Scholar]
- 14.Chau MN, El Touny LH, Jagadeesh S, Banerjee PP. Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3. Carcinogenesis. 2007;28:2282–90. doi: 10.1093/carcin/bgm148. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Touny LH, Banerjee PP. Genistein induces the metastasis suppressor kangai-1 which mediates its anti-invasive effects in TRAMP cancer cells. Biochem Biophys Res Commun. 2007;361:169–75. doi: 10.1016/j.bbrc.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai B, Rogers MJ, Chellaiah MA. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer. 2007;6:18. doi: 10.1186/1476-4598-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentor-Marcel R, Lamartiniere CA, Eltoum IA, Greenberg NM, Elgavish A. Dietary genistein improves survival and reduces expression of osteopontin in the prostate of transgenic mice with prostatic adenocarcinoma (TRAMP) J Nutr. 2005;135:989–95. doi: 10.1093/jn/135.5.989. [DOI] [PubMed] [Google Scholar]
- 19.El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–74. doi: 10.1016/j.cytogfr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Vatanparast H, Chilibeck PD. Does the effect of soy phytoestrogens on bone in postmenopausal women depend on the equol-producing phenotype? Nutr Rev. 2007;65:294–9. doi: 10.1301/nr.2007.jun.294-299. [DOI] [PubMed] [Google Scholar]
- 21.Limer JL, Parkes AT, Speirs V. Differential response to phytoestrogens in endocrine sensitive and resistant breast cancer cells in vitro. Int J Cancer. 2006;119:515–21. doi: 10.1002/ijc.21863. [DOI] [PubMed] [Google Scholar]
- 22.Wietrzyk J, Mazurkiewicz M, Madej J, et al. Genistein alone or combined with cyclophosphamide may stimulate 16/C transplantable mouse mammary cancer growth. Med Sci Monit. 2004;10:BR414–9. [PubMed] [Google Scholar]
- 23.Moore AB, Castro L, Yu L, et al. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22:2623–31. doi: 10.1093/humrep/dem185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper CR, Pienta KJ. Cell adhesion and chemotaxis in prostate cancer metastasis to bone: a minireview. Prostate Cancer Prostatic Dis. 2000;3:6–12. doi: 10.1038/sj.pcan.4500387. [DOI] [PubMed] [Google Scholar]
- 25.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan-Lefko PJ, Chen TM, Ittmann MM, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–37. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuck AB, O’Malley FP, Singhal H, et al. Osteopontin and p53 expression are associated with tumor progression in a case of synchronous, bilateral, invasive mammary carcinomas. Arch Pathol Lab Med. 1997;121:578–84. [PubMed] [Google Scholar]
- 29.Kolb A, Kleeff J, Guweidhi A, et al. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol Ther. 2005;4:740–6. doi: 10.4161/cbt.4.7.1821. [DOI] [PubMed] [Google Scholar]
- 30.Shimada Y, Watanabe G, Kawamura J, et al. Clinical significance of osteopontin in esophageal squamous cell carcinoma: comparison with common tumor markers. Oncology. 2005;68:285–92. doi: 10.1159/000086961. [DOI] [PubMed] [Google Scholar]
- 31.Hu Z, Lin D, Yuan J, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res. 2005;11:4646–52. doi: 10.1158/1078-0432.CCR-04-2013. [DOI] [PubMed] [Google Scholar]
- 32.Behrend EI, Craig AM, Wilson SM, Denhardt DT, Chambers AF. Reduced malignancy of ras-transformed NIH 3T3 cells expressing antisense osteopontin RNA. Cancer Res. 1994;54:832–7. [PubMed] [Google Scholar]
- 33.Frey AB, Wali A, Pass H, Lonardo F. Osteopontin is linked to p65 and MMP-9 expression in pulmonary adenocarcinoma but not in malignant pleural mesothelioma. Histopathology. 2007;50:720–6. doi: 10.1111/j.1365-2559.2007.02675.x. [DOI] [PubMed] [Google Scholar]
- 34.Rangaswami H, Bulbule A, Kundu GC. Nuclear factor inducing kinase: a key regulator in osteopontin- induced MAPK/IkappaB kinase dependent NF-kappaB-mediated promatrix metalloproteinase-9 activation. Glycoconj J. 2006;23:221–32. doi: 10.1007/s10719-006-7927-1. [DOI] [PubMed] [Google Scholar]
- 35.Zirngibl RA, Chan JS, Aubin JE. Estrogen receptor-related receptor alpha (ERRalpha) regulates osteopontin expression through a non-canonical ERRalpha response element in a cell context-dependent manner. J Mol Endocrinol. 2008;40:61–73. doi: 10.1677/JME-07-0114. [DOI] [PubMed] [Google Scholar]
- 36.Craig AM, Denhardt DT. The murine gene encoding secreted phosphoprotein 1 (osteopontin): promoter structure, activity, and induction in vivo by estrogen and progesterone. Gene. 1991;100:163–71. doi: 10.1016/0378-1119(91)90362-f. [DOI] [PubMed] [Google Scholar]
- 37.Shao J, Washington MK, Saxena R, Sheng H. Heterozygous disruption of the PTEN promotes intestinal neoplasia in APCmin/+ mouse: roles of osteopontin. Carcinogenesis. 2007;28:2476–83. doi: 10.1093/carcin/bgm186. [DOI] [PubMed] [Google Scholar]
- 38.Seo HS, DeNardo DG, Jacquot Y, et al. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006;99:121–34. doi: 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- 39.Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–82. [PubMed] [Google Scholar]
- 40.Lakshman M, Xu L, Ananthanarayanan V, et al. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–32. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 41.Hillman GG, Wang Y, Kucuk O, et al. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3:1271–9. [PubMed] [Google Scholar]
- 42.Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8:2369–77. [PubMed] [Google Scholar]
- 43.Grossini E, Molinari C, Mary DA, et al. Intracoronary genistein acutely increases coronary blood flow in anesthetized pigs through beta-adrenergic mediated nitric oxide release and estrogenic receptors. Endocrinology. 2008;149:2678–87. doi: 10.1210/en.2007-1361. [DOI] [PubMed] [Google Scholar]
- 44.Kousidou OC, Mitropoulou TN, Roussidis AE, Kletsas D, Theocharis AD, Karamanos NK. Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int J Oncol. 2005;26:1101–9. doi: 10.3892/ijo.26.4.1101. [DOI] [PubMed] [Google Scholar]
- 45.Chiaverotti T, Couto SS, Donjacour A, et al. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236–46. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huss WJ, Gray DR, Tavakoli K, et al. Origin of androgen-insensitive poorly differentiated tumors in the transgenic adenocarcinoma of mouse prostate model. Neoplasia. 2007;9:938–50. doi: 10.1593/neo.07562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.